Abstract
Background: Danggui Buxue Tang (DBT), a traditional Chinese medicine decoction, has been proven to have satisfactory effects on treating diabetic nephropathy (DN). In this study, we explored the potential underlying mechanism of DBT in DN treatment. Methods: The DBT-containning serum was prepared by intragastric administration with DBT for rats. The levels of fibronectin (FN), laminin (LN) and collagen IV (COL IV) and TGF-β1 protein secreted in cell culture medium were determined by ELISA assay. The mRNA and protein expression of related molecule was measured using qRT-PCR and western blotting. MTT assay was applied to test MCs proliferation. Results: DBT has a negative effect on the high glucose (HG)-induced proliferation and extracellular matrix (ECM) accumulation of mesangial cells (MCs). Further research showed that DBT reduced the acetylation level of histone H3 at the site of PVT1 promoter to promote PVT1 downregulation, which was accompanied by a decrease in TGF-β and c-myc expression. Moreover, PVT1 overexpression significantly enhanced cell viability and promoted the expression levels of TGF-β1 and c-myc. Furthermore, PVT1 overexpression significantly reversed the inhibition of DBT on HG-induced cell viability and ECM accumulation and also lifted the effect of DBT on TGF-β1 and c-myc expression. Conclusion: DBT inhibited TGF-β1 and c-myc expression through downregulating PVT1, and thus attenuated MCs excessive proliferation and ECM accumulation in DN.
Keywords: Danggui buxue tang (DBT), diabetic nephropathy (DN), PVT1, extracellular matrix (ECM) accumulation, proliferation
Introduction
Diabetic nephropathy (DN) is a clinical common disease in microvascular complications, and remains one of the leading causes of death [1]. With the rapid increase in the prevalence of diabetes in the world, DN accounts for 15% of end stage renal diseases (ESRD) [2]. At present, the key of the clinical treatment is to control blood sugar and proteinuria in the earlier period that prevents continuous deteriorative condition. The therapy of traditional Chinese medicine is more easily accepted by the patients, because it takes great effect on effectively reducing blood sugar and proteinuria, improving renal function, with fewer side effects. Pharmacological research on treatment of DN with single herbs, traditional Chinese medicine monomer and decoction has been studied and researched widely in several decades, and made some achievements.
The development of glomerulosclerosis in diabetic is mainly achieved by a network of multiple cytokines. Transforming growth factor-β (TGF-β) expression exists in normal kidney tissue. Local TGF-β overproduction appears to participate in the pathogenesis of DN, as a center factor in the complex cytokine network [3]. TGF-β induced renal tubular epithelial cell hypertrophy and extracellular matrix (ECM) accumulation, so it was considered to be a key cytokine for glomerulosclerosis [4]. Eexisting research has confirmed that TGF-β induced by the excessive proliferation of mesangial cells, promoted ECM protein synthesis, which resulted in the deposition of collagen, fibronectin and laminin in glomerular [5]. TGF-β also promoted the synthesis of plasminogen activator inhibitor (PAI) and tissue inhibitor metalloproteinase-1 (TIMP-1) [6] and inhibited the generation and activation of matrix metalloprotein 9 (MMP-9), and led eventually to ECM excessive deposition [7].
As a protooncogene, c-myc is widely distributed in human normal cells with low-level expression. The renal local overexpression of c-myc promotes cell proliferation in interacting, and further leads to renal hypertrophy and renal fibrosis. C-myc has been shown to be highly expressed in rat kidney with diabetes and was related with the proliferation and ECM hypertrophy of the cells in the kidney [8]. Mosel HL et al. found that TGF-β could directly regulate the expression of c-myc in vitro [9]. Long noncoding RNAs (lncRNAs), length larger than 200 nt, are non-coding RNAs to regulate target gene expression. A number of lncRNAs proved to be involved in the pathogenesis of DN such as PVT1 [10]. The expression of lncRNA PVT1 increased greatly under high glucose conditions, about 5 times of control, which may promote the genesis and development of DN via several mechanisms [11]. Studies have shown that the expression of ECM associated proteins in glomerular was decreased by knockdown of PVT1 [10]. In addition, recent studies identified PVT1 as a key regulator of MYC protein [12].
Danggui Buxue Tang (DBT), a traditional Chinese medicine decoction, is consists of huang-qi and dang-gui in a traditional ratio of 5:1. DBT is traditionally used for tonifying blood and improving haematopoietic function in Chinese medicine [13]. But it was also found that DBT inhibited the proliferation of glomerular mesangial cells and attenuated ECM accumulation which were induced by high glucose [14], indicating its therapeutic effect to DN. However, its specific mechanisms of DBT therapy on DN still need to be further explored.
