Abstract
Objectives
The aims of this study were to identify the causes of severe lipemia in an academic medical center patient population and to determine the relationship between lipemia and hemolysis.
Design and methods
Retrospective study was done on the data from the core clinical laboratory at an academic medical center. Lipemic indices were available for all chemistry specimens analyzed over a 16-month period (n=552,029 specimens) and for serum/plasma triglycerides concentrations ordered for clinical purposes over a 16-year period (n=393,085 specimens). Analysis was performed on Roche Diagnostics cobas 8000 analyzers. Extensive chart review was done for all specimens with lipemic index greater than 500 (severely lipemic) and for all specimens with serum/plasma triglycerides greater than 2000 mg/dL. We also determined the relationship between lipemia and hemolysis.
Results
The most frequent suspected causes of very high lipemic index (>500) were found to be lipid-containing intravenous infusions (54.4% of total; fat emulsions for parenteral nutrition – 47%; propofol −7.4%) and diabetes mellitus (25% of total, mainly type 2). The most frequent suspected causes of very elevated serum/plasma triglycerides (>2000 mg/dL) was diabetes mellitus (64%, mainly type 2) and hyperlipidemia (16.9%). The frequency of hemolysis increased with increasing lipemic index.
Conclusions
Intravenous lipid infusions and type 2 diabetes were the most common causes of severe lipemia in this study at an academic medical center. Given that iatrogenic factors are the most common cause of severe lipemia, education and intervention may be helpful in reducing frequency of severe lipemia in patient specimens.
Keywords: Clinical chemistry tests, Hyperlipidemias, Intravenous fat emulsions, Intravenous infusions, Parenteral nutrition, Propofol
Highlights
-
•
Intravenous lipids and type 2 diabetes were most common causes of severe lipemia.
-
•
The frequency of hemolysis increased with increasing lipemic index.
-
•
Diabetes type 2 was the most common cause of extreme hypertriglyceridemia.
-
•
Education and intervention may be helpful in reducing frequency of lipemia.
1. Introduction
Endogenous interferences can be a significant source of error with clinical laboratory testing [1], [2], [3], [4], [5], [6]. Lipemia is one of the most common pre-analytical interferences and results from sample turbidity caused by accumulation of lipoprotein particles [4], [7], [8]. Lipemia can interfere with analysis by several mechanisms [4], [7], [9]. Lipemia can cause increased absorption of light, affecting tests that use spectrophotometric methods [4], [7], [8]. Lipemia can also cause interferences by volume displacement, especially impacting analysis of electrolytes [9]. Other possible issues with lipemic specimens include sample non-homogeneity (disrupting how analyzers sense and pipette specimens) and alterations in serum protein electrophoretic patterns [4], [7], [9]. The relationship between lipemia and hemolysis is not well characterized, but increasing lipid concentrations have been associated with frequency of hemolysis [10], [11]. While low levels of lipemia usually do not significantly affect clinical laboratory testing, the presence of severe lipemia is likely to impact laboratory analysis [4], [7], [9]. As such, there is interest in identifying which iatrogenic and patient factors are most likely to be associated with severe lipemia.
A variety of factors can lead to sample lipemia. The most common pre-analytical cause of lipemia is not fasting prior to blood collection [4], [12], [13]. The other main cause is hypertriglyceridemia, either resulting from a primary disorder (e.g., Fredrickson type I, IV, or V hyperlipidemia) or secondary cause. Common secondary causes of hypertriglyceridemia include diabetes mellitus, alcoholism, renal disease, nonalcoholic fatty liver disorder, HIV infections, and medications. Some intravenous infusions cause lipemia directly by containing lipid emulsions, either for parenteral nutrition [14], [15], as antidote for poisonings of certain medications (e.g., local anesthetics) [4], [7], or as the diluent for poorly water-soluble medications (e.g., propofol injectable suspended in Intralipid) [16]. Blood collection in patients who have recently received intravenous lipid emulsion therapy can result in samples that are extremely lipemic [4], [7]. Medications that can indirectly cause lipemia include glucocorticoids, antiretroviral medications (especially protease inhibitors), and non-selective beta-adrenergic antagonists. Ketogenic diets used in treatment of epilepsy can lead to development of hypercholesterolemia and hypertriglyceridemia [17].
