Abstract
Caragaphenol A (CAA) is a novel resveratrol trimer isolated from the roots of Caraganastenophylla. However, the biological activity of CAA is still unknown. In the present study, we investigated the anticancer effects of CAA on gastric cancer cells. CAA selectively inhibited cell growth of human gastric cancer cells. Moreover, CAA potently induced cell cycle arrest at G2/M phase and apoptosis with the increased intracellular reactive oxidative species (ROS) level. Inhibition of ROS could partially rescue CAA-induced cell apoptosis. Additionally, DNA is not the target of CAA. CAA in combination with DDP or 5FU synergistically inhibited the growth of human gastric cancer cells. Altogether, our study provides the evidence for the potential therapeutic application of CAA on human gastric cancer.
Keywords: Caragaphenol A, resveratrol trimer, reactive oxidative species, apoptosis, gastric cancer
Introduction
Gastric cancer is the fifth most common cancer and the third leading cause of cancer-related mortality worldwide with 951,600 new cases and 723,100 deaths per year [1], becoming a great threat to human and public health. Adjuvant chemotherapy is an essential strategy of comprehensive treatment in gastric cancer [2-4]. However, drug resistance exists in gastric cancer cells enormously limits the effectiveness of the chemotherapy. Therefore, it’s urgently necessary to develop a novel therapeutic agent against gastric cancer.
Resveratrol is a polyphenolic compound found in more than 72 plant species (distributed in 31 genera and 12 families) and widely present in edible foods and beverages such as peanuts, mulberries, grapes, and red wine [5], exerting a beneficial influence on multiple diseases including neurodegenerative disease, vascular disease, cardiovascular disease, and aging [6-8]. Recently, lots of researches have been focusing on resveratrol oligomers, derivatives of resveratrol, such as resveratrol dimers, resveratrol trimers and resveratrol tetramers [9]. Caragaphenol A (CAA), a novel resveratrol trimer, is isolated from the roots of Caraganastenophylla [10]. Although multitudinous studies reported resveratrol oligomers exhibit widely biochemical and pharmacological properties on multiple cancer cells [11-19], the biological activity of CAA is still unknown. In the present study, we investigated the anticancer effects of CAA on gastric cancer cells.
Materials and methods
Cells, cell culture, and reagents
Human gastric cancer cell linesBGC-823, MGC-803, MKN-45, SGC-7901, AGS and its 5FU-resistant line AGS-5FU, and normal human gastric epithelium cell line GES-1 were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml ) and streptomycin (100 ng/ml) in a humidified incubator at 37°C with 5% CO2. AGS-5FU cells were generated by continuous exposure to increasing concentration of 5FU. Doxorubicin (DOX), 5-FU and DDP were ordered from LC Laboratories. N-acetly-L-cysteine (NAC) and dihydroethidium (DHE) were purchased from Sigma-Aldrich. Methylthiazolyldiphenyl-tetrazolium bromide (MTT) was from ApexBioTechnology. Propidium iodide (PI) and other chemicals were purchased from Shanghai Sangon Biotech. CAA (Figure 1A) was dissolved in DMSO as the stock concentration of 10 mM. Anti-PARP (9542) antibodies were from Cell Signaling Technologies. Anti-Bcl-2 (SC-492), Anti-Cdk2 (SC-163) and Anti-Cdk4 (SC-260) antibodies were from Santa Cruz Biotechnology. Anti-CyclinB1 (610219), Anti-CyclinD1 (554181), Anti-CyclinE (551159), Anti-p27 (610241), Anti-Cdk1 (610037) and Anti-XIAP (61076) antibodies were from BD Biosciences. Anti-Cdk6 (3524-1) antibody was from Abcam. Anti-GAPDH (LK9002T) antibody was from Tianjin Sungene Biotech.
Figure 1.
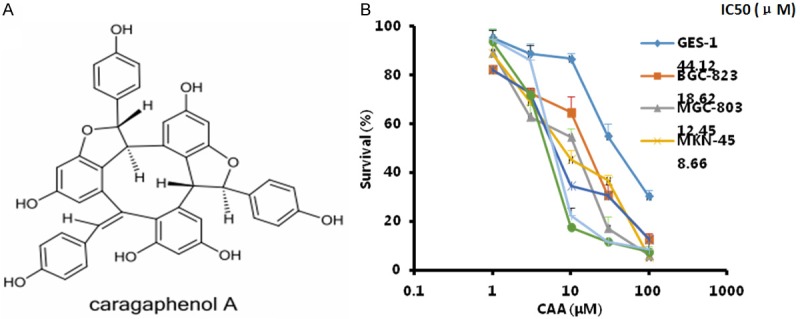
CAA suppresses the growth of human gastric cancer cells. The chemical structure of CAA (A), the representative growth curves and IC50 values of the indicated cells treated with CAA (B) were shown. Cells were treated with the indicated concentrations of CAA for 72 h, and cell survival was determined by MTT assay.
