Abstract
Objective: This study aims to observe the expression of YKL-40 in prostate cancer and whether YKL-40 can affect the migration and invasion of tumor cells by regulating epithelial mesenchymal transition. Methods: We collected 14 cases of prostate cancer tissues and adjacent tissues in this study. The expression levels of YKL-40 in the tissues were analyzed by western blotting and immunohistochemical methods. The expression of YKL-40 in human prostate cancer cell line DU145 and PC3 was detected by fluorescence quantitative PCR and western blotting methods. The expression levels of YKL-40 in different cells were up-regulated or down- regulated by lentivirus to observe the changes of cell migration and invasion. The expression levels of EMT related genes were analyzed by RT-PCR and Western blotting methods. Results: The expression level of YKL-40 in prostate cancer tissues was significantly higher than that in adjacent tissues (P<0.01), and it was higher in DU145 cells than that in PC3 cells (P<0.05). The expression level of YKL-40 was positively correlated with cell migration and invasion. YKL-40 can regulate the expression of EMT related genes (Twist, Snail, Slug, N-cadherin, Vimentin and E-cadherin). Conclusions: The expression level of YKL-40 was positively correlated with the migration and invasion of prostate cells, it affects cancer metastasis by regulating EMT.
Keywords: YKL-40, prostate cancer, epithelial mesenchymal transition (EMT), RT-PCR, western blotting, immunohistochemistry
Introduction
Prostate cancer (PCa) is one of the most common malignant tumors in male genitourinary system, its incidence in Europe and the United States is high. PCa is the second malignant tumors of the European men [1]. Invasion and metastasis are the most closely related to the prognosis of patients with prostate cancer. More than 70% of all prostate cancer cases die from bone metastases and other sites each year [2,3], which seriously affect the prognosis and quality of life. At present, surgery or hormone therapy is the most effective treatment. Although endocrine therapy or radiation therapy can prevent and delay the recurrence and progression of prostate cancer, but for advanced and metastatic prostate cancer, the majority of prostate cancer has been converted to hormone refractory prostate cancer after endocrine therapy and the median survival time was only 12 to 18 months [4].
YKL-40 protein is highly expressed in many malignant tumors, its value in the diagnosis of disease, evaluation of disease and prognosis receives more and more attention. Francescone found that YKL-40 can enhance the expression of vascular endothelial growth factor (VEGF) in malignant glioma cell line U87, the median survival time of glioma patients undergoing surgery with YKL-40 low expression and high expression was 14.6 and 5.9 months respectively (P<0.05) [5]. Faibish found that YKL-40 antibody could significantly inhibit tumor growth and angiogenesis in mice, and prolong the survival time of mice [6]. Libreros [7] and Ku [8] found that the expression of YKL-40 in breast cancer cells can promote the synthesis and secretion of MMP-9 by tumor associated macrophages, thereby enhancing the invasiveness of breast cancer. The level of YKL-40 in patients with prostate cancer serum was significantly higher than that in patients with benign prostatic hyperplasia. Johansen found that YKL-40 level is an independent indicator of prognosis in patients with metastatic prostate cancer after endocrine therapy [9]. Özdemir found that YKL-40 may be a predictor of tumor burden and metastasis in prostate cancer [10]. These studies suggested that YKL-40 may play an important role in the progression and metastasis of prostate cancer, but its mechanism is still unclear.
In this study, we compared the expression levels of YKL-40 in prostate cancer and normal prostate tissues, the expression level of YKL-40 and its correlation with metastasis and invasion in prostate cancer cells were analyzed, the regulation of YKL-40 on EMT in prostate cancer cells was observed by up regulation and down regulation of YKL-40 expression to elucidate the role of YKL-40 in the metastasis and invasion of prostate cancer.
Materials and methods
Specimens
A total of 11 surgical resection specimens of patients with prostate cancer were collected from Department of Urology, Shanxi Cancer Hospital, each specimen consisted of cancer tissues and its adjacent tissues. All tissues were divided into two parts, one was preserved in liquid nitrogen for the extraction of total protein for Western blot detection; one was prepared for paraffin sections after being fixed with 4% paraformaldehyde for immunohistochemical detection. The informed consent was obtained from patients and the study was approved by the ethics committee of Shanxi Cancer Hospital.
