Abstract
Objectives
The aim of this study was to compare the operational impact of using vanadate oxidase versus diazo direct bilirubin assays for an academic medical center patient population.
Design and methods
Retrospective study was done over an approximately 3.5 year period. The main automated chemistry instrumentation was a Roche Diagnostics cobas 8000 line. The Roche Direct Bilirubin assay was compared to Diazyme Laboratories Direct Bilirubin Assay and Randox Laboratories Direct Bilirubin assay using manufacturer's guidelines for hemolysis index, lipemia index, and analytical measurement range (AMR).
Results
Retrospective data was analyzed for 47,333 serum/plasma specimens that had clinical orders for direct bilirubin. A total of 5943 specimens (12.6%) exceeded the hemolysis index limit for the Roche method compared to only 0.2% and 0.05% of specimens for the Diazyme and Randox methods, respectively. The impact was particularly large on patients less than 2 years old, for which 51.3% of specimens exceeded the hemolysis index for the Roche method. A total of 1671 specimens (3.5%) exceeded the lipemia index limit for the Roche method compared to less than 0.1% for the Randox method. Lastly, 988 (2.1%) of specimens had direct bilirubin concentrations exceeding the upper AMR limit of 10 mg/dL [171 µmol/L] for the Roche assay compared to less than 1% of specimens for the vanadate oxidase methods.
Conclusions
Vanadate oxidase direct bilirubin methods offer advantages over diazo methods in terms of less interference by hemolysis and lipemia, as well as wider AMR. The advantages are particularly evident for neonatal and infant populations.
Keywords: Bilirubin, Clinical chemistry tests, Hemolysis, Hyperlipidemias, Jaundice, Photometry
1. Introduction
Bilirubin is the major metabolite of heme, a key component of hemoglobin, myoglobin, and cytochromes [1]. Measurement of direct bilirubin (DBIL, also known as conjugated bilirubin) and total bilirubin (TBIL) is widely used in clinical medicine, especially in the diagnosis and management of disorders affecting the hematologic and hepatobiliary systems [1], [2], [3]. Bilirubin can be measured in body fluids by a variety of analytical methods including chromatography, electrophoresis, and spectrophotometry [1]. Given that DBIL and TBIL are among the most commonly ordered laboratory tests, clinical laboratories typically utilize automated clinical chemistry assays. The most widely used methods for measurement of different forms of bilirubin employ the diazo method, originally developed by Ehrlich in 1883 [4], [5] and modified multiple times over the years [1], [6], [7], [8], [9]. Conjugated bilirubin reacts directly with the diazo reagent (thus the designation of conjugated bilirubin as “directly reacting” or “direct” bilirubin) [1].
In a typical automated diazo method, serum/plasma is incubated with diazo reagent at approximately pH 1.7–2.0 to form a diazonium salt [8]. The resulting product then reacts with bilirubin to form isomers of azobilirubin. In DBIL assays, the conjugated bilirubin is the predominant form converted by the diazotized sulfanilic acid (approximately 5% of unconjugated bilirubin may react as well). The intensity of the red color of azobilirubin is measured photometrically at approximately 600 nm and is proportional to DBIL concentration. Unconjugated bilirubin reacts with diazo reagents following the addition of accelerants (e.g., caffeine, sodium benzoate, or methanol), thus allowing for the determination of the concentration of TBIL. The difference between TBIL and DBIL measurements allows for the estimation of the concentration of unconjugated bilirubin (hence the designation of this difference as “indirect” bilirubin).
While the diazo DBIL methods are inexpensive and readily automated, they have some key limitations, including interference by low levels of hemolysis and less commonly by lipemia or paraproteins [1], [10], [11], [12]. The interference by hemolysis can be especially problematic in the neonatal and infant populations, where sample collection may be challenging and lead to suboptimal quality of specimens. While TBIL is the laboratory test primarily used for assessment and management of neonatal hyperbilirubinemia, DBIL measurements can be very useful in the differential diagnosis and clinical management of neonates whose hyperbilirubinemia is due to a cause other than physiologic jaundice of the newborn [3], [13], [14]. A recent study has shown that hemolysis interference with a diazo DBIL method can lead to unpredictable bias even at low levels of hemolysis [15].
The mechanism by which hemolysis interferes with diazo DBIL assays is complex and incompletely understood [15]. The interference is influenced by composition of DBIL assay reagents, primary/sub-wavelength used, type of chemical reaction (e.g, rate vs. end-point), assay pH, serum/plasma albumin concentration, medications, and presence of low molecular weight molecules. A detailed analysis of hemolysis interference is described by Devgun and Richardson, who utilized hemolysis thresholds to better define the extent of interference on diazo DBIL analysis [15]. Depending on patient population, a sizable number of specimens may have hemolysis that exceeds the recommended hemolysis limit for diazo DBIL assays given that hemolysis is the most common endogenous interference [16], [17], [18], [19], [20]. Although the mechanisms of hemolysis interference with DBIL are incompletely understood, several studies have shown predominantly negative interference [15], [21]. The sensitivity of diazo DBIL assays to even low degrees of hemolysis places the clinical laboratory in a difficult position - either cancel the testing if hemolysis is present or, alternatively, report with a disclaimer that the results may be impacted by hemolysis. The former approach can lead to clinician and patient dissatisfaction, while the latter approach risks reporting inaccurate results.
Paraproteins (especially IgM monoclonal proteins) have also been reported to interfere with the diazo DBIL and TBIL methods, producing either positive or negative interferences [10], [11], [12], [22]. Paraprotein interference is method specific, with significant variability between assays even for the same specimen. The concentration and isotype of the paraprotein does not correlate well with presence or degree of interference. Paraprotein interference may be detected by unusual results such as DBIL concentration much greater than the TBIL concentration, unusual reaction kinetics or absorbance readings (sometimes resulting in error flags on instruments), or a significant discrepancy between serum icterus index and either DBIL or TBIL concentration. Proposed mechanisms for paraprotein interference include increased turbidity, interference with reaction kinetics, and/or alterations in spectrophotometric readings [10], [11], [12], [22].