Cell viability assay
Cells were firstly seeded into a 96-well plate at a density of 5000 cells per well, and incubated with drugs in three parallel wells for 72 h. Then 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) was added to each well at a final concentration of 0.5 mg/ml. After incubation for 4 h, formazan crystals were dissolved in 100 mL of DMSO, and absorbance at 570 nm was measured by plate reader. The concentrations required to inhibit growth by 50% (IC50) were calculated from survival curves using the Bliss method [20,21]. For drug combination experiments, cells were co-treated with different concentrations of CAA and DDP or 5FU for 72 h. The data were analyzed by CompuSyn software with the results showed as combination index (CI) values according to the median-effect principle, where CI < 1, =1, and > 1 indicate synergism, additive effect, and antagonism, respectively [22,23].
Cell cycle analysis
Cells were harvested and washed twice with cold phosphate buffered saline (PBS), then fixed with ice-cold 70% ethanol for 30 min at 4°C. After centrifugation at 200 × g for 10 min, cells were washed twice with PBS and resuspended with 0.5 ml PBS containing PI (50 μg/ml), 0.1% Triton X-100, 0.1% sodiumcitrate, and DNase-free RNase (100 μg/ml), and detected by FCM after 15 min incubation at room temperature in the dark. Fluorescence was measured at an excitation wavelength of 480 nm through a FL-2filter (585 nm). Data were analyzed using ModFit LT 3.0 software (Becton Dickinson) [24,25].
Apoptosis assay
Cell apoptosis was examined with flow cytometry (FCM) assay. Briefly, cells were harvested and washed twice with PBS, stained with Annexin V-FITC and propidium iodide (PI) in the binding buffer, and detected by FACSCaliburFCM (BD, CA, USA) after 15 min incubation at room temperature in the dark. Fluorescence was measured at an excitation wave length of 480 nm through FL-1 (530 nm) and FL-2 filters (585 nm). The early apoptotic cells (Annexin V positive only) and late apoptotic cells (Annexin V and PI positive) were quantified [26,27].
Western blot analysis
Cells were harvested and washed twice with cold PBS, then resuspended and lysed in RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 ng/ml PMSF, 0.03% aprotinin, 1 μM sodium orthovanadate) at 4°C for 30 min. Lysates were centrifuged for 10 min at 14,000 × g and supernatants were stored at -80°C as whole cell extracts. Total protein concentrations were determined with Bradford assay. Proteins were separated on 12% SDS-PAGE gels and transferred to polyvinylidenedifluoride membranes. Membranes were blocked with 5% BSA and incubated with the indicated primary antibodies. Corresponding horseradish peroxidase-conjugated secondary antibodies were used against each primary antibody. Proteins were detected using the chemiluminescent detection reagents and films [28,29].
Reactive oxygen species (ROS) assay
Cells were incubated with 10 µM of DHE for 30 min at 37°C, washed twice with PBS and immediately photographed under fluorescent microscope (Olympus, Japan). For each well, 5 fields were taken randomly [30,31].
DNA binding assay
One microgram pcDNA3.1 was incubated with the different concentrations of CAA or DOX at 37°C for the indicated time points. After incubation, the reaction mixture was electrophoresed on 0.8% agarose gel and visualized by ethidium bromide staining [32].
Statistical analysis
All results are expressed as mean ± standard deviation (SD). Statistical analysis of the difference between treated and untreated groups was performed with Student’s t-test. Values of P < 0.05 were considered as significant differences.
Results
CAA suppresses the growth of gastric cancer cells
To assess the effect of CAA on gastric cancer cells, human gastric cancer cell lines BGC-823, MGC-803, MKN-45, SGC-7901, AGS and its 5FU-resistant line AGS-5FU, and normal human gastric epitheliumcell line GES-1 were treated with increasing concentrations of CAA for 72 h. As shown in Figure 1B, CAA significantly inhibited the growth of these cells in a dose-dependent manner. The IC50 values of CAA in BGC-823, MGC-803, MKN-45, SGC-7901, AGS and AGS-5FU cells are 18.62, 12.45, 8.66, 7.18, 5.80 and 6.98 μM, respectively, which is clearly lower than that in normal human gastric epitheliumcell line GES-1 44.12 μM. These results suggest that CAA is more cytotoxic to gastric cancer cells than normal gastric epithelium cells. In addition, CAA showed the similar cytotoxic in AGS and 5FU-resistant AGS-5FU cells, indicating that CAA suppresses the growth of not only gastric cancer cells but also their resistant cells.