Cell culture
Human prostate cancer cell line DU145 and PC3 cells were purchased from China infrastructure of cell line resources. The DU145 cells were cultured in RPMI 1640 containing 10% fetal bovine serum (FBS; Gibco, Invitrogen), 100 U/mL penicillin (GIBCO) and 100 U/mL of streptomycin in a humidified atmosphere with 5% CO2 at 37°C. The PC3 cells were cultured F12K medium containing 10% fetal bovine serum (FBS; Gibco, Invitrogen), 100 U/mL penicillin (GIBCO) and 100 U/mL of streptomycin in a humidified atmosphere with 5% CO2 at 37°C.
Immunohistochemical detection
The paraffin embedded prostate tissue was made into 2 μm serial sections. They were de-waxed in xylene for 2 times (5 min/times) and hydrated in gradient dilution of ethanol. Then they were incubated in H2O2 for 20 min in order to block the endogenous peroxidase, microwave repair the antigen for 5 min in citrate buffer solution and repeat it. 10% normal goat serum prepared with 0.01 M PBS buffer was used to seal the non-specific antibody binding site and incubated for 1 h at room temperature. Washed them with distilled water and PBS for 5 min respectively and repeat it. Drop-adding the 1st antibody (YKL-40, 1:400) and incubated at 37°C in water bath for 2 h, then washed them with PBS every time for 2 min, a total of 3 times; Drop-adding the 2nd antibody (Biotin labeled Goat anti mouse IgG, 1:500) and incubated at 37°C in water bath for 30 min, washed them with PBS every time for 2 min, a total of 3 times. Developed with the DAB solution and observe them under microscope, flushed them completely, hematoxylin counterstained them, washed with water, dehydration, transparency, mo-unt and observed under microscope. They were analyzed with Image J software.
Construction of PC3 cell line stably expressing YKL-40
The Lentivirus solution containing a full length of YKL-40 cDNA was purchased from Shanghai Ji Ma Pharmaceutical Technology Co., Ltd., the titer was 5×108 TU/ml. 2×105 PC3 cells were seeded in each well of 24-well plate and incubated at 37°C with 5% CO2 for 24 h. The slow virus solution was diluted with fresh medium for MOI=5, polybrene was added into them and the final concentration was 6 μg/ml, and 2 ml was added to each well of the plate and incubated at 37°C with 5% CO2 for 24 h. The culture media containing lentivirus were replaced with fresh medium. A fresh culture medium containing a final concentration of 4 g/ml puromycin was added into them after infection for 72 h and they were cultured continually. The cells with puromycin resistance (PC3-YKL-40) were screened for subsequent experiments. Lentivirus infected cells without target fragments were used as the control group.
Construction of DU145 cell line stably expressing down regulated YKL-40
Lentivirus solution capable of inhibiting YKL-40 expression was purchased from Shanghai Ji Ma Pharmaceutical Technology Co., Ltd., its titer was 3×108 TU/ml. 2×105 DU145 cells were seeded in each well of 24-well plate and incubated at 37°C with 5% CO2 for 24 h. The lentivirus was diluted with fresh medium for MOI=5, polybrene was added into them and the final concentration was 6 μg/ml and 2 ml was added to each well of the plate and incubated at 37°C with 5% CO2 for 24 h. The culture media containing lentivirus were replaced with fresh medium. A fresh culture medium containing a final concentration of 4 g/ml puromycin was added into them after infection for 72 h and they were cultured continually. The cells with puromycin resistance (DU145-KD) were screened for subsequent experiments. Lentivirus infected cells without target fragments were used as the control group.
Stably down-regulated the expression of YKL-40 in PC3-YKL-40 cells and stably up-regulated the expression of YKL-40 in DU145-KD cells
PC3-YKL-40-KD cell line stably expressing down regulated YKL-40 was screened by infection of PC3-YKL-40 cells with lentivirus which could inhibit the expression of YKL-40. DU145-KD- YKL-40 cell line stably expressing up regulated YKL-40 was screened by infection of DU145-KD cells with lentivirus containing a full length of YKL-40 cDNA. The methods were the same with the above. Lentivirus infected cells without target fragments were used as the control group.
