Abstract
Introduction
Previous studies have examined the correlation between hyperandrogenemia and non-alcoholic fatty liver disease (NAFLD) in women and showed contradictory results. Therefore, we aimed to evaluate the relationship between testosterone level and Fatty Liver Index (FLI), as a surrogate marker for NAFLD, in a cohort of postmenopausal women.
Material and methods
A total of 150 postmenopausal women were included in this cross-sectional study. Anthropometric and biochemical parameters, as well as blood pressure, were obtained. Non-alcoholic fatty liver disease is assessed by FLI, an algorithm based on body mass index, waist circumference, triglycerides and γ-glutamyl transferase, as a simple and accurate predictor of hepatic steatosis. Women were divided into three groups (FLI < 30, n = 80; 30 ≤ FLI < 60, n = 44; FLI ≥ 60, n = 26). Homeostasis model assessment of insulin resistance (HOMA-IR) as a surrogate marker of insulin resistance was calculated.
Results
Multiple linear regression analysis revealed that the best model consisted of 4 parameters (e.g., bioavailable testosterone (β = 0.288, p = 0.001), log HOMA-IR (β = 0.227, p = 0.005), log high-sensitivity C-reactive protein (β = 0.322, p < 0.001), and retinol-binding protein 4 (β = 0.226, p < 0.001)). Adjusted R2 for the best model was 0.550, which means that as much as 55.0% of variation in FLI could be explained with this model.
Conclusions
Bioavailable testosterone is independently associated with FLI in postmenopausal women.
Keywords: fatty liver, hyperandrogenemia, insulin resistance, obesity, postmenopausal
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the commonest form of a broad spectrum of conditions ranging from simple steatosis to inflammation and fibrosis, resulting in non-alcoholic steatohepatitis and cirrhosis, and affects 20–30% of the general population [1].
Liver disease is an early predictor of diabetes mellitus type 2 (DM2) [2] and cardiovascular disease (CVD) [3].
Recent reports show that the prevalence of NAFLD is higher in postmenopausal women than those in a premenopausal state [4, 5] and that postmenopause is a risk factor for NAFLD [6]. One possible explanation may lie in the fact that women after menopause have increased insulin resistance (IR) and visceral abdominal fat, which are the major contributors to NAFLD development [4, 7].
Postmenopausal women experience estrogen deficiency and relative androgen excess, which might lead to redistribution of total body fat toward increase of visceral fat with consequent development of IR [8]. It is speculated that not estrogen deficiency by itself, but rather androgen excess, might be the main culprit for such pathophysiological changes, since exogenous administration of androgens to women has been demonstrated to lead to development of IR [8, 9]. On the other hand, administration of estradiol in postmenopausal women did not show any benefits [10, 11]. In line with this, the increased prevalence of NAFLD in patients with polycystic ovarian syndrome (PCOS) is explained by androgen excess, also [12].
Due to its invasive diagnostic nature, liver biopsy as the gold standard for NAFLD [13] has been replaced with abdominal ultrasonography, as the commonest technique for NAFLD assessment in clinical trials. In line with this, Bedogni et al. [14] developed the Fatty Liver Index (FLI), a simple and accurate predictor of hepatic steatosis that was derived in a general population-based study which highly correlated with abdominal ultrasonography. It has been reported that NAFLD assessed by FLI was an independent risk factor for new-onset hypertension [1], DM2 [15], and CVD [16].
Previous studies have examined the correlation between hyperandrogenemia and NAFLD and showed either no relationship [17, 18] or an independent association between testosterone level and NAFLD [19, 20].
Taking all these contradictory results into account, the aim of our study was to evaluate the relationship between testosterone level (e.g., total, free and bioavailable form) and FLI, as a surrogate marker for NAFLD, in a cohort of postmenopausal women. In addition, we aimed to examine a cluster of other biomarkers (e.g., adipokines, inflammation markers, insulin resistance markers and sex hormones) that might make a significant contribution to fatty liver assessment.
Material and methods
Population
The study enrolled a total of 150 postmenopausal women (mean age: 56.6 ±4.8 years) who volunteered to participate in the study. Participants were consecutively recruited in the study when seeking gynecologic healthcare in the Primary Health Care Center in Podgorica, Montenegro for their regular check-up, in the period from October 2012 to May 2013. Menopause is defined as the absence of menstrual bleeding for more than 1 year. All the participants completed a questionnaire including demographic characteristics, somatic illnesses, smoking history and current medications use. Medical history and clinical examinations were carried out on the same day.
Inclusion criteria were: menopausal status, no hormone replacement therapies, no signs and symptoms of acute inflammatory disease, no history or the presence of malignancy, and non-smoking. Exclusion criteria were: ethanol consumption > 20 g/day, liver disease (cirrhosis, viral hepatitis, autoimmune hepatitis, primary sclerosing cholangitis, primary biliary cirrhosis and overlap syndromes, drug-induced liver disease, hemochromatosis, Wilson’s disease, α1-antitrypsin deficiency), diabetes mellitus, hypothyroidism or hyperthyroidism, renal dysfunction, cardiovascular disorders, and medications use (antihypertensive, lipid-lowering, hypoglycemic, anti-inflammatory medications, hormonal replacement therapy or any medications that can affect liver function) in the last 6 months.