The aim of the present study was to demonstrate that DBT inhibited the expression of TGF-β1 and c-myc through downregulating lncRNA PVT1, thereby alleviating DN.
Material and methods
Preparation of decoction
Danggui and Huangqi were purchased from The affiliated hospital of Changchun university of Chinese medicine, and identified by Professor Xiuge Wang. The preparation of DBT was conducted as the reported method [15]. In brief, The crude material (Danggui:Huangqi, 1:5) was immersed in water for 1 h and then was extracted thrice with reflux (2 h per time). The extract was got by the filtration, enrichment, and was placed at 4°C overnight. After centrifuged in 3000 r, the impurities were removed from the crude extract. DBT was extracted from the supernatant with macroporous silica gel D101. Alcohol (40%) was used to discarded the residual impurities, and DBT was dissolved in 80% alcohol. After freeze-drying, the yield of DBT was approximately 28.0% (w/w). It has been demonstrated that the main active constituents of DBT were ferulic acid, astragaloside IV and polysaccharides [16], and the contents of them were detected in our study, 32 Ug/g, 265.0 ìg/g, 56.7 mg/g, respectively.
Preparation of serum containing DBT
All animal experiments were performed in accordance with the Guidelines of the Care and Use of Laboratory Animals of The affiliated hospital of Changchun university of Chinese medicine. The experiments were approved by the Ethics Committees at The affiliated hospital of Changchun university of Chinese medicine. Thirty male Sprague Dawley rats (weighing 180-200 g) were purchased from the animal center of The affiliated hospital of Changchun university of Chinese medicine and were feed routinely. The rats were randomly divided into control (n=10) and DBT group (n=20). The DBT group was administered DBT (3.6 g/kg.d) for 7 days, and the control was given distilled water of the same dose. All rats were anesthetized with ketamine/xylazine (50/6 mg/kg, i.p) after 2 h since last intragastric administration, and then blood samples were obtained from abdominal aorta. Serum specimens centrifuged from blood samples, and then devitalized by water bath at 56°C for 30 min, filter sterilization with 0.22 μm filter, stored at -80°C.
Cell culture and treatment
HBZY-1, rat glomerular mesangial cell line, was provided by the Cell Center of Wuhan University. Cells were cultured in DMEM (Hyclone) supplemented with 10% fetal bovine serum (FBS, Gibco), 100 μg/ml streptomycin and 100 U/ml penicillin (HyClone) with 5% CO2 at 37°C. The cells with good growth condition were divided into 5 groups. Cells in experimental group were incubated with high glucose (30 mM) and different concentrations of DBT-containning serum (0, 5%, 15%, 20%) for 0, 24, 48, 72 h. In control group, cells were cultured in conventional medium supplemented with 20% FBS.
MTT assay
MCs were cultured at 104 cells/well in 96-well plates for 24 h, and then the cells were treated with high glucose and different concentrations of DBT-containning serum (0, 5%, 15%, 20%) for 0, 24, 48, 72 h. MTT colorimetric assay was used to examine cell proliferation. MTT solution (5 mg/ml) was prepared with PBS and added into cell medium. After 4 h incubation, the medium was discarded, and then 100 μl of DMSO were added to dissolve the formazan crystals. Absorbance of the solution was detected at 590 nm with a microplate reader (BioRad).
Detection of LN, FN and Col IV
The levels of LN, FN, Col IV (Mercodia) and TGF-β1 (Boster) in cell culture supernatants were detected by commercial ELISA kits according to manufacturer’s instructions.
Quantitative real-time PCR
Total RNA of cells was extracted using TRIzol (Invitrogen) according to the manufacturer’s instructions. The concentration of RNA was determined by NanoDrop 2000 (Thermo Fisher Scientific) at 260 nm. Total RNA was used for reverse transcription reaction by a cDNA reverse transcription kit (Thermo Fisher Scientific). Quantitative RT-PCR was performed using PrimeScript RT Master Mix (Takara) on an ABI7700 Real Time PCR system (Applied Biosystems). All reactions were conducted in triplicate and normalized to GAPDH expression. Data were calculated using 2-ΔΔCT. The primers used in this study were designed and provided by Invitrogen.