RNA extraction and real-time PCR
Total RNA was extracted from PC3, PC3-YKL-40, DU145 and DU145-KD cells using Trizol plus RNA purification kit (Invitrogen China) according to the manufacturer’s manual. Their concentration and purity were detected with Agilent 2100 Bioanalyzer. 1 μg RNA was subjected to reverse transcription using Transcriptor First Strand cDNA Synthesis Kit (Roche China). Real-time PCR were performed using FastStart Universal SYBR Green Master. The primers used in this study were shown in Table 1. Reaction parameters were 95°C for 45 sec, 95°C for 5 sec and 60°C for 30 sec with 40 cycles. The relative expression of genes was calculated using 2(-ΔΔCT) method.
Table 1.
Primers in this study
| Primer sense | Primer sequence (5’→3’) | Size of PCR products (bp) | |
|---|---|---|---|
| YKL-40 | F | GAAGACTCTCTTGTCTGTCGGA | 108 |
| R | AATGGCGGTACTGACTTGATG | ||
| E-cadherin | F | ATTTTTCCCTCGACACCCGAT | 109 |
| R | TCCCAGGCGTAGACCAAGA | ||
| N-cadherin | F | TTTGATGGAGGTCTCCTAACACC | 120 |
| R | ACGTTTAACACGTTGGAAATGTG | ||
| Vimentin | F | AGTCCACTGAGTACCGGAGAC | 98 |
| R | CATTTCACGCATCTGGCGTTC | ||
| Twist | F | GCCTAGAGTTGCCGACTTATG | 123 |
| R | TGCGTTTCCTGTTAAGGTAGC | ||
| Snail | F | TCGGAAGCCTAACTACAGCGA | 140 |
| R | AGATGAGCATTGGCAGCGAG | ||
| Slug | F | CGAACTGGACACACATACAGTG | 87 |
| R | CTGAGGATCTCTGGTTGTGGT | ||
| GAPDH | F | TGTGGGCATCAATGGATTTGG | 116 |
| R | ACACCATGTATTCCGGGTCAAT |
Cell migration and invasion assays
Cell migration and invasion assays were performed using a Trans-well Chamber (Millipore, Billerica, MA, USA) with 8-μm pore size. Briefly, 1×106 cells were seeded into the upper chamber. After 24 h of incubation, the cells migrating through the pores or invading through the Matrigel were fixed and stained with 0.5% crystal violet. The cells on the lower surface of the chamber were counted in 6 randomly selected fields. The test was repeated three times.
Western blotting analysis
The cells were harvested and lysed with RIPA lysis buffer on ice for 30 min with shaking at 12 000 rpm/min. Total cellular protein was collected and the concentration was determined by BCA Kit. They were analyzed with SDS-PAGE electrophoresis. Then it was electrotransferred to the PVDF membrane. After the transmembrane, PVDF membrane was rinsed with TBS for 10 to 15 min, placed in TBS/T blocking buffer containing 5% (w/v) skimmed milk powder and shook at room temperature for one hour. It was incubated at 4°C overnight after added with appropriate dilution degree of primary antibodies. Then the membrane was washed with TBST for three times (5 minutes each time). The membrane was incubated at 37°C for one hour with HRP labeled secondary antibody (1:10000) diluted with TBST containing 0.05% (w/v) skimmed milk powder. It was developed with ECL for 5 minutes. The protein bands were quantified as a ratio to β-actin using Image J software.
Statistical analysis
All analyses were conducted using SPSS 16.0 software (SPSS Inc., Chicago, USA). The data are expressed as the mean values ± standard deviations. Differences between two groups were assessed using t test. P values <0.05 were considered indicative of a significant difference.
Results
The expression level of YKL-40 in prostate cancer tissues was significantly higher than that in adjacent tissues
Western blotting results showed that the expression level of YKL-40 in prostate cancer tissues was significantly higher than that in adjacent tissues. Immunohistochemical results showed that YKL-40 was expressed in cytoplasm, it also showed that the expression level of YKL-40 in prostate cancer tissues was significantly higher than that in adjacent tissues (Figure 1).
Figure 1.