Participants were instructed not to perform any vigorous physical activity the day before the blood samples were taken. All the participants provided written informed consent. The study protocol was approved by the Ethical Committee of Primary Health Care Center in Podgorica, Montenegro, and the research was carried out in compliance with the Declaration of Helsinki [21].
Anthropometric measurements
Basic anthropometric measurements – body height (cm), body weight (kg) and waist circumference (WC) (cm) – were obtained in the morning. Weight was measured to the nearest 0.1 kg on a balance beam scale, with the subjects barefoot and with light clothing. Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer, without shoes. Waist circumference was measured with non-stretchable tape over the unclothed abdomen at the midpoint between the lowest rib and the iliac crest. Measurements were made at the end of normal expiration. The tape was parallel to the floor and did not compress the skin. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2).
Although our obese postmenopausal women comprised slightly obese (overweight, 25 ≤ BMI < 30 kg/m2) and moderately obese subjects (30 ≤ BMI < 40 kg/m2), for reasons of simplicity, we referred to them as overweight/obese women.
Blood pressure was measured with a sphygmomanometer after the subject had been seated for 15 min. The average of three measurements taken on the right arm was recorded. All measurements were taken by the same trained evaluator.
Participants who met at least three of the following conditions were diagnosed with metabolic syndrome (MetS): WC ≥ 80 cm, hyperglycemia ≥ 5.6 mmol/l; high-density lipoprotein cholesterol (HDL-c) < 1.30 mmol/l; triglycerides ≥ 1.70 mmol/l and hypertension: systolic blood pressure (SBP) or diastolic blood pressure (DBP) ≥ 130/85 mm Hg [22].
Non-alcoholic fatty liver disease is assessed by the FLI, an algorithm based on body mass index, waist circumference, triglycerides and γ-glutamyl transferase, as a simple and accurate predictor of hepatic steatosis. The FLI was calculated for each individual subject in accordance with the score sheet available as an on-line calculator at http://www.medicalalgorithms.com/fatty-liver-index-fli-of-bedogni-et-al-for-predicting-hepatic-steatosis.
Women were divided into three groups (FLI < 30, n = 80; 30 ≤ FLI < 60, n = 44; FLI ≥ 60, n = 26) [14].
Biochemical analyses
The blood samples were taken between 7 and 9 a.m., after at least 8 h of overnight fast. Samples were left to clot for 30 min and then centrifuged at 3000 rpm for 10 min. Serum samples were divided into aliquots and stored at –80°C, without prior thawing and re-freezing before analyses, except for glucose, which was determined immediately after the blood was drawn. Serum levels of glucose, total cholesterol (TC), HDL-c, low-density lipoprotein cholesterol (LDL-c), triglycerides (TG), albumin, uric acid, aspartate aminotransferase (AST), alanine aminotransferase (ALT) and γ-glutamyl transferase (GGT) were measured using standardized enzymatic procedures, spectrophotometrically (Roche Cobas 400, Mannheim, Germany). Cystatin C, RBP4, and high-sensitivity C-reactive protein (hsCRP) levels were determined using a nephelometric assay (Behring Nephelometer Analyzer, Marburg, Germany). Sex-hormone binding globulin (SHBG), total estradiol, total testosterone and insulin were measured by chemiluminescent immunometric assay (Immulite 2000, Siemens, Munich, Germany). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated: HOMA-IR = fasting glucose (mmol/l) × fasting insulin [µIU/l]/22.5 [23]. Bioavailable testosterone was calculated, also (http://www.issam.ch/freetesto.htm) [24].
Statistical analysis
Statistical analysis was performed using the SPSS statistical package (version 15.0 for Windows, SPSS, Chicago, IL, USA). Data are presented as mean ± standard deviation, or median (interquartile range), or counts and percentages. Differences between groups were evaluated with Student’s t test for normally or the Mann-Whitney test for non-normally distributed parameters or one-way analysis of variance (ANOVA) and Kruskal-Wallis non-parametric analysis of variance, where appropriate. The χ2 test was used to analyze the differences in categorical data. Pearson’s (r) correlation coefficient was used to determine the relationships between FLI and other variables. Due to skewed distribution, log transformed HOMA-IR and hsCRP were used. Multiple linear regression (MLR) analysis was performed to identify independent determinants of FLI. Receiver operating characteristic (ROC) curve analysis was used with the purpose of testing the discriminatory potential of a group of parameters selected in MLR analysis, with FLI as the dependent variable. Construction of a model consisting of FLI formula independent parameters by using logistic regression analysis was also performed. In all analyses a p-value of < 0.05 was considered as statistically significant.