CAA enhances the population of subG1 and G2/M phase in gastric cancer cells
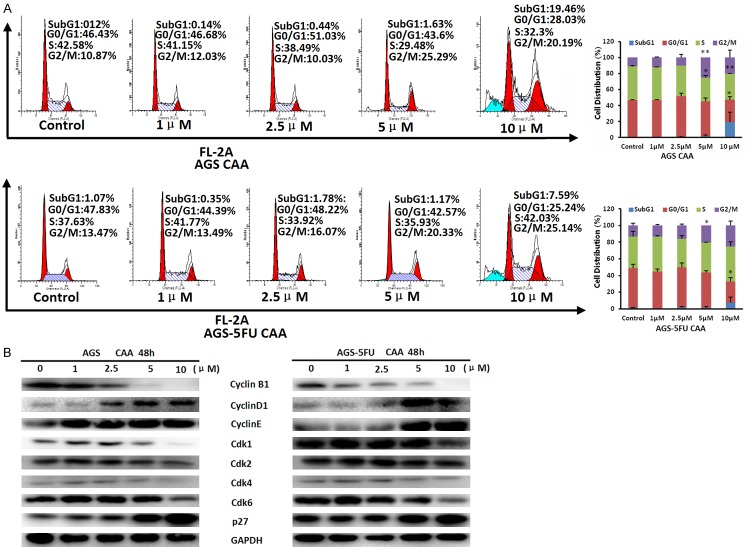
To examine the effect of CAA on cell cycle distribution of gastric cancer cells, AGS and AGS-5FU cells were treated with CAA (1, 2.5, 5 and 10 μM) for 48 h, stained with PI, and examined by FCM. The cell cycle distribution was calculated using ModFit LT 3.0 software. As shown in Figure 2A, CAA enhanced the population of subG1 and G2/M phase in a dose-dependent manner in both cells. Furthermore, the results of Western blot showed that CAA dose-dependently upregulated the protein levels of CyclinD1, CyclinE and p27 and downregulated the protein levels of CyclinB1, Cdk1, Cdk2, Cdk4 and Cdk6 in both cells (Figure 2B). In conclusion, these results suggested that CAA can induce cell cycle arrest at G2/M phase in human gastric cancer cells.
Figure 2.
CAA enhances the population of subG1 and G2/M phase in gastric cancer cells. AGS and AGS-5FU cells were treated with CAA for 48 h in the indicated concentrations, and the distribution of cell cycle was detected by FCM with PI staining. The percentages of subG1, G1/G0, S, G2/M phase were calculated using ModFit LT 3.0 software. The protein expression was examined by Western blot after lysing cells, and GAPDH was used as loading control. The representative charts, quantified results (A) and Western blot results (B) of three independent experiments were shown. *P < 0.05 and **P < 0.01 vs. corresponding control.
CAA induced apoptosis in gastric cancer cells
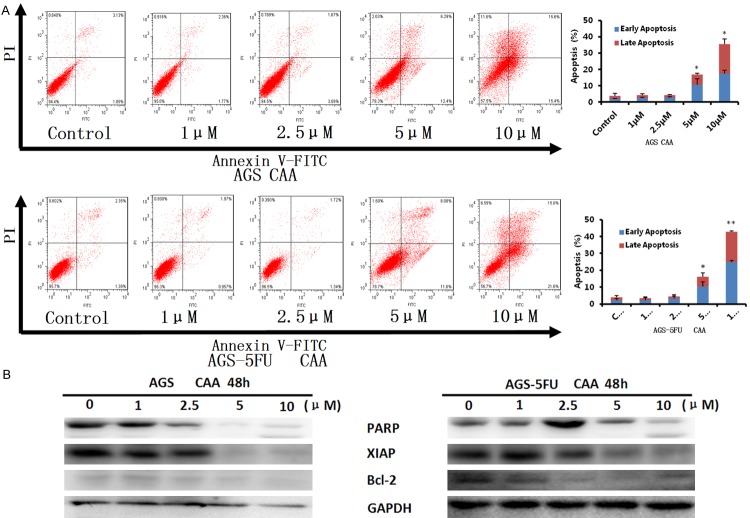
To evaluate whether CAA can induces apoptosis in gastric cancer cells, AGS and AGS-5FU cells were treated with CAA (1, 2.5, 5, and 10 μM) for 48 h, stained with Annexin V/PI, and examined by FCM. As shown in Figure 3A, CAA induced early apoptosis (Annexin V+/PI-) and late apoptosis (Annexin V+/PI+) in a dose-dependent manner in both cells. Moreover, the results of Western blot showed that CAA dose-dependently upregulated the protein levels of cleaved PARP and downregulated the protein levels of XIAP and Bcl-2 in both cells (Figure 3B). Altogether, these data indicated that CAA is able to induce apoptosis in human gastric cancer cells.
Figure 3.
CAA induces apotosis in gastric cancer cells. AGS and AGS-5FU cells were treated with CAA for 48 h in the indicated concentrations, and the apoptosis was detected by FCM Annexin V/PI staining. The proportions of Annexin V+/PI- and Annexin V+/PI+ cells indicated the early and late stage of apoptosis, respectively. The protein expression was examined by Western blot after lysing cells, and GAPDH was used as loading control. The representative charts, quantified results (A) and Western blot results (B) of three independent experiments were shown. *P < 0.05 and **P < 0.01 vs. corresponding control.
CAA stimulates the generation of intracellular ROS in gastric cancer cells
ROS plays a pivotal role in the anticancer effect of most anticancer agents via inducing cell apotosis [33,34]. To measure the impact of CAA on ROS in gastric cancer cells, AGS and AGS-5FU cells were treated with CAA (1, 2.5, 5, and 10 μM) for 12, 24 and 48 h, stained with dihydroethidium (DHE) as ROS fluorescent probe, and examined by microscopy. As shown in Figure 4, the fluorescent intensities of DHE were enhanced in the dose- and time-dependent manners in both cells after CAA treatment, suggesting that CAA can stimulate the generation of intracellular ROS in gastric cancer cells.
Figure 4.