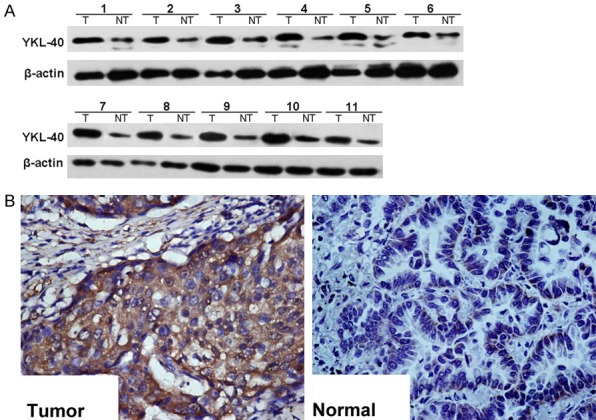
YKL-40 expression in prostate cancer tissues and their adjacent tissues. The expression level of YKL-40 in prostate cancer tissues was significantly higher than that in adjacent tissues. A: Western blotting results; B: Immunohistochemical results.
YKL-40 expression level was correlated with the migration and invasion of prostate cancer cells
RT-PCR and Western blotting results showed that the expression level of YKL-40 in DU145 cells was significantly higher than that of PC3 cells (P<0.01). Trans-well migration and invasion analysis showed that the migration and invasion ability of DU145 cells was significantly higher than that of PC3 cells (Figure 2).
Figure 2.
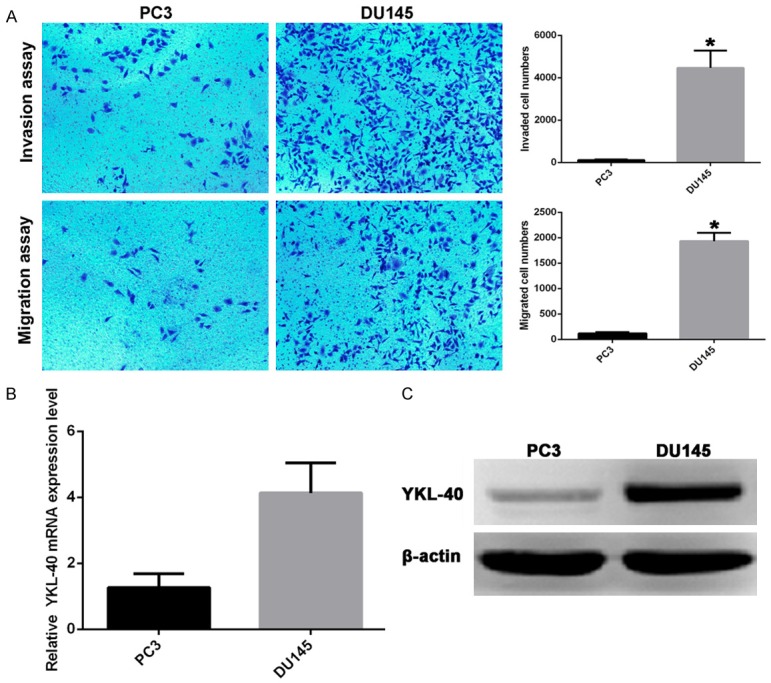
YKL-40 expression level was correlated with the migration and invasion of prostate cancer cells. A: The migration and invasion of DU145 and PC3 cells; B: RT-PCR results; C: Western blotting results; *P<0.01.
Effects of YKL-40 expression level on cell migration and invasion in DU145 and PC3 cells
In order to study the effect of YKL-40 expression on migration and invasion of prostate cancer cells, we constructed stably upregulated YKL-40 expression with PC3-YKL-40 cells and stably downregulated YKL-40 expression with DU145-KD cells. After that, the PC3-YKL-40-KD cells stably expressing down-regulated YKL-40 mediated by lentiviral vector was established in PC3-YKL-40 cells and DU145-KD-YKL-40 cells stably expressing up-regulated YKL-40 mediated by lentiviral vector was established in DU145-KD cells. The expression level of YKL-40 increased 510%, migration ability increased 230% and invasive capacity increased 155% in PC3-YKL-40 cells compared with PC3 cells. When the expression level of YKL-40 in the PC3-YKL-40 cells was down regulated, their migration and invasion ability returned to the original level. The expression level of YKL-40 decreased 19%, migration ability decreased 35% and invasive capacity decreased 22% in DU145-KD cells compared with DU145 cells. When the expression level of YKL-40 in the DU145-KD cells was down regulated, their migration and invasion ability also returned to the same level as that of DU145 cells (Figure 3).
