Abstract
Introduction
Fluid overload is one of the most important, yet modifiable, risk factors associated with worse outcomes in hemodialysis (HD) patients. However, its precise assessment in clinical practice is still under investigation.
Material and methods
This is an observational prospective study which included 285 stable patients with end-stage renal disease on standard thrice-weekly HD therapy. Overhydration was assessed by the combination of relative fluid overload (RFO), using bioimpedance spectroscopy, and N-terminal pro-B-type natriuretic peptide (NT-proBNP). The outcome of interest was all-cause mortality.
Results
The median values for NT-proBNP and RFO were 4595 pg/ml and 6.9%, respectively. We divided the study population into four groups according to these median levels: group 1 – low NT-proBNP and low RFO; group 2 – high NT-proBNP and low RFO; group 3 – low NT-proBNP and high RFO; group 4 – high NT-proBNP and high RFO. During the follow-up (mean: 41.1, median: 48.7 months), 89 (31.2%) patients died. In the univariable Cox survival analysis only patients in group 4, and not those from group 2 or 3, had significantly higher HRs as compared to those in group 1 (HR = 1.5, 95% CI: 0.8–2.8, HR = 1.6, 95% CI: 0.8–2.9 and HR = 2.4, 95% CI: 1.3–4.2, for group 2, 3 and 4, respectively). Furthermore, these results were maintained in the multivariable Cox analysis.
Conclusions
Including both bioimpedance and NT-proBNP monitoring in a more comprehensive fluid status assessment could improve the diagnosis of fluid overload with a final improvement in patients’ outcome.
Keywords: fluid overload, bioimpedance spectroscopy, NT-proBNP, all-cause mortality, hemodialysis
Introduction
End-stage renal disease (ESRD) is associated with an increased mortality rate [1], much higher than that observed in heart failure or many forms of cancer. Among the numerous characteristics that contribute to this excess death rate, fluid overload is one of the most important modifiable factor associated with this outcome. Since clinical evaluation for assessing fluid status in dialysis patients lacks sensibility and specificity, numerous efforts have been made to find alternative methods to better assess patients’ volume status [2], including measurement of inferior vena cava diameter [3], bioimpedance analysis [4], biomarkers of volume overload (such as natriuretic peptides) [5] or lung ultrasonography for detection of B-lines [6].
Bioimpedance spectroscopy defines the individual fluid status/overload on the basis of an individual’s normal extracellular volume, taking into account the individual’s body composition. Recent studies show that fluid overload, as assessed by this technique, is one of the most important predictors of mortality in hemodialysis (HD) patients [4, 7]. It can also improve the predictive abilities for mortality above different clinical and biological parameters [8]. More importantly, the use of this method to achieve normohydration is associated with an improvement in blood pressure and arterial stiffness [9, 10] and even with reduced all-cause mortality [10].
The use of brain-type natriuretic peptide (BNP) for fluid overload evaluation in HD is still controversial [11]. There are numerous papers that show a positive relationship between BNP levels and fluid overload in this population [5, 12–14] and also that these increased levels are associated with worse outcomes [14–19].
Bioimpedance analysis does not differentiate between intravascular and interstitial extracellular water (ECW) and serum BNP does not reflect interstitial/tissue water content. The differentiation of “wet BNP” (bioimpedance-determined excessive water content plus high BNP values induced by volume overload) from “dry BNP” (bioimpedance-determined euvolemia plus high BNP values) has been suggested to improve detailed fluid status evaluation in clinical practice [20]. Therefore, we hypothesized that a combination of these two methods (bioimpedance and N-terminal-proBNP (NT-proBNP)) could potentially provide complementary information about the relationship between fluid overload and survival in HD patients.
Material and methods
Patients
Between 1 April and 1 October 2012, we invited all patients who were undergoing HD for at least 3 months in two dialysis units to take part in this study. We excluded patients under 18 years old, with systemic infections and terminal neoplasia; subjects with metallic joint prostheses, cardiac pacemakers or stents, decompensated cirrhosis and limb amputations were also excluded, since accurate bioimpedance assessment cannot be performed in patients with these conditions.
From an overall eligible 337 HD patients we excluded 52 patients because of limb amputation (n = 10), decompensated cirrhosis (n = 6) or presence of a cardiac pacemaker or stent (n = 11). Twenty-five additional patients did not provide informed consent and were not included in the study. Details of the final patient population (n = 285) are presented in Table I. All included patients performed intermittent HD (4 h per session, three times per week), using high-flux membrane dialyzers.
Table I.