Results
Table I shows the general clinical and biochemical characteristics of apparently healthy postmenopausal women involved in this study. Significantly higher BMI and WC (p < 0.001, respectively), fasting glucose (p = 0.003), insulin and HOMA-IR (p < 0.001, respectively), LDL-c (p = 0.015), triglycerides, GGT and ALT activity, SBP, and DBP (p < 0.001, respectively), but lower HDL-c (p < 0.001), were observed in women with a higher FLI level as compared with the low FLI level group. Furthermore, we also found a significant difference in several other cardiometabolic parameters, which are independent of FLI calculation, i.e., a significantly higher level of uric acid, hsCRP, cystatin C (p < 0.001, respectively), and RBP4 (p = 0.001) among women in the higher FLI level group, as compared with women in the low FLI level group. Moreover, a significantly higher number of obese individuals (χ2 = 57.29; p < 0.001), as well as individuals with MetS (χ2 = 53.24; p < 0.001), was found in the group with the highest FLI. There was no difference with respect to age, TC level, AST activity, albumin, total estradiol or testosterone level between groups.
Table I.
General characteristics of studied postmenopausal women divided according to Fatty Liver Index level
| Parameter | FLI < 30(n = 80) | 30 ≤ FLI < 60(n = 44) | FLI ≥ 60(n = 26) | P-value* |
|---|---|---|---|---|
| Age [years] | 56.0 ±4.90 | 57.3 ±4.39 | 57.5 ±5.22 | 0.203 |
| BMI [kg/m²] | 23.6 ±2.93aaa,bbb | 27.9 ±1.88aaa | 32.8 ±2.70 | < 0.001 |
| WC [cm] | 80.2 ±7.40aaa,bbb | 95.7 ±5.80aaa | 104.5 ±8.29 | < 0.001 |
| Glucose [mmol/l] | 5.24 ±0.39aaa | 5.39 ±0.53 | 5.63 ±0.72 | 0.003 |
| TC [mmol/l] | 6.31 ±0.99a | 6.60 ±1.18 | 6.76 ±0.97 | 0.104 |
| HDL-c [mmol/l] | 1.85 ±0.43aaa,bb | 1.62 ±0.32aaa | 1.34 ±0.27 | < 0.001 |
| LDL-c [mmol/l] | 4.09 ±0.95aa,bb | 4.51 ±1.16 | 4.65 ±0.84 | 0.015 |
| TG [mmol/l]# | 1.06 (0.79–1.41)aaa,bb | 1.42 (0.99–1.83)aaa | 2.31 (1.40–2.45) | < 0.001 |
| Insulin [µIU/l]# | 4.90 (3.89–5.98)aaa,bbb | 8.92 (6.53–10.79)a | 10.05 (7.88–14.40) | < 0.001 |
| HOMA-IR# | 1.10 (0.90–1.42)aaa,bbb | 2.01 (1.54–2.78)a | 2.67 (1.81–3.64) | < 0.001 |
| SBP [mm Hg] | 119 ±23.1aaa,bbb | 139 ±20.0 | 148 ±18.0 | < 0.001 |
| DBP [mm Hg] | 57.8 ±14.2aaa,bbb | 91.2 ±9.7 | 106 ±10.4 | < 0.001 |
| AST [U/l] | 18 (16–20) | 18 (16–21) | 18 (15–22) | 0.986 |
| ALT [U/l] | 16 (13–20)aaa,bbb | 21 (15–24) | 23 (17–28) | < 0.001 |
| GGT [U/l] | 10.0 (8.0–12.5)aaa,bbb | 13.0 (10.0–16.0)aa | 15.5 (13.0–21.0) | < 0.001 |
| RBP4 [mg/l] | 38.8 ±9.34aaa | 42.1 ±8.63a | 46.0 ±6.38 | 0.001 |
| Cystatin C [mg/l] | 0.73 ±0.09aaa,bbb | 0.79 ±0.10aaa | 0.85 ±0.10 | < 0.001 |
| HsCRP [mg/l] | 0.53 (0.28–1.20)aaa,bbb | 1.39 (0.94–2.56)a | 1.91 (1.16–3.23) | < 0.001 |
| Uric acid [µmol/l] | 234 ±54.6aaa,bbb | 273 ±54.5aaa | 330 ±53.1 | < 0.001 |
| Albumin [g/l] | 46.6 ±2.34 | 47.4 ±2.67 | 47.0 ±2.73 | 0.125 |
| Estradiol [pmol/l]# | 53.5 (39.6–68.1) | 54.5 (41.0–69.0) | 53.0 (41.0–69.0) | 0.961 |
| Total T [nmol/l]# | 1.00 (0.77–1.24) | 1.01 (0.81–1.33) | 1.10 (0.81–1.23) | 0.942 |
| Free T (%)# | 1.04 (0.90–1.18)aaa,bbb | 1.32 (1.13–1.63)aa | 1.58 (1.39–1.80) | < 0.001 |
| Bioavailable T (%)# | 25.5 (23.0–29.7)aaa,bbb | 34.15 (29.4–40.4)aa | 39.9 (36.2–48.8) | < 0.001 |
| SHBG [nmol/l] | 74.67 ±17.93aaa,bbb | 51.37 ±17.02aa | 38.81 ±13.35 | < 0.001 |
| Overweight/obese, n (%) | 30 (37.5) | 44 (100) | 26 (100) | χ2 = 53.23 < 0.001 |
| Normal weight, n (%) | 50 (62.5) | 0 (0) | 0 (0) | |
| MetS (+), n (%) | 5 (6.3) | 26 (59) | 20 (77) | χ2 = 57.29 < 0.001 |
| MetS (–), n (%) | 75 (93.7) | 18 (41) | 6 (23) |
p < 0.001
p < 0.01
p < 0.05 vs. third group
p < 0.001
p < 0.01
p < 0.05 vs. Second group