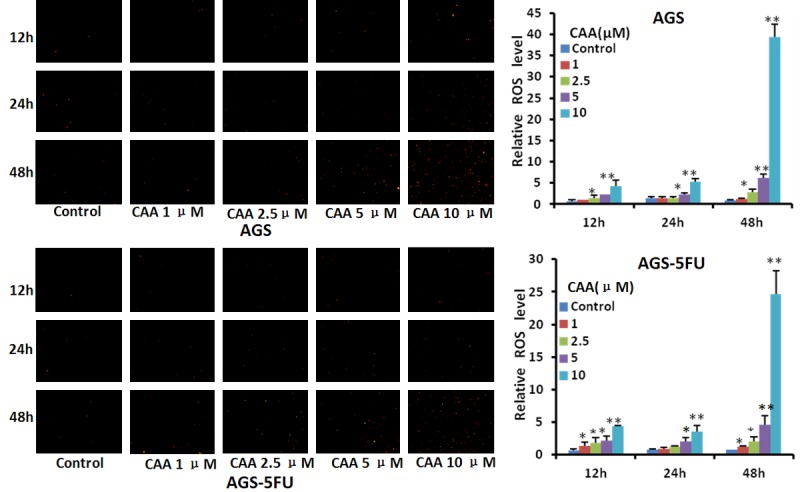
CAA stimulates the generation of intracellular ROS in gastric cancer cells. AGS and AGS-5FU cells were treated with CAA in the indicated concentrations and times, stained with DHE and photographed under florescent microscope. The representative micrographs and quantified results were shown. *P < 0.05 and **P < 0.01 vs. corresponding control.
Inhibition of ROS partially rescues CAA-induced apoptosis in gastric cancer cells
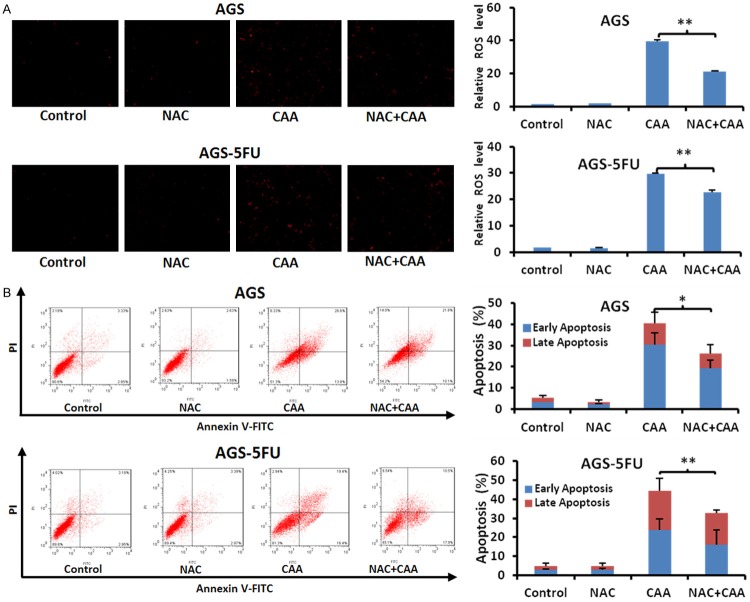
To further study the role of ROS in CAA-induced apoptosis in gastric cancer cells, AGS and AGS-5FU cells were treated with CAA at 10 μM for 48 h in the presence or absence of the ROS inhibitor NAC at 5 mM pretreated for 1 h. The CAA-stimulated DHE fluorescent signals were obviously decreased by NAC in both cells (Figure 5A). Additionally, the CAA-induced apoptosis were also partially rescued by NAC (Figure 5B), indicating that CAA is able to induce both ROS dependent and independent apoptosis in gastric cancer cells.
Figure 5.
Inhibition of ROS partially rescues CAA-induced apoptosis in gastric cancer cells. AGS and AGS-5FU cells were treated with 10 μM CAA for 48 h in the presence or absence of 5 mM NAC pretreatment for 1 h, stained with DHE and photographed under florescent microscope. The apoptosis was detected by FCM with Annexin V/PI staining. The representative micrographsor charts and quantified results (A, B) of three independent experiments were shown. *P < 0.05 and **P < 0.01 versus corresponding control.
DNA is not the target of CAA
DNA is one of common targets of many anticancer drugs. To investigate whether CAA can directly target DNA, we mixed DNA with various concentrations of CAA and the positive control doxorubicin. As shown in Figure 6, CAA could not induce DNA degradation at the concentration up to 25 μM for 6 h, while doxorubicin at 10 μM significantly induced DNA degradation at a dose-dependent manner. These results indicated that DNA is not the target of CAA.
Figure 6.
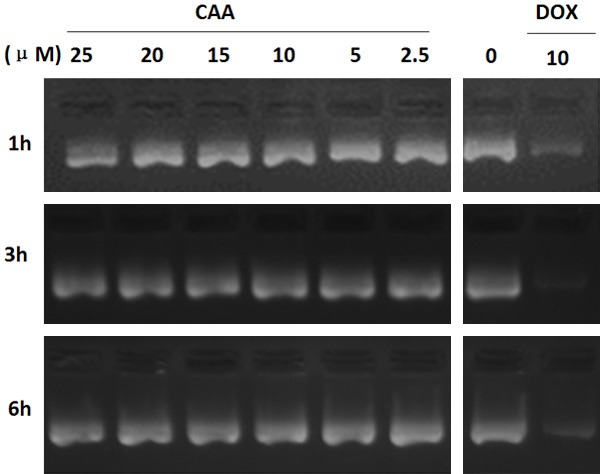
DNA is not the target of CAA. One microgram pcDNA3.1 was incubated with the different concentrations of CAA or DOX at 37°C for the indicated time points. After incubation, the reaction mixture was electrophoresed on 0.8% agarose gel and visualized by ethidium bromide staining. The representative micrographs of three independent experiments were shown.