Western blot analysis
After treatment, cells were incubated with RIPA buffer (Thermo Fisher Scientific) to obtain total protein. The concentrations of samples were determined by the BCA Protein kit (Jiancheng biotech) according to the manufacturer’s instructions. Total protein (25 μg per sample) was separated by SDS-PAGE, and target proteins were transferred to PVDF membranes with gel transfer device (Bio-Rad). PVDF membranes were immersed in 5% nonfat dry milk for 1 h and incubated with primary antibodies, anti-TGF-β1 antibody (1:1000, Abcam), anti-c-myc antibody (1:10000, Abcam), anti-AcH3 antibody (1:1000, Santa cruz), anti-GAPDH antibody (1:2500, Abcam), anti-β-Actin antibody (1:1000, Abcam) overnight at 4°C. After washing, PVDF membranes were incubated with HRP-conjugated secondary antibodies for 1 h. Target proteins were observed by enhanced chemiluminescent (ECL) kit (Thermo Fisher Scientific) and recorded by X-ray film.
Detection of the acetylation level of histone H3
The ChIP analysis was conducted with the Acetyl-Histone H3 Immunoprecipitation Assay Kit (Millipore) according to the manufacturer’s instructions. Immunoprecipitated protein was used for western blotting, and the product from Input was used as the internal control. The result was expressed as percent of the internal control. Independent experiments were carried out for four times.
Statistical analysis
All data were obtained from 3 independent experiments and expressed as mean ± standard deviation (SD). The statistical analyses and graphing were performed using SPSS17.0 software system. The resulting data using Student’s t-test to test for differences. P < 0.05 was considered to be statistically significant.
Results
DBT inhibited the high glucose induced-MCs proliferation and ECM accumulation
MCs were cultured with 5.6 mM glucose (NG) or 30 mM glucose (HG) or 30 mM glucose with three concentrations of rats serum containing DBT (5%, 10%, 20%), respectively. MCs proliferation was determined with MTT assay at 0, 24, 48, 72 hours after culture, and the inhibition rate of proliferation was calculated. As compared with NG, MCs were observed to rapidly proliferate under HG condition; however, DBT significantly reduced the HG-induced proliferation, and the inhibitory rate of DBT on MCs proliferation increased as DBT concentration increasing (Figure 1A, 1B). It was also found that HG treatment dramatically promoted LN, FN and Col IV secretion of MCs, but DBT could inhibit ECM accumulation induced by HG in a dose-dependent manner (Figure 1C).
Figure 1.
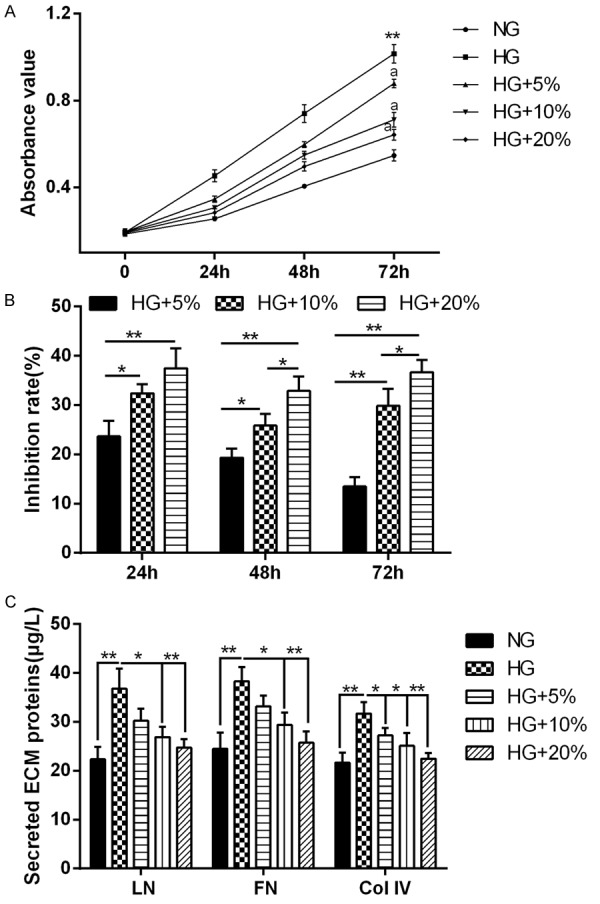
DBT inhibited the HG-induced proliferation and ECM accumulation in MCs. MCs were cultured with 5.6 mM glucose (NG) or 30 mM glucose (HG) or 30 mM glucose with three concentrations of rats serum containing DBT (5%, 10%, 20%), respectively. A: MCs proliferation was determined with MTT assay at 0, 24, 48, 72 hours after culture. DBT obviously suppressed the HG-induced proliferation of MCs. **P < 0.01 vs. NG, aP < 0.01 vs. HG. B: The inhibition rate of proliferation was also calculated. *P < 0.05, **P < 0.01 comparison between two groups. C: LN, FN and Col IV levels in the culture medium of MCs were assessed by ELISA after culture for 72 hours. HG dramatically increased LN, FN and Col IV secretion of MCs, but DBT could inhibit this increase in a dose-dependent manner. *P < 0.05, **P < 0.01 comparison between two groups.