Data are presented as mean ± standard deviation or #data with non-Gaussian distribution are shown as median values (interquartile range), or counts and percentages
p-value from one-way ANOVA or Kruskal-Wallis non-parametric analysis of variance, followed by non-parametric Mann-Whitney U test, where appropriate; FLI – fatty liver index, BMI – body mass index, WC – waist circumference, HOMA-IR – homeostasis model assessment of insulin resistance, TC – total cholesterol, HDL-c – high-density lipoprotein cholesterol, LDL-c – low-density lipoprotein cholesterol, TG – triglycerides, AST – aspartate aminotransferase, ALT – alanine aminotransferase, HsCRP – high-sensitivity C-reactive protein, SBP – systolic blood pressure, DBP – diastolic blood pressure, T – testosterone, SHBG – sex-hormone binding globulin, MetS (+) – postmenopausal women with metabolic syndrome, MetS (–) – postmenopausal women without metabolic syndrome.
Next, we performed Pearson’s correlation in order to examine the potential relationship between FLI level and cardiometabolic parameters independent of FLI calculation (LDL-c, glucose, insulin, HOMA-IR, cystatin C, RBP4, hsCRP, uric acid, ALT, free and bioavailable testosterone, SHBG, SBP and DBP) in the whole group of apparently healthy postmenopausal women.
Pearson’s correlation revealed a significant positive relationship between FLI and LDL-c (p = 0.002), log HOMA-IR, cystatin C, RBP4, uric acid, bioavailable testosterone, SHBG, ALT activity and SBP (p < 0.001, respectively), as well as a significant negative relationship between FLI and HDL-c (p < 0.001) (Table II).
Table II.
Pearson’s correlation (r) of log transformed Fatty Liver Index and examined parameters independent of FLI calculation
| Variable | r | P-value |
|---|---|---|
| Age [years] | 0.107 | 0.192 |
| Glucose [mmol/l] | 0.347 | < 0.001 |
| Log HOMA-IR | 0.600 | < 0.001 |
| TC [mmol/l] | 0.155 | 0.058 |
| LDL-c [mmol/l] | 0.255 | 0.002 |
| HDL-c [mmol/l] | –0.575 | < 0.001 |
| Uric acid [µmol/l] | 0.543 | < 0.001 |
| Log hsCRP [mg/l] | 0.526 | < 0.001 |
| Cystatin C [mg/l] | 0.387 | < 0.001 |
| RBP4 [mg/l] | 0.332 | < 0.001 |
| ALT [U/l] | 0.318 | < 0.001 |
| Albumin [g/l] | 0.045 | 0.581 |
| SBP [mm Hg] | 0.497 | < 0.001 |
| DBP [mm Hg] | 0.485 | < 0.001 |
| Total estradiol [pmol/l] | –0.050 | 0.544 |
| Total testosterone [nmol/l] | –0.067 | 0.417 |
| Free testosterone (%) | 0.622 | < 0.001 |
| Bioavailable testosterone (%) | 0.631 | < 0.001 |
| SHBG [nmol/l] | –0.641 | < 0.001 |
Log HOMA-IR – logarithmically transformed homeostasis model assessment of insulin resistance, TC – total cholesterol, HDL-c – high-density lipoprotein cholesterol, LDL-c – low-density lipo-protein cholesterol, ALT – alanine aminotransferase, Log hsCRP – logarithmically transformed high sensitivity C-reactive protein, RBP4 – retinol-binding protein 4, SBP – systolic blood pressure, SHBG – sex-hormone binding globulin.
Multiple linear regression (MLR) analysis was performed to identify which of the measured markers have the best association with FLI. Namely, all variables found to have a significant predictive value in Pearson’s correlation (e.g., ALT, SBP, LDL-c, bioavailable testosterone, HOMA-IR, uric acid, hsCRP, cystatin C and RBP4) were further analyzed in MLR analysis for FLI prediction. Backward selection enabled us to find the best model consisting of 4 parameters (e.g., bioavailable testosterone (β = 0.288, p = 0.001), log HOMA-IR (β = 0.227, p = 0.005), log hsCRP (β = 0.322, p < 0.001), and RBP4 (β = 0.226, p < 0.001)). Adjusted R2 for the best model was 0.550, which means that as much as 55.0% of variation in FLI could be explained with this model (Tables III and IV).
Table III.
