Abstract
Introduction
Data on relationship between lipoprotein(a) (Lp(a)) and non-ischemic heart dysfunction are limited. This study is aimed to assess the association between Lp(a) and left ventricular systolic dysfunction in a Chinese population of patients with hypertension and without coronary artery disease (CAD).
Material and methods
This cross-sectional study included 1611 patients with hypertension and without CAD in China. The factors associated with left ventricular ejection fraction (LVEF) were evaluated using univariate and multivariate analysis.
Results
A higher percentage of hypertensive patients with LVEF < 50% were men, and had lower plasma high-density lipoprotein cholesterol, but higher plasma Lp(a), serum creatinine, and hemoglobin levels than those with LVEF ≥ 50% using univariate analysis. When participants were classified as four groups according to Lp(a) quartiles, LVEF was decreased with increased Lp(a) levels. The prevalence of LVEF < 50% was increased with Lp(a) quartiles. Multiple linear regression analysis indicated that plasma Lp(a) levels, man, and serum creatinine levels were independently correlated with LVEF in hypertensive patients. Multiple logistic regression analysis indicated that plasma Lp(a) levels (OR = 5.566, 95% CI: 1.745–17.758, p = 0.004) or Lp(a) quartiles (quartile 4: OR = 3.234, 95% CI: 1.290–8.105, quartile 1 as reference, p = 0.012) was independently correlated with LVEF < 50% with adjustment for other potential confounders. Ordinal logistic regression analysis demonstrated that Lp(a) (OR = 5.760, 95% CI: 1.831–18.120, p = 0.003) was independently correlated with different LVEF categories (≥ 50%, 35–49%, and < 35%) in hypertensive patients.
Conclusions
Left ventricular ejection fraction is decreased with increased plasma Lp(a) levels. Lipoprotein(a) is independently correlated with left ventricular systolic dysfunction in patients with hypertension and without CAD.
Keywords: hypertension, coronary artery disease, lipoprotein(a), left ventricular systolic dysfunction
Introduction
There is mounting evidence that elevated plasma lipoprotein(a) [Lp(a)] levels contribute significantly to the incidence of cardiovascular diseases (CVDs) including coronary artery disease (CAD) [1–3]. Lipoprotein(a) exhibits strong atherogenic properties and can cause more severe and diffused coronary artery lesions [4]. Numerous studies demonstrate that elevated plasma Lp(a) levels can increase the risk of myocardial infarction (MI) and mortality in CAD patients [5]. Elevated plasma Lp(a) levels are also associated with impaired heart function in CAD and MI patients. Patients with high Lp(a) have worse regional wall motion and decreased left ventricular ejection fraction (LVEF) [6]. However, data on the relationship between Lp(a) and non-ischemic heart dysfunction are limited to date.
Hypertension remains a significant risk factor for development of heart failure, with various mechanisms contributing to both systolic and diastolic dysfunction. The pathogenesis of heart dysfunction includes left ventricular hypertrophy and fibrosis, increased arterial stiffness, activation of the sympathetic nervous system and renin-angiotensin system, etc [7, 8]. Increased arterial stiffness will possibly increase cardiac afterload and further risk of heart failure [8]. Elevated plasma Lp(a) levels are commonly detected in hypertensive patients and are correlated with target organ damage such as CAD, stroke, and renal failure [9]. The atherogenic effect of high Lp(a) levels may possibly also lead to aortic arteriosclerosis and increased arterial stiffness in hypertensive patients. Therefore high Lp(a) levels are speculated to be possibly associated with cardiac dysfunction. However, whether Lp(a) is associated with heart systolic dysfunction in hypertensive patients without CAD remains to be elucidated. Thus, we present this study aimed to assess the association between Lp(a) and left ventricular systolic dysfunction in a Chinese population of patients with hypertension and without CAD.