There are a variety of methods to assess lipemia in laboratory samples. The simplest is visual inspection of the specimen; however, there is significant inaccuracy and inter-individual variation in this type of assessment [4], [7]. Measurement of triglycerides in serum or plasma can also give a rough assessment of degree of lipemia, but the degree of turbidity does not correlate well with triglyceride concentration, with a complex and non-linear relationship [7], [18]. Many clinical chemistry platforms have the ability to determine a lipemic index [7], [19]. For automatic detection of lipemic index, dilution of the patient's sample is made in saline or buffer and then specific wavelengths are measured [7]. Different manufacturers use various wavelengths to detect lipemic index; for example, Roche Diagnostics cobas analyzers use 660/700 nm and Beckman Coulter chemistry analyzers use 660/800 nm. There are both advantages and disadvantages of automatic detection. Low cost, high speed, increased reproducibility and rapid analysis time are some of the advantages of automatic detection, whereas some of the disadvantages are lack of standardization among manufacturers in reporting lipemic index and false positives due to sample turbidity not caused by accumulation of lipid.
The aim of this study was to assess the causes of severe lipemia and to better define the relationship between lipemia and hemolysis. To this end, we utilized a large body of retrospective data from the centralized core laboratory at an academic medical center. We focused our chart review on specimens with markedly elevated lipemic indices, reasoning that these specimens have the highest likelihood of causing clinically significant interference. The lipemic index data was drawn from all clinical chemistry testing performed during an approximately 16 month period. We also analyzed data on serum/plasma triglycerides for those patients on which it was clinically ordered, utilizing a longer retrospective time period to achieve a large dataset.
2. Materials and methods
The study was conducted at the University of Iowa Hospitals and Clinics (UIHC), a 734-bed tertiary care academic medical center with inpatient and outpatient services, level one trauma center, and multiple intensive care units (ICUs). The electronic health record (EHR) is currently Epic (Epic systems, Inc., Madison, WI, USA) which contains historic data covering the entire period of retrospective analysis. The Department of Pathology manages pathology services throughout the medical center, including a centralized core clinical laboratory providing clinical chemistry and hematology services for all UIHC clinical units. [20] The data in this study is from the core laboratory clinical chemistry section and was collected as part of two retrospective studies approved by the University of Iowa Institutional Review Board (protocols # 201603710 and 201606722) covering the time period from January 1, 2000 to May 31, 2016. As described below, the entire retrospective time period was used to evaluating serum/plasma triglycerides orders. A more limited timeframe (January 15, 2015 to May 31, 2016) was used to evaluate lipemia and hemolysis indices using middleware data. Data for these indices was incompletely available prior to January 15, 2015. This study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). As described in our previous studies [21], [22], Reporting Workbench functionality within Epic allowed for retrieval of past laboratory results. Specimen accession numbers allowed for explicit linking of tests performed on specific blood tubes.
The main automated clinical chemistry instrumentation was from Roche Diagnostics (Indianapolis, IN, USA). The original configuration consisted of Modular P and Modular E170 analyzers. In 2013, a cobas 8000 system with two c702, three c502, and five e602 analyzers replaced the Modular P and E170 analyzers. The core laboratory currently runs 131 Roche assays and 14 non-Roche assays on the automated chemistry line. All serum/plasma assays run on the Roche analyzers have hemolysis, icterus, and lipemia indices determined by spectrophotometry which are used in autoverification rules for all chemistry tests [20].