Multiple linear regression R coefficients in the best-fit model for the association of several parameters with log FLI as dependent variable
| Model summary | ||||
|---|---|---|---|---|
| Model | R | R2 | Adjusted R2 | Std. error of the estimate |
| 1 | 0.631a | 0.398 | 0.394 | 0.34468 |
| 2 | 0.691b | 0.478 | 0.471 | 0.32206 |
| 3 | 0.733c | 0.538 | 0.528 | 0.30404 |
| 4 | 0.750d | 0.562 | 0.550 | 0.29679 |
Predictors: (Constant), Bioavailable testosterone.
Predictors: (Constant), Bioavailable testosterone, logCRP.
Predictors: (Constant), Bioavailable testosterone, logCRP, RBP4.
Predictors: (Constant), Bioavailable testosterone, logCRP, RBP4, logHOMA-IR.
Table IV.
Multiple linear regression standardized β coefficients and p-values for the parameters in the best-fit model for the association of several parameters with log FLI as dependent variable
| Coefficientsa | |||||
|---|---|---|---|---|---|
| Model | Unstandardized coefficients | Standardized coefficients β | t | P-value | |
| B | Std. error | ||||
| 1 (Constant) | 0.348 | 0.102 | 3.402 | 0.001 | |
| Bioavailable testosterone | 0.031 | 0.003 | 0.631 | 9.885 | < 0.001 |
| 2 (Constant) | 0.557 | 0.105 | 5.292 | < 0.001 | |
| Bioavailable testosterone | 0.024 | 0.003 | 0.497 | 7.526 | < 0.001 |
| Log hsCRP | 0.312 | 0.066 | 0.313 | 4.745 | < 0.001 |
| 3 (Constant) | 0.146 | 0.137 | 1.067 | 0.288 | |
| Bioavailable testosterone | 0.021 | 0.003 | 0.438 | 6.865 | < 0.001 |
| Log hsCRP | 0.338 | 0.062 | 0.339 | 5.424 | < 0.001 |
| RBP4 | 0.012 | 0.003 | 0.251 | 4.352 | < 0.001 |
| 4 (Constant) | 0.350 | 0.152 | 2.311 | 0.022 | |
| Bioavailable testosterone | 0.014 | 0.004 | 0.288 | 3.551 | 0.001 |
| Log hsCRP | 0.321 | 0.061 | 0.322 | 5.247 | < 0.001 |
| RBP4 | 0.011 | 0.003 | 0.226 | 3.968 | < 0.001 |
| Log HOMA-IR | 0.421 | 0.147 | 0.227 | 2.867 | 0.005 |
Dependent variable: log FLI, Log HOMA-IR – logarithmically transformed homeostasis model assessment of insulin resistance, Log hsCRP – logarithmically transformed high-sensitivity C-reactive protein, RBP4 – retinol-binding protein 4.
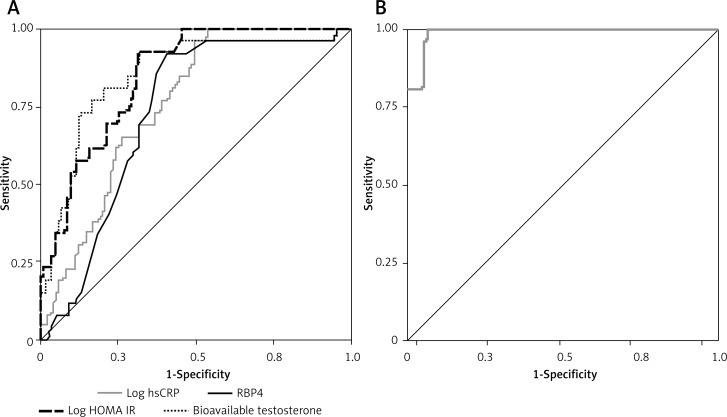
After that, we conducted a receiver operating characteristic (ROC) analysis of selected parameters to test their discriminatory ability regarding FLI level (low vs. higher level). Additionally, we constructed a model consisting of those 4 parameters (bioavailable testosterone, log HOMA-IR, log hsCRP and RBP4) by using logistic regression analysis generated predictive probabilities. Figure 1 shows the ROC curve graph and Table V shows the most important ROC parameters: area under the curve (AUC) with 95% confidence interval (CI) of selected parameters and the model. Table V also shows sensitivities, specificities and cut-off values for selected parameters.
Figure 1.
A – ROC curves of selected parameters’, B – ROC curve of model consisting of 4 selected parameters’ discriminatory ability regarding Fatty Liver Index level (low vs. higher level)
Table V.