Material and methods
Study subjects
It was a cross-sectional study which focused on patients with hypertension and without CAD in China. All the participants (n = 1797) aged over 20 years old had been consecutively hospitalized in the cardiology or hypertension division of Shanghai Rui Jin Hospital between January and December 2014. Hypertension was diagnosed when systolic blood pressure ≥ 140 mm Hg, or diastolic blood pressure ≥ 90 mm Hg, or when actively treated with anti-hypertension drugs. The hypertensive participants in this study were hospitalized to perform clinical assessment and also evaluate whether cardiovascular risk factors, target organ damage or accompanying cardiovascular diseases were present. All participants who had ever received coronary angiography (CAG) or coronary computed tomography (CT) angiography and CAD patients were excluded from this study. Coronary artery disease was diagnosed when diameter stenosis was > 50% in at least one main coronary artery, or when there was a history of confirmed acute or old myocardial infarction, or a history of revascularization by percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG). Other exclusion criteria included congenital heart disease, acute infectious diseases, liver failure, hypothyroidism, pregnancy, mental disorder, or cancer. After the subjects with exclusion criteria or incomplete data were removed, there were 1611 participants left.
The study complied with the Declaration of Helsinki. It was also approved by the ethics committee of Shanghai Jiao Tong University and informed consent was obtained from all the participants prior to enrollment.
Blood sampling and laboratory test
The blood samples were collected from each patient on the next day after admission. Plasma Lp(a) levels were measured using an immunoturbidimetric assay (Wako Chemicals USA, Inc. Richmond, VA) with a Hitachi 912 autoanalyzer (Roche Diagnostics, Basel, Switzerland). Meanwhile, the levels of plasma total cholesterol, total triglyceride, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), plasma glucose, and serum creatinine were analyzed by the automatic biochemical analyzer. Blood cell tests were executed using an automated blood cell counter (Beckman Coulter LH750, CA, USA). Laboratory test results were generated by personnel blinded to the clinical characteristics of the study participants.
Blood pressure and echocardiographic measurements
Blood pressure was measured using a standardized automatic electronic sphygmomanometer (HEM-741C; Omron, Tokyo, Japan). One experienced physician performed 3 blood pressure measurements using an American Heart Association protocol after study participants had at least a 5 min rest in the sitting position. Study participants avoided eating, drinking alcohol, drinking coffee, smoking, exercising, and bathing for 30 min before taking these measurements. The mean of 3 blood pressure values was calculated and used for further analysis.
One echocardiographer blinded to the biochemical examination results of the study participants performed all echocardiographic measurements using the Phillips IE33 device according to the American Society of Echocardiography (ASE) recommendations. M-mode, two-dimensional, and color Doppler images were first recorded, and then analyzed offline. Left ventricular ejection fraction assessment was based on two-dimensional echocardiography using the quantitative two-dimensional biplane volumetric Simpson method from 4- and 2-chamber views. Intraobserver reproducibility was assessed among 25 randomly selected patients. No significant difference was found (intra-observer: mean difference: 1.4 ±0.20%, p = 0.701).
Clinical data collection
A case report form was developed to assess the general characteristics, clinical diagnosis, medical history, medical treatment, and biochemical examination. Blood pressure measurement recorded in the form was obtained on admission. Current smoking was determined when subjects were smoking currently and had smoked more than one cigarette daily for at least one year continuously. Diabetes mellitus was diagnosed by a fasting plasma glucose test showing ≥ 7.0 mmol/l, or by a random plasma glucose test showing ≥ 11.1 mmol/l, or when they were actively receiving therapy using insulin or oral medications for diabetes. Medical therapy including statins, angiotensin converting enzyme inhibitors (ACEI), angiotensin receptor antagonists (ARB), calcium antagonists, and β-blockers was also included in the case report form.
Statistical analysis
Data entry and management were performed on Epidata software, version 3.1 (Epidata Association, Odense, Denmark). Data were analyzed using the software program SPSS 13.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as the mean ± standard deviation, and categorical variables as a percentage. The χ2 test and trend test were used to compare categorical variables among several groups. The independent-sample t-test was used to compare continuous variables between two groups. The trend test was used to compare continuous variables among more than two groups. Multiple linear regression analysis which included variables identified as statistically significant in the univariate analysis was used to assess the independence of the association between LVEF and Lp(a). Multiple logistic regression analysis was used to assess the independence of the association between LVEF < 50% and Lp(a). Ordinal logistic regression analysis was used to assess the association between different LVEF categories and Lp(a). Two-sided p < 0.05 was considered significant.
