Abstract
Introduction
The aim of the study was to evaluate the impact of individual training on the level of physical capacity and echocardiographic parameters in patients with systolic heart failure (SHF), NYHA III and an implantable cardioverter-defibrillator (ICD).
Material and methods
The study included 84 patients with SHF, randomly assigned to one of two groups: with regular training (ICD-Ex) and a control group (ICD-control). The ICD-Ex group participated in a hospital rehabilitation program which after discharge was individually continued for 6 months in an outpatient setting. The ICD-control group participated in a training program during hospitalization, but after discharge did not perform any controlled activities. Prior to discharge, at 6 and 18 months cardiopulmonary exercise testing (CPX), standard echocardiographic examination and the 6-minute walk test (6-MWT) were performed in all patients.
Results
After 18 months in the ICD-Ex group most of the CPX parameters improved significantly (VO2 peak, ml/kg/min: 13.0 ±4.1 vs. 15.9 ±6.1, p < 0.0017; VCO2 peak, l/min: 1.14 ±0.34 vs. 1.58 ±0.65, p < 0.0008; Watt: 74.5 ±29.7 vs. 92.6 ±39.1, p < 0.0006; METs 3.72 ±1.81 vs. 4.35 ±1.46, p < 0.0131). In the ICD-control group no significant improvement of any parameter was observed. Left ventricular systolic dimensions remained significantly lower at 18 months only in the ICD-Ex group (49.5 ±11.0 vs. 43.4 ±10.0, p < 0.011). Left ventricular ejection fraction in both groups significantly increased at 6 and 18 months compared to baseline (ICD-Ex: 25.07 ±5.4 vs. 31.4 ±9.2, p < 0.001, vs. 30.9 ±8.9, p < 0.002, ICD-C: 25.1 ±8.3 vs. 29.2 ±7.7, p < 0.012 vs. 30.1 ±9.1, p < 0.005). Distance of the 6-MWT was significantly improved after 6 and 18 months in the ICD-Ex group and was overall longer than in the ICD-control group (491 ±127 vs. 423 ±114 m, p < 0.04).
Conclusions
An individual, 6-month training program, properly controlled in patients with SHF and an implanted ICD, was safe and resulted in a significant improvement of exercise tolerance and capacity and echocardiographic parameters.
Keywords: systolic heart failure, implantable cardioverter-defibrillator, training programs
Introduction
European Society of Cardiology (ESC) guidelines recommend systematic physical activity, together with medical treatment and optional device implantation, as an integral part of systolic heart failure (SHF) therapy [1]. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity and quality of life in patients with chronic heart failure [2]. Deficiency of individually tailored, systematic physical activity may lead to further limitation of patients’ physical performance and worsen the prognosis. Furthermore, modern therapy of SHF often includes implantable medical devices: cardiac resynchronization therapy (CRT) and implantable cardioverter-defibrillators (ICD) [3]. The effectiveness of rehabilitation in these patients has already been studied, but the issue of safety and optimal training methods needs to be further clarified, especially in patients with implantable devices. Cardiopulmonary exercise testing (CPX) is a valuable clinical tool of heart failure patients’ evaluation [4]. Treatment of these patients should be complex and associated with everyday care. The aim of the present study was to evaluate the safety and impact of individual training on the level of physical capacity and echocardiographic parameters in patients with SHF in functional class III of the New York Heart Association (NYHA) and an implanted cardioverter-defibrillator in primary prevention.
Material and methods
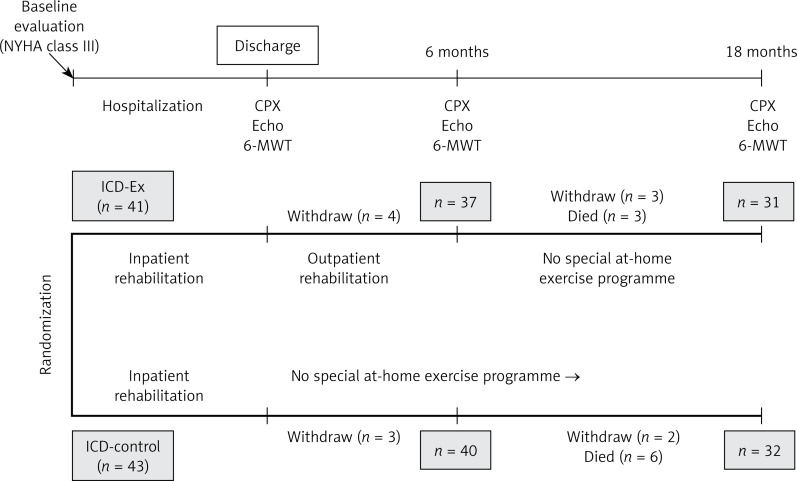
The study initially included 84 patients with SHF and ICD (primary prevention), mean time 2.1 years, hospitalized for worsening of SHF to NYHA III/IV and NYHA IV (Figure 1). According to the New York Heart Association the definitions of NYHA classes are as follows: NYHA III, marked limitation of physical activity, comfortable at rest, less than ordinary activity causes fatigue, palpitation, or dyspnea; NYHA IV, unable to carry on any physical activity without discomfort, symptoms of heart failure at rest. There were no pacemaker-dependent patients in the study groups. The inclusion criteria were: SHF of ischemic or non-ischemic etiology, implanted ICD, low left ventricular ejection fraction (LVEF ≤ 35%), controlled hypertension and diabetes. All patients provided signed informed consent to participate in the study. Exclusion criteria were: resynchronization therapy, possible indications for CRT in the future (left bundle branch block, QRS > 150 ms), severe musculoskeletal conditions which preclude physical rehabilitation (orthopedic, neurological), planned cardiac surgery or percutaneous coronary interventions, cardiac surgery or coronary angioplasty within the last 3 months, acute coronary syndromes, stroke or transient ischemic attack (TIA) within the last 6 months, venous thrombosis or