Area under the curve, 95% confidence interval and standard error for the parameters of selected parameters’ discriminatory ability regarding Fatty Liver Index level (low vs. higher risk); pairwise comparison of the areas under ROC curves (AUCs) for model and separate parameters
| Parameter | AUC | 95% CI | SE | Sensitivity (%) | Specificity (%) | Cut-off value (%) | P-value* |
|---|---|---|---|---|---|---|---|
| Model (4 parameters) | 0.990 | 0.977–1.003 | 0.007 | – | – | – | < 0.001 |
| Bioavailable testosterone | 0.868 | 0.803–0.933 | 0.033 | 96 | 55 | 27.65 | < 0.001 |
| Log HOMA-IR | 0.850 | 0.783–0.917 | 0.034 | 96 | 45 | 1.43 | < 0.001 |
| Log hsCRP | 0.758 | 0.676–0.840 | 0.042 | 96 | 53 | –0.14 | < 0.001 |
| RBP4 | 0.729 | 0.639–0.819 | 0.046 | 96 | 94 | 26.50 | < 0.001 |
AUC – area under ROC curve, CI – confidence interval, SE – standard error, Model: Bioavailable testosterone, Log HOMA-IR – logarithmically transformed homeostasis model assessment of insulin resistance, Log hsCRP – logarithmically transformed high sensitivity C-reactive protein, RBP4 – retinol-binding protein 4.
P from pairwise comparison for AUC differences between Model and separate parameter.
Comparison of ROC curves showed that all separate curves have comparable discriminatory capability towards FLI level status. Construction of a model consisting of those 4 FLI formula independent parameters (bioavailable testosterone, log HOMA-IR, log hsCRP and RBP4) by using logistic regression analysis showed that the new ROC curve had outstanding discriminatory capability (AUC = 0.990, according to Hosmer and Lemeshow’s rules) [25].
Discussion
To our knowledge, this is the first study to examine a cluster of biomarkers (e.g., adipokines, inflammation markers, insulin resistance markers and sex hormones) that can make a significant contribution to fatty liver assessment. We found several biomarkers that are independently associated with FLI, such as bioavailable testosterone, insulin resistance as measured by HOMA-IR, RBP4 and hsCRP, showing that as much as 55.0% of variation in FLI could be explained with this model. Furthermore, the current study has demonstrated that the multimarker approach could be of great benefit in order to identify those postmenopausal women with higher FLI. Namely, construction of a model consisting of those 4 FLI formula independent parameters (bioavailable testosterone, log HOMA-IR, log hsCRP and RBP4) by using logistic regression analysis showed that the new ROC curve had outstanding discriminatory capability, according to Hosmer and Lemeshow’s rules (Figure 1 B).
The NAFLD represents the hepatic manifestation of MetS [1]. In line with this, in our study we reported a significantly higher number of obese postmenopausal women, as well as women with MetS status, in the highest FLI group, as compared to the low FLI group. Moreover, all normal weight women were in the low FLI group, suggesting that obese state may greatly influence the hepatic steatosis state.
Visceral adipose tissue is a significant source of free fatty acids which reach the liver through the portal vein, thus having a great influence on fatty liver development [12]. In addition, IR and compensatory hyperinsulinemia also contribute to increased hepatic lipogenesis. The enlargement of visceral adipose tissue is also accompanied by chronic low-grade inflammation and monocyte infiltration, leading to dysregulation of secretion of pro-inflammatory cytokines (tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), CRP) [3, 26] and adipokines (e.g., increased levels of leptin and RBP4 and decreased levels of adiponectin) that modulate insulin sensitivity [12].
Inflammation plays a key role in the pathogenesis of NAFLD [3]. Bhatia et al. [27] demonstrated that an increase in CRP was an independent risk factor for NAFLD, which is in line with our results.
Even though RBP4 originates from the liver and is closely related to adiposity, IR and liver fat [28], no clear link has been established between RBP4 and NAFLD severity [29, 30].
The current study demonstrated an independent association between RBP4 and FLI, which is also consistent with the results in a pediatric population [31].
Although we did not report any difference in total estradiol and total testosterone level between groups, we observed higher free and bioavailable testosterone in the high FLI group. Our results were similar to the results obtained by Polyzos et al. [32], who also reported no difference in serum total estradiol and testosterone level between women with NAFLD and the control group, but reported higher free and bioavailable testosterone levels in postmenopausal women with NAFLD. All these data demonstrated that androgen excess, which is the main feature of PCOS and postmenopause, and is interrelated to IR, may be an additional contributing factor to NAFLD development [12].
Previous studies have examined the correlation between hyperandrogenemia and NAFLD and showed either no relationship [17, 18] or an independent association between testosterone level and NAFLD [19, 20].
Macut et al. [17] reported no independent correlation between free androgen index and NAFLD in PCOS women, suggesting that the association between elevated androgen levels and NAFLD is mediated by IR. They speculated that IR aggravates hyperandrogenemia by increasing ovarian androgen synthesis and by down-regulating hepatic SHBG production, establishing a positive feedback loop between increased circulating levels of free androgens and more pronounced IR in patients with PCOS.
In concordance with Macut et al. [17], IR evaluated with the HOMA-IR was independently associated with NAFLD in our population. IR is associated with impaired suppression of lipolysis in the adipose tissue, leading to an increased influx of free fatty acids to the liver and consequent hepatic steatosis [33].
However, in our study in MLR, bioavailable testosterone was independently associated with FLI, suggesting that androgen excess in postmenopausal women could be an additional fatty liver risk factor, independently of IR.