Figure 3.
Effects of YKL-40 expression level on cell migration and invasion. A: PC3 cells; B: DU-145 cells; *P<0.05; **P<0.01.
YKL-40 affects migration and invasion in prostate cancer cells by regulating the expression of EMT related genes
The expression levels of EMT related genes such as Twist, Snail, Slug and Vimentin and adhesion molecules such as E-cadherin and N-cadherin in PC3, PC3-YKL-40, PC3-YKL-40-KD, DU145, DU145-KD and DU145-KD-YKL-40 cells were determined respectively. The results showed that the markers of interstitial cells N-cadherin and Vimentin was upregulated after upregulation of YKL-40 expression in PC3 cells, while the epithelial marker E-cadherin was down regulated, the expression of Twist, Snail and Slug was up-regulated. When the expression of YKL-40 was down regulated, the expression levels of all genes were reversed. In DU145 cells, the markers of interstitial cells N-cadherin and Vimentin was down regulated after down-regulation of YKL-40 expression, the epithelial marker E-cadherin was up regulated, the expression of Twist, Snail and Slug was down regulated. When the expression of YKL-40 was up regulated, the expression levels of all genes were reversed (Figure 4).
Figure 4.

Effects of YKL-40 on the expression of EMT related genes. A: PC3 cells; B: DU-145 cells; *P<0.05; **P<0.01; ***P<0.001.
Discussion
YKL-40, also named human cartilage glycoprotein -39 (HCgp-39), is a kind of secreted protein, it was first found in the culture of human osteosarcoma cell line MG63 in vitro. Human YKL-40 gene is located on q32.1 of chromosome 1, it contains 10 exons and 7948 base pairs. It was named as YKL-40 because the first 3 amino acids in the end of a polypeptide chain are tyrosine, lysine and leucine (the symbol is Y, K and L), and the molecular weight is 40 kd. The biological function of YKL-40 is still not fully clear. YKL-40 was highly expressed in inflammation, angiogenesis and tumor tissues in the development and progression of the musculoskeletal system according to clinical trial [11]. YKL-40 has synergistic effect with insulin-like growth factor, which can activate MAPK and PI3K signaling pathways and ERK1/2 and AKT to promote cell proliferation and fibrosis [12]. Overexpression of YKL-40 in human astrocytes can make the cells acquire the ability to invade like tumor cells, and have radiation and low serum tolerance at the same time, which suggested that YKL-40 may be a protective factor for cell survival [13]. YKL-40 can inhibit the apoptosis signal kinase-1 (SAK-1), phosphorylation of p38 and JNK1/2 signaling pathways and apoptosis induced by TNF-α by phosphorylation of PI3K/AKT signaling pathway [14]. YKL-40 can also promote vascular endothelial cells and smooth muscle cells adhesion, migration and rearrangement in order to form new blood vessels [15,16]. Recent clinical studies have shown that the expression level of YKL-40 is positively correlated with tumor metastasis and patient survival [17]. However, the mechanism of YKL-40 in tumor metastasis is not clear.
In this study, we investigated the relationship between YKL-40 and tumor migration and invasion in human prostate. It was found that the expression of YKL-40 in human prostate cancer tissues was significantly higher than that in adjacent tissues. The migration and invasion of DU145 cells with high expression of YKL-40 was significantly higher than that of PC3 cells with low expression of YKL-40. Down regulation of YKL-40 expression in DU145 cells decreased their migration and invasion ability, while up regulation of YKL-40 expression in PC3 cells enhanced their migration and invasion ability. These results suggested that the expression level of YKL-40 was closely related to the migration and invasion of prostate cancer cells.