Results
Study population characteristics
In total, 1611 participants with hypertension and without CAD were included in the final statistical analysis. General characteristics of these participants are shown in Table I. Their mean age was 62.4 ±9.67 years. Eight hundred and two (49.8%) participants were men. A total of 1300 study subjects (80.7%) had received antihypertensive drugs. Furthermore, 506 (31.4%) subjects, 970 (60.2%) subjects, and 330 subjects (20.5%) were treated with β-blockers, ACEI or ARB, and calcium antagonists, respectively. The average LVEF was 65.3 ±7.09%. The prevalence of LVEF < 50% was 3.6%. A higher percentage of hypertensive patients with LVEF < 50% were men than those with LVEF ≥ 50% (p < 0.001). The hypertensive patients with LVEF < 50% had lower HDL-C levels, but higher plasma Lp(a), serum creatinine, and hemoglobin levels than those with LVEF ≥ 50% (all p < 0.05).
Table I.
Clinical characteristics of hypertensive patients with LVEF ≥ 50% or < 50%
| Variables | All(n = 1611) | EF ≥ 50% (n = 1553) | EF < 50%(n = 58) | P-value |
|---|---|---|---|---|
| Age [years] | 62.4 ±9.67 | 62.5 ±9.65 | 60.4 ±10.0 | 0.103 |
| Men, n (%) | 802 (49.8) | 755 (48.6) | 47 (81.0) | < 0.001 |
| Current smoking, n (%) | 249 (15.5) | 238 (15.3) | 11 (19.0) | 0.451 |
| Diabetes mellitus, n (%) | 391 (24.3) | 378 (24.3) | 13 (22.4) | 0.737 |
| BMI [kg/m2] | 25.3 ±3.34 | 25.2 ±3.32 | 25.6 ±3.55 | 0.469 |
| SBP [mm Hg] | 139 ±19.0 | 139 ±18.7 | 137 ±22.8 | 0.278 |
| DBP [mm Hg] | 77.0 ±12.1 | 77.0 ±11.9 | 76.2 ±14.3 | 0.340 |
| Total cholesterol [mmol/l] | 4.25 ±0.96 | 4.26 ±0.96 | 4.12 ±1.00 | 0.274 |
| Total triglyceride [mmol/l] | 1.73 ±1.04 | 1.73 ±1.02 | 1.67 ±1.55 | 0.663 |
| LDL-C [mmol/l] | 2.53 ±0.80 | 2.53 ±0.79 | 2.50 ±0.84 | 0.781 |
| HDL-C [mmol/l] | 1.18 ±0.31 | 1.18 ±0.31 | 1.09 ±0.34 | 0.034 |
| Lp(a) [g/l] | 0.19 ±0.18 | 0.19 ±0.18 | 0.25 ±0.20 | 0.016 |
| HbA1c (%) | 6.13 ±1.02 | 6.12 ±1.03 | 6.31 ±0.98 | 0.232 |
| Fasting plasma glucose [mmol/l] | 5.27 ±1.28 | 5.26 ±1.26 | 5.30 ±1.75 | 0.838 |
| 2 h plasma glucose [mmol/l] | 8.22 ±3.27 | 8.21 ±3.30 | 8.54 ±2.38 | 0.566 |
| Fasting plasma insulin [μIU/ml] | 11.6 ±20.7 | 11.7 ±21.0 | 8.81 ±6.39 | 0.467 |
| Serum creatinine [μmol/l] | 75.7 ±34.7 | 75.0 ±34.6 | 94.5 ±32.0 | < 0.001 |
| Hemoglobin [g/l] | 134 ±15.2 | 133 ±15.1 | 140 ±18.5 | 0.003 |
| LVEF (%) | 65.3 ±7.09 | 66.3 ±4.79 | 38.7 ±7.16 | < 0.001 |
| Statin use, n (%) | 306 (19.0) | 294 (18.9) | 12 (20.7) | 0.737 |
| β-blockers, n (%) | 506 (31.4) | 485 (31.2) | 21 (36.2) | 0.423 |
| ACEI or ARB, n (%) | 970 (60.2) | 931 (59.9) | 39 (67.2) | 0.265 |
| Calcium antagonist, n (%) | 330 (20.5) | 322 (20.7) | 8 (13.8) | 0.198 |
BMI – body mass index, SBP – systolic blood pressure, LDL-C – low-density lipoprotein cholesterol, HDL-C – high-density lipoprotein cholesterol, Lp(a) – lipoprotein(a), HbA1c – glycated hemoglobin, LVEF – left ventricular ejection fraction, ACEI – angiotensin-converting enzyme inhibitors, ARB – angiotensin receptor antagonists. Values are means ± SD or numbers with percentage in parentheses.