In a population of men with NAFLD, lower total and free testosterone levels were independently associated with NAFLD [34], thus confirming the paradox in understanding the relationship of circulating androgen levels with metabolic disorders and NAFLD. Namely, low total and free testosterone are also associated with MetS and DM2 in men, whereas high total and free androgens are associated with MetS and IR in women with PCOS [35]. Similarly, increased free testosterone was related to higher risk of MetS and is strongly related to abdominal obesity in postmenopausal women [36].
Bioavailable testosterone may play an important role in menopause-related redistribution of fat towards the central abdominal region. Janssen et al. [37] demonstrated that women with more rapidly changing bioavailable testosterone experienced a larger increase in adiposity compared with their more stable counterparts, suggesting that the relationship between increased bioavailable testosterone and adipose tissue may be key in the understanding of hormonal changes observed during the menopausal transition that links obesity to fatty liver risk.
Our study has some limitations. The small number of postmenopausal women included in the current cross-sectional study may affect the results. Furthermore, the causal relationship between FLI and bioavailable testosterone in postmenopausal women could not be established. Furthermore, we excluded the potential influence of medications on the obtained results, since we included only those women who did not receive any medications in the last 6 months. However, it could be interesting in some future studies to examine the influence of medications on the results obtained in the current study.
In conclusion, bioavailable testosterone is independently associated with the FLI in postmenopausal women. Prospective studies are needed for a better understanding of the mechanisms of the relationship between increased androgenicity in postmenopausal women and non-alcoholic fatty liver disease in order to find better targeted therapy for decreased risk and consequences of fatty liver status in this population group.
Acknowledgments
This work was financially supported in part by a grant from the Ministry of Education, Science and Technological Development, Republic of Serbia (Project number OI 175035).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Huh JH, Ahn SV, Koh SB, Choi E, Kim JY, Sung KC. A prospective study of fatty liver index and incident hypertension: the KoGES-ARIRANG Study. PLoS One. 2015;10:e0143560. doi: 10.1371/journal.pone.0143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non-alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096–108. doi: 10.1016/j.metabol.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu H, Lu HY. Nonalcoholic fatty liver disease and cardiovascular disease. World J Gastroenterol. 2014;20:8407–15. doi: 10.3748/wjg.v20.i26.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung GE, Yim JY, Kim D, et al. The influence of metabolic factors for nonalcoholic fatty liver disease in women. BioMed Res Int. 2015;2015:131528. doi: 10.1155/2015/131528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim MK, Ahn CW, Nam JS, Kang S, Park JS, Kim KR. Association between nonalcoholic fatty liver disease and coronary artery calcification in postmenopausal women. Menopause. 2015;22:1323–7. doi: 10.1097/GME.0000000000000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Florentino GS, Cotrim HP, Vilar CP, Florentino AV, Guimarães GM, Barreto VS. Nonalcoholic fatty liver disease in menopausal women. Arq Gastroenterol. 2013;50:180–5. doi: 10.1590/S0004-28032013000200032. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues MH, Bruno AS, Nahas-Neto J, Sandrim VC, Muniz LG, Nahas EA. Evaluation of clinical and inflammatory markers of nonalcoholic fatty liver disease in postmenopausal women with metabolic syndrome. Metab Syndr Relat Disord. 2014;12:330–8. doi: 10.1089/met.2013.0140. [DOI] [PubMed] [Google Scholar]
- 8.Torrens JI, Sutton-Tyrrell K, Zhao X, et al. Relative androgen excess during the menopausal transition predicts incident metabolic syndrome in midlife women: study of Women’s Health Across the Nation. Menopause. 2009;16:257–64. doi: 10.1097/gme.0b013e318185e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braunstein GD. Androgen insufficiency in women: summary of critical issues. Fertil Steril. 2002;77(Suppl 4):S94–9. doi: 10.1016/s0015-0282(02)02962-x. [DOI] [PubMed] [Google Scholar]
- 10.Anderson L, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the women’s health initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 11.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 12.Vassilatou E. Nonalcoholic fatty liver disease and polycystic ovary syndrome. World J Gastroenterol. 2014;20:8351–63. doi: 10.3748/wjg.v20.i26.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieckowska A, Feldstein A. Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasive. Semin Liver Dis. 2008;28:386–95. doi: 10.1055/s-0028-1091983. [DOI] [PubMed] [Google Scholar]