The analyzers take an aliquot of the patient specimen and dilute in 0.9% sodium chloride saline to measure the absorbances for lipemia at 660 nm (primary wavelength) and 700 nm (secondary wavelength), for hemolysis at 570 nm (primary wavelength) and 600 nm (secondary wavelength), and for icterus at 480 nm (primary wavelength) and 505 nm (secondary wavelength). Interfacing throughout the laboratory is provided by Middleware software (Instrument Manager) from Data Innovations (Burlington, VA) [20], [23]. Lipemia and hemolysis indices were extracted from Instrument Manager data in the time period of January 15, 2015 to May 31, 2016 using specimen accession numbers, allowing for linkage to patient data and the laboratory testing ordered on the accession number.
3. Results
3.1. Lipemic Index
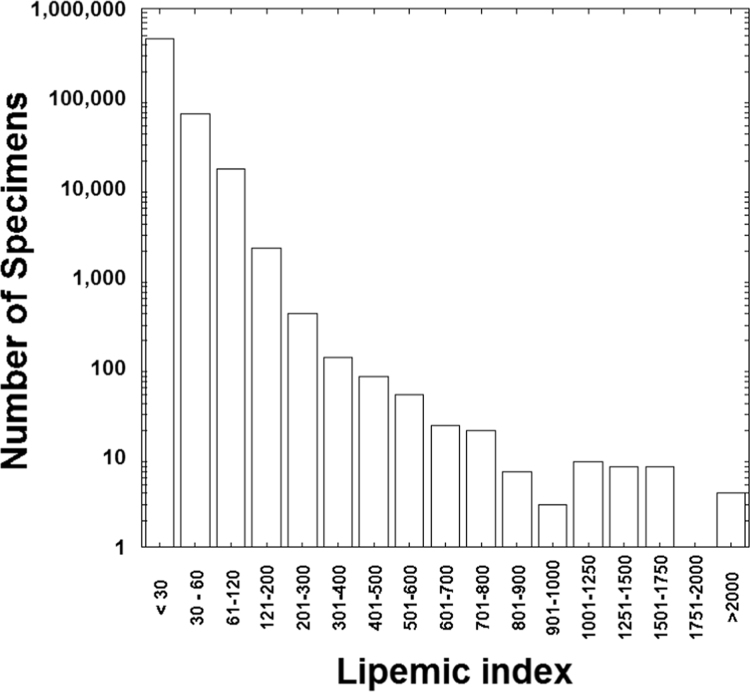
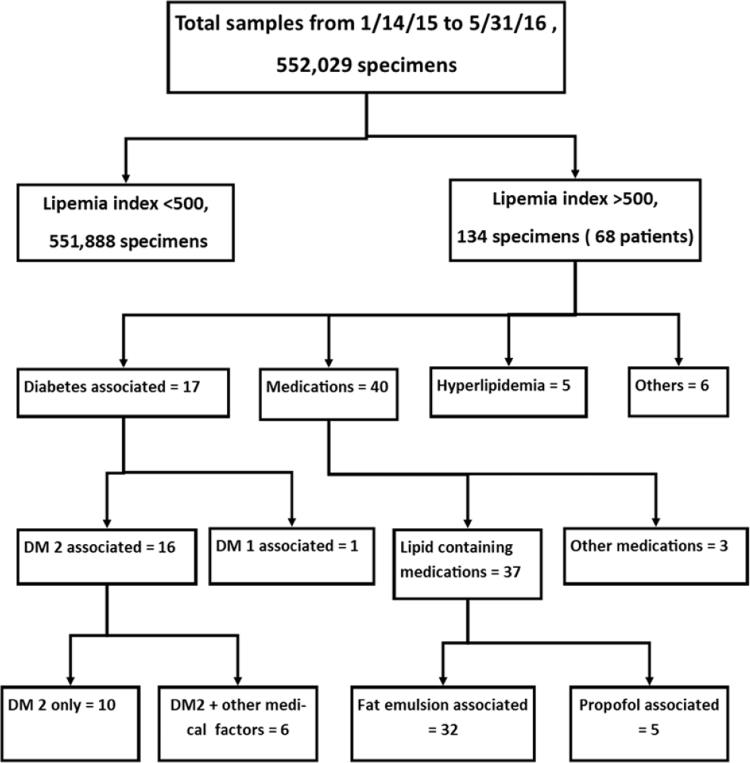
Data for interference indices (including hemolysis and lipemia) from the UIHC core clinical chemistry laboratory over a nearly 16-month period was available for 552,029 specimens. Detailed chart review was performed in all cases with lipemic index greater than 500, encompassing 134 specimens from 68 patients. Fig. 1 shows the distribution of lipemic indices. The flow diagram for the suspected causes of lipemia greater than 500 is shown in Fig. 2.