Baseline characteristics of the study population
| Parameter | All(N = 285) | Group 1 (n = 81) | Group 2 (n = 62) | Group 3 (n = 62) | Group 4(n = 80) | P-value* |
|---|---|---|---|---|---|---|
| Age [years] | 58.9 ±14.1 | 57.9 ±14.9 | 62.0 ±14.0 | 55.4 ±13.9 | 60.1 ±13.2 | 0.06 |
| Weight [kg] | 71.3 ±14.7 | 75.4 ±13.9 | 70.7 ±16.3 | 70.1 ±12.9 | 68.6 ±14.9 | 0.02 |
| Dialysis vintage [years] | 52.6(18.7–97.5) | 27.8(11.3–62.2) | 51.6(17.0–93.5) | 81.5(22.7–147.2) | 72.8(37.2–117.4) | < 0.001 |
| Male, n (%) | 136 (47.7) | 35 (43.2) | 25 (40.3) | 37 (59.7) | 39 (48.8) | 0.13 |
| Hypertension, n (%) | 213 (74.7) | 56 (69.1) | 50 (80.6) | 47 (75.8) | 60 (75.0) | 0.47 |
| Systolic pressure [mm Hg] | 134.9 ±14.9 | 135.1 ±14.5 | 137.1 ±13.4 | 133.7 ±15.1 | 134.2 ±15.2 | 0.57 |
| Diastolic pressure [mm Hg] | 70.7 ±10.9 | 70.1 ±12.5 | 70.7 ±10.2 | 72.6 ±10.9 | 70.1 ±10.0 | 0.51 |
| Diabetes, n (%) | 50 (17.5) | 15 (18.5) | 11 (17.7) | 11 (17.7) | 13 (16.3) | 0.99 |
| NYHA class 3–4, n (%) | 15 (5.3) | 1 (1.2) | 4 (6.5) | 2 (3.2) | 8 (10.0) | 0.07 |
| Peripheral arterial disease, n (%) | 45 (15.8) | 13 (16.0) | 13 (21.0) | 5 (8.1) | 14 (17.5) | 0.24 |
| Coronary artery disease, n (%) | 50 (17.5) | 12 (14.8) | 13 (21.0) | 6 (9.7) | 19 (23.8) | 0.13 |
| Stroke, n (%) | 25 (8.8) | 5 (6.2) | 5 (8.1) | 8 (12.9) | 7 (8.8) | 0.57 |
| Anuric, n (%) | 152 (53.3) | 36 (44.4) | 38 (61.3) | 36 (58.1) | 42 (52.5) | 0.19 |
| NT-proBNP [pg/ml] | 4595.0(1826.5–13342.0) | 1615.0(1052.0–3331.5) | 8684.5(5627.5–16691.0) | 2016.5(1007.7–2780.0) | 18944.0(8206.6–30109.3) | < 0.001 |
| Hemoglobin [g/dl] | 11.5 ±1.5 | 11.3 ±1.5 | 11.5 ±1.5 | 11.6 ±1.4 | 11.5 ±1.6 | 0.73 |
| CRP [mg/dl] | 5.3(2.1–12.3) | 4.2(2.1–8.5) | 8.4(3.3–17.1) | 4.7(1.4–13.1) | 5.8(1.8–12.7) | 0.04 |
| Albumin [g/dl] | 3.9 ±0.3 | 3.9 ±0.2 | 3.8 ±0.3 | 3.9 ±0.3 | 3.9 ±0.3 | 0.24 |
| Calcium [mg/dl] | 8.6 ±0.7 | 8.5 ±0.6 | 8.5 ±0.7 | 8.6 ±0.7 | 8.6 ±0.7 | 0.47 |
| Phosphate [mg/dl] | 5.1(3.9–6.2) | 5.2(4.1–6.4) | 5.3(4.6–6.3) | 4.9(3.3–6.6) | 4.7(3.5–5.8) | 0.11 |
| TBW [l] | 33.9 ±6.3 | 33.7 ±6.2 | 33.0 ±6.4 | 35.7 ±6.8 | 33.8 ±5.9 | 0.09 |
| ECW [l] | 16.2 ±2.9 | 15.8 ±2.6 | 15.3 ±2.9 | 17.2 ±3.1 | 16.4 ±2.8 | 0.001 |
| ICW [l] | 17.8 ±3.7 | 17.9 ±3.9 | 17.7 ±3.7 | 18.5 ±3.9 | 17.3 ±3.3 | 0.32 |
| AFO [l] | 1.2 ±1.3 | 0.3 ±0.7 | 0.1 ±0.8 | 2.3 ±1.1 | 2.1 ±0.8 | < 0.001 |
| RFO, % | 7.1 ±7.6 | 1.6 ±4.2 | 0.8 ±5.8 | 13.4 ±5.1 | 12.8 ±4.3 | < 0.001 |
Data are expressed as mean ± SD, median with IQR, or percent frequency, as appropriate. AFO – absolute fluid overload, CRP – C-reactive protein, ECW – extracellular water, ICW – intracellular water, NT-proBNP – N-terminal pro-B-type natriuretic peptide, NYHA – New York Heart Association, RFO – relative fluid overload, TBW – total body water.
Comparison between groups
All laboratory parameters were determined pre-dialysis, once in each patient at the inclusion of the study before a midweek HD session. NT-proBNP in serum samples were collected at the same time and were analyzed centrally using the Roche Elecsys kit, an electro-chemiluminescence ‘sandwich’ immunoassay based on polyclonal antibodies against NT-proBNP.