DBT inhibited HG-induced PVT1, TGF-β1, c-myc expression in MCs
To gain further insight into the mechanism of the DBT-induced inhibition on MCs proliferation and ECM accumulation, we investigated the effects of DBT on ECM accumulation and proliferation associated molecules. The results showed that PVT1 (Figure 2A) and TGF-β1 (Figure 2B) mRNA expression, the secreting level of TGF-β1 (Figure 2C), relative protein expression of c-myc (Figure 2D) and TGF-β1, c-myc protein expression (Figure 2E) were increased dramatically in MCs stimulated by HG. However, DBT inhibited the increased expression of ECM accumulation and proliferation associated molecules (Figure 2B-E) in MCs treated with HG in a concentration-dependent fashion as well as PVT1 expression (Figure 2A).
Figure 2.
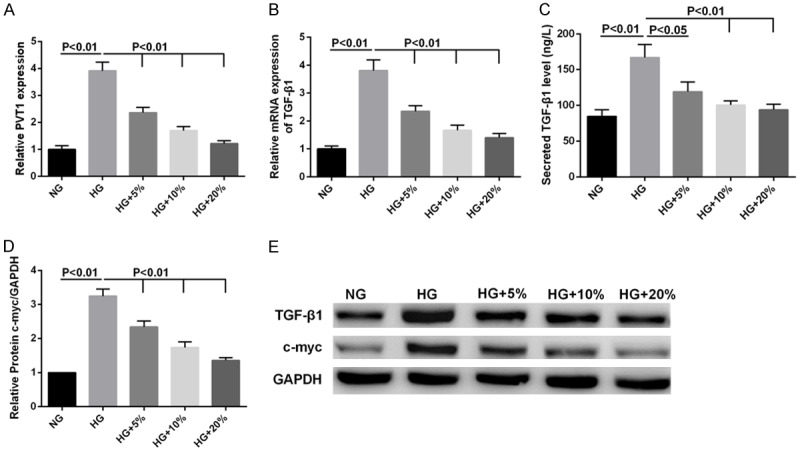
Effects of DBT on the HG-induced PVT1, TGF-β1, c-myc expression in MCs. MCs were cultured with 5.6 mM glucose (NG) or 30 mM glucose (HG) or 30 mM glucose with three concentrations of rats serum containing DBT (5%, 10%, 15%), respectively. A, B: The mRNA levels of PVT1 and TGF-β1 as determined by qRT-PCR were dramatically increased by HG, but DBT inhibited PVT1 and TGF-β1 expression. C: The content of TGF-β1 in the culture medium of MCs was detected by ELISA. D: The relative protein expression of c-myc was calculated using GAPDH as internal control. E: Western blot was carried out to analyze the expression of TGF-β1 and c-myc at protein level.
DBT inhibited the acetylation level of histone H3 at the promoter of PVT1
We aimed to explore the regulation mechanism of DBT on PVT1. First, it was observed that with increasing TSA concentration, PVT1 expression significantly increased (Figure 3A) as well as the expression of acetyl-histone H3 protein in MCs (Figure 3B). Meanwhile, the acetylation level of PVT1 promoter also was enhanced by TSA (Figure 3C), suggesting that PVT1 expression was positively regulated by the acetylation level of histone H3. However, the acetylation level of PVT1 promoter was significantly reduced by DBT (Figure 3D).
Figure 3.