Fig. 1.
Distribution of lipemic index of 552,029 specimens. The number of specimens is in logarithmic scale.
Fig. 2.
Flow chart showing the breakdown of lipemic indices and the suspected causes of samples with lipemic index greater than 500.
Table 1 summarizes the likely factors contributing to markedly elevated lipemic indices (>500). The most common likely causes of elevated lipemic index were lipid-containing intravenous medications (fat emulsion for parenteral nutrition; propofol) and diabetes mellitus (mainly type 2).
Table 1.
Breakdown of likely factors contributing to elevated lipemic index greater than 500.
| Factor | Number of patients |
|---|---|
| Diabetes mellitus | 17 |
| Diabetes type 1 + recurrent pancreatitis | 1 |
| Diabetes type 2 with no other factors identified | 10 |
| Diabetes type 2 with one or more other factors | |
| Diabetes type 2 + alcohol abuse | 1 |
| Diabetes type 2 + Type IV/V hyperlipidemiaa | 1 |
| Diabetes type 2 + Type V hyperlipidemia | 1 |
| Diabetes type 2 + HIV medicationsb | 1 |
| Diabetes type 2 + pancreatitis | 2 |
| Intravenous fat emulsion for parenteral nutrition | 32 |
| Fat emulsion with no other factors identified | 14 |
| Fat emulsion with one of more other factors | |
| Fat emulsion + acute kidney injury | 5 |
| Fat emulsion + pancreatitis | 1 |
| Fat emulsion + alcohol abuse | 1 |
| Fat emulsion + glucocorticoids | 2 |
| Fat emulsion + glucocorticoids + propranolol | 1 |
| Fat emulsion + hydrochlorothiazide | 1 |
| Fat emulsion + glucocorticoids + diabetes type 2 | 1 |
| Fat emulsion + diabetes type 1 | 2 |
| Fat emulsion + diabetes type 2 | 4 |
| Propofol infusion + one or more other factors | 6 |
| Propofol + diabetes type 2 | 1 |
| Propofol + diabetes type 2 + glucocorticoids | 1 |
| Propofol + alcohol abuse + acute kidney injury + pancreatitis | 1 |
| Propofol + acute kidney injury | 1 |
| Propofol + seizures | 1 |
| Propofol + alcohol abuse | 1 |
| Hyperlipidemia | 4 |
| Type I | 1 |
| Type IV + valacyclovir | 1 |
| Type V | 2 |
| Medications/treatments including ketogenic diet | 5 |
| Ketogenic diet | 2 |
| Glucocorticoids | 1 |
| Pegasparagase, methotrexate, vincristine, cytarabine, doxorubicin | 1 |
| Hydrochlorothiazide + chronic kidney disease | 1 |
| Other medical conditions | 4 |
| Generic diagnosis of hyperlipidemia | 1 |
| Alcohol abuse | 1 |
| Alcohol abuse + fatty infiltration of liver | 1 |
| Pancreatitis | 1 |
| Total | 68 |
Patient had lipoprotein analysis showing features intermediate between Type IV and Type V hyperlipidemia.
Includes teofovir, raltegravir, abacavir, lamivudine, and efavirez.
3.2. Association between lipemia and hemolysis