Included patients were followed up for time-to-event analysis until occurrence of death. Patients were censored at the last follow-up (1 August 2016) or if they moved to another dialysis unit, switched to peritoneal dialysis or received a kidney transplant. All procedures performed in this study were in accordance with the 1964 Declaration of Helsinki and its later amendments. The study protocol was approved by the Ethics Committee of University Hospital ‘Dr C.I. Parhon’ (Iasi, Romania).
Bioimpedance spectroscopy
The hydration state and the body composition were estimated using a portable whole body bioimpedance spectroscopy device (BCM–Fresenius Medical Care D GmbH). This device measures the impedance spectroscopy at 50 frequencies. Measurements were performed before a midweek dialysis session, at the same time as the serum samples collection.
All measurements were performed by two trained physicians blinded to patients’ daily management. The ECW, intracellular water (ICW) and total body water (TBW) were determined as previously described [21]. Absolute fluid overload (AFO) was defined as the difference between the expected patient’s ECW under normal physiological conditions and the actual ECW, whereas the relative fluid overload (RFO), used to facilitate comparison between patients, was defined as the absolute fluid overload to extracellular water ratio (AFO/ECW).
Statistical analysis
Data are expressed as mean ± SD, median with inter-quartile range (IQR) or as percent frequency, as appropriate. Comparisons between groups were performed with the one-way analysis of variance (ANOVA) for normally distributed variables, Kruskal-Wallis test for non-normally distributed variables and by the χ2 test for categorical data. For the pairwise multiple comparisons analysis we used the Bonferroni post-hoc test and the Mann-Whitney test with Bonferroni correction for normally distributed and non-normally distributed variables, respectively. The normality of the distribution of the variables was tested with the Shapiro-Wilk test. Logarithmic conversion was performed for non-normally distributed variables. The association between NT-proBNP and RFO levels was investigated by the Pearson product moment correlation coefficient.
Survival between different groups was compared using the Kaplan-Meier log-rank test for statistical significance and Cox analysis. The multivariable Cox models included all the variables that showed an association with the outcome at a p-value < 0.10. Proportional hazards assumptions were checked using the Schoenfeld residuals test. From Cox models including all univariable variables that showed an association with the outcome at a p-value < 0.10 with and without continuous NT-proBNP and RFO we evaluated the C statistic difference, continuous net reclassification index (NRI), and integrated discrimination improvement index (IDI) using methods accounting for censoring [22, 23].
The Bayesian information criterion (BIC) and the Akaike information criterion (AIC) scores were calculated for each model; the model with the lower BIC and AIC scores indicates a better model.
All statistical analyses were performed with SPSS 19.0 (SPSS Inc., Chicago, IL) and the R (version 3.2.0) package for statistical analysis (Foundation for Statistical Computing, Vienna, Austria). A p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics
Two hundred and eighty-five chronic HD patients were included in this study: 136 (47.7%) males, with a mean age of 58.9 years and a median dialysis vintage of 52.6 months. Two hundred and thirteen (74.7%) and 50 (17.5%) patients had hypertension and diabetes, respectively (Table I). Other demographic, clinical and biological characteristics of the entire population are presented in Tables I and II.
Table II.
Univariable COX analysis for all-cause mortality
| Parameter | HR | 95% CI | P-value |
|---|---|---|---|
| NT-proBNP – RFO Groups: | |||
| Group 1 | Reference | ||
| Group 2 | 1.45 | 0.75–2.82 | 0.27 |
| Group 3 | 1.55 | 0.81–2.96 | 0.19 |
| Group 4 | 2.36 | 1.32–4.23 | 0.004 |
| Log NT-proBNP [pg/ml]* | 1.48 | 1.19–1.83 | < 0.001 |
| RFO (%) | 1.03 | 1.01–1.06 | 0.02 |
| Age [years] | 1.04 | 1.02–1.06 | < 0.001 |
| Diabetes (0 – no, 1 – yes) | 1.56 | 0.95–2.54 | 0.08 |
| Gender (1 – male, 2 – female) | 0.69 | 0.45–1.05 | 0.08 |
| Severe NYHA Class (0 – Class 1 and 2, 1 – Class 3 and 4) | 3.87 | 2.05–7.28 | < 0.001 |
| Hemoglobin [g/dl] | 1.18 | 1.03–1.35 | 0.02 |
| Log CRP [mg/dl]* | 1.40 | 1.13–1.74 | 0.002 |
| Log phosphorus [mg/dl]* | 0.80 | 0.69–0.92 | 0.002 |
CRP – C-reactive protein, NT-proBNP – N-terminal pro-B-type natriuretic peptide, NYHA – New York Heart Association, RFO – relative fluid overload.
Hazard ratio for each increase in 1 SD in log of the variable.