pulmonary embolism in the past, significant valvular disease, history of malignancy. All patients enrolled in the study were randomly assigned to a 6-month rehabilitation program (ICD-Ex group) and standard SHF care (ICD-control). Before the discharge patients received guidelines on a healthy lifestyle (physical activity, diet). The hospital rehabilitation program included: dynamic exercise of small and large muscle groups, isometric exercise of small muscle groups, coordination, stretching, breathing, exercise to reduce swelling of the lower extremities (according to Ratschow and Buerger protocols) and walking. The duration of exercise lasted from 5 to 20 min. Exercises were carried out 5 times a week, the time depending on the capability of the patient (4–8 repetitions of exercises, with 5–30-second pauses between exercises). The positions of exercises (sitting on the bed, sitting with legs lowered) depended on the efficiency and patient’s preference. ICD-Ex patients underwent interval workouts with a cycloergometer and conditioning exercises with elements of resistance exercises (3 times a week) after discharge. In each patient evaluation of exercise intensity in endurance training was based on maximal heart rate. Training heart rate was evaluated according to the Karvonen formula. In patients with atrial fibrillation the load percentage was compared with the Borg scale. If the subjective patient evaluation of workload was 5 on a 10-point scale, training was stopped. Cycloergometer training lasted on average 30 min. Patients exercised with a speed of 55 to 64 revolutions per minute. Resistance training lasted from 5 to 10 min, with exercise involving a single muscle group of one limb – alternately – with a load of up to 50% of muscle strength. During the working phase lasting 1 min, the patients performed 10–12 repetitions in one cycle. Prior to discharge, at 6 and 18 months after discharge, CPX was performed using a bicycle ergometer according to the Ramp 10 W protocol in all patients (load increase of 10 watts per 1 min – 1 watt per 6 s). The analysis included intensity of exercise which was measured in metabolic equivalents (METs, 1 MET: the amount of energy expended at rest or 3.5 ml of oxygen per kilogram per minute), work load (watts, W), duration of the test (min), peak oxygen consumption (peak VO2), peak carbon dioxide excretion (peak VCO2), ventilation equivalent for oxygen (VE/VO2) and carbon dioxide (VE/VCO2), and ventilation anaerobic threshold (VAT). Standard echocardiographic examination and the 6-minute walk test (6-MWT) were performed in each patient.
Figure 1.
Study design
ICD-Ex – rehabilitation group, ICD-control – control group.
The study was supported by the State Committee for Scientific Research (grant NN 404171934) and performed at the Institute of Cardiology, Warsaw, Poland, between January 2008 and December 2011. The design and protocol of the study were accepted by the Institutional Review Board (registration number IK-NP-0021-5/996/07).
Statistical analysis
Since continuous variables are normally distributed, they are expressed as means and standard deviations. Categorical variables are reported as absolute frequencies and percentages.
Comparisons according to demographic and clinical profile were made using the χ2 test and Student’s t-test. The impact of individual training on the level of physical capacity in CPX and echocardiographic parameters was assessed by multiple analysis of variance (MANOVA). If the results of analysis yielded statistical significance, preplanned comparisons were performed. We focused more on two specific comparisons, not on every possible one, according to our pre-planned a priori hypothesis. Simple contrast was used, where the first measures were at the reference levels. All probability values were calculated from two-sided tests, and a p-value < 0.005 was considered statistically significant. All statistical analyses were performed using SAS software.
Results
The study groups did not differ in terms of demographic and clinical data. The mean age of the patients was relatively low (63.7; 61.1 years, NS). Cardiovascular risk factors were typical. Ischemic etiology of SHF was diagnosed in both groups with a comparable incidence: ICD-Ex: 87.8% vs. ICD-control: 69.8%, NS. Non-ischemic etiology of SHF was present in, respectively, 12.2% and 30.2% of patients, ns. Study groups did not differ in the incidence of comorbidities, coronary artery disease risk factors and interventions, or echocardiographic and physical activity parameters (Table I). In the ICD-Ex group 14 (34.1%) and in the ICD-control group 23 (53.5%) patients had atrial fibrillation (AF). All patients were optimally treated, according to current guidelines (Table I). Medical therapy was not significantly modified during the study period. At baseline the ICD-Ex group consisted of 41 patients, 5 of them women, and the ICD-Control group consisted of 43 patients, 3 of them women.