CAA synergizes with DDP or 5FU to inhibit the growth of gastric cancer cells
DDP and 5FU currently are the common chemotherapeutic drugs for gastric cancer in clinic. To test the effect of CAA on the anticancer activity of DDP or 5FU in gastric cancer cells, AGS and AGS-5FU cells were treated with CAA (1, 3 and 10 μM) in combination with DDP (10, 30 and 100 μM) or 5FU (3, 10, and 30 μM), and cell survival was detected by MTT assay. As shown in Figure 7, almost all CI values of both combination were < 1, suggesting that the anticancer effects of combination of CAA with DDP or 5FU is synergistic.
Figure 7.
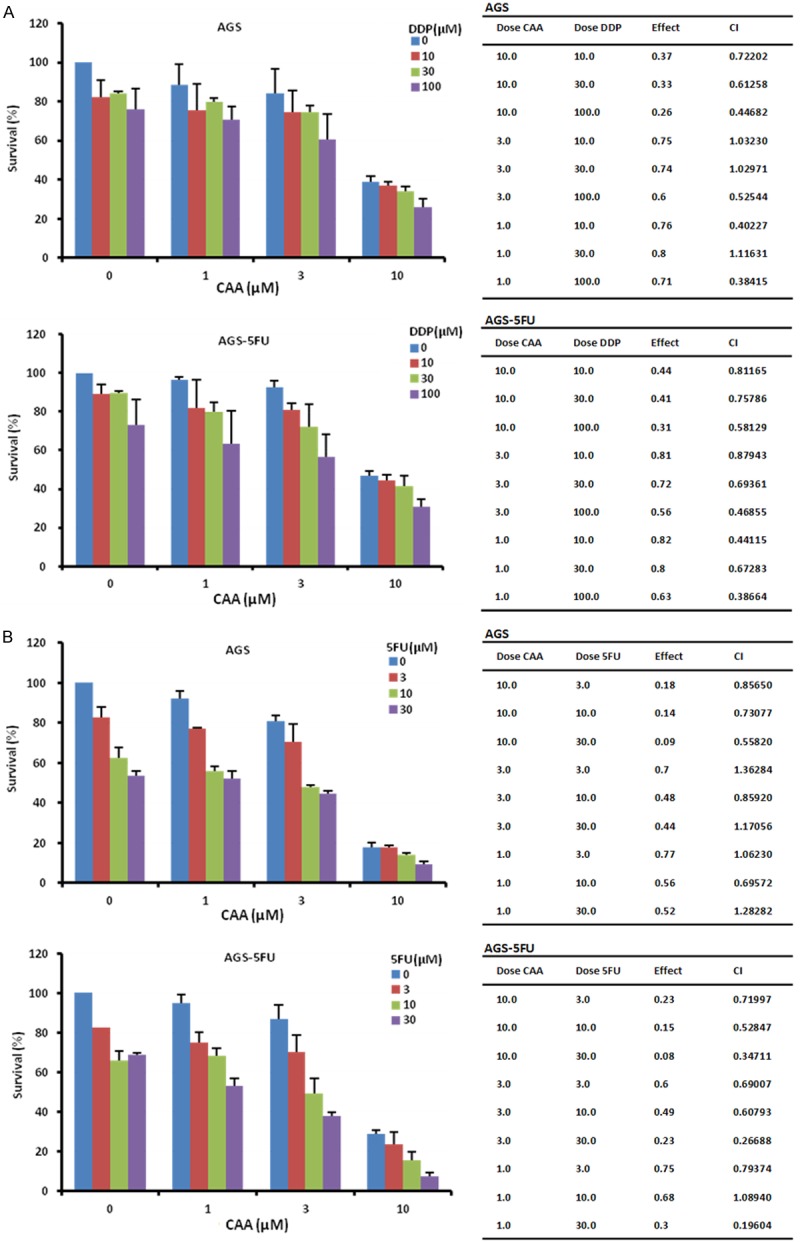
CAA synergizes with DDP or 5FU to inhibit the growth of gastric cancer cells. AGS and AGS-5FU cells were treated with the indicated concentrations of CAA and DDP (A) or 5FU (B) for 72 h, and cell survival was detected by MTT assay. The data were analyzed by CompuSyn software with the results shown as growth histogram, and CI values.