Epithelial mesenchymal transition (EMT) is a physiological phenomenon in the development process. In view of the fact that EMT is an effective way for the migration of epithelial cells, more than 90% of the invasive and metastatic pathways of malignant tumors are related to EMT [18]. In vivo and in vitro experiments showed that EMT was the main mode of primary infiltration and secondary metastasis of breast cancer, colon cancer, lung cancer, prostate cancer, liver cancer and pancreatic cancer [19-22]. In patients with primary liver cancer, EMT cells increase tumor cell infiltration [23]. Epithelial cell specific E-cadherin expressed in non invasive epithelial tumor cells. When EMT occurs, the expression level of E-cadherin decreased or disappeared, and the cancer cells became invasive. The decreased expression level of E-cadherin and EMT may be a reversible process caused by tumor microenvironment [24]. In this study, we found that YKL-40 could induce the occurrence of EMT in prostate cancer. The expression level of E-cadherin was significantly lower in the cells with high expression level of YKL-40, while the expression levels of mesenchymal cell markers of N-cadherin and Vimentin decreased. The expression levels of EMT related transcription factors Snail, Slug and Twist in the cells with high expression levels of YKL-40 were higher than those with low expression level of YKL-40. These results indicated that the expression level of YKL-40 was positively correlated with the expression level of EMT related genes, which suggested that YKL-40 can promote the migration and invasion of tumor cells by regulating EMT. Previous studies have shown that YKL-40 is involved in tumor survival, transformation, migration and invasion by regulating the PI3K/AKT/mTOR signaling pathway and Ras/Raf/MEK/ERK signaling pathway [12,14]. It was found that activation of PI3K/AKT signaling pathway is one of the important characteristics of EMT, which was associated with tumor migration and invasion [18].
In summary, YKL-40 is an important gene that affects the metastasis of prostate cancer, it may induce EMT in prostate cancer cells by activating PI3K/AKT signaling pathway and affect the metastasis of cancer cells by regulating EMT. The expression level of YKL-40 was positively correlated with the migration and invasion of prostate cells, YKL-40 may be a potential therapeutic target for tumor cell invasion and metastasis.
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 3.Tu SM, Millikan RE, Mengistu B, Delpassand ES, Amato RJ, Pagliaro LC, Daliani D, Papandreou CN, Smith TL, Kim J, Podoloff DA, Logothetis CJ. Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet. 2001;357:336–341. doi: 10.1016/S0140-6736(00)03639-4. [DOI] [PubMed] [Google Scholar]
- 4.Van Allen EM, Ryan CJ. Novel secondary hormonal therapy in advanced prostate cancer: an update. Curr Opin Urol. 2009;19:315–321. doi: 10.1097/MOU.0b013e328329b73a. [DOI] [PubMed] [Google Scholar]
- 5.Francescone RA, Scully S, Faibish M, Taylor SL, Oh D, Moral L, Yan W, Bentley B, Shao R. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. J Biol Chem. 2011;286:15332–15343. doi: 10.1074/jbc.M110.212514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faibish M, Francescone R, Bentley B, Yan W, Shao R. A YKL-40-neutralizing antibody blocks tumor angiogenesis and progression: a potential therapeutic agent in cancers. Mol Cancer Ther. 2011;10:742–751. doi: 10.1158/1535-7163.MCT-10-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libreros S, Garcia-Areas R, Shibata Y, Carrio R, Torroella-Kouri M, Iragavarapu-Charyulu V. Induction of proinflammatory mediators by CHI3L1 is reduced by chitin treatment: decreased tumor metastasis in a breast cancer model. Int J Cancer. 2012;131:377–386. doi: 10.1002/ijc.26379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ku BM, Lee YK, Ryu J, Jeong JY, Choi J, Eun KM, Shin HY, Kim DG, Hwang EM, Yoo JC, Park JY, Roh GS, Kim HJ, Cho GJ, Choi WS, Paek SH, Kang SS. CHI3L1 (YKL-40) is expressed in human gliomas and regulates the invasion, growth and survival of glioma cells. Int J Cancer. 2011;128:1316–1326. doi: 10.1002/ijc.25466. [DOI] [PubMed] [Google Scholar]