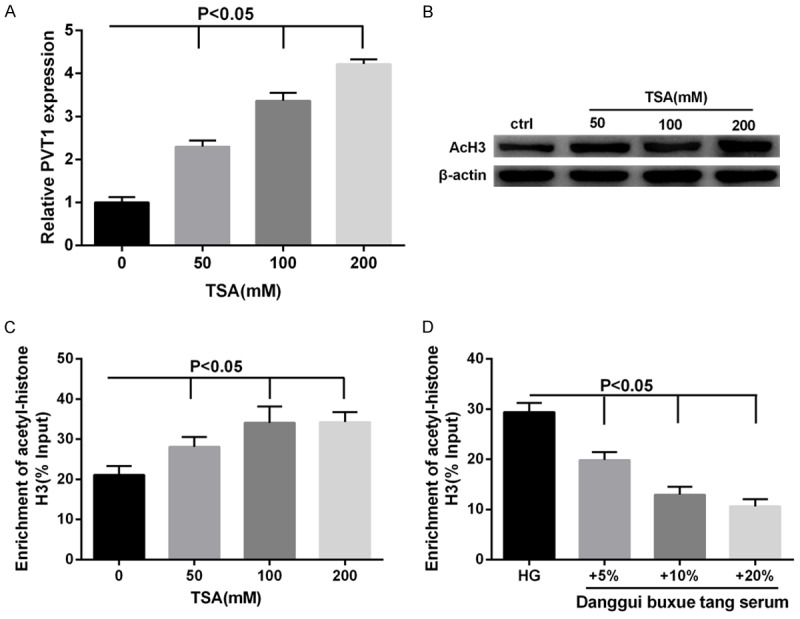
Effects of DBT on the acetylation level of histone H3 at the site of PVT1 promoter in MCs. Cells were treated with TSA at the indicated concentrations (0, 50, 100, 200 mM). A: TSA significantly increased the expression PVT1 mRNA in MCs. B: The expression of acetyl-histone H3 protein was increased with increasing TSA concentration in MCs. C: The level of histone acetylation at the site of PVT1 promoter was elevated by TSA. D: Cells were cultured with 30 mM glucose (HG) or 30 mM glucose with three concentrations of rats serum containing DBT (5%, 10%, 20%), respectively. The level of histone acetylation at the site of PVT1 promoter was suppressed by DBT.
PVT1 regulated TGF-β1 and c-myc expression
To investigate the role of PVT1 in MCs proliferation and ECM accumulation, PVT1 expression was modified by si-PVT1 or pcDNA-PVT1. PVT1 knockdown decreased MCs viability significantly (Figure 4A) and suppressed TGF-β1 mRNA and protein expression (Figure 4B, 4D) and c-myc protein expression (Figure 4C, 4D); contrarily, the over-expressed PVT1 enhanced the cell viability (Figure 4A) and promoted TGF-β1 and c-myc expression (Figure 4B-D).
Figure 4.
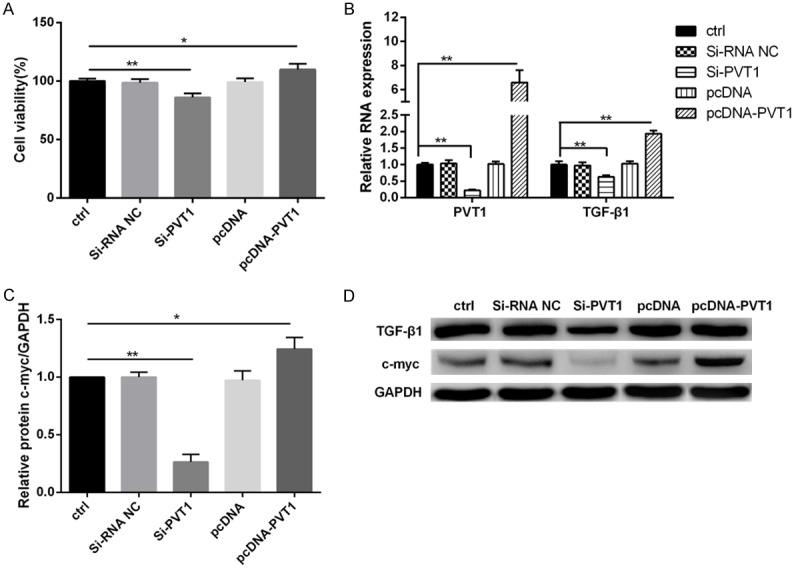
PVT1 positively regulated TGF-β1 and c-myc expression. MCs were transfected with si-PVT1 or pcDNA-PVT1 or their negative controls. A: PVT1 could increase cell viability of MCs. B-D: Moreover, PVT1 overexpression significantly promoted the expression of TGF-β1 and c-myc both at mRNA and protein level in MCs. ctrl, control. *P < 0.05, **P < 0.01 vs. ctrl.
DBT inhibited MCs proliferation and ECM accumulation through regulating PVT1
We have discovered the effects of DBT on MCs, and confirmed that PVT1 was involved in MCs proliferation and ECM accumulation. We also analyzed the role of PVT1 in the mechanism of DBT by using pcDNA-PVT1. MCs were transfected with pcDNA-PVT1 or pcDNA and cultured in HG or HG with 20% rat serum containing DBT. We found that DBT could effectively inhibit MCs viability (Figure 5A) and ECM accumulation, as shown by the decrease in expression levels of LN, FN, Col IV (Figure 5B), TGF-β1 secretion (Figure 5E), PVT1 (Figure 5C), TGF-β1 (Figure 5D, 5G) and c-myc (Figure 5F, 5G) expression. Whereas, PVT1 overexpression significantly reversed the effects of DBT on MCs viability and ECM accumulation under HG condition (Figure 5A-G).