Table I.
Demographic and clinical characteristics of study groups at inclusion (ICD-Ex: n = 41, ICD-control: n = 43) and after completion of the study (ICD-Ex: n = 31, ICD-control: n = 32)
| Parameter | ICD-Ex (n = 41) | ICD-control (n = 43) | P-value | ICD-Ex (n = 31) | ICD-control (n = 32) | P-value |
|---|---|---|---|---|---|---|
| Age [years] | 63.7 ±9.5 | 61.1 ±9.7 | 0.2074 | 62.4 ±8.8 | 61.9 ±8.1 | 0.7956 |
| Men, n (%) | 36 (87.8) | 40 (93.0) | 0.4779 | 27 (87.1) | 30 (93.8) | 0.4258 |
| Body mass [kg] | 86.0 ±17.7 | 83.8 ±16.6 | 0.5528 | 84.2 ±18.3 | 85.6 ±17.4 | 0.7566 |
| BMI [kg/m2] | 28.3 ±5.1 | 28.2 ±5.0 | 0.8770 | 27.7 ±4.9 | 28.7 ±5.2 | 0.4485 |
| Coronary disease, n (%) | 36 (87.8) | 30 (69.8) | 0.0440 | 27 (87.1) | 23 (71.9) | 0.1355 |
| Heart infarct, n (%) | 32 (78.1) | 26 (60.5) | 0.0814 | 22 (71.0) | 19 (59.4) | 0.3346 |
| PCI, n (%) | 24 (58.5) | 19 (44.2) | 0.1884 | 16 (51.6) | 15 (46.9) | 0.7069 |
| CABG, n (%) | 6 (14.6) | 6 (14.0) | 0.9290 | 5 (16.1) | 5 (15.6) | 1.0000 |
| Hypertension, n (%) | 27 (65.9) | 24 (55.8) | 0.3463 | 20 (64.5) | 17 (53.1) | 0.3585 |
| Diabetes, n (%) | 13 (31.7) | 15 (34.9) | 0.7576 | 9 (29.0) | 12 (37.5) | 0.4760 |
| Hyperlipidemia, n (%) | 27 (65.9) | 24 (55.8) | 0.3463 | 19 (61.3) | 21 (65.6) | 0.7209 |
| Stroke, n (%) | 4 (9.8) | 0 (0) | 0.0525 | 3 (9.7) | 0 (0) | 0.1132 |
| TIA, n (%) | 2 (4.9) | 3 (7.0) | 1.0000 | 2 (6.4) | 2 (6.3) | 1.0000 |
| Atrial fibrillation, n (%): | ||||||
| Paroxysmal | 9 (21.9) | 16 (37.2) | 0.1930 | 7 (22.6) | 12 (37.5) | 0.1692 |
| Permanent | 5 (12.2) | 7 (16.3) | 3 (9.7) | 6 (18.8) | ||
| β-Blockers, n (%) | 41 (100) | 43 (100) | 1.0000 | 31 (100) | 32 (100) | 1.0000 |
| ACE-I, n (%) | 33 (80.5) | 37 (86.1) | 0.4944 | 24 (77.4) | 27 (84.4) | 0.4821 |
| ARB, n (%) | 8 (19.5) | 7 (16.3) | 0.6989 | 7 (22.6) | 5 (15.6) | 0.4821 |
| Calcium antagonists, n (%) | 8 (19.5) | 5 (11.6) | 0.3179 | 5 (16.1) | 4 (12.5) | 0.7323 |
| Diuretics, n (%) | 39 (95.1) | 39 (90.7) | 0.6764 | 30 (96.8) | 30 (93.7) | 1.0000 |
| Loop diuretics, n (%) | 34 (82.9) | 36 (83.7) | 0.9222 | 26 (83.9) | 28 (87.5) | 0.7323 |
| Thiazide diuretics, n (%) | 6 (14.6) | 7 (16.3) | 0.8349 | 4 (12.9) | 5 (15.6) | 1.0000 |
| Spironolactone, n (%) | 25 (61.0) | 28 (65.1) | 0.6942 | 20 (64.5) | 22 (68.7) | 0.7215 |
| Eplerenone, n (%) | 10 (24.4) | 7 (16.3) | 0.3550 | 8 (25.8) | 5 (15.6) | 0.3181 |
| Statins, n (%) | 35 (85.4) | 30 (69.8) | 0.0876 | 25 (80.6) | 25 (78.1) | 0.8048 |
| Fibrates, n (%) | 4 (9.8) | 3 (7.0) | 0.7096 | 3 (9.7) | 1 (3.1) | 0.3547 |