Discussion
In this study, we firstly demonstrated that CAA selectively mediated time- and concentration-dependent anti-growth effects on human gastric cancer cells. The IC50 values after 72 h treatment with CAA were ranged from 5.80 to 18.62 μM in five human gastric cancer cell lines. CAA showed the similar growth inhibition of not only AGS gastric cancer cells but also their resistant cells AGS-5FU. The results of FCM analysis revealed that CAA could enhance the population of subG1 and G2/M phase and induce apoptosis in both AGS and AGS-5FU cells. Furthermore, inhibition of CAA-induced intercellular ROS accumulation by NAC could partially reverse CAA-induced apoptosis. ROS plays a critical role in controlling cell growth, differentiation or death by alteration of the cellular redox condition [35]. Except ROS dependent apoptosis, there are ROS independent apoptosis induced by some stimuli, such as emodin-induced apoptosis in promyeloleukemic cells [36], etoposide-induced apoptosis in B lymphoma cells [37]. Similarly with our study, resveratrol has been reported to induce apoptosis in SGC-7901 human gastric cancer cells with the increased generation of ROS, and co-incubation with superoxide dismutaseor catalase decreased resveratrol-induced apoptosis [38]. Resveratrol can also induce apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells and A549 human lung cancer cells [39,40]. Therefore, ROS may play important roles in resveratrol, CAA or other resveratrol analogues induced apoptosis in cancer cells.
Combination therapy is a routine strategy for gastric cancer chemotherapy, which has significant advantages such as synergistically enhancing the bioavailability against the cancer, decreasing toxicity or side effects of agents, and delaying the development of drug resistance [41,42]. Lots of groups have reported the effects of combination treatment of resveratrol with DDP or 5FU on various cancer models. For example, resveratrol can enhance the anticancer effects of DDP on human non-small cell lung cancer H838 and H520 cells through inducing mitochondrial dysfunction and cell apoptosis [43]. The combination treatment of resveratrol and DDP synergistically induce apoptosis through oxidative mitochondrial damage in malignant mesothelioma MSTO-211H cells [44]. Resveratrol can also potentiate the cytotoxicity of 5FU in HT-29 and SW-620 colon cancer cells [45]. In the murine hepatoma models, resveratrol is able to enhance the antitumor effect of 5-FU in vivo [46]. Our data showed that CAA synergistically enhanced the anti-growth effect of DDP or 5FU in both AGS and AGS-5FU cells, suggesting that CAA may be a potential chemosensitizer for gastric cancer chemotherapy. However, the antitumor effects of combination treatment of CAA with DDP or 5FU on gastric cancer in vivo need to be further investigated in the future.
In summary, our study provided the first evidence that CAA potently induce cell growth, cell cycle arrest at G2/M phase and apoptosis with the increased intracellular ROS level in human gastric cancer cells. Moreover, CAA in combination with DDP or 5FU synergistically inhibited the growth of human gastric cancer cells, suggesting this beneficial combinational therapy may provide a promising strategy for the treatment of gastric cancer.
Acknowledgements
This work was supported by funds from the National Natural Science Foundation of China Nos. 31271444, 81201726, 8161101021 (Z. Shi) and 81303305 (K. Cheng), the Lishui Science and Technology Bureau Research Fund No. 20140212037 (K. Cheng), the Guangdong Natural Science Funds for Distinguished Young Scholar No. 2014A030306001 (Z. Shi), the Science and Technology Program of Guangdong No. 2016A050502027 (Z. Shi), the Science and Technology Program of Guangzhou No. 201704030058 (Z. Shi), and the Foundation for Research Cultivation and Innovation of Jinan University No. 21616119 (Z. Shi).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Ilson DH. Adjuvant treatment for gastric cancer: too much is not enough. Lancet Oncol. 2014;15:788–789. doi: 10.1016/S1470-2045(14)70293-1. [DOI] [PubMed] [Google Scholar]
- 3.Group G, Paoletti X, Oba K, Burzykowski T, Michiels S, Ohashi Y, Pignon JP, Rougier P, Sakamoto J, Sargent D, Sasako M, Van Cutsem E, Buyse M. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 4.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 5.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 6.Kovacic P, Somanathan R. Multifaceted approach to resveratrol bioactivity: focus on antioxidant action, cell signaling and safety. Oxid Med Cell Longev. 2010;3:86–100. doi: 10.4161/oxim.3.2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rege SD, Kumar S, Wilson DN, Tamura L, Geetha T, Mathews ST, Huggins KW, Broderick TL, Babu JR. Resveratrol protects the brain of obese mice from oxidative damage. Oxid Med Cell Longev. 2013;2013:419092. doi: 10.1155/2013/419092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harikumar KB, Aggarwal BB. Resveratrol: a multi targeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 9.Xue YQ, Di JM, Luo Y, Cheng KJ, Wei X, Shi Z. Resveratrol oligomers for the prevention and treatment of cancers. Oxid Med Cell Longev. 2014;2014:765832. doi: 10.1155/2014/765832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu HX, Lin WH, Yang JS. Oligomericstilbenes from the root of Caraganastenophylla. Chem Pharm Bull (Tokyo) 2004;52:1339–1341. doi: 10.1248/cpb.52.1339. [DOI] [PubMed] [Google Scholar]