Factors associated with plasma Lp(a) levels
We also explored the factors associated with plasma Lp(a) levels using univariate and multivariate analysis. The data indicated that hypertensive patients with Lp(a) > 0.13 g/l (median value) had higher LDL-C levels (2.62 ±0.80 mmol/l, n = 774, vs. 2.44 ±0.78 mmol/l, n = 837, p < 0.001), plasma total cholesterol levels (4.35 ±0.96 mmol/l, n = 774, vs. 4.16 ±0.95 mmol/l, n = 837, p < 0.001), but lower LVEF (64.7 ±7.78, n = 774, vs. 65.9 ±6.33, n = 837, p < 0.001) than those with Lp(a) ≤ 0.13 g/l in the univariate analysis. Furthermore, independent factors associated with higher Lp(a) (> 0.13 g/l) were evaluated using multiple logistic regression analysis. Variables identified as statistically significant in the univariate analysis and other important demographic characteristics including age, sex, and diabetes mellitus entered in the regression equation. The results showed that LDL-C (OR = 1.440, 95% CI: 1.040–1.994, p = 0.028) and LVEF (OR = 0.971, 95% CI: 0.957–0.986, p < 0.001) were independent factors correlated with higher Lp(a) in patients with hypertension and without CAD.
Association between LVEF and Lp(a)
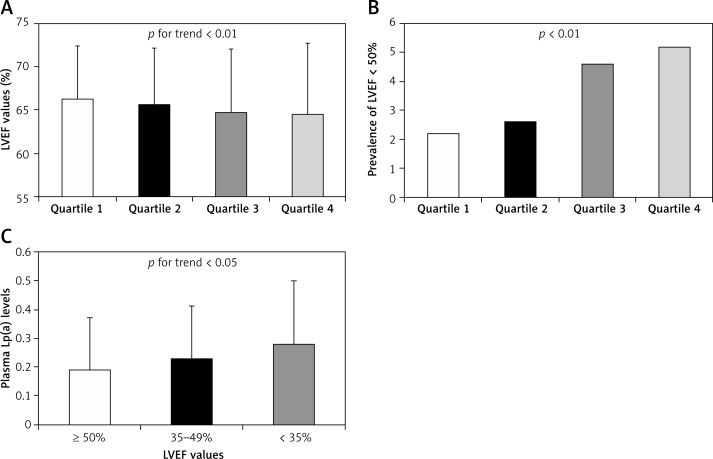
The participants were classified into four groups according to plasma Lp(a) levels. The Lp(a) ranges in quartile 1, quartile 2, quartile 3, and quartile 4 were < 0.07 g/l, 0.07–0.13 g/l, 0.14–0.24 g/l, and > 0.24 g/l, respectively. The LVEF decreased with increasing plasma Lp(a) levels (66.3 ±6.19% vs. 65.7 ±6.43% vs. 64.8 ±7.30% vs. 64.5 ±8.21%, p for trend = 0.002, Figure 1 A). At the same time, the prevalence of LVEF < 50% increased with Lp(a) quartiles (2.2% vs. 2.6% vs. 4.6% vs. 5.2%, p for trend = 0.008, Figure 1 B). The prevalence of LVEF < 50% in patients of quartile 4 was increased by 2.4-fold compared with that in patients of quartile 1.
Figure 1.
Association between LVEF and Lp(a). A – LVEF values in patients with different Lp(a) quartiles. The Lp(a) ranges in quartile 1, quartile 2, quartile 3, and quartile 4 were < 0.07 g/l, 0.07–0.13 g/l, 0.14–0.24 g/l, and > 0.24 g/l, respectively. N = 367 for quartile 1, 470 for quartile 2, 370 for quartile 3, and 404 for quartile 4. P for trend < 0.01. B – Prevalence of LVEF < 50% in patients with different Lp(a) quartiles. P < 0.01 among four group. C – Plasma Lp(a) levels in patients with different LVEF categories (< 35%, 35–49%, ≥ 50%). N = 1553 for LVEF < 35%, 38 for LVEF of 35–49%, and 20 for LVEF ≥ 50%. P for trend < 0.05
In order to explore the plasma Lp(a) levels with different severity of heart dysfunction, we divided the participants into three groups according to LVEF (≥ 50%, 35–49%, and < 35%). We found that plasma Lp(a) levels were further increased with more severely impaired LVEF (0.19 ±0.18 g/l vs. 0.23 ±0.18 g/l vs. 0.28 ±0.22 g/l, p = 0.031, Figure 1 C).
