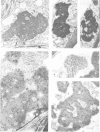
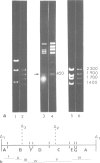
Abstract
We have used electron microscopy of thin sections and experiments on isolated viroplasms to compare the properties of four strains of cauliflower mosaic virus (CaMV), three of which were partially or completely deleted in open reading frame (ORF) II. Our results confirm that this gene is required for aphid transmissibility and show that the product of ORF II influences the firmness with which virions are held within the viroplasm. Analysis of the proteins in the viroplasms showed that a mutant with a partial deletion in ORF II produced a protein smaller than the normal ORF product. This smaller protein was non-functional with respect both to aphid transmissibility and properties of the viroplasms.
Keywords: cauliflower mosaic virus, inclusion body, gene, electron microscopy, ELISA
Full text
PDF
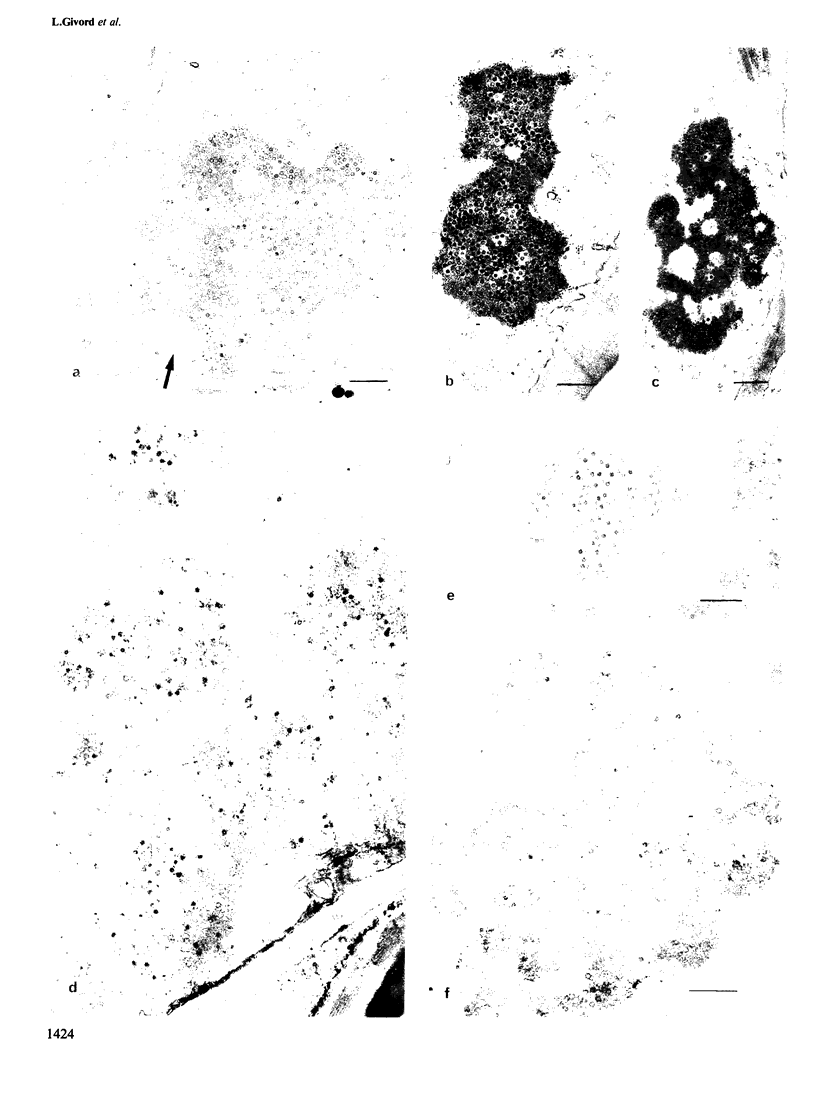

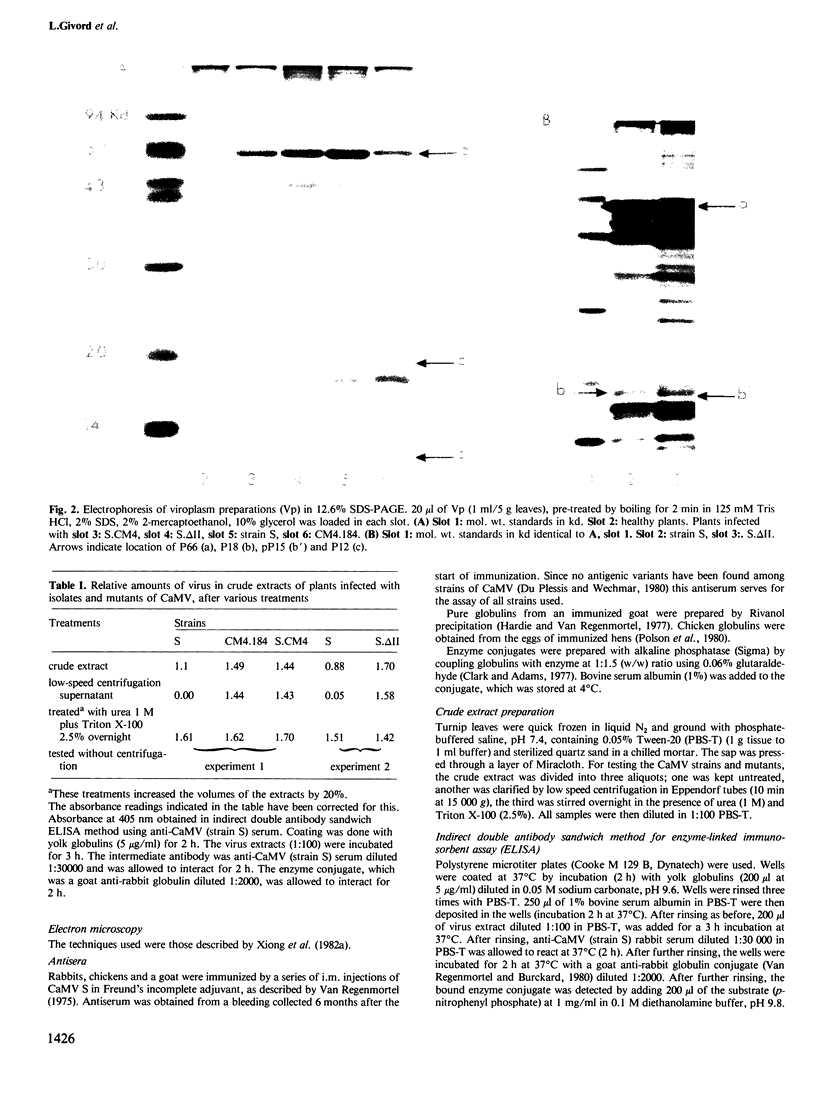

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balàzs E., Guilley H., Jonard G., Richards K. Nucleotide sequence of DNA from an altered-virulence isolate D/H of the cauliflower mosaic virus. Gene. 1982 Oct;19(3):239–249. doi: 10.1016/0378-1119(82)90013-0. [DOI] [PubMed] [Google Scholar]
- Clark M. F., Adams A. N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol. 1977 Mar;34(3):475–483. doi: 10.1099/0022-1317-34-3-475. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. II. Nature of the defect. J Mol Biol. 1973 May 25;76(3):325–343. doi: 10.1016/0022-2836(73)90508-1. [DOI] [PubMed] [Google Scholar]