- 9.Johansen JS, Jensen BV, Roslind A, Nielsen D, Price PA. Serum YKL-40, a new prognostic biomarker in cancer patients? Cancer Epidemiol Biomarkers Prev. 2006;15:194–202. doi: 10.1158/1055-9965.EPI-05-0011. [DOI] [PubMed] [Google Scholar]
- 10.Ozdemir E, Cicek T, Kaya MO. Association of serum YKL-40 level with tumor burden and metastatic stage of prostate cancer. Urol J. 2012;9:568–573. [PubMed] [Google Scholar]
- 11.Libreros S, Iragavarapu-Charyulu V. YKL-40/CHI3L1 drives inflammation on the road of tumor progression. J Leukoc Biol. 2015;98:931–936. doi: 10.1189/jlb.3VMR0415-142R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang H, Sun Y, Shi Z, Huang H, Fang Z, Chen J, Xiu Q, Li B. YKL-40 induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-kappaB pathways, causing bronchial smooth muscle proliferation and migration. J Immunol. 2013;190:438–446. doi: 10.4049/jimmunol.1201827. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W, Kawanishi M, Miyake K, Kagawa M, Kawai N, Murao K, Nishiyama A, Fei Z, Zhang X, Tamiya T. Association between YKL-40 and adult primary astrocytoma. Cancer. 2010;116:2688–2697. doi: 10.1002/cncr.25084. [DOI] [PubMed] [Google Scholar]
- 14.El-Galaly TC, Bilgrau AE, Gaarsdal E, Klausen TW, Pedersen LM, Nielsen KR, Baech J, Bogsted M, Dybkaer K, Johansen JS, Johnsen HE. Circulating tumor necrosis factor-alpha and YKL-40 level is associated with remission status following salvage therapy in relapsed non-Hodgkin lymphoma. Leuk Lymphoma. 2015;56:2476–2478. doi: 10.3109/10428194.2014.1001984. [DOI] [PubMed] [Google Scholar]
- 15.Shao R. YKL-40 acts as an angiogenic factor to promote tumor angiogenesis. Front Physiol. 2013;4:122. doi: 10.3389/fphys.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francescone R, Ngernyuang N, Yan W, Bentley B, Shao R. Tumor-derived mural-like cells coordinate with endothelial cells: role of YKL-40 in mural cell-mediated angiogenesis. Oncogene. 2014;33:2110–2122. doi: 10.1038/onc.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burman J, Raininko R, Blennow K, Zetterberg H, Axelsson M, Malmestrom C. YKL-40 is a CSF biomarker of intrathecal inflammation in secondary progressive multiple sclerosis. J Neuroimmunol. 2016;292:52–57. doi: 10.1016/j.jneuroim.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3’ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 19.Matysiak M, Kapka-Skrzypczak L, Jodlowska-Jedrych B, Kruszewski M. EMT promoting transcription factors as prognostic markers in human breast cancer. Arch Gynecol Obstet. 2017;295:817–825. doi: 10.1007/s00404-017-4304-1. [DOI] [PubMed] [Google Scholar]
- 20.Elaskalani O, Razak NB, Falasca M, Metharom P. Epithelial-mesenchymal transition as a therapeutic target for overcoming chemoresistance in pancreatic cancer. World J Gastrointest Oncol. 2017;9:37–41. doi: 10.4251/wjgo.v9.i1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pradella D, Naro C, Sette C, Ghigna C. EMT and stemness: flexible processes tuned by alternative splicing in development and cancer progression. Mol Cancer. 2017;16:8. doi: 10.1186/s12943-016-0579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito T, Kudoh S, Ichimura T, Fujino K, Hassan WA, Udaka N. Small cell lung cancer, an epithelial to mesenchymal transition (EMT)-like cancer: significance of inactive Notch signaling and expression of achaete-scute complex homologue 1. Hum Cell. 2017;30:1–10. doi: 10.1007/s13577-016-0149-3. [DOI] [PubMed] [Google Scholar]
- 23.van Zijl F, Zulehner G, Petz M, Schneller D, Kornauth C, Hau M, Machat G, Grubinger M, Huber H, Mikulits W. Epithelial-mesenchymal transition in hepatocellular carcinoma. Future Oncol. 2009;5:1169–1179. doi: 10.2217/fon.09.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaquero J, Guedj N, Claperon A, Nguyen Ho-Bouldoires TH, Paradis V, Fouassier L. Epithelial-mesenchymal transition in cholangiocarcinoma: from clinical evidence to regulatory networks. J Hepatol. 2017;66:424–441. doi: 10.1016/j.jhep.2016.09.010. [DOI] [PubMed] [Google Scholar]