Independent correlation between Lp(a) and LVEF
Multiple linear regression analysis was used to evaluate the independent factors associated with LVEF. The plasma Lp(a) levels entered the regression analysis as a linear variable. Other variables identified as statistically significant in the univariate analysis (EF < 50% vs. EF ≥ 50%) also entered the regression equation. Age and sex were also included as important demographic characteristics. Furthermore, factors associated with plasma Lp(a) levels also entered the regression equation. Finally, various factors including age, male sex, plasma Lp(a) levels, serum creatinine levels, plasma HDL-C levels, plasma LDL-C levels, and hemoglobin were included in the multiple linear regression analysis. The results indicated that plasma Lp(a) levels, male sex, and serum creatinine levels were independent factors correlated with LVEF in patients with hypertension and without CAD (Table II).
Table II.
Factors associated with LVEF using multiple linear regression analysis
| Variables | Standard β coefficient | β coefficient | β coefficient’s 95% CI | P-value |
|---|---|---|---|---|
| Age [years] | 0.034 | 0.025 | –0.012 to 0.062 | 0.188 |
| Male (1 = yes, 0 = no) | –0.116 | –1.616 | –2.473 to –0.759 | < 0.001 |
| Serum creatinine [μmol/l] | –0.064 | –0.013 | –0.023 to –0.003 | 0.013 |
| HDL-C [mmol/l] | 0.010 | 0.228 | –0.919 to 1.375 | 0.697 |
| LDL-C [mmol/l] | 0.018 | 0.160 | –0.278 to 0.599 | 0.474 |
| Hemoglobin [g/l] | –0.019 | –0.009 | –0.035 to 0.018 | 0.525 |
| Lp(a) [g/l] | –0.081 | –3.106 | –4.970 to –1.242 | 0.001 |
HDL-C – high-density lipoprotein cholesterol, LDL-C – low-density lipoprotein cholesterol, Lp(a) – lipoprotein(a), HbA1c – glycated hemoglobin.
Independent correlation between Lp(a) and LVEF < 50%
Independent factors associated with LVEF < 50% were evaluated using multiple logistic regression analysis. The plasma Lp(a) levels entered the logistic regression analysis as a linear variable or as quartiles respectively (model 1 or model 2). Age, sex, and other variables identified as statistically significant in the univariate analysis were also included in the regression equation. The regression analysis in model 1 indicated that plasma Lp(a) levels (OR = 5.566, 95% CI: 1.745–17.758, p = 0.004) and male sex were independent factors correlated with LVEF < 50% in hypertensive patients. At the same time, plasma Lp(a) levels (quartile 4: OR = 3.234, 95% CI: 1.290–8.105, p = 0.012, quartile 1 as reference), male sex, and serum creatinine levels were independently correlated with LVEF < 50% in model 2 (Table III).
Table III.
Adjusted OR of factors associated with LVEF < 50% using multiple logistic regression analysis
| Variables | OR (95% CI) | P-value | Variables | OR (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Model 1: | Model 2: | |||||
| Age [years] | 0.993 (0.964–1.022) | 0.628 | Age [years] | 0.992 (0.963–1.022) | 0.588 | |
| Male (1 = yes, 0 = no) | 3.996 (1.800–8.868) | 0.001 | Male (1 = yes, 0 = no) | 3.681 (1.666–8.135) | 0.001 | |
| Serum creatinine [μmol/l] | 1.004 (1.000–1.008) | 0.055 | Serum creatinine [μmol/l] | 1.004 (1.000–1.008) | 0.036 | |
| HDL-C [mmol/l] | 0.784 (0.283–2.170) | 0.639 | HDL-C [mmol/l] | 0.715 (0.257–1.990) | 0.521 | |
| LDL-C [mmol/l] | 0.980 (0.689–1.394) | 0.910 | LDL-C [mmol/l] | 0.963 (0.676–1.371) | 0.834 | |
| Hemoglobin [g/l] | 1.006 (0.987–1.026) | 0.528 | Hemoglobin [g/l] | 1.007 (0.988–1.027) | 0.468 | |
| Lp(a) [g/l]: | 5.566 (1.745–17.758) | 0.004 | Lp(a): | – | 0.030 | |
| – | Quartile 1 | Reference | ||||
| – | Quartile 2 | 1.487 (0.560–3.951) | 0.426 | |||
| – | Quartile 3 | 2.746 (1.083–6.963) | 0.033 | |||
| – | Quartile 4 | 3.234 (1.290–8.105) | 0.012 | |||
HDL-C – high-density lipoprotein cholesterol, LDL-C – low-density lipoprotein cholesterol, Lp(a) – lipoprotein(a), HbA1c – glycated hemoglobin. Lp(a) entered the regression equation as a continuous variable (g/l) in model 1. Lp(a) quartiles also entered the regression equation as a categorical variable in model 2 (quartile 1 as reference).