| Digoxin, n (%) | 13 (31.7) | 12 (27.9) | 0.7034 | 8 (25.8) | 8 (25.0) | 0.9414 |
| Oral anti-coagulants, n (%) | 11 (26.8) | 20 (46.5) | 0.0617 | 10 (32.3) | 17 (53.1) | 0.0943 |
| Oral antidiabetic drugs, n (%) | 11 (26.8) | 9 (20.9) | 0.5257 | 7 (22.6) | 6 (18.8) | 0.7072 |
| Insulin, n (%) | 1 (2.4) | 8 (18.6) | 0.0298 | 1 (3.2) | 8 (25.0) | 0.0265 |
| Antiplatelets drugs, n (%) | 32 (78.1) | 27 (62.8) | 0.1263 | 23 (74.2) | 17 (53.1) | 0.0825 |
| ASA, n (%) | 31 (75.6) | 24 (55.8) | 0.0565 | 23 (74.2) | 14 (43.8) | 0.0141 |
| Ticlopidine, n (%) | 0 (0.0) | 0 (0.0) | NS | 0 (0.0) | 0 (0.0) | NS |
| Clopidogrel, n (%) | 11 (26.8) | 10 (23.31) | 0.7054 | 7 (22.6) | 7 (21.9) | 0.9463 |
ICD-Ex – rehabilitation group, ICD-control – control group, BMI – body mass index, PCI – percutaneous coronary intervention, CABG – coronary artery bypass graft, TIA – transient ischemic attack, ACE-I – angiotensin converting enzyme-inhibitors, ARB – angiotensin receptor blockers, ASA – acetylsalicylic acid.
In the ICD-Ex group 31 patients completed the study (from the initial 41) due to withdrawing consent (7 patients), and deaths (3 patients). In the ICD-control group 32 patients completed the study from the initial 43 – there were 5 cases of withdrawing consent and 6 deaths (5 cardiovascular).
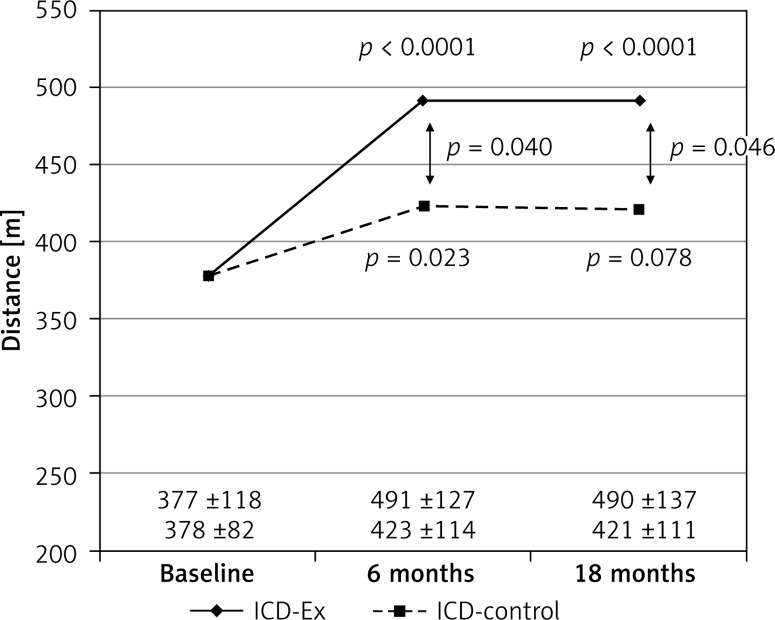
At baseline the study groups did not differ in walking distance. In the ICD-Ex group walking distance was significantly higher after 6 months compared to baseline, but at 18 months it remained at the same level. In the control group, walking distance did not change after 6 and 18 months (Figure 2). Baseline CPX results did not differ between groups. After 6 months of training and 18 months later, most of the CPX parameters improved significantly in the ICD-Ex group: peak VO2, p = 0.017, peak VO2 (%), p = 0.0025, peak VCO2, p = 0.0002, work time, p = 0.0083, work load, p = 0.0057, METs, p = 0.047 and after 18 months VE/VCO2, p = 0.044 (Table II). In the ICD-control group there was no significant improvement in any of the studied parameters. In conclusion, there were significant differences in favor of the ICD-Ex group in peak VO2, peak VO2 (%), VCO2 peak, exercise time, and intensity of exercise (METs) both at 6 and 18 months (Table II).
Figure 2.
Comparison of walking distance in 6-MWT before and after training program (6 months) and after 18-month follow-up
ICD-Ex – rehabilitation group, ICD-control – control group, 6-MWT – 6-minute walk test.
Table II.