An alternative approach to measurement of DBIL are enzymatic methods utilizing bilirubin oxidase, an enzyme that catalyzes the oxidation of bilirubin to biliverdin [23], [24], [25], [26], [27], [28]. The vanadate oxidase method for determination of DBIL utilizes vanadate as the oxidizing agent for the conversion of bilirubin (yellow colored) to biliverdin, which may then be further oxidized to colorless products. Although the vanadate oxidase method for determination of DBIL has been known for several decades, there is relatively little published literature on the application of this method to clinical samples except for the recent study referenced above [15] and a veterinary publication analyzing samples from dog, monkey, and rat [29]. For the vanadate oxidase methods, specimen is mixed with reagent at approximately pH 3, converting DBIL to biliverdin, thereby decreasing the absorbance of yellow (main wavelength 450 nm, sub-wavelength 546 nm) [27], [28]. There is very little interference from hemolysis for the vanadate oxidase DBIL methods [15]. There are several vanadate oxidase DBIL methods available for automated clinical chemistry analyzers, although these usually need to be run on open analyzer channels given that the major clinical chemistry instrumentation vendors generally offer their own diazo DBIL methods as the default choice [1].
The aim of the present study was to estimate the operational impact of using a vanadate oxidase DBIL method at an academic medical center. The initial impetus for the study was clinician complaints from cancellation of testing for hemolyzed specimens, especially in the neonatal and infant population. The primary aim was therefore to estimate how often the vanadate oxidase assays allowed for analysis of specimens that exceeded the hemolysis limit specified by the manufacturer of the diazo DBIL method. We also examined the impact of vanadate oxidase DBIL assays on lipemia interference and analytical measurement range (AMR). We utilized a 3.5 year retrospective time period where we had complete data on hemolysis index (HI), lipemia index, and patient demographics for all samples for which DBIL was ordered clinically.
2. Materials and methods
2.1. Study setting and population
The study was conducted at the University of Iowa Hospitals and Clinics (UIHC), a 734-bed pediatric and adult tertiary/quaternary care academic medical center with level one trauma center, inpatient units (including multiple intensive care units), and outpatient clinics. The electronic health record is currently Epic (Epic Systems, Inc., Madison, WI, USA) which contains historic data covering the entire period of retrospective analysis. The data in this study is from the UIHC core laboratory and was collected as part of a retrospective study approved by the University of Iowa Institutional Review Board (protocol # 201608719) covering the time period from January 1, 2013 to July 31, 2016. This study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). As described in our previous studies, Reporting Workbench functionality within Epic allowed for retrieval of past laboratory results and associated patient demographics [30], [31].
The main automated clinical chemistry instrumentation in the UIHC core clinical laboratory is from Roche Diagnostics (Indianapolis, IN, USA) and consists of a cobas 8000 system with two c702, three c502, and five e602 analyzers. All serum/plasma assays run on the Roche analyzers have HI, icterus index, and lipemia index determined by spectrophotometry (SI2 Serum Index Gen.2, currently version 0586707002V2) which are used in autoverification rules for all chemistry tests [32], [33]. The analyzers take an aliquot of the patient specimen and dilute in 0.9% sodium chloride solution to measure the absorbances for lipemia at 660 nm (primary wavelength) and 700 nm (secondary wavelength), for hemolysis at 570 nm (primary wavelength) and 600 nm (secondary wavelength), and for icterus at 480 nm (primary wavelength) and 505 nm (secondary wavelength). In the core laboratory, specimens exceeding established thresholds for HI or lipemia index for a particular assay are cancelled. The serum indices officially have no units. However, the hemolysis and icterus indices correspond approximately to serum hemoglobin and bilirubin concentrations, respectively, in mg/dL. The serum lipemia index measures turbidity and correlates weakly with triglyceride concentration.
Interfacing throughout the laboratory is provided by Middleware software (Instrument Manager) from Data Innovations (Burlington, VA) [32], [33]. HI and lipemia index were extracted from Instrument Manager data in the time period of January 1, 2013 to July 31, 2016 using specimen accession numbers, allowing for linkage to patient data and the laboratory testing ordered on the accession number. The dataset over 3.5 years included 47,333 serum/plasma specimens for which DBIL was ordered clinically and with complete data for DBIL concentrations, HI, lipemia index, and patient demographics. This allowed us to estimate how many samples would have exceeded AMR, HI, and lipemia index limits for the three different DBIL assays considered in the study, even though all three assays were not run concurrently throughout the entire retrospective time period.
2.2. Direct bilirubin assays
All analysis reported here is of serum or plasma specimens. During the retrospective time period, three different DBIL assays were run for serum/plasma specimens: Roche cobas 8000 DBILI (Direct Bilirubin, currently version 2016-02, V5), Diazyme Laboratories (Poway, CA) Direct Bilirubin Assay (catalog DZ151A-K), and Randox (Kearneysville, WV) Direct Bilirubin (revised June 7, 2016, Rev. 004). The Diazyme DBIL assay was used at the UIHC core clinical laboratory throughout the retrospective time period of this study but has since become unavailable on the United States market. The primary method for clinical orders of DBIL was the Roche method. However, any specimens with HI exceeding 30 (Roche package insert limit) and/or a DBIL concentration exceeding the upper limit of the AMR for the Roche assay (10 mg/dL) automatically reflexed to the vanadate oxidase method (originally Diazyme and then Randox after the Diazyme method became commercially unavailable in the United States). The manufacturer guidelines for HI limit, lipemia index limit, AMR, and reference range for the three DBIL assays are summarized in Table 1.