- Conti G. G., Vegetti G., Bassi M., Favali M. A. Some ultrastructural and cytochemical observations on Chinese cabbage leaves infected with cauliflower mosaic virus. Virology. 1972 Mar;47(3):694–700. doi: 10.1016/0042-6822(72)90559-4. [DOI] [PubMed] [Google Scholar]
- Dixon L. K., Koenig I., Hohn T. Mutagenesis of cauliflower mosaic virus. Gene. 1983 Nov;25(2-3):189–199. doi: 10.1016/s0378-1119(83)80001-8. [DOI] [PubMed] [Google Scholar]
- Franck A., Guilley H., Jonard G., Richards K., Hirth L. Nucleotide sequence of cauliflower mosaic virus DNA. Cell. 1980 Aug;21(1):285–294. doi: 10.1016/0092-8674(80)90136-1. [DOI] [PubMed] [Google Scholar]
- Gardner R. C., Howarth A. J., Hahn P., Brown-Luedi M., Shepherd R. J., Messing J. The complete nucleotide sequence of an infectious clone of cauliflower mosaic virus by M13mp7 shotgun sequencing. Nucleic Acids Res. 1981 Jun 25;9(12):2871–2888. doi: 10.1093/nar/9.12.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie G., van Regenmortel M. H. Isolation of specific antibody under conditions of low ionic strength. J Immunol Methods. 1977;15(4):305–314. doi: 10.1016/0022-1759(77)90092-8. [DOI] [PubMed] [Google Scholar]
- Hohn T., Hohn B., Lesot A., Lebeurier G. Restriction map of native and cloned cauliflower mosaic virus DNA. Gene. 1980 Oct;11(1-2):21–31. doi: 10.1016/0378-1119(80)90083-9. [DOI] [PubMed] [Google Scholar]
- Lebeurier G., Hirth L., Hohn B., Hohn T. In vivo recombination of cauliflower mosaic virus DNA. Proc Natl Acad Sci U S A. 1982 May;79(9):2932–2936. doi: 10.1073/pnas.79.9.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeurier G., Hirth L., Hohn T., Hohn B. Infectivities of native and cloned DNA of cauliflower mosaic virus. Gene. 1980 Dec;12(1-2):139–146. doi: 10.1016/0378-1119(80)90024-4. [DOI] [PubMed] [Google Scholar]