Independent correlation between Lp(a) and LVEF categories
In order to investigate which factors were independently correlated with the severity of left ventricular systolic dysfunction, ordinal logistic regression analysis was used to evaluate the correlation between various factors and different LVEF categories (≥ 50%, 35–49%, and < 35%). Age, male sex, plasma Lp(a) levels, serum creatinine levels, plasma HDL-C levels, plasma LDL-C levels, and hemoglobin (as in the above-described multiple linear regression analysis) were included in the ordinal logistic regression analysis. The results showed that plasma Lp(a) levels (OR = 5.760, 95% CI: 1.831–18.120, p = 0.003), male sex, and serum creatinine levels were independently correlated with different LVEF categories (Table IV).
Table IV.
Adjusted OR of factors associated with different LVEF levels using ordinal logistic regression analysis
| Variables | OR (95% CI) | P-value |
|---|---|---|
| Age [years] | 0.992 (0.964–1.021) | 0.603 |
| Male (1 = yes, 0 = no) |
4.007 (1.808–8.891) | 0.001 |
| Serum creatinine [μmol/l] | 1.004 (1.000–1.007) | 0.030 |
| HDL-C [mmol/l] | 0.772 (0.279–2.138) | 0.618 |
| LDL-C [mmol/l] | 0.984 (0.693–1.398) | 0.928 |
| Hemoglobin [g/l] | 1.006 (0.987–1.026) | 0.517 |
| Lp(a) [g/l] | 5.760 (1.831–18.120) | 0.003 |
HDL-C – high-density lipoprotein cholesterol, LDL-C – low-density lipoprotein cholesterol, Lp(a) – lipoprotein(a), HbA1c – glycated hemoglobin. Lp(a) was included in the regression equation as a continuous variable [g/l] to evaluate the association with different LVEF categories (variable assignment: ≥ 50% as 0, 35–49% as 1, and < 35% as 2).
Discussion
The incidence, prevalence of and overall mortality from ischemic heart disease and chronic heart failure (CHF) have been increasing in recent decades [8, 10–13]. Hypertension, alone or in combination with ischemic heart disease, precedes the development of heart failure and causes more deaths. The relative risk of CHF among patients with hypertension is significantly higher than in the general population [14]. Hypertension is one of the most important modifiable risk factors for CHF [15]. Hypertension and CHF are both major public health problems in China. The prevalence of LVEF < 50% in Chinese hypertensive patients without CAD is 3.6% in this study. Thus, heart dysfunction in hypertensive patients should receive more attention.
In this study, a higher percentage of hypertensive patients with LVEF < 50% were men, and had lower plasma HDL-C levels, but higher plasma Lp(a), serum creatinine, and hemoglobin levels than those with EF ≥ 50%. The data from the Framingham Heart Study also indicated that male sex significantly predicted the new onset of heart failure and reduced ejection fraction (HFREF) during an 8-year follow-up [16]. Chronic kidney disease (CKD) was very common in CHF and was independently associated with long-term adverse outcomes in some cohort studies [17, 18]. Large epidemiological studies revealed that more than 30% of patients hospitalized for decompensated HF have a glomerular filtration rate (GFR) < 60 ml/min/1.73 m2 [19, 20]. Numerous studies have confirmed the role of inflammation in both ischemic and non-ischemic heart failure. The HDL-C is anti-inflammatory and may help to improve heart failure. A previous study showed that HDL-C was decreased in patients with advanced heart failure waiting for heart transplant [21]. Framingham Heart Study also suggested that low HDL-C and a high hematocrit can predict incident cardiac failure during long periods of follow-up [22].