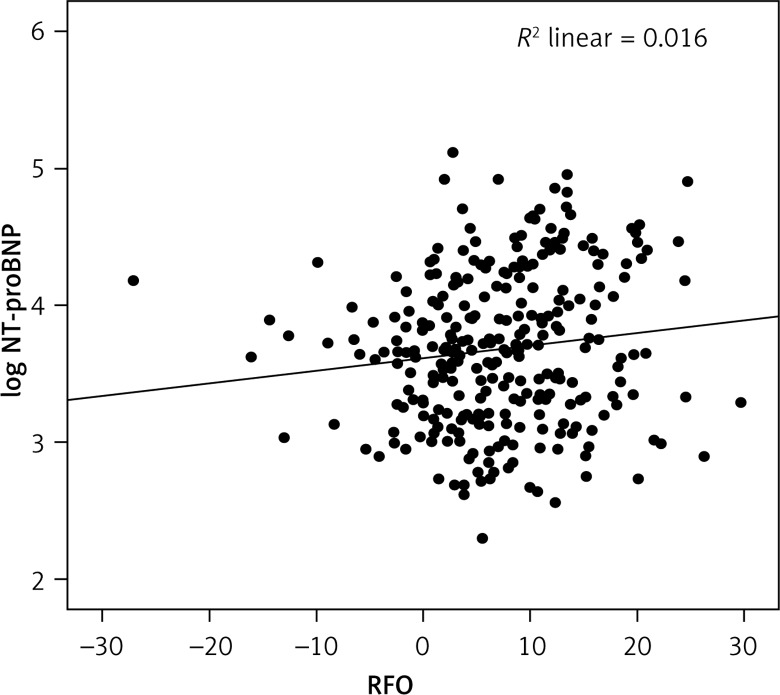
The median values for NT-proBNP and RFO were 4595 pg/ml and 6.9%, respectively. As per our aims we divided the study population into four groups according to median NT-proBNP and RFO levels: group 1 – low NT-proBNP (< 4595 pg/ml) and low RFO (< 6.9%); group 2 – high NT-proBNP (≥ 4595 pg/ml) and low RFO (< 6.9%); group 3 – low NT-proBNP (< 4595 pg/ml) and high RFO (≥ 6.9%); group 4 – high NT-proBNP (≥ 4595 pg/ml) and high RFO (≥ 6.9%). Patients from group 1 had a higher weight than those in group 4, but also lower dialysis vintage than those in group 3 and 4 (Table I). As also reported in Table I these patients also had lower CRP levels than those from group 2. We also found a significant, but weak, positive correlation between NT-proBNP and RFO values (r = 0.13, p = 0.04, Figure 1).
Figure 1.
Regression analysis of RFO and log NT-proBNP
Survival analysis
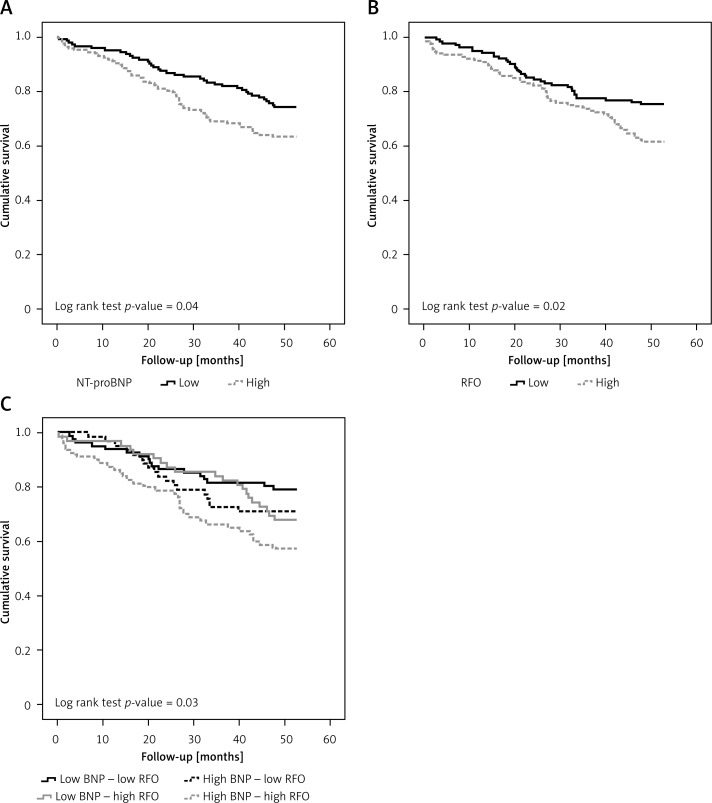
During the follow-up (mean: 41.1, median: 48.7 months), 89 (31.2%) patients died. Patients who had either increased NT-proBNP (more than 4595 pg/ml) or increased RFO (more than 6.9%) levels had an augmented risk for mortality (Figure 2 A and B, respectively). However, as shown in Figure 2 C, combining these two variables, we observed that patients in group 1 (low NT-proBNP – low RFO) had the lowest, while those in group 4 (high NT-proBNP – high RFO) had the highest risk for the outcome. These findings were confirmed in the univariable Cox survival analysis (Table II), where only patients in group 4, and not those from group 2 (high NT-proBNP – low RFO) or 3 (low NT-proBNP – high RFO), had significantly higher HRs as compared to those in group 1 (HR = 1.5, 95% CI: 0.8–2.8, HR = 1.6, 95% CI: 0.8–2.9 and HR = 2.4, 95% CI: 1.3–4.2, for group 2, 3 and 4, respectively). These results were maintained even after adjustment for all the univariable associates of the outcome (Table III).