- 11.Ito T, Akao Y, Yi H, Ohguchi K, Matsumoto K, Tanaka T, Iinuma M, Nozawa Y. Antitumor effect of resveratrol oligomers against human cancer cell lines and the molecular mechanism of apoptosis induced by vaticanol C. Carcinogenesis. 2003;24:1489–1497. doi: 10.1093/carcin/bgg105. [DOI] [PubMed] [Google Scholar]
- 12.Tian CY, Hu CQ, Xu G, Song HY. Assessment of estrogenic activity of natural compounds using improved E-screen assay. Acta Pharmacol Sin. 2002;23:572–576. [PubMed] [Google Scholar]
- 13.Bala AE, Kollmann A, Ducrot PH, Majira A, Kerhoas L, Leroux P, Delorme R, Einhorn J. Cis epsilon-viniferin: a new antifungal resveratrol dehydrodimer from Cyphostemmacrotalarioides roots. Journal of Phytopathology-Phytopathologische Zeitschrift. 2000;148:29–32. [Google Scholar]
- 14.Barjot C, Tournaire M, Castagnino C, Vigor C, Vercauteren J, Rossi JF. Evaluation of antitumor effects of two vine stalk oligomers of resveratrol on a panel of lymphoid and myeloid cell lines: comparison with resveratrol. Life Sci. 2007;81:1565–1574. doi: 10.1016/j.lfs.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 15.Wibowo A, Ahmat N, Hamzah AS, Sufian AS, Ismail NH, Ahmad R, Jaafar FM, Takayama H. Malaysianol A, a new trimer resveratrol oligomer from the stem bark of Dryobalanopsaromatica. Fitoterapia. 2011;82:676–681. doi: 10.1016/j.fitote.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 16.He S, Jiang L, Wu B, Pan Y, Sun C. Pallidol, a resveratrol dimer from red wine, is a selective singlet oxygen quencher. Biochem Biophys Res Commun. 2009;379:283–287. doi: 10.1016/j.bbrc.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 17.Lim KG, Gray AI, Pyne S, Pyne NJ. Resveratrol dimers are novel sphingosine kinase 1 inhibitors and affect sphingosine kinase 1 expression and cancer cell growth and survival. Br J Pharmacol. 2012;166:1605–1616. doi: 10.1111/j.1476-5381.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhury SA, Kishino K, Satoh R, Hashimoto K, Kikuchi H, Nishikawa H, Shirataki Y, Sakagami H. Tumor-specificity and apoptosisinducing activity of stilbenes and flavonoids. Anticancer Res. 2005;25:2055–2063. [PubMed] [Google Scholar]
- 19.Kim HJ, Saleem M, Seo SH, Jin C, Lee YS. Two new antioxidant stilbene dimers, parthenostilbenins A and B from parthenocissus tricuspidata. Planta Med. 2005;71:973–976. doi: 10.1055/s-2005-871229. [DOI] [PubMed] [Google Scholar]
- 20.Shi Z, Liang YJ, Chen ZS, Wang XW, Wang XH, Ding Y, Chen LM, Yang XP, Fu LW. Reversal of MDR1/P-glycoprotein-mediated multidrug resistance by vector-based RNA interference in vitro and in vivo. Cancer Biol Ther. 2006;5:39–47. doi: 10.4161/cbt.5.1.2236. [DOI] [PubMed] [Google Scholar]
- 21.Shi Z, Peng XX, Kim IW, Shukla S, Si QS, Robey RW, Bates SE, Shen T, Ashby CR Jr, Fu LW, Ambudkar SV, Chen ZS. Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res. 2007;67:11012–11020. doi: 10.1158/0008-5472.CAN-07-2686. [DOI] [PubMed] [Google Scholar]
- 22.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 23.Gong LH, Chen XX, Wang H, Jiang QW, Pan SS, Qiu JG, Mei XL, Xue YQ, Qin WM, Zheng FY, Shi Z, Yan XJ. Piperlongumine induces apoptosis and synergizes with cisplatin or paclitaxel in human ovarian cancer cells. Oxid Med Cell Longev. 2014;2014:906804. doi: 10.1155/2014/906804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Gong L, Ou R, Zheng Z, Chen J, Xie F, Huang X, Qiu J, Zhang W, Jiang Q, Yang Y, Zhu H, Shi Z, Yan X. Sequential combination therapy of ovarian cancer with cisplatin and gamma-secretase inhibitor MK-0752. Gynecol Oncol. 2016;140:537–544. doi: 10.1016/j.ygyno.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Lv M, Qiu JG, Zhang WJ, Jiang QW, Qin WM, Yang Y, Zheng DW, Chen Y, Huang JR, Wang K, Wei MN, Cheng KJ, Shi Z. Wallichinine reverses ABCB1-mediated cancer multidrug resistance. Am J Transl Res. 2016;8:2969–2980. [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Z, Park HR, Du Y, Li Z, Cheng K, Sun SY, Fu H, Khuri FR. Cables1 complex couples survival signaling to the cell death machinery. Cancer Res. 2015;75:147–158. doi: 10.1158/0008-5472.CAN-14-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Z, Li Z, Li ZJ, Cheng K, Du Y, Fu H, Khuri FR. Cables1 controls p21/Cip1 protein stability by antagonizing proteasome subunit alpha type 3. Oncogene. 