Figure 5.
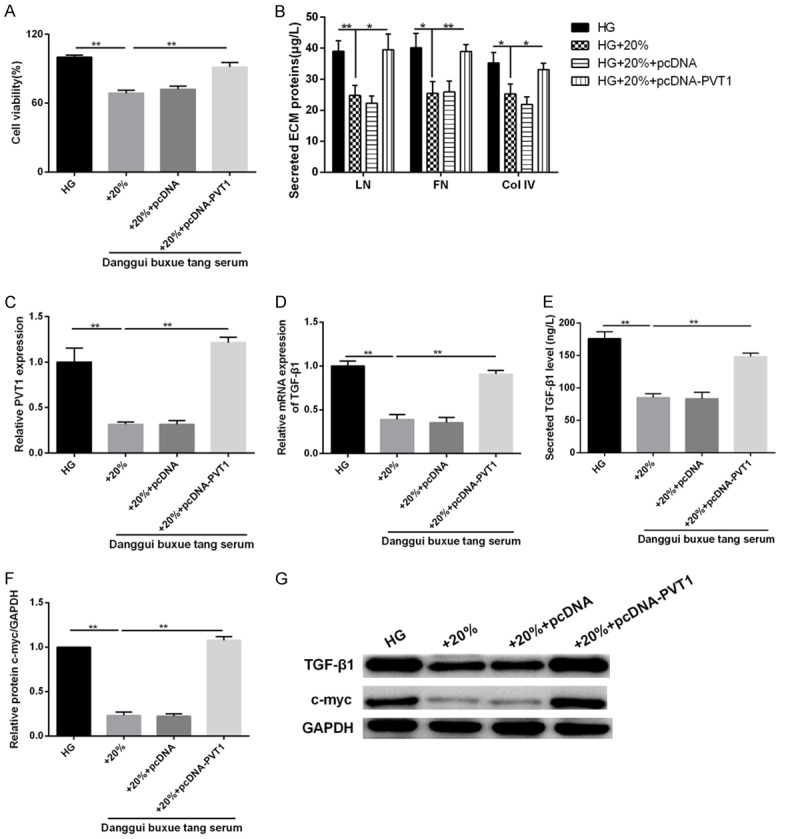
Effects of PVT1 overexpression on high glucose-induced MCs proliferation and ECM accumulation. MCs were transfected with pcDNA-PVT1 or pcDNA and cultured in 30 mM glucose (HG) or 30 mM glucose with 20% rat serum containing DBT. (A) The cell viability was assessed using MTT assay. (B) The secretion levels of LN, FN, and Col IV were determined using the commercial ELISA kits. (C) qRT-PCR analysis of PVT1 and (D) TGF-β1 expression in MCs. (E) The content of TGF-β1 in the culture medium of MCs was detected by ELISA assay. (F) The relative protein expression of c-myc and (G) TGF-β1, c-myc protein expression were examined using western blot analysis. *P < 0.05, **P < 0.01 vs. HG; *P < 0.05, **P < 0.01 vs. HG+20%.
Discussion
Based on the mechanism analyses, it was found that Danggui Buxue Tang (DBT) could ameliorate diabetic renal failure, slowing the progression of diabetic nephropathy (DN) [17], indicating that it can be used as an adjuvant therapy for the control of diabetes and symptoms. Our study discovered that DBT has a negative effect on the HG-induced proliferation and extracellular matrix (ECM) accumulation of mesangial cells (MCs). Further research showed that DBT reduced the acetylation level of histone H3 of PVT1 promoter to result in PVT1 downregulation, which was accompanied by the decrease of TGF-β and c-myc expression. Finally, the result demonstrated that DBT inhibited TGF-β1 and c-myc expression through downregulating PVT1, and thus attenuated MCs excessive proliferation and ECM accumulation in DN.