Figure 2.
Kaplan-Meier analysis for the all-cause mortality outcome according to the median values for NT-proBNP (A) and RFO (B) levels and for the four predefined groups of patients (group 1 – low NT-proBNP and low RFO; group 2 – high NT-proBNP and low RFO; group 3 – low NT-proBNP and high RFO; group 4 – high NT-proBNP and high RFO) (C)
Table III.
Multivariable COX analysis for all-cause mortality (using NR-proBNP – RFO Groups)
| Parameter | HR | 95% CI | P-value |
|---|---|---|---|
| NT-proBNP – RFO Groups: | |||
| Group 1 | Reference | ||
| Group 2 | 1.16 | 0.59–2.26 | 0.67 |
| Group 3 | 1.40 | 0.72–2.75 | 0.32 |
| Group 4 | 2.00 | 1.11–3.62 | 0.02 |
| Age [years] | 1.04 | 1.02–1.06 | < 0.001 |
| Diabetes (0 – no, 1 – yes) | 1.02 | 0.62–1.69 | 0.94 |
| Gender (1 – male, 2 – female) | 0.76 | 0.49–1.18 | 0.76 |
| Severe NYHA Class (0 – Class 1 and 2, 1 – Class 3 and 4) | 2.04 | 1.06–3.95 | 0.03 |
| Hemoglobin [g/dl] | 1.22 | 1.06–1.39 | 0.004 |
| Log CRP [mg/dl]* | 1.30 | 1.03–1.64 | 0.03 |
| Log phosphorus [mg/dl]* | 0.83 | 0.69–0.99 | 0.04 |
CRP – C-reactive protein, NT-proBNP – N-terminal pro-B-type natriuretic peptide, NYHA – New York Heart Association, RFO – relative fluid overload.
Hazard ratio for each increase in 1 SD in log of the variable.
Although dialysis vintage was not associated with the outcome in the univariable survival analysis (HR = 1.12, 95% CI: 0.89–1.38 for each increase in 1 SD in the log of the dialysis vintage), due to the differences in its values observed between the 4 RFO-NT-proBNP groups (Table I) we also performed an additional survival analysis (Table IV). Including dialysis vintage in the final model, patients in group 4 (high NT-proBNP – high RFO) had a significantly higher risk for the outcome as compared with those in group 1 (low NT-proBNP – low RFO) (HR = 1.83, 95% CI: 1.02–3.54).
Table IV.
Multivariable COX analysis for all-cause mortality (including dialysis vintage)
| Parameter | HR | 95% CI | P-value |
|---|---|---|---|
| NT-proBNP – RFO Groups: | |||
| Group 1 | Reference | ||
| Group 2 | 1.12 | 0.56–2.21 | 0.75 |
| Group 3 | 1.34 | 0.67–2.68 | 0.40 |
| Group 4 | 1.83 | 1.02–3.54 | 0.04 |
| Age [years] | 1.04 | 1.02–1.06 | < 0.001 |
| Diabetes (0 – no, 1 – yes) | 1.07 | 0.63–1.81 | 0.81 |
| Gender (1 – male, 2 – female) | 0.76 | 0.49–1.18 | 0.76 |
| Severe NYHA Class (0 – Class 1 and 2, 1 – Class 3 and 4) | 2.06 | 1.06–3.99 | 0.03 |
| Hemoglobin [g/dl] | 1.21 | 1.05–1.39 | 0.01 |
| Log CRP [mg/dl]* | 1.31 | 1.03–1.65 | 0.03 |
| Log phosphorus [mg/dl]* | 0.82 | 0.68–0.99 | 0.04 |
| Log dialysis vintage [months]* | 1.08 | 0.83–1.41 | 0.56 |
CRP – C-reactive protein, NT-proBNP – N-terminal pro-B-type natriuretic peptide, NYHA – New York Heart Association, RFO – relative fluid overload.
Hazard ratio for each increase in 1 SD in log of the variable.
Since these results would suggest that a high NT-proBNP-high RFO phenotype could predict a worse outcome, we further tested the potential incremental prognostic value of adding NT-pro-BNP and RFO to a model including classical risk factors, i.e. all the univariable Cox associates for all-cause mortality (with p < 0.10 in the univariable analysis).
The addition of NT-proBNP (Model 2), RFO (Model 3) or both biomarkers (Model 4) to the baseline model (Model 1) did not increase the discrimination power of the model (Table V). All models showed good calibration, but the BIC and AIC scores were the lowest in the model that included both biomarkers (Table V). The reclassification abilities were increased only when both NT-proBNP and RFO were added to the baseline model (IDI = 3.1%, NRI = 16.8%), but not when only NT-proBNP (IDI = 1.7%, NRI = 12%) or RFO (IDI = 1.9%, NRI = 10.7%) was included in the initial model (Table IV).
Table V.