Spiroergometric parameters baseline, 6 and at 18 months of follow-up
| Parameter | ICD-Ex | ICD-Control | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (1) | 6 months (2) | 18 months (3) | MANOVA | (2) vs. (1) | (3) vs. (1) | Baseline (1) | 6 months (2) | 18 months (3) | MANOVA | (2) vs. (1) | (3) vs. (1) | |
| VO2 peak [ml/kg/min] | 13.0 ±4.1 | 15.6 ±6.0 | 15.9 ±6.1 | 0.0026 | 0.0017 | 0.0017 | 12.5 ±4.5 | 12.5 ±4.4* | 12.4 ±4.3* | 0.986 | 0.941 | 0.966 |
| VO2 peak (%) | 50.6 ±14.7 | 61.3 ±22.3 | 61.3 ±22.6 | 0.0068 | 0.0025 | 0.0088 | 47.1 ±19.0 | 48.0 ±18.1* | 48.6 ±17.8* | 0.918 | 0.800 | 0.687 |
| VCO2 peak [l/min] | 1.14 ±0.34 | 1.47 ±0.58 | 1.58 ±0.65 | 0.0007 | 0.0002 | 0.0008 | 0.97 ±0.32 | 1.01 ±0.37** | 1.05 ±0.42* | 0.691 | 0.601 | 0.409 |
| VE/VCO2 | 37.6 ±5.7 | 35.6 ±7.9 | 32.0 ±9.0 | 0.118 | 0.246 | 0.044 | 38.8 ±8.1 | 33.1 ±6.0 | 31.6 ±5.6 | 0.160 | 0.150 | 0.052 |
| AT [ml/kg/min] | 13.2 ±4.2 | 14.2 ±6.1 | 13.0 ±4.1 | 0.792 | 0.562 | 0.891 | 13.7 ±3.4 | 12.3 ±2.2 | 11.5 ±3.2 | 0.403 | 0.198 | 0.214 |
| AT %VO2 | 47.8 ±13.4 | 52.6 ±21.1 | 48.6 ±15.9 | 0.722 | 0.416 | 0.880 | 45.9 ±14.7 | 42.3 ±11.2 | 40.3 ±14.7 | 0.599 | 0.304 | 0.400 |
| Time [min] | 9.4 ±4.1 | 10.9 ±4.7 | 9.90 ±4.0 | 0.0219 | 0.0083 | 0.416 | 6.9 ±3.0* | 7.9 ±3.2* | 7.6 ±2.7* | 0.234 | 0.095 | 0.321 |
| Work load [W] | 74.5 ±29.7 | 89.8 ±38.1 | 92.6 ±39.1 | 0.0033 | 0.0057 | 0.0006 | 65.2 ±22.4 | 72.9 ±27.2 | 74.5 ±28.1 | 0.245 | 0.090 | 0.116 |
| METs | 3.72 ±1.81 | 4.23 ±1.52 | 4.35 ±1.46 | 0.0494 | 0.0477 | 0.0131 | 3.57 ±1.41 | 3.38 ±1.07* | 3.43 ±1.03* | 0.692 | 0.430 | 0.599 |
ICD-Ex – rehabilitation group, ICD-control – control group.
p < 0.05
p < 0.005 ICD-EX vs. ICD-control, appropriately 6 and 18 months. VO2 peak – peak oxygen uptake, VCO2 peak – peak carbon dioxide elimination, AT – anaerobic threshold, VE/VCO2 – ventilatory equivalent ratio for carbon dioxide, METs – metabolic equivalents, HR – heart rate.
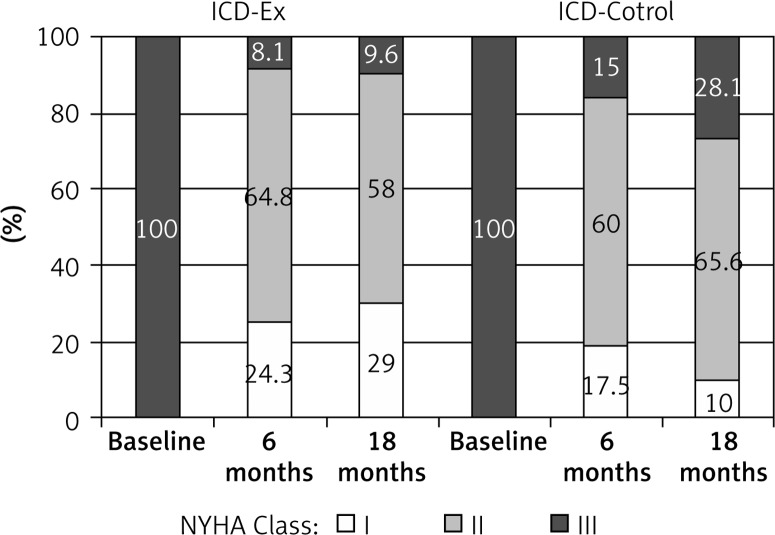
In the ICD-Ex and ICD-Control groups a significant decrease of systolic and diastolic left ventricular (LV) diameters (after 6 months) was observed. The LV systolic diameter was significantly lower at 18 months in the ICD-Ex group (p = 0.114) but not in the ICD-control group. Right ventricular diastolic dimension in the trained group increased significantly after 6 months (p = 0.035), but after 18 months of the follow-up it returned to baseline. No significant differences between groups were observed (Table III). An increase in LVEF was observed in both groups (ICD-Ex, p = 0.0001, ICD-control p = 0.02). The LVEF remained significantly higher after 18 months in comparison to the baseline (ICD-Ex, p = 0.002, ICD-control, p = 0.005). After 6 and 18 months more patients from the ICD-Ex group were NYHA class I and fewer NYHA class III in comparison to ICD-Control patients (Figure 3). In the ICD-Ex group one incident of appropriate ICD shock (ventricular tachycardia) occurred during observation. No shock occurred during training sessions or exercise tests. In the ICD-control group 5 appropriate ICD shocks (ventricular tachycardia) were reported in 5 patients. There were no significant differences in the incidence of deaths and hospitalizations in the studied groups in 18-month follow-up. In the ICD-Ex group there were 3 cardiac deaths and 50 hospitalizations (in 22 persons, 52.4%). In the ICD-control group there were 6 deaths, including 5 cardiac deaths, and 35 hospitalizations (in 19 patients, 43.2%) during follow-up. Medical therapy was optimal and no major changes had been made during follow-up. Also no differences were detected between groups (NS).