The major pathological changes of DN are glomerular basement membrane, ECM hyperplasy and nodular or diffuse glomerulosclerosis. The ECM imbalance of anabolic and catabolic changes and the subsequent excessive deposition is the direct cause of glomerulosclerosis [18]. ECM accumulation is the primary pathological basis of glomerular fibrosis, and is also the key step leading to the end stage renal diseases (ESRD). However, the excessive proliferation of MCs is thought to be one of the main feature of DN, and is also ECM accumulation basis. In our study, high glucose (HG) significantly promoted MCs proliferation and induced the predominant components of ECM, including fibronectin (FN), laminin (LN) and collagen IV (COL IV); while DBT attenuated the HG-induced cell proliferation and ECM accumulation in a dose-dependent manner, which is consistent with previous study [14]. Moreover, DBT also downregulated the increase of PVT1, TGF-β1 and c-myc expression induced by HG in MCs. Both the renal local overexpression of c-myc and TGF-β1 can promote cell proliferation, resulting in ECM accumulation. Additionally, recent studies indicated that the decrease in degradation ability of ECM could be more important during the development of DN, and gradually become a new hot spot. The matrix metalloproteinases (MMPs), a major degradative enzyme system for the regulation of ECM degradation in renal cells, could specifically degrade almost all of the ECM component except polysaccharides [19]. TGF-β1 can promote the synthesis of plasminogen activator inhibitor (PAI) and inhibited the generation and activation of matrix metalloprotein 9 (MMP-9), and led eventually to ECM excessive deposition [7]. Therefore, we hypothesized that DBT suppressed the expression of TGF-β1 and c-myc via regulating PVT1, and it is our next research focus.
Firstly, we confirmed that DBT reduced the acetylation level of histone H3 of PVT1 promoter to result in PVT1 downregulation in MCs. Studies have shown that a variety of histone modifications, including acetylation, phosphorylation and methylation, could induce or inhibit gene expression [20]. The epigenetic regulation was confirmed to be involved in the pathogenesis of many diseases [21]. Our results also suggested that DBT regulated PVT1 by the acetylation level of histone H3 of PVT1 promoter. To evaluate the effect of PVT1 on MCs, the expression level of PVT1 was modified by si-PVT1 or pcDNA-PVT1. The results indicated that PVT1 overexpression significantly enhanced cell viability. Furthermore, the expression levels of TGF-β1 and c-myc were increased with the increase of PVT1. Hence, PVT1 served as a regulatory factor for TGF-β1 and c-myc in DN, but the concrete regulation mechanism needs to be studied further. In the final section, we further investigated the role of PVT1 in DBT improving DN and revealed that PVT1 overexpression significantly reversed the inhibition of DBT on the HG-induced MCs viability and ECM accumulation. Meanwhile, PVT1 overexpression also lifted the effect of DBT on TGF-β1 and c-myc expression.
In summary, our results confirmed that DBT inhibited TGF-β1 and c-myc expression through downregulating PVT1, and thus attenuated MCs proliferation and ECM accumulation. This study provides the theoretical basis for DBT used to treat DN in clinic, which has certain practical instructive significance.
Disclosure of conflict of interest
None.
References
- 1.Kato M, Natarajan R. Diabetic nephropathy--emerging epigenetic mechanisms. Nat Rev Nephrol. 2014;10:517–530. doi: 10.1038/nrneph.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mauer M, Drummond K. The early natural history of nephropathy in type 1 diabetes: I. Study design and baseline characteristics of the study participants. Diabetes. 2002;51:1580–1587. doi: 10.2337/diabetes.51.5.1572. [DOI] [PubMed] [Google Scholar]