2015;34:2538–2545. doi: 10.1038/onc.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang WJ, Li Y, Wei MN, Chen Y, Qiu JG, Jiang QW, Yang Y, Zheng DW, Qin WM, Huang JR, Wang K, Wang YJ, Yang DH, Chen ZS, Shi Z. Synergistic antitumor activity of regorafenib and lapatinib in preclinical models of human colorectal cancer. Cancer Lett. 2017;386:100–109. doi: 10.1016/j.canlet.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Qiu JG, Li Y, Di JM, Zhang WJ, Jiang QW, Zheng DW, Chen Y, Wei MN, Huang JR, Wang K, Shi Z. Targeting ABCB1-mediated tumor multidrug resistance by CRISPR/Cas9-based genome editing. Am J Transl Res. 2016;8:3986–3994. [PMC free article] [PubMed] [Google Scholar]
- 30.Mei XL, Yang Y, Zhang YJ, Li Y, Zhao JM, Qiu JG, Zhang WJ, Xue YQ, Zheng DW, Chen Y, Qin WM, Wei MN, Shi Z. Sildenafil inhibits the growth of human colorectal cancer in vitro and in vivo . Am J Cancer Res. 2015;5:3311–3324. [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang QW, Cheng KJ, Mei XL, Qiu JG, Zhang WJ, Xue YQ, Qin WM, Yang Y, Zheng DW, Chen Y, Wei MN, Zhang X, Lv M, Chen MW, Wei X, Shi Z. Synergistic anticancer effects of triptolide and celastrol, two main compounds from thunder god vine. Oncotarget. 2015;6:32790–32804. doi: 10.18632/oncotarget.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang XH, Jia DZ, Liang YJ, Yan SL, Ding Y, Chen LM, Shi Z, Zeng MS, Liu GF, Fu LW. Lgf-YL-9 induces apoptosis in human epidermoid carcinoma KB cells and multidrug resistant KBv200 cells via reactive oxygen species-independent mitochondrial pathway. Cancer Lett. 2007;249:256–270. doi: 10.1016/j.canlet.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–425. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, Stern AM, Mandinova A, Schreiber SL, Lee SW. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen YC, Shen SC, Lee WR, Hsu FL, Lin HY, Ko CH, Tseng SW. Emodin induces apoptosis in human promyeloleukemic HL-60 cells accompanied by activation of caspase 3 cascade but independent of reactive oxygen species production. Biochem Pharmacol. 2002;64:1713–1724. doi: 10.1016/s0006-2952(02)01386-2. [DOI] [PubMed] [Google Scholar]
- 37.Senturker S, Tschirret-Guth R, Morrow J, Levine R, Shacter E. Induction of apoptosis by chemotherapeutic drugs without generation of reactive oxygen species. Arch Biochem Biophys. 2002;397:262–272. doi: 10.1006/abbi.2001.2681. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Li W, Meng X, Jia B. Resveratrol induces gastric cancer cell apoptosis via reactive oxygen species, but independent of sirtuin1. Clin Exp Pharmacol Physiol. 2012;39:227–232. doi: 10.1111/j.1440-1681.2011.05660.x. [DOI] [PubMed] [Google Scholar]
- 39.Juan ME, Wenzel U, Daniel H, Planas JM. Resveratrol induces apoptosis through ROSdependent mitochondria pathway in HT-29 human colorectal carcinoma cells. J Agric Food Chem. 2008;56:4813–4818. doi: 10.1021/jf800175a. [DOI] [PubMed] [Google Scholar]
- 40.Lucas IK, Kolodziej H. Trans-resveratrol induces apoptosis through ROS-triggered mitochondria-dependent pathways in A549 human lung adenocarcinoma epithelial cells. Planta Med. 2015;81:1038–1044. doi: 10.1055/s-0035-1546129. [DOI] [PubMed] [Google Scholar]
- 41.Bozic I, Reiter JG, Allen B, Antal T, Chatterjee K, Shah P, Moon YS, Yaqubie A, Kelly N, Le DT, Lipson EJ, Chapman PB, Diaz LA Jr, Vogelstein B, Nowak MA. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. 2013;2:e00747. doi: 10.7554/eLife.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh CK, George J, Ahmad N. Resveratrolbased combinatorial strategies for cancer management. Ann N Y Acad Sci. 2013;1290:113–121. doi: 10.1111/nyas.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma L, Li W, Wang R, Nan Y, Wang Q, Liu W, Jin F. Resveratrol enhanced anticancer effects of cisplatin on non-small cell lung cancer cell lines by inducing mitochondrial dysfunction and cell apoptosis. Int J Oncol. 2015;47:1460–1468. doi: 10.3892/ijo.2015.3124. [DOI] [PubMed] [Google Scholar]
- 44.Lee YJ, Lee GJ, Yi SS, Heo SH, Park CR, Nam HS, Cho MK, Lee SH. Cisplatin and resveratrol induce apoptosis and autophagy following oxidative stress in malignant mesothelioma cells. Food Chem Toxicol. 2016;97:96–107. doi: 10.1016/j.fct.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 45.Santandreu FM, Valle A, Oliver J, Roca P. Resveratrol potentiates the cytotoxic oxidative stress induced by chemotherapy in human colon cancer cells. Cell Physiol Biochem. 2011;28:219–228. doi: 10.1159/000331733. [DOI] [PubMed] [Google Scholar]
- 46.Wu SL, Sun ZJ, Yu L, Meng KW, Qin XL, Pan CE. Effect of resveratrol and in combination with 5-FU on murine liver cancer. World J Gastroenterol. 2004;10:3048–3052. doi: 10.3748/wjg.v10.i20.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]