Table III.
Comparison of echocardiographic parameters between the two groups (ICD-Ex) and ICD-control, baseline, 6 and at 18 months of follow-up
| Parameter | ICD-Ex | ICD-Control | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (1) | 6 months (2) | 18 months (3) | MANOVA | P (1) vs. (2) | P (1) vs. (3) | Baseline (1) | 6 months (2) | 18 months(3) | MANOVA | P (1) vs. (2) | P (1) vs. (3) | |
| LVDD [mm] | 64.6 ±9.6 | 61.6 ±7.0 | 61.1 ±10.1 | 0.114 | 0.040 | 0.067 | 64.8 ±7.0 | 61.6 ±6.0 | 62.8 ±6.8 | 0.060 | 0.023 | 0.230 |
| LVSD [mm] | 49.5 ±11.0 | 42.0 ±8.0 | 43.4 ±10.0 | 0.005 | 0.001 | 0.011 | 50.6 ±8.2 | 43.9 ±6.7 | 45.5 ±9.5 | 0.006 | 0.002 | 0.062 |
| IVSD [mm] | 11.5 ±2.4 | 11.0 ±2.1 | 12.2 ±4.8 | 0.183 | 0.203 | 0.481 | 11.3 ±1.9 | 12.0 ±1.9 | 12.0 ±3.9 | 0.265 | 0.100 | 0.455 |
| RVDD [mm] | 29.0 ±4.9 | 31.9 ±4.9 | 31.9 ±5.8 | 0.054 | 0.035 | 0.075 | 30.0 ±6.6 | 31.8 ±6.1 | 31.7 ±6.2 | 0.158 | 0.089 | 0.202 |
| LA [mm] | 46.6 ±5.8 | 45.9 ±7.2 | 45.7 ±7.2 | 0.764 | 0.613 | 0.508 | 47.0 ±7.0 | 44.2 ±6.1 | 44.9 ±7.1 | 0.113 | 0.042 | 0.180 |
| Aorta [mm] | 35.7 ±5.9 | 35.6 ±4.5 | 36.3 ±5.3 | 0.502 | 0.885 | 0.447 | 34.0 ±4.5 | 36.5 ±4.4 | 35.2 ±4.1 | 0.012 | 0.003 | 0.127 |
| EF (%) | 25.07 ±5.4 | 31.4 ±9.2 | 30.9 ±8.9 | 0.001 | 0.001 | 0.002 | 25.1 ±8.3 | 29.2 ±7.7 | 30.1 ±9.1 | 0.017 | 0.012 | 0.005 |
ICD-Ex – rehabilitation group, ICD-control – control group, LVDD – left ventricular end-diastolic dimension, LVSD – left ventricular end-systolic dimension, IVSD – inter-ventricular septal dimension, RVDD – right ventricular end-diastolic dimension, LA – left atrial end-systolic diameter, aorta – ascending aorta diameter, EF – left ventricular ejection fraction.
Figure 3.
NYHA class in study groups (ICD-Ex and ICD-control), evaluated in consecutive control visits
ICD-Ex – rehabilitation group, ICD-control – control group.