Performance of the models for predicting all-cause mortality
| Variable | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Discrimination: | ||||
| Δ C statistics, 95% CI | Reference | 0.005 (–0.015 to 0.025) | 0.005 (–0.013 to 0.024) | 0.010 (–0.013 to 0.033) |
| Calibration: | ||||
| H-L | χ2 = 15.14 p = 0.08 | χ2 = 15.45 p = 0.08 | χ2 = 13.06 p = 0.16 | χ2 = 15.46 p = 0.08 |
| AIC | 939.29 | 934.58 | 937.23 | 933.94 |
| BIC | 956.71 | 954.49 | 957.14 | 956.34 |
| Reclassification: | ||||
| IDI, 95% CI | Reference | 0.017 (–0.001 to 0.056) | 0.019 (–0.001 to 0.056) | 0.31 (0.003 to 0.082) |
| NRI, 95% CI | Reference | 0.120 (–0.036 to 0.235) | 0.107 (–0.073 to 0.266) | 0.168 (0.004 to 0.312) |
C statistic with only conventional predictors was 0.749. AIC – Akaike information criterion, BIC – Bayesian information criterion, H-L – Hosmer and Lemeshow test, NRI – net reclassification improvement. Model 1 – age, gender, smoking status, diabetes, systolic blood pressure, HDL and total cholesterol. Model 2 – Model 1 + NT-proBNP. Model 3 – Model 1 + RFO. Model 4 – Model 1 + NT-proBNP + RFO.
Discussion
This study shows for the first time that, in HD patients, using two complementary methods for fluid estimation (bioimpedance and NT-proBNP) could improve patients’ outcome prediction. Utilizing the four categories of fluid status according to the two parameters evaluated could better discriminate hydration status and therefore might improve treatment prescription and individualization.
Numerous studies have shown the diagnostic abilities of bioimpedance in HD patients. Wizemann et al. demonstrated for the first time in HD patients that fluid overload, as assessed by bioimpedance, is associated with mortality [4]. These findings were later confirmed in another observational study which showed an increased risk for death in patients with fluid overload, above and independent of hypertension – the main parameter of clinical fluid assessment in clinical practice [24]. Our group recently took this approach a step further and showed that bioimpedance-assessed fluid overload and its association with all-cause mortality are independent even of underlying (echo)cardiac parameters (7) – recognized predictors of worse outcomes in HD patients [25]. Most importantly, two randomized controlled studies proved that using bioimpedance for dry weight estimation improved blood pressure control, arterial stiffness [9, 10], left ventricular mass index [9] and even survival [10].
However, although the beneficial effects of this method are well supported, it is far from being perfect or unanimously accepted. Recently, Raimann et al. showed in a well-conducted validation study that bioimpedance analysis could significantly facilitate body fluid assessment, but is associated with evident precision and accuracy errors [26]. Firstly, bioimpedance spectroscopy estimates fluid compartments from the resistance and reactance of a current passing through different body regions. When an alternating current is applied to tissues, the measurement of resistance is inversely proportional to the total content (which includes both ECW and ICW) between the two electrodes placed at distance on the skin, while the reactance is proportional to the cell mass in the same tissue volume [27]. Therefore, the derived information about body compartments is only an indirect measure of tissue water content and distribution. Although there is a very good agreement between bioimpedance spectroscopy and the gold-standard isotope dilution techniques for TBW and ECW assessment, the 95% CI in the agreement with ECW is ±2.8 l, which is approximately 17% of the TBW, and it may be larger in some HD patients [21, 27]. Furthermore, Raimann et al. demonstrated that, as compared with direct estimation methods, there is slightly better accuracy for ECW estimation using multi- over single-frequency bioimpedance and for ICW estimation using single- over multi-frequency bioimpedance. However, bioimpedance analysis and direct estimation methods have minimal differences in precision, suggesting that none could serve as a true gold standard with absolute accuracy for body fluid assessment, at least in HD patients [26]. Secondly, when estimating ECW, bioimpedance analysis does not differentiate between intravascular and interstitial water content. Therefore, other methods addressing the intravascular water compartment would be of interest.
NT-proBNP is one of the most important biomarkers for diagnostic and prognostic assessment of patients with heart failure. In HD patients, elevated NT-proBNP levels are associated with an increased risk for all-cause mortality [14–16, 18, 19], cardiovascular mortality [15] or a composite outcome of death and cardiovascular events [17]. However, in HD patients there is currently a debate whether its increased levels could serve as a marker of cardiac dysfunction [11, 28] or fluid overload [5, 12, 13]. Moreover, studies have shown a direct relationship between change in NT-pro-BNP levels and both changes in left ventricular mass [29] and/or changes in volume status [30, 31].
Combining these two methods (bioimpedance and NT-proBNP) for fluid status evaluation has been used before in ICU patients [20]. Chen et al. included 98 patients at the start of the renal replacement therapy and found that in those patients with both higher NT-proBNP and fluid overload there was an increased risk for all-cause mortality. Although this association was lost in the multivariable regression analysis, the study had limited statistical power with only 29 deaths during the follow-up. By comparison, in our study this association remained statistically significant in the multivariable survival analysis. These results could be related to the different characteristics of the included patients (one analyzed stable HD patients, while the other one analyzed critically ill patients, but who were younger and with different baseline comorbidities), but also to the difference in the bioimpedance method used for fluid assessment. Furthermore, we also demonstrated that by adding these two markers of fluid status into a baseline model we can obtain an improvement in the reclassification abilities of that model.