- Martelli G. P., Castellano M. A. Light and electron microscopy of the intracellular inclusions of cauliflower mosaic virus. J Gen Virol. 1971 Oct;13(1):133–140. doi: 10.1099/0022-1317-13-1-133. [DOI] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Polson A., von Wechmar M. B., van Regenmortel M. H. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol Commun. 1980;9(5):475–493. doi: 10.3109/08820138009066010. [DOI] [PubMed] [Google Scholar]
- Shortle D., Nathans D. Local mutagenesis: a method for generating viral mutants with base substitutions in preselected regions of the viral genome. Proc Natl Acad Sci U S A. 1978 May;75(5):2170–2174. doi: 10.1073/pnas.75.5.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Regenmortel M. H. Antigenic relationships between strains of tobacco mosaic virus. Virology. 1975 Apr;64(2):415–420. doi: 10.1016/0042-6822(75)90118-x. [DOI] [PubMed] [Google Scholar]
- Volovitch M., Drugeon C., Yot P. Studies on the single-stranded discontinuities of the cauliflower mosaic virus genome. Nucleic Acids Res. 1978 Aug;5(8):2913–2925. doi: 10.1093/nar/5.8.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolston C. J., Covey S. N., Penswick J. R., Davies J. W. Aphid transmission and a polypeptide are specified by a defined region of the cauliflower mosaic virus genome. Gene. 1983 Jul;23(1):15–23. doi: 10.1016/0378-1119(83)90212-3. [DOI] [PubMed] [Google Scholar]
- Xiong C., Muller S., Lebeurier G., Hirth L. Identification by immunoprecipitation of cauliflower mosaic virus in vitro major translation product with a specific serum against viroplasm protein. EMBO J. 1982;1(8):971–976. doi: 10.1002/j.1460-2075.1982.tb01280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]