- 3.Lu A, Miao M, Schoeb TR, Agarwal A, Murphy-Ullrich JE. Blockade of TSP1-dependent TGF-β activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am J Pathol. 2011;178:2573–2586. doi: 10.1016/j.ajpath.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Jim B, Ziyadeh FN. Diabetic nephropathy and transforming growth factor-beta: transforming our view of glomerulosclerosis and fibrosis build-up. Semin Nephrol. 2003;23:532–543. doi: 10.1053/s0270-9295(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 5.Xing M, Luo W, Sun J, Yang N, Zhang LW, Zhao Z, Zhang Z, Wu H. Usp2-69 overexpression slows down the progression of rat anti-Thy1.1 nephritis. Exp Mol Pathol. 2016;101:249–258. doi: 10.1016/j.yexmp.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Baghy K, Dezso K, László V, Fullár A, Péterfia B, Paku S, Nagy P, Schaff Z, Iozzo RV, Kovalszky I. Ablation of the decorin gene enhances experimental hepatic fibrosis and impairs hepatic healing in mice. Lab Invest. 2011;91:439–451. doi: 10.1038/labinvest.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall MC, Young DA, Waters JG, Rowan AD, Chantry A, Edwards DR, Clark IM. The comparative role of AP1 and Smad factors in the regulation of Timp-1 and MMP-1 gene expression by TGFβ1. J Biol Chem. 2003;278:10304–10313. doi: 10.1074/jbc.M212334200. [DOI] [PubMed] [Google Scholar]
- 8.Okada M, Takemura T, Yanagida H, Yoshioka K. Response of mesangial cells to low-density lipoprotein and angiotensin II in diabetic (OLETF) rats. Kidney Int. 2002;61:113–124. doi: 10.1046/j.1523-1755.2002.00107.x. [DOI] [PubMed] [Google Scholar]
- 9.Pietenpol JA, Stein RW, Moran E, Yaciuk P, Schlegel R, Lyons RM, Pittelkow MR, Münger K, Howley PM, Moses HL. TGF-beta 1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell. 1990;61:777–785. doi: 10.1016/0092-8674(90)90188-k. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez ML, DiStefano JK. Functional characterization of the plasmacytoma variant translocation 1 gene (PVT1) in diabetic nephropathy. PLoS One. 2011;6:e18671. doi: 10.1371/journal.pone.0018671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez ML, Khosroheidari M, Eddy E, Kiefer J. Role of microRNA 1207-5P and its host gene, the long non-coding RNA Pvt1, as mediators of extracellular matrix accumulation in the kidney: implications for diabetic nephropathy. Plos One. 2013;8:1175–1177. doi: 10.1371/journal.pone.0077468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tseng YY, Moriarity BS, Gong W, Akiyama R, Tiwari A, Kawakami H, Ronning P, Reuland B, Guenther K, Beadnell TC. PVT1 dependence in cancer with MYC copy-number increase. Nature. 2014;512:82–86. doi: 10.1038/nature13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang GC, Chen SY, Tsai PW, Ganzon JG, Lee CJ, Shiah HS, Wang CC. Effects of Dang-Gui-Bu-Xue-Tang, an herbal decoction, on iron uptake in iron-deficient anemia. Drug Des Devel Ther. 2016;10:949–957. doi: 10.2147/DDDT.S94309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ke H, Zhang Y, Zhou B, Zhen R. Effects of Danggui Buxue Tang, a traditional Chinese herbal decoction, on high glucose-induced proliferation and expression of extracellular matrix proteins in glomerular mesangial cells. Nat Prod Res. 2012;26:1022–1026. doi: 10.1080/14786419.2010.546796. [DOI] [PubMed] [Google Scholar]
- 15.Zhao P, Zhou WC, Li DL, Mo XT, Xu L, Li LC, Cui WH, Gao J. Total glucosides of Danggui buxue tang attenuate BLM-induced pulmonary fibrosis via regulating oxidative stress by inhibiting NOX4. Oxid Med Cell Longev. 2015;2015:645814. doi: 10.1155/2015/645814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong TT, Zhao KJ, Gao QT, Ji ZN, Zhu TT, Li J, Duan R, Cheung AW, Tsim KW. Chemical and biological assessment of a Chinese herbal decoction containing radix astragali and radix angelicae sinensis: determination of drug ratio in having optimized properties. J Agric Food Chem. 2006;54:2767–2774. doi: 10.1021/jf053163l. [DOI] [PubMed] [Google Scholar]
- 17.Ye TS, Zhang YW, Zhang XM. Protective effects of Danggui buxue tang on renal function, renal glomerular mesangium and heparanase expression in rats with streptozotocin-induced diabetes mellitus. Exp Ther Med. 2016;11:2477–2483. doi: 10.3892/etm.2016.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyata T, Inagi R, Nangaku M, Imasawa T, Sato M, Izuhara Y, Suzuki D, Yoshino A, Onogi H, Kimura M, Sugiyama S, Kurokawa K. Overexpression of the serpin megsin induces progressive mesangial cell proliferation and expansion. J Clin Invest. 2002;109:585–593. doi: 10.1172/JCI14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nichik TE, Lin’kova NS, Kraskovskaia NA, Dudkov AV, Khavinson VKh. [Molecular aspects of renal desease] . Usp Fiziol Nauk. 2015;45:49–56. [PubMed] [Google Scholar]
- 20.Grant PA. A tale of histone modifications. Genome Biol. 2001;2:REVIEWS0003. doi: 10.1186/gb-2001-2-4-reviews0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muhonen P, Holthofer H. Epigenetic and microRNA-mediated regulation in diabetes. Nephrol Dial Transplant. 2009;24:1088–1096. doi: 10.1093/ndt/gfn728. [DOI] [PMC free article] [PubMed] [Google Scholar]