In this study, Lp(a) was one of the significant factors associated with left ventricular dysfunction. Lp(a) was independently correlated with EF, EF < 50%, or different EF categories respectively after adjusting for other potential confounders. The Lp(a) was proved to be strongly associated with CAD and MI [4, 5], which can be promoted by hypertension and can increase the onset of heart failure. Thus, Lp(a) might be correlated with left ventricular dysfunction in hypertensive patients by promoting the development of ischemic heart disease. However, we excluded CAD and MI patients in this study. It means that the association between Lp(a) and heart dysfunction in this Chinese population of hypertensive patients is possibly independent from ischemic heart disease. In fact, a previous study showed that the Lp(a) levels in age- and sex-matched normal people were significantly lower than those of either the CHF or the heart transplant recipients, who included both ischemic and non-ischemic heart disease patients [21]. But the sample size was small in that study, and multiple regression analysis was not used to evaluate the independent correlation between Lp(a) and heart failure. However, we evaluated the independent association between Lp(a) and non-ischemic heart systolic dysfunction in hypertensive patients in this study. Also, a recently published study demonstrated that elevated Lp(a) levels and corresponding LPA risk genotypes were associated with an increased risk of heart failure consistent with a causal association in a general population. The association appeared to be partly mediated by myocardial infarction and aortic valve stenosis [23, 24]. However, the authors could not exclude an additional contribution from Lp(a), also acting via other presently unknown mechanisms that affect cardiac function independently of CAD and aortic valve stenosis. They also speculated that high Lp(a) levels may possibly lead to increased arterial stiffness, including vascular noncompliance in the aorta, which will increase afterload, and which had been strongly associated with increased risk of HF. Thus, we speculate that the association between high Lp(a) and low LVEF was possibly mediated by increased arterial stiffness, aortic valve stenosis, or other unknown mechanisms in our study population.
Our study should also be interpreted within the context of its limitations. First, this is a cross-sectional study, so a causal relationship between Lp(a) and heart systolic dysfunction remains to be confirmed. Pathophysiological mechanisms of the association between Lp(a) and LVEF are unknown and need further studies. Second, although the overall population size is reasonable, the sample size of subjects with low LVEF is rather small, which might impair the power. Third, subjects had received hypotensive drugs before blood pressure was measured. Thus, blood pressure in this study cannot be considered as a good index of hypertension severity. It is a limitation that we did not include any measure of the severity of hypertension.
In conclusion, LVEF decreases with increasing plasma Lp(a) levels. Lp(a) is independently correlated with left ventricular systolic dysfunction in patients with hypertension and without CAD.
Acknowledgments
Yong Wang and Heng Ma contributed equally to this work.
We are grateful to the subjects who participated in the study, and for the physicians’ assistance in this study.
This study was sponsored by the National Natural Science Foundation of China (81500335, 81401385, and 81571636), Shanghai Natural Science Foundation (13ZR1433500), and the 56th Project Funded by the China Postdoctoral Science Foundation (2014M560346).