Table 1.
Manufacturer specifications (package insert) for the direct bilirubin methods.
| Method | Hemolysis index limit | Lipemia index limit | Linearity of method (mg/dL) | Dilution protocol | Reference range (mg/dL) |
|---|---|---|---|---|---|
| Roche diagnostics | 30 | 60 | 0.2–10.0 | Manual | 0.0–0.3 |
| Diazyme laboratories | 500 | Not listed | 0.1–20.0 | Auto-dilution | 0.0–0.2 |
| Randox laboratories | 1000 | 1000 | 0.1–15.4 | Auto-dilution | 0.0–0.2 |
The Roche DBIL method is based on the Jendrassik-Grof diazo procedure [7] and is standardized against the manual DBIL procedure of Lo and Wu [8]. Acidified sodium nitrite produces nitrous acid, which reacts with sulfanilic acid to form a diazonium salt. The diazotized sulfanilic acid then reacts with bilirubin to form isomers of azobilirubin. The intensity of the red color of azobilirubin is measured photometrically (main wavelength 570 nm, sub-wavelength 660 nm). The Diazyme Direct Bilirubin Assay (currently unavailable commercially in the United States) and Randox Direct Bilirubin methods both use a bilirubin oxidase method with vanadate-containing reagent [27], [28]. Specimen is mixed with reagent at approximately pH 3, converting DBIL to biliverdin, thereby decreasing the absorbance of yellow (main wavelength 450 nm, sub-wavelength 546 nm). Middleware rules flag unusual results for technologist manual review such as TBIL or DBIL concentration much greater than icterus index [32].
The Roche DBIL assay was run on the cobas c702 analyzers. The two vanadate oxidase methods were run as open channels on a single cobas c502 analyzer. Comparisons between the diazo and vanadate oxidase assays on the different Roche analyzers were performed at least every six months. In addition, quality control was run routinely for both the diazo and vanadate oxidase methods, as these were both used for clinical testing. Sample volumes for the three assays are as follows: Roche, 6.0 μL (no onboard dilution possible); Diazyme, 5.0 μL for normal run/2.5 μL for onboard dilution; Randox, 3.5 μL for normal run/1.8 μL for onboard dilution. Dead volumes for the three assays were 100 μL.
2.3. Verification of interference
To verify manufacturer interference claims, pooled plasma samples from lithium heparin separator tubes were prepared as test samples. Hemolysate was prepared by washing packed red blood cells and then subjecting to freezing and thawing. This hemolysate was added to simulate various degrees of hemolysis. Lipemic samples were prepared by adding Intralipid® (Baxter, Deerfield, IL). DBIL was measured before and after addition of interferent and the percent change noted.
3. Results
3.1. Correlation between direct bilirubin methods
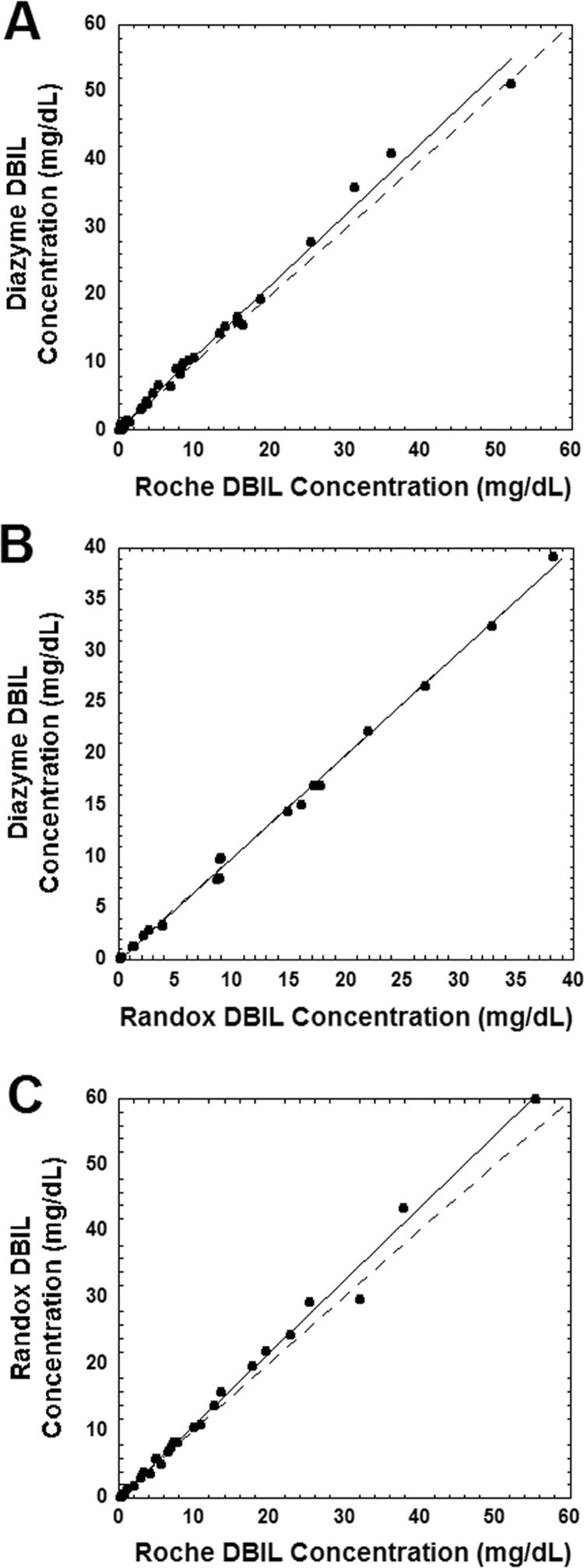
Fig. 1A shows the correlation between the Diazyme and Roche DBIL methods for specimens with HI less than 30. The two methods correlate well up to 20 mg/dL [342 µmol/L], with a slight positive bias for the Diazyme compared to the Roche method more noticeable above 20 mg/dL (leading to a slope slightly but significantly greater than 1.0). Fig. 1B shows the correlation between the two vanadate oxidase methods (Randox and Diazyme). There is a close correlation between these two methods throughout all concentrations tested. Fig. 1C shows the correlation between the Randox and Roche DBIL methods for specimens with HI less than 30. Similar to the correlation between the Diazyme and Roche methods, the Randox and Roche methods correlate well up to 20 mg/dL [342 µmol/L], with a slight positive bias for the Diazyme compared to the Roche method more noticeable above 20 mg/dL (leading to a slope slightly but significantly greater than 1.0). Note that DBIL concentrations exceeding 10 mg/dL [342 µmol/L] require manual dilution for the Roche method. Specimens exceeding DBIL upper AMR limit for the Diazyme and Randox assays undergo auto-dilution protocols.