Our study has limitations. Firstly, being an observational study no cause-effect inferences can be made. Secondly, we did not perform an echocardiographic assessment of the patients, although this could have helped to better define the relationship between NT-proBNP, fluid status and cardiac function. However, it was previously shown that NT-proBNP levels are strongly associated with cardiac function [32, 33]. Thirdly, due to logistic issues, we were not able to assess the specific causes of death (such as cardiovascular causes), though it would have added important information regarding the specific implication of fluid overload. Fourthly, the results obtained in this study come from only a single region of Romania, and should be confirmed in larger, multinational, studies. Fifthly, the analysis was performed with baseline and not with serial measurements, which could have been extremely relevant to better understand the relationship between overhydration and survival.
In conclusion, different types of fluid distribution can be observed in HD patients. Including both bioimpedance and NT-proBNP monitoring in a more comprehensive fluid status assessment could improve the diagnosis of fluid overload with a final improvement in patients’ outcome. However, further studies, which should also include parameters of cardiac function in the analysis, are required to better define and understand the relationship between cardiac abnormalities, fluid status and hard outcomes in HD patients.
Acknowledgments
This study was partially funded by the UEFISCDI, Grant number IDEI–PCE 2011, PN-II-ID-PCE-2011-3-0637. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
Prof. Dr. Adrian Covic is an honorary speaker for Fresenius Medical Care. Fresenius Medical Care is the manufacturer of the BCM device and was not involved in any way in the study. Other authors declare no conflict of interest.
References
- 1.Ortiz A, Covic A, Fliser D, et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet. 2014;383:1831–43. doi: 10.1016/S0140-6736(14)60384-6. [DOI] [PubMed] [Google Scholar]
- 2.Ishibe S, Peixoto AJ. Methods of assessment of volume status and intercompartmental fluid shifts in hemodialysis patients. Implications in clinical practice. Semin Dial. 2004;17:37–43. doi: 10.1111/j.1525-139x.2004.17112.x. [DOI] [PubMed] [Google Scholar]
- 3.Cheriex EC, Leunissen KM, Janssen JH, Mooy JM, van Hooff JP. Echography of the inferior vena cava is a simple and reliable tool for estimation of ‘dry weight’ in haemodialysis patients. Nephrol Dial Transplant. 1989;4:563–8. [PubMed] [Google Scholar]
- 4.Wizemann V, Wabel P, Chamney P, et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant. 2009;24:1574–9. doi: 10.1093/ndt/gfn707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fagugli RM, Palumbo B, Ricciardi D, et al. Association between brain natriuretic peptide and extracellular water in hemodialysis patients. Nephron Clin Pract. 2003;95:c60–6. doi: 10.1159/000073669. [DOI] [PubMed] [Google Scholar]
- 6.Noble VE, Murray AF, Capp R, Sylvia-Reardon MH, Steele DJ, Liteplo A. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest. 2009;135:1433–9. doi: 10.1378/chest.08-1811. [DOI] [PubMed] [Google Scholar]
- 7.Onofriescu M, Siriopol D, Voroneanu L, et al. Overhydration, cardiac function and survival in hemodialysis patients. PloS One. 2015;10:e0135691. doi: 10.1371/journal.pone.0135691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siriopol D, Voroneanu L, Hogas S, et al. Bioimpedance analysis versus lung ultrasonography for optimal risk prediction in hemodialysis patients. Int J Cardiovasc Imaging. 2016;32:263–70. doi: 10.1007/s10554-015-0768-x. [DOI] [PubMed] [Google Scholar]
- 9.Hur E, Usta M, Toz H, et al. Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis. 2013;61:957–65. doi: 10.1053/j.ajkd.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Onofriescu M, Hogas S, Voroneanu L, et al. Bioimpedance-guided fluid management in maintenance hemodialysis: a pilot randomized controlled trial. Am J Kidney Dis. 2014;64:111–8. doi: 10.1053/j.ajkd.2014.01.420. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal R. B-type natriuretic peptide is not a volume marker among patients on hemodialysis. Nephrol Dial Transplant. 2013;28:3082–9. doi: 10.1093/ndt/gft054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tapolyai M, Faludi M, Réti V, et al. Volume estimation in dialysis patients: the concordance of brain-type natriuretic peptide measurements and bioimpedance values. Hemodial Int. 2013;17:406–12. doi: 10.1111/hdi.12023. [DOI] [PubMed] [Google Scholar]
- 13.Nongnuch A, Panorchan K, Davenport A. Predialysis NTproBNP predicts magnitude of extracellular volume overload in haemodialysis patients. Am J Nephrol. 2014;40:251–7. doi: 10.1159/000368376. [DOI] [PubMed] [Google Scholar]