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–53. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dube JB, Boffa MB, Hegele RA, Koschinsky ML. Lipoprotein(a): more interesting than ever after 50 years. Curr Opin Lipidol. 2012;23:133–40. doi: 10.1097/MOL.0b013e32835111d8. [DOI] [PubMed] [Google Scholar]
- 3.Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 4.Wang XL, Tam C, McCredie RM, Wilcken DE. Determinants of severity of coronary artery disease in Australian men and women. Circulation. 1994;89:1974–81. doi: 10.1161/01.cir.89.5.1974. [DOI] [PubMed] [Google Scholar]
- 5.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–9. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 6.Aksoy M, Kepekçi Y, Göktekin O, et al. Relation of plasma lipoprotein(a) with myocardial viability and left ventricular performance in survivors of myocardial infarction. Jpn Heart J. 1999;40:703–13. doi: 10.1536/jhj.40.703. [DOI] [PubMed] [Google Scholar]
- 7.Kannan A, Janardhanan R. Hypertension as a risk factor for heart failure. Curr Hypertens Rep. 2014;16:447. doi: 10.1007/s11906-014-0447-7. [DOI] [PubMed] [Google Scholar]
- 8.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60:1455–69. doi: 10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 9.Catena C, Novello M, Lapenna R, et al. New risk factors for atherosclerosis in hypertension: focus on the prothrombotic state and lipoprotein(a) J Hypertens. 2005;23:1617–31. doi: 10.1097/01.hjh.0000178835.33976.e7. [DOI] [PubMed] [Google Scholar]
- 10.Qi X, Xu M, Yang H, et al. Comparing mortality and myocardial infarction between coronary artery bypass grafting and drug-eluting stenting in patients with diabetes mellitus and multivessel coronary artery disease: a meta-analysis. Arch Med Sci. 2014;10:411–8. doi: 10.5114/aoms.2014.43734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Qu P, Zhao J, Chang Y. NLRP3 and downstream cytokine expression elevated in the monocytes of patients with coronary artery disease. Arch Med Sci. 2014;10:791–800. doi: 10.5114/aoms.2014.44871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodani S, Dong L, Guirgis FW, Reddy ST. Carotid intima media thickness and low high-density lipoprotein (HDL) in South Asian immigrants: could dysfunctional HDL be the missing link? Arch Med Sci. 2014;10:870–9. doi: 10.5114/aoms.2014.46208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liszka J, Haberka M, Tabor Z, Finik M, Gąsior Z. Two-dimensional speckle-tracking echocardiography assessment of left ventricular remodeling in patients after myocardial infarction and primary reperfusion. Arch Med Sci. 2014;10:1091–100. doi: 10.5114/aoms.2014.47821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorobantu M, Tautu OF, Darabont R, et al. Objectives and methodology of Romanian SEPHAR II Survey. Project for comparing the prevalence and control of cardiovascular risk factors in two East-European countries: Romania and Poland. Arch Med Sci. 2015;11:715–23. doi: 10.5114/aoms.2015.53290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANESI epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 16.Meredith PA, Ostergren J. From hypertension to heart failure are there better primary prevention strategies? J Renin Angiotensin Aldosterone Syst. 2006;7:64–73. doi: 10.3317/jraas.2006.012. [DOI] [PubMed] [Google Scholar]
- 17.Ho JE, Lyass A, Lee DS, et al. Predictors of new-onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–86. doi: 10.1161/CIRCHEARTFAILURE.112.972828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, et al. Chronic kidney disease as an independent risk for long-term adverse outcomes in patients hospitalized with heart failure in Japan. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J. 2009;73:1442–7. doi: 10.1253/circj.cj-09-0062. [DOI] [PubMed] [Google Scholar]
- 19.Zamora E, Lupón J, Vila J, et al. Estimated glomerular filtration rate and prognosis in heart failure: value of the Modification of Diet in Renal Disease Study-4, chronic kidney disease epidemiology collaboration, and Cockroft-Gault formulas. J Am Coll Cardiol. 2012;59:1709–15. doi: 10.1016/j.jacc.2011.11.066. [DOI] [PubMed] [Google Scholar]
- 20.Heywood JT, Fonarow GC, Costanzo MR, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–30. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Fonarow GC, Abraham WT, Albert NM, et al. Influence of a performance-improvement initiative on quality of care for patients hospitalized with heart failure: results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF) Arch Intern Med. 2007;167:1493–502. doi: 10.1001/archinte.167.14.1493. [DOI] [PubMed] [Google Scholar]
- 22.Cooke GE, Eaton GM, Whitby G, et al. Plasma atherogenic markers in congestive heart failure and posttransplant (heart) patients. J Am Coll Cardiol. 2000;36:509–16. doi: 10.1016/s0735-1097(00)00756-7. [DOI] [PubMed] [Google Scholar]
- 23.Kannel WB, Cupples A. Epidemiology and risk profile of cardiac failure. Cardiovasc Drugs Ther. 1988;2(Suppl 1):387–95. [PubMed] [Google Scholar]
- 24.Kamstrup PR, Nordestgaard BG. Elevated lipoprotein(a) levels, LPA risk genotypes, and increased risk of heart failure in the general population. JACC Heart Fail. 2016;4:78–87. doi: 10.1016/j.jchf.2015.08.006. [DOI] [PubMed] [Google Scholar]