- 14.Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jäger S, Jacobs S, Kröger J, et al. Association between the Fatty Liver Index and risk of type 2 diabetes in the EPIC-Potsdam Study. PLoS One. 2015;10:e0124749. doi: 10.1371/journal.pone.0124749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Lido Study Group. Fatty liver is an independent predictor of early carotid atherosclerosis. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Macut D, Tziomalos K, Božić-Antić I, et al. Non-alcoholic fatty liver disease is associated with insulin resistance and lipid accumulation productin women with polycystic ovary syndrome. Hum Reprod. 2016;31:1347–53. doi: 10.1093/humrep/dew076. [DOI] [PubMed] [Google Scholar]
- 18.Hua X, Sun Y, Zhong Y, et al. Low serum sex hormone-binding globulin is associated with nonalcoholic fatty liver disease in type 2 diabetic patients. Clin Endocrinol (Oxf) 2014;80:877–83. doi: 10.1111/cen.12360. [DOI] [PubMed] [Google Scholar]
- 19.Vassilatou E, Lafoyianni S, Vryonidou A, et al. Increased androgen bioavailability is associated with non-alcoholic fatty liver disease in women with polycystic ovary syndrome. Hum Reprod. 2010;25:212–20. doi: 10.1093/humrep/dep380. [DOI] [PubMed] [Google Scholar]
- 20.Jones H, Sprung VS, Pugh CJ, et al. Polycystic ovary syndrome with hyperandrogenism is characterized by an increased risk of hepatic steatosis compared to nonhyperandrogenic PCOS phenotypes and healthy controls, independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97:3709–16. doi: 10.1210/jc.2012-1382. [DOI] [PubMed] [Google Scholar]
- 21.World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–6. [PubMed] [Google Scholar]
- 22.Alberti KGMM, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 25.Hosmer D, Lemeshow S, Sturdivant RX. Applied logistic regression. 3rd ed. New York, NY: John Wiley & Sons Inc; 2013. [Google Scholar]
- 26.Klisic AN, Vasiljevic ND, Simic TP, Djukic TI, Maksimovic MZ, Matic MG. Association between C-reactive protein, anthropometric and lipid parameters among healthy normal weight and overweight postmenopausal women in Montenegro. Lab Med. 2014;45:12–6. doi: 10.1309/lmi6i2rn7ampeuul. [DOI] [PubMed] [Google Scholar]
- 27.Bhatia LS, Curzen NP, Byrne CD. Nonalcoholic fatty liver disease and vascular risk. Curr Opin Cardiol. 2012;27:420–8. doi: 10.1097/HCO.0b013e328354829c. [DOI] [PubMed] [Google Scholar]
- 28.Papaetis GS, Papakyriakou P, Panagiotou TN. Central obesity, type 2 diabetes and insulin: exploring a pathway full of thorns. Arch Med Sci. 2015;11:463–82. doi: 10.5114/aoms.2015.52350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terra X, Auguet T, Broch M, et al. Retinol binding protein-4 circulating levels were higher in nonalcoholic fatty liver disease vs. histologically normal liver from morbidly obese women. Obesity (Silver Spring) 2013;21:170–7. doi: 10.1002/oby.20233. [DOI] [PubMed] [Google Scholar]
- 30.Cengiz C, Ardicoglu Y, Bulut S, Boyacioglu S. Serum retinol-binding protein 4 in patients with nonalcoholic fatty liver disease: does it have a significant impact on pathogenesis? Eur J Gastroenterol Hepatol. 2010;22:813–9. doi: 10.1097/MEG.0b013e32833283cb. [DOI] [PubMed] [Google Scholar]
- 31.Huang SC, Yang YJ. Serum retinol-binding protein 4 is independently associated with pediatric NAFLD and fasting triglyceride level. J Pediatr Gastroenterol Nutr. 2013;56:145–50. doi: 10.1097/MPG.0b013e3182722aee. [DOI] [PubMed] [Google Scholar]
- 32.Polyzos SA, Kountouras J, Tsatsoulis A, et al. Sex steroids and sex hormone-binding globulin in postmenopausal women with nonalcoholic fatty liver disease. Hormones (Athens) 2013;12:405–16. doi: 10.1007/BF03401306. [DOI] [PubMed] [Google Scholar]
- 33.Tziomalos K, Athyros VG, Karagiannis A. Non-alcoholic fatty liver disease in type 2 diabetes: pathogeneis and treatment options. Curr Vasc Pharmacol. 2012;10:162–72. doi: 10.2174/157016112799305012. [DOI] [PubMed] [Google Scholar]
- 34.Sumida Y, Fukui M, Soh J, et al. The association of low free testosterone with histological severity of nonalcoholic fatty liver disease in Japanese men. Gastroenterol Hepatol Open Access. 2015;2:00052. [Google Scholar]
- 35.Wang C, Swerdloff RS. Comments on ‘Low serum sex hormone binding globulin is associated with nonalcoholic fatty liver disease in type 2 diabetic patients’. Clin Endocrinol (Oxf) 2014;80:874–6. doi: 10.1111/cen.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olszanecka A, Kawecka-Jaszcz K, Czarnecka D. Association of free testosterone and sex hormone binding globulin with metabolic syndrome and subclinical atherosclerosis but not blood pressure in hypertensive perimenopausal women. Arch Med Sci. 2016;12:521–8. doi: 10.5114/aoms.2016.59925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janssen I, Powell LH, Jasielec MS, Kazlauskaite R. Covariation of change in bioavailable testosterone and adiposity in midlife women. Obesity (Silver Spring) 2015;23:488–94. doi: 10.1002/oby.20974. [DOI] [PMC free article] [PubMed] [Google Scholar]