Discussion
The belief that a “rest and keep in bed” lifestyle is mandatory in the treatment of systolic heart failure is no longer valid. Existing reports and clinical experience suggest that individually tailored programs of physical rehabilitation in patients with SHF improve the functioning of patients by affecting the central and peripheral mechanisms of cardiovascular disease. According to the current guidelines of the European Society of Cardiology, regular aerobic exercise to improve exercise capacity and tolerance (class I/A) and to reduce the risk of re-hospitalization (class I/A) is recommended in all patients with heart failure, including SHF [1]. Evaluation of the safety and effectiveness of physical training and the development of kinesiotherapeutic techniques in patients with SHF require further studies. The SHF patients’ care should be comprehensive and consist of optimal medical therapy, individually tailored training programs, psychological care and counseling with the recommendations of healthy lifestyle and secondary prevention. Patients with SHF and ICD may also have problems with the implanted devices. Episodes of arrhythmia and ICD interventions along with fear of their recurrence lead to avoidance of physical activity and psychological problems. It is therefore important to persuade the patient to perform supervised, secure and regular physical activity. Exercise may not only improve physical performance but also reduce the level of anxiety and depression and support the feeling of well being. The results of controlled studies in various aspects of physical training in patients with SHF have confirmed the benefits of these forms of therapy. In these studies overall efficiency, neurohormonal biomarker concentrations, and central and peripheral hemodynamic changes were analyzed [5–8]. A meta-analysis of 81 studies in SHF patients (LVEF < 40%) subjected to physical training (cycloergometers and marches) showed the safety of rehabilitation and significant improvement in physical endurance, measured with oxygen uptake, anaerobic threshold and the 6-MWT distance. Smaller benefits were observed in resistance training [9]. In patients who performed regular training, significant improvement in the peak excretion of carbon dioxide (VCO2 peak), duration of exercise and achieved load was observed [6, 9–11]. A few studies confirmed the beneficial effect of exercise to increase oxygen uptake and maximum load and also safety of training (low risk of arrhythmias and ICD interventions) in patients with SHF and a relatively short follow-up [8–15]. But only a few included patients with ICD. In the current study, the results of 6 months of endurance training (interval training) and general conditioning exercises with elements of resistance showed a mean 15% improvement in exercise capacity and peak oxygen uptake compared to the control group. Patients in the ICD-Ex group also achieved a significant improvement in the elimination of carbon dioxide during exercise, time of the exercise and the gained load. In the ICD-control group these parameters deteriorated or remained at the baseline level. The present results also showed a significant increase in the walking distance in the 6-MWT in the ICD-Ex group compared to the ICD-control. The parameters of CPX testing and the results of the 6-MWT confirmed that ICD-Ex patients followed the instructions and training regime for a longer time. After 18 months some parameters in the ICD-Ex group remained at the same significantly higher levels as after 6 months, whereas in the ICD-control group these parameters had not changed. Significant differences in favor of the training group were observed in VO2 peak, peak VO2 (%), VCO2 peak, exercise duration and the value of METs between the groups. An improvement in NYHA class occurred in both groups, but it was larger in the ICD-Ex group. The good clinical performance of ICD-control group patients may be due not only to regular, comprehensive medical care but also to following healthy lifestyle guidelines received during the hospital stay. Significant reduction of systolic and diastolic LV dimensions and LVEF increase were observed in both study groups after completion of training, partly due to optimal medical therapy and good compliance. This is consistent with the results presented by other authors. Hambrecht et al. observed an increase in LVEF from 30% to 35% and a decrease in LV dimensions in patients with SHF after 6 months of training. The control group achieved an improvement only in LVEF [2, 7]. On the other hand, ICD presence always implies more strict and careful therapy control, including more frequent outpatient visits, optimization of medical therapy and possibilities of introducing telemonitoring solutions. It was also proved that resistance training improves LVEF and fractional shortening [16, 17]. Palevo et al. observed a significant increase in LVEF from 32% to 37% after 8 weeks of isotonic training [17]. It was confirmed by other authors [18]. The results of the randomized controlled trial HF-ACTION in 2331 outpatients with SHF showed significant improvement in health status after training compared with usual care which persisted over time. Exercise training resulted in nonsignificant reductions in the primary end point of all-cause mortality or hospitalization and in key secondary clinical end points. After adjustment for highly prognostic predictors of the primary end point modest significant reductions for both all-cause mortality or hospitalization and cardiovascular mortality or heart failure hospitalization were observed, but patients with ICD were not treated as a prospectively evaluated end-point group [19, 20]. In the retrospective analysis of 1053 patients with an ICD there was no evidence of increased ICD shocks, and they trained to a similar degree as patients without ICDs. The exercise training appeared to be safe, but the study was characterized by a high dropout rate [21]. The complexity of clinical problems, including severe SHF (NYHA class III), implanted ICD, repeated cardiopulmonary testing and 6-MWT, along with a long follow-up, account for the novelty of the present study. Medical therapy was optimal, no differences were detected between groups, and no major changes were made during follow-up. On the other hand, the percentage of hospitalizations was similar (NS). This indicates the complexity of heart failure and the lack of impact of exercise on clinical outcome assessment, including hospitalizations.
The results of this study confirm the results of other authors, but go further and confirm maintenance of positive effects of training, such as improving the overall physical fitness and training safety regarding the implanted ICD. The current data support widespread implementation of physical activity in patients with SHF. Proper application of these recommendations may extend patients’ life in better psycho-physical comfort. Therefore, physiotherapy should be considered as an important part of the treatment strategy in patients with SHF and implanted devices.
In conclusion, an individual, 6-month training program, properly controlled in patients with systolic heart failure and an implanted ICD, was safe and resulted in a significant improvement of exercise tolerance, capacity and echocardiographic parameters. It is further evidence for overall positive results of exercise training in patients with advanced systolic heart failure and an implanted ICD, although these effects did not improve the prognosis.