- 14.Sivalingam M, Vilar E, Mathavakkannan S, Farrington K. The role of natriuretic peptides in volume assessment and mortality prediction in haemodialysis patients. BMC Nephrol. 2015;16:218. doi: 10.1186/s12882-015-0212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández-Reyes MJ, Mon C, Heras M, et al. Predictive value of troponin T levels for ischemic heart disease and mortality in patients on hemodialysis. J Nephrol. 2004;17:721–7. [PubMed] [Google Scholar]
- 16.Satyan S, Light RP, Agarwal R. Relationships of N-terminal pro-B-natriuretic peptide and cardiac troponin T to left ventricular mass and function and mortality in asymptomatic hemodialysis patients. Am J Kidney Dis. 2007;50:1009–19. doi: 10.1053/j.ajkd.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sommerer C, Beimler J, Schwenger V, et al. Cardiac biomarkers and survival in haemodialysis patients. Eur J Clin Invest. 2007;37:350–6. doi: 10.1111/j.1365-2362.2007.01785.x. [DOI] [PubMed] [Google Scholar]
- 18.McGill D, Talaulikar G, Potter JM, Koerbin G, Hickman PE. Over time, high-sensitivity TnT replaces NT-proBNP as the most powerful predictor of death in patients with dialysis-dependent chronic renal failure. Clin Chim Acta. 2010;411:936–9. doi: 10.1016/j.cca.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Voroneanu L, Siriopol D, Nistor I, et al. Superior predictive value for NTproBNP compared with high sensitivity cTnT in dialysis patients: a pilot prospective observational study. Kidney Blood Press Res. 2014;39:636–47. doi: 10.1159/000368452. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Wu B, Gong D, Liu Z. Fluid overload at start of continuous renal replacement therapy is associated with poorer clinical condition and outcome: a prospective observational study on the combined use of bioimpedance vector analysis and serum N-terminal pro-B-type natriuretic peptide measurement. Crit Care. 2015;19:135. doi: 10.1186/s13054-015-0871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moissl UM, Wabel P, Chamney PW, et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas. 2006;27:921–33. doi: 10.1088/0967-3334/27/9/012. [DOI] [PubMed] [Google Scholar]
- 22.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–23. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 23.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of newbiomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chazot C, Wabel P, Chamney P, Moissl U, Wieskotten S, Wizemann V. Importance of normohydration for the long-term survival of haemodialysis patients. Nephrol Dial Transplant. 2012;27:2404–10. doi: 10.1093/ndt/gfr678. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Zhang DL, Guo W, Cui WY, Liu WH. Left ventricular mass index and aortic arch calcification score are independent mortality predictors of maintenance hemodialysis patients. Hemodial Int. 2012;16:504–11. doi: 10.1111/j.1542-4758.2012.00700.x. [DOI] [PubMed] [Google Scholar]
- 26.Raimann JG, Zhu F, Wang J, et al. Comparison of fluid volume estimates in chronic hemodialysis patients by bioimpedance, direct isotopic, and dilution methods. Kidney Int. 2014;85:898–908. doi: 10.1038/ki.2013.358. [DOI] [PubMed] [Google Scholar]
- 27.Davies SJ, Davenport A. The role of bioimpedance and biomarkers in helping to aid clinical decision-making of volume assessments in dialysis patients. Kidney Int. 2014;86:489–96. doi: 10.1038/ki.2014.207. [DOI] [PubMed] [Google Scholar]
- 28.Mallamaci F, Zoccali C, Tripepi G, et al. Diagnostic potential of cardiac natriuretic peptides in dialysis patients. Kidney Int. 2001;59:1559–66. doi: 10.1046/j.1523-1755.2001.0590041559.x. [DOI] [PubMed] [Google Scholar]
- 29.Choi SY, Lee JE, Jang EH, et al. Association between changes in N-terminal pro-brain natriuretic peptide levels and changes in left ventricular mass index in stable hemodialysis patients. Nephron Clin Pract. 2008;110:c93–100. doi: 10.1159/000157622. [DOI] [PubMed] [Google Scholar]
- 30.Gutiérrez OM, Tamez H, Bhan I, et al. N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations in hemodialysis patients: prognostic value of baseline and follow-up measurements. Clin Chem. 2008;54:1339–48. doi: 10.1373/clinchem.2007.101691. [DOI] [PubMed] [Google Scholar]
- 31.Chazot C, Vo-Van C, Zaoui E, et al. Fluid overload correction and cardiac history influence brain natriuretic peptide evolution in incident haemodialysis patients. Nephrol Dial Transplant. 2011;26:2630–4. doi: 10.1093/ndt/gfq804. [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki M, Ogawa T, Tamei N, Ando Y, Nitta K. Relation of N-terminal pro-B-type natriuretic peptide (NT-proBNP) and left atrial volume index to left ventricular function in chronic hemodialysis patients. Heart Vessel. 2011;26:421–7. doi: 10.1007/s00380-010-0066-4. [DOI] [PubMed] [Google Scholar]
- 33.David S, Kümpers P, Seidler V, Biertz F, Haller H, Fliser D. Diagnostic value of N-terminal pro-B-type natriuretic peptide (NT-proBNP) for left ventricular dysfunction in patients with chronic kidney disease stage 5 on haemodialysis. Nephrol Dial Transplant. 2008;23:1370–7. doi: 10.1093/ndt/gfm700. [DOI] [PubMed] [Google Scholar]