Fig. 1.
Correlation between the DBIL assays for samples with hemolysis index of less than 30. The dashed line in the three plots indicates the line of identity, while the solid line is from linear regression. Slope and y-intercept are presented with 95% confidence intervals in parentheses. (A) Comparison of the Diazyme vanadate oxidase DBIL assay with the Roche Diagnostics diazo DBIL assay (n=38). Linear regression statistics: r2=0.992, slope=1.05 (1.02, 1.08), y-intercept =0.26 (−0.19, 0.71). Slope was significantly greater than 1.0 (p<0.01). (B) Comparison of the Randox and Diazyme DBIL assays (n=27). Linear regression statistics: r2=0.998, slope=1.00 (0.98, 1.02), y-intercept =0.08 (−0.35, 0.18). (C) Comparison of the Roche and Randox DBIL assay (n=29). Linear regression statistics: r2=0.993, slope=1.09 (1.05, 1.12), y-intercept =0.10 (−0.50, 0.71). Slope was significantly greater than 1.0 (p<0.01). DBIL in mg/dL may be converted to µmol/L by multiplying by 17.1.
3.2. Hemolysis index
The manufacturer package insert limits for HI are summarized in Table 1 (HI corresponds approximately to serum hemoglobin concentration in mg/dL). We verified these claims by spiking pooled plasma with hemolysate as described in Methods. For the Roche DBIL method (with a package insert HI limit of 30), a HI of 40 produced an average decrease of 15.1+/−3.3% in DBIL concentration compared to baseline (n=4). The negative interference worsened at an HI of 100 with an average decrease of 36.7+/−6.9% in DBIL concentration compared to baseline (n=4). We observed less than 2% average decrease in DBIL concentration for the Diazyme DBIL method at an HI of 500 and for the Randox DBIL method at an HI of 1000 (each n=4).
We then estimated how often the manufacturer HI limits would have been exceeded for the three DBIL assays for clinical orders at our medical center. In the retrospective period of analysis, there were a total of 47,333 DBIL orders from 18,491 unique patients that had data for DBIL concentration and HI. Note that in this time period, the three different DBIL assays were not used concurrently. The analysis simply examined which specimens had HI exceeding the package insert limits for the Roche, Diazyme, and Randox DBIL methods.
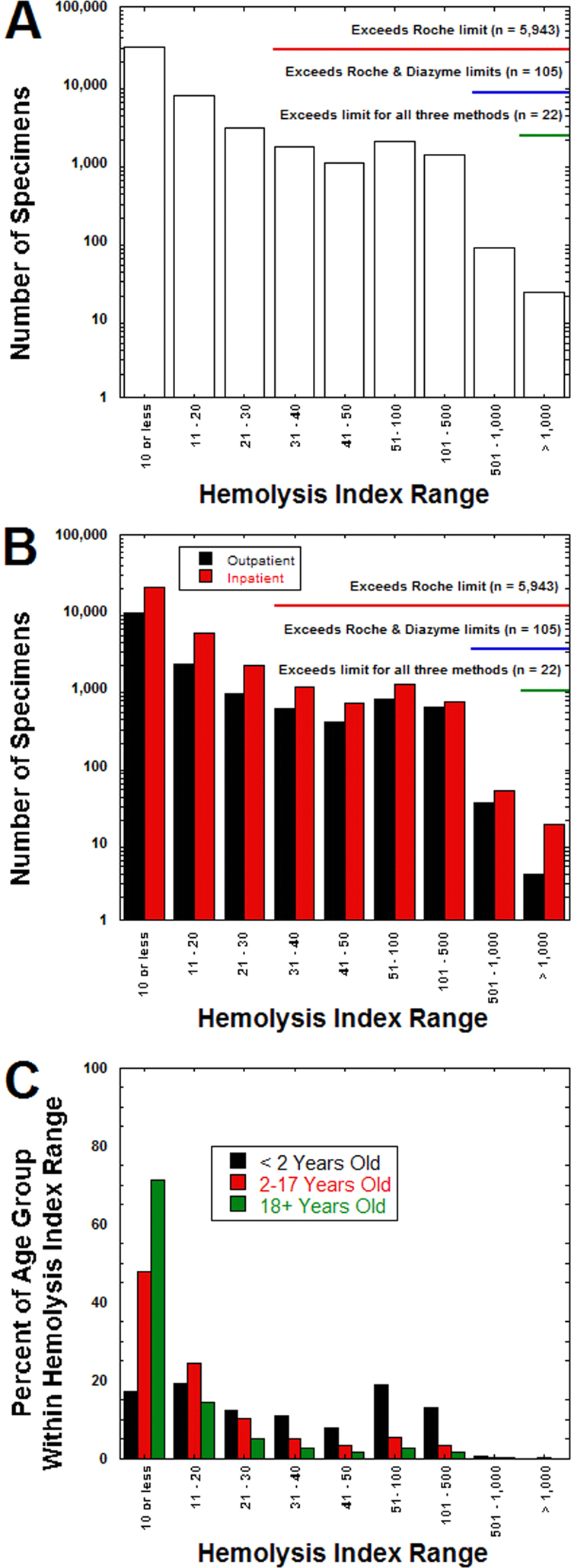
For clinical orders for DBIL for the 18,491 unique patients, the average patient age was 46.4±23.0 years (median: 52.5 years; range: neonate – 99.8 years old), with 24,684 specimens (52.1%) from male patients and 22,649 specimens (47.9%) from female patients. A total of 5943 specimens (12.6%) had HI exceeding 30, the package insert limit for the Roche method (see Table 1). There were also 2887 specimens (6.1%) with HI between 21 and 30. In contrast, only 105 specimens (0.2%) exceeded the Diazyme HI package insert limit of 500, and only 22 specimens (0.05%) exceeded the Randox package insert HI limit of 1000 (Fig. 2A). Within all HI ranges, there were similar proportions of specimens from inpatient units and outpatient clinics (Fig. 2B). Only at the very highest HI range (1000 or greater) was there a noticeable shift in proportion towards inpatient samples. Specimens from young children less than 2 years old comprised a disproportionate fraction of the hemolyzed specimens (Fig. 2C). Overall, specimens from patients in this age range accounted for 1508 of the 5943 (25.4%) specimens with HI greater than 30, even though children less than 2 year old comprised only 2943 (6.2%) of all specimens submitted for DBIL analysis. This meant that 51.2% of specimens from children less than 2 year old were hemolyzed enough to exceed manufacturer specifications for the Roche diazo DBIL method.
Fig. 2.
Hemolysis index (HI) in specimens submitted for DBIL analysis. (A) The bar graph shows total number of specimens with HI values within the specified ranges. The annotation highlights which specimens exceed the manufacturer package insert limits for HI for the three DBIL assays analyzed in this report. (B) Bar graph shows same data as in (A) but broken down into whether specimens were drawn in outpatient clinic (including emergency treatment center and outpatient phlebotomy sites) or inpatient unit. (C) Bar graph shows HI data broken down into three broad age categories (<2 years old, 2–17 years old, and 18 years or older), with the percent of specimens within that age group having HI within the limits. Whereas the majority of specimens for the 2–17 years and 18 years or older groups have HI less than 20, a majority of the specimens for the less than 2 years old group have HI exceeding 30 (limit for the Roche DBIL assay). HI values correspond to an approximate serum hemoglobin concentration in mg/dL.
3.3. Lipemia index
The manufacturer package insert limits for lipemia index are summarized in Table 1 (lipemia index is officially unit-less but is a measure of turbidity). The package insert for the Roche diazo DBIL assay states a lipemia index limit of only 60. The Diazyme DBIL assay package insert did not list a lipemic index limit, while the Randox DBIL assay package insert stated a lipemia index limit of 1000. We assessed these claims by spiking pooled plasma with Intralipid as described in Methods. For the Roche DBIL method (with a package insert lipemia index limit of 60), greater than 10% in baseline DBIL was not observed until a lipemia index of 300, which produced an average increase of 13.3+/−2.8% in DBIL concentration compared to baseline (n=4). We observed less than 2% average decrease in DBIL concentration for the Diazyme and Randox DBIL method even at a lipemia index of 1000 (each n=4).
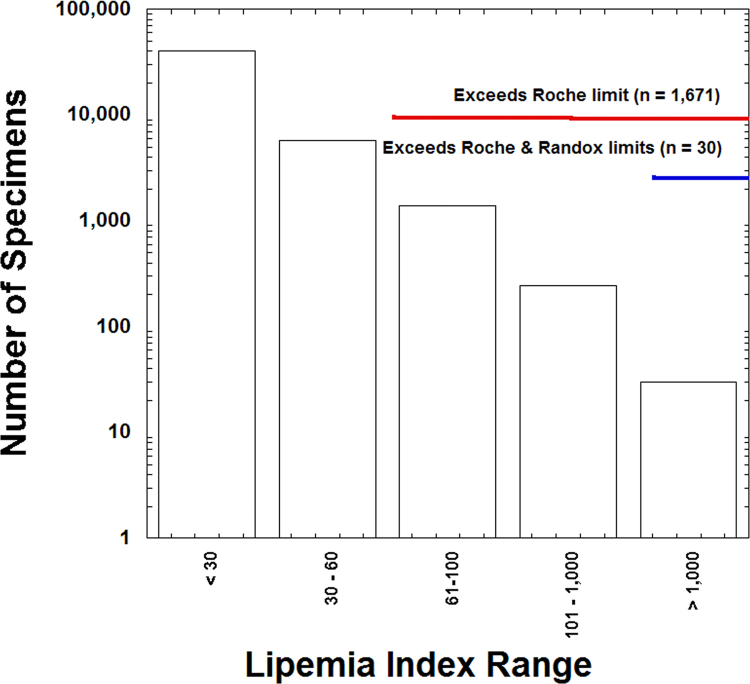
In the retrospective period of analysis, there were a total of 47,333 DBIL orders from 18,491 unique patients that had data for DBIL concentration and lipemia index. Out of the 47,333 specimens, a total of 1671 specimens (3.5%) had a lipemia index exceeding 60 (exceeding the Roche package insert limit), while only 30 specimens (0.06%) had indices exceeding 1000 (Randox package insert; Fig. 3).
Fig. 3.
Lipemia index in specimens submitted for DBIL analysis. The bar graph shows total number of specimens (logarithmic scale) with lipemia index within the specified ranges. The annotation highlights which specimens exceed the manufacturer package insert limits for lipemia index. Lipemia index is a measure of turbidity and is unit-less.
3.4. Analytical measurement range
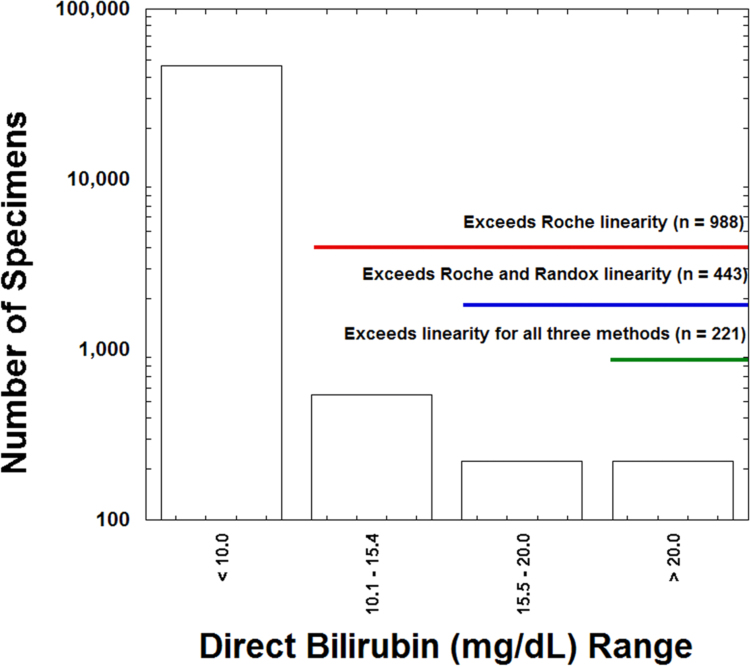
Both of the vanadate oxidase DBIL methods included in the study have AMRs wider than that for the Roche assay (Table 1). Of the total of 47,333 specimens analyzed for DBIL, 988 (2.1%) exceeded the upper linearity limit of 10 mg/dL [171 µmol/L] for the Roche assay, while 443 specimens (0.9%) exceeded the Randox linearity limit of 15.4 mg/dL [263.3 µmol/L] and only 221 specimens (0.5%) exceeded the Diazyme linearity limit of 20.0 mg/dL [342 µmol/L] (Fig. 4). The Roche assay requires manual dilution for samples that exceed the linearity, whereas the Diazyme and Randox methods can use auto-dilution protocols on the cobas analyzers.
Fig. 4.
Analytical measurement range and DBIL values. The bar graph shows total number of specimens (logarithmic scale) whose quantitative DBIL concentrations fall within the specified ranges. The annotation highlights which specimens exceed the upper AMR limits for the three DBIL assays shown.
4. Discussion
Diazo-based assays have been the most common approach for DBIL measurement for clinical purposes [1], [6], [7], [8], [9]. Diazo DBIL methods have the advantage of low-cost and easy adaptation to automated clinical chemistry analyzers. However, a significant limitation of diazo DBIL methods is interference from even low levels of hemolysis [1]. Other limitations include interference by lipemia, relatively narrow AMR, and, rarely, spurious results caused by the presence of paraproteins [1], [10], [11], [12].
Vanadate oxidase methods represent an alternative approach to measurement of DBIL [23], [24], [25], [26], [27], [28]. There are now a number of such assays available for automated clinical chemistry platforms, although these generally require set up as an open channel on the analyzer (provided this is allowed for the particular assay and analyzer combination). As shown in the present study at an academic medical center, two vanadate oxidase DBIL assays would be able to analyze a significant number of specimens that would otherwise exceed the HI limit for the Roche Diagnostics diazo DBIL assay. In comparing the Roche and Randox methods, the Randox assay could tolerate 12.5% of total specimens that exceeded the Roche package insert HI limit of 30 but not the much higher HI limit of 1000 for the Randox method. These numbers only reflect the HI limit in the package insert. Recent data demonstrates that hemolysis interference with diazo DBIL methods can be variable and unpredictable, potentially causing issues even at low levels of hemolysis [15]. In our study, there were also 2887 specimens (6.1%) with HI between 21 and 30 that may be at risk for causing some level of interference with diazo-based DBIL assays.
For lipemia interference, the Randox assay could handle 3.4% of total specimens that exceeded the Roche package insert lipemia index limit of 60. Furthermore, 2.1% of specimens exceeded the upper AMR limit for the Roche assay (10 mg/dL, 171 µmol/L) and would otherwise have required manual dilution, thereby delaying turnaround time and adding a possible source of error from additional manipulation of the specimen. An additional risk with specimens whose DBIL concentration exceeds the upper AMR limit is running out of specimen for dilution analysis, especially with minimum draws from pediatric patients. Both of the vanadate oxidase DBIL methods used in the present study have auto-dilution capabilities for the small percentage of samples that exceed their upper linearity limit, thereby minimizing risk of dilution error and reducing manual technologist time. A previous study has shown that the bias between diazo and vanadate oxidase DBIL increases when specimens are diluted [15].
The patient population most impacted in our study were children less than 2 years old. The present study was performed at an academic medical center with a tertiary care children's hospital, including a neonatal intensive care unit that provides specialty neonatology for a wide geographic region. For children less than 2 years old, 51% of specimens submitted for DBIL analysis in our retrospective time period had HI indices greater than 30. This reflects the challenge of specimen collection in this population of patients [34], [35]. Further, measurement of DBIL is often focused on the subgroup of infants that have elevated TBIL and are undergoing more detailed workup [3], [13], [14]. Inability to perform DBIL analysis in this subgroup of patients can delay exact diagnosis and require additional iatrogenic blood loss from repeat phlebotomy. Laboratories may also consider using the vanadate oxidase method for specimens with very low sample volume, especially from neonates. With a wider AMR, vanadate oxidase DBIL assays have a greater chance of avoiding the situation where a final result cannot be obtained because the initial result exceeds the upper AMR limit but with insufficient remaining specimen to perform dilution and further analysis. The results of our study would thus likely translate best to clinical laboratories that receive a significant number of neonatal and infant specimens for DBIL analysis.
The current main limitations to adoption of vanadate oxidase DBIL assays are availability, cost, and the additional effort and feasibility of setting up these assays as open channels on automated clinical chemistry analyzers. At our medical center, we have chosen to continue to use the Roche diazo method as our primary DBIL method, reflexing to a vanadate oxidase method for specimens that are hemolyzed, lipemic, and/or exceed the upper AMR limit for the diazo method. Although the Diazyme method analyzed in the study is currently unavailable in the United States market, we utilized this vanadate oxidase DBIL method as a secondary method for clinical testing for several years. Following the unavailability of the Diazyme DBIL assay, we switched to the Randox assay as our secondary DBIL method. Both the Diazyme and Randox DBIL methods performed similarly in all respects in our study and, at least with respect to hemolysis, were also similar to a report on a third commercially available DBIL assay [15]. We estimate the direct costs of the Diazyme and Randox assays to be approximately double that of the Roche diazo assay, when factoring in not just reagent costs but also expenses for calibrators, open channel cassettes, and additional staff effort in manual reagent maintenance and quality control for these open channel assays.
5. Conclusions
Vanadate oxidase DBIL methods offer advantages over diazo methods in terms of lower interference by hemolysis and lipemia, as well as wider analytical measurement range. The advantages are particularly evident for the neonatal and infant population, where specimen hemolysis is common.
Conflict of interest
None of the authors have any conflict to report.
Acknowledgements
None.
Contributor Information
Neha Dhungana, Email: neha-dhungana@uiowa.edu.
Cory Morris, Email: cory-morris@uiowa.edu.
Matthew D. Krasowski, Email: mkrasows@healthcare.uiowa.edu.
References
- 1.Higgins T., Eckfeldt J.H., Barton J.C., Doumas B.T. Hemoglobin, iron, and bilirubin. In: Burtis C.A., Ashwood E.R., Bruns D.E., editors. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. Elsevier Saunders; St. Louis, MO: 2012. pp. 1016–1030. [Google Scholar]
- 2.Devgun M.S., Chan M.K., El-Nujumi A.M., Abara R., Armbruster D., Adeli K. Clinical decision limits for interpretation of direct bilirubin--a CALIPER study of healthy multiethnic children and case report reviews. Clin. Biochem. 2015;48(1–2):93–96. doi: 10.1016/j.clinbiochem.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Fevery J. Bilirubin in clinical practice: a review. Liver Int. 2008;28(5):592–605. doi: 10.1111/j.1478-3231.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- 4.Ehrlich P. Verhalten des Harns zu Sulfodiazobenzol [behavior of the urine to sulphodiazobenzene] Z. Anal. Chem. [German] 1883;22(301) [Google Scholar]
- 5.Ehrlich P. Sulfodiazobenzol als Reagens auf bilirubin [Sulfodiazobenzene as a reagent on bilirubin] Z. Anal. Chem. [German] 1884;22:275–276. [Google Scholar]
- 6.Cherian A.G., Soldin S.J., Hill J.G. Automated Jendrassik-Grof method for measurement of bilirubin in serum with the Greiner Selective Analyzer (GSA II D), and comparison with the method involving diazotized 2,4-dichloroaniline. Clin. Chem. 1981;27(5):748–752. [PubMed] [Google Scholar]
- 7.Jendrassik L., Grόf P. Vereinfachte photometrische methoden zur bestimmung des blutbilirubins [Simplified photometric methods for the determination of blood bilirubin] Biochem. Z. [German] 1938;297:82–89. [Google Scholar]
- 8.Lo D.H., Wu T.W. Assessment of the fundamental accuracy of the Jendrassik-Grof total and direct bilirubin assays. Clin. Chem. 1983;29(1):31–36. [PubMed] [Google Scholar]
- 9.Malloy H.T., Evelyn K.L. The determination of bilirubin with the photoelectric colorimetry. J. Biol. Chem. 1937;199:481–490. [Google Scholar]
- 10.Cascavilla N., Falcone A., Sanpaolo G., D'Arena G. Increased serum bilirubin level without jaundice in patients with monoclonal gammopathy. Leuk. Lymphoma. 2009;50(8):1392–1394. doi: 10.1080/10428190903046730. [DOI] [PubMed] [Google Scholar]
- 11.Pantanowitz L., Horowitz G.L., Upalakalin J.N., Beckwith B.A. Artifactual hyperbilirubinemia due to paraprotein interference. Arch. Pathol. Lab. Med. 2003;127(1):55–59. doi: 10.5858/2003-127-55-AHDTP. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y., Howanitz P.J., Howanitz J.H., Gorfajn H., Wong K. Paraproteins are a common cause of interferences with automated chemistry methods. Arch. Pathol. Lab. Med. 2008;132(2):217–223. doi: 10.5858/2008-132-217-PAACCO. [DOI] [PubMed] [Google Scholar]
- 13.Ahlfors C.E., Wennberg R.P., Ostrow J.D., Tiribelli C. Unbound (free) bilirubin: improving the paradigm for evaluating neonatal jaundice. Clin. Chem. 2009;55(7):1288–1299. doi: 10.1373/clinchem.2008.121269. [DOI] [PubMed] [Google Scholar]
- 14.Lippi G. Systematic assessment of the hemolysis index: pros and cons. Adv. Clin. Chem. 2015;71:157–170. doi: 10.1016/bs.acc.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Devgun M.S., Richardson C. Direct bilirubin in clinical practice – interpretation and haemolysis interference guidance reassessed. Clin. Biochem. 2016;49(18):1351–1353. doi: 10.1016/j.clinbiochem.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal S., Vargas G., Nordstrom C., Tam E., Buffone G.J., Devaraj S. Effect of interference from hemolysis, icterus and lipemia on routine pediatric clinical chemistry assays. Clin. Chim. Acta. 2015;438:241–245. doi: 10.1016/j.cca.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Atay A., Demir L., Cuhadar S., Saglam G., Unal H., Aksun S., Arslan B., Ozkan A., Sutcu R. Clinical biochemistry laboratory rejection rates due to various types of preanalytical errors. Biochem. Med. 2014;24(3):376–382. doi: 10.11613/BM.2014.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimeski G. Interference testing. Clin. Biochem. Rev. 2008;29(Suppl 1):S43–S48. [PMC free article] [PubMed] [Google Scholar]
- 19.Kroll M., McCudden C. De Gruyter; Berlin: 2013. Endogenous Interferences in Clinical Laboratory Tests. [Google Scholar]
- 20.Lippi G., Blanckaert N., Bonini P., Green S., Kitchen S., Palicka V., Vassault A.J., Plebani M. Haemolysis: an overview of the leading cause of unsuitable specimens in clinical laboratories. Clin. Chem. Lab. Med. 2008;46(6):764–772. doi: 10.1515/CCLM.2008.170. [DOI] [PubMed] [Google Scholar]
- 21.Ji J.Z., Meng Q.H. Evaluation of the interference of hemoglobin, bilirubin, and lipids on Roche Cobas 6000 assays. Clin. Chim. Acta. 2011;412(17–18):1550–1553. doi: 10.1016/j.cca.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Gonzalez E., Gonzalez-Tarancon R., Aramendia M., Rello L. Analytical interference by monoclonal immunoglobulins on the direct bilirubin AU Beckman Coulter assay: the benefit of unsuspected diagnosis from spurious results. Clin. Chem. Lab. Med. 2016;54(8):1329–1335. doi: 10.1515/cclm-2015-0608. [DOI] [PubMed] [Google Scholar]
- 23.Andreu Y., Galban J., de Marcos S., Castillo J.R. Determination of direct-bilirubin by a fluorimetric-enzymatic method based on bilirubin oxidase. Fresenius J. Anal. Chem. 2000;368(5):516–521. doi: 10.1007/s002160000503. [DOI] [PubMed] [Google Scholar]
- 24.Doumas B.T., Perry B., Jendrzejczak B., Davis L. Measurement of direct bilirubin by use of bilirubin oxidase. Clin. Chem. 1987;33(8):1349–1353. [PubMed] [Google Scholar]
- 25.Kurosaka K., Senba S., Tsubota H., Kondo H. A new enzymatic assay for selectively measuring conjugated bilirubin concentration in serum with use of bilirubin oxidase. Clin. Chim. Acta. 1998;269(2):125–136. doi: 10.1016/s0009-8981(97)00194-0. [DOI] [PubMed] [Google Scholar]
- 26.Mullon C.J., Langer R. Determination of conjugated and total bilirubin in serum of neonates, with use of bilirubin oxidase. Clin. Chem. 1987;33(10):1822–1825. [PubMed] [Google Scholar]
- 27.Murao S., Tanaka N. A new enzyme "bilirubin oxidase"produced by Myrothecium carrucaria MT-1. Agric. Biol. Chem. 1981;45:2383–2384. [Google Scholar]
- 28.Tokuda J., Tanimoto K. New method of measuring serum bilirubin using vanadic acid. Jpn. J. Clin. Chem. 1993;22(2):116–122. [Google Scholar]
- 29.Ameri M., Schnaars H., Sibley J., Honor D. Comparison of the vanadate oxidase method with the diazo method for serum bilirubin determination in dog, monkey, and rat. J. Vet. Diagn. Investig. 2011;23(1):120–123. doi: 10.1177/104063871102300121. [DOI] [PubMed] [Google Scholar]
- 30.Krasowski M.D., Chudzik D., Dolezal A., Steussy B., Gailey M.P., Koch B., Kilborn S.B., Darbro B.W., Rysgaard C.D., Klesney-Tait J.A. Promoting improved utilization of laboratory testing through changes in an electronic medical record: experience at an academic medical center. BMC Med. Inform. Decis. Mak. 2015;15:11. doi: 10.1186/s12911-015-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson L.S., Davis S.R., Humble R.M., Kulhavy J., Aman D.R., Krasowski M.D. Impact of add-on laboratory testing at an academic medical center: a five year retrospective study. BMC Clin. Pathol. 2015;15:11. doi: 10.1186/s12907-015-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krasowski M.D., Davis S.R., Drees D., Morris C., Kulhavy J., Crone C., Bebber T., Clark I., Nelson D.L., Teul S., Voss D., Aman D., Fahnle J., Blau J.L. Autoverification in a core clinical chemistry laboratory at an academic medical center. J. Pathol. Inform. 2014;5(1):13. doi: 10.4103/2153-3539.129450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krasowski M.D., Wilford J.D., Howard W., Dane S.K., Davis S.R., Karandikar N.J., Blau J.L., Ford B.A. Implementation of epic beaker clinical pathology at an academic medical center. J. Pathol. Inform. 2016;7:7. doi: 10.4103/2153-3539.175798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bush R.A., Mueller T., Sumwalt B., Cox S.A., Hilfiker M.L. Assessing pediatric trauma specimen integrity. Clin. Lab Sci. 2010;23(4):219–222. [PubMed] [Google Scholar]
- 35.Meites S., Lin S.S., Thompson C. Studies on the quality of specimens obtained by skin puncture of children 1. Tendency to hemolysis, and hemoglobin and tissue fluid as contaminants. Clin. Chem. 1981;27(6):875–878. [PubMed] [Google Scholar]