The study was conducted in one cardiology center in relatively small-sized groups of SHF patients. The study group was difficult to work with, so a substantial number of dropouts occurred. Further effective motivation of patients to take up regular long-term exercise was another challenge that required careful consideration. Availability of rehabilitation programs in our area for this specific patient group was sufficient.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology Developed in collaboration with the Heart Failure Association (HFA) Eur Heart J. 2012;33:1787–847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 2.Hambrecht R, Fiehn E, Weigl C, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients chronic heart failure. Ciculation. 1998;98:2709–15. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 3.2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2013;34:2281–329. doi: 10.1093/eurheartj/eht150. [DOI] [PubMed] [Google Scholar]
- 4.Albouaini K, Egred M, Alahmar A, Wright DJ. Cardiopulmonary exercise testing and its application. Postgrad Med J. 2007;83:675–82. doi: 10.1136/hrt.2007.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Task Force on Heart Failure of European Society of Cardiology. Guidelines for the diagnosis of heart failure. Eur Heart J. 1995;16:741–51. [PubMed] [Google Scholar]
- 6.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life and clinical outcome. Circulation. 1999;99:1173–82. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 7.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripherial resistance in patients with chronic heart failure: a randomized trial. JAMA. 2000;283:3095–101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 8.Tyni-Lenne R, Gordon A, Jensen-Urstad M, Dencker K, Jansson E, Sylven C. Aerobic training involving a minor muscle mass shows greater efficiency than training involving a major muscle mass in chronic heart failure patients. J Card Fail. 1999;5:300–7. doi: 10.1016/s1071-9164(99)91334-9. [DOI] [PubMed] [Google Scholar]
- 9.Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med. 2004;116:693–706. doi: 10.1016/j.amjmed.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 10.Holzapfel N, Zugck C, Muller-Tasch T, et al. Routine screening for depression and quality of life in outpatients with congestive heart failure. Psychosomatics. 2007;48:112–6. doi: 10.1176/appi.psy.48.2.112. [DOI] [PubMed] [Google Scholar]
- 11.Martensson J, Dracup K, Canary C, Fridlund B. Living with heart failure: depression and quality of life in patients and spouses. J Heart Lung Transplant. 2003;22:460–7. doi: 10.1016/s1053-2498(02)00818-5. [DOI] [PubMed] [Google Scholar]
- 12.Smart N, Haluska B, Jeffriess L, Marwick TH. Predictors of sustained response to exercise training in patients with chronic heart failure: a telemonitoring study. Am Heart J. 2005;150:1240–7. doi: 10.1016/j.ahj.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Fitchet A, Doherty PJ, Bundy C, Bell W, Fitzpatrick AP, Garrat CJ. Comprehensive cardiac rehabilitation programme for implanted cardioverter-defibrillator patients: a randomised controlled trial. Heart. 2003;89:155–60. doi: 10.1136/heart.89.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davids JS, McPherson CA, Earley C, Batsford WP, Lampert R. Benefits of cardiac rehabilitation in patients with implantable cardioverter-defibrillators: a patients’ survey. Arch Phys Med Rehabil. 2005;86:1924–28. doi: 10.1016/j.apmr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Isaksen K, Morken IM, Munk PS, Larsen AI. Exercise training and cardiac rehabilitation in patients with implantable cardioverter defibrillators: a review of current literature focusing on safety, effects of exercise training, and the psychological impact of programme participation. Eur J Prev Cardiol. 2012;19:804–12. doi: 10.1177/1741826711414624. [DOI] [PubMed] [Google Scholar]
- 16.Hussein NA, Thomas MA. Rehabilitation of patients with implantable cardioverter/defibrillator: a literature review. Acta Cardiol. 2008;63:249–57. doi: 10.2143/AC.63.2.2029535. [DOI] [PubMed] [Google Scholar]
- 17.Palevo G, Keteyian SJ, Kang M, Caputo JL. Resistance exercise training improves heart function and physical fitness in stable patients with heart failure. J Cardiopulm Rehab Prev. 2009;29:294–8. doi: 10.1097/HCR.0b013e3181ac784b. [DOI] [PubMed] [Google Scholar]
- 18.Levinger I, Bronks R, Cody DV, Linton I, Davie A. The effect of resistance training on left ventricular function and structure of patients with chronic heart failure. Int J Cardiol. 2005;105:159–63. doi: 10.1016/j.ijcard.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Flynn KE, Piña IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: findings from the HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–9. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connor CM, Whellan DJ, Lee KL, et al. the HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION Randomized Controlled Trial. JAMA. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piccini JP, Hellkamp AS, Whellan DJ, et al. HF-ACTION Investigators. Exercise training and implantable cardioverter-defibrillator shocks in patients with heart failure: results from HF-ACTION (Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing) JACC Heart Fail. 2013;1:142–8. doi: 10.1016/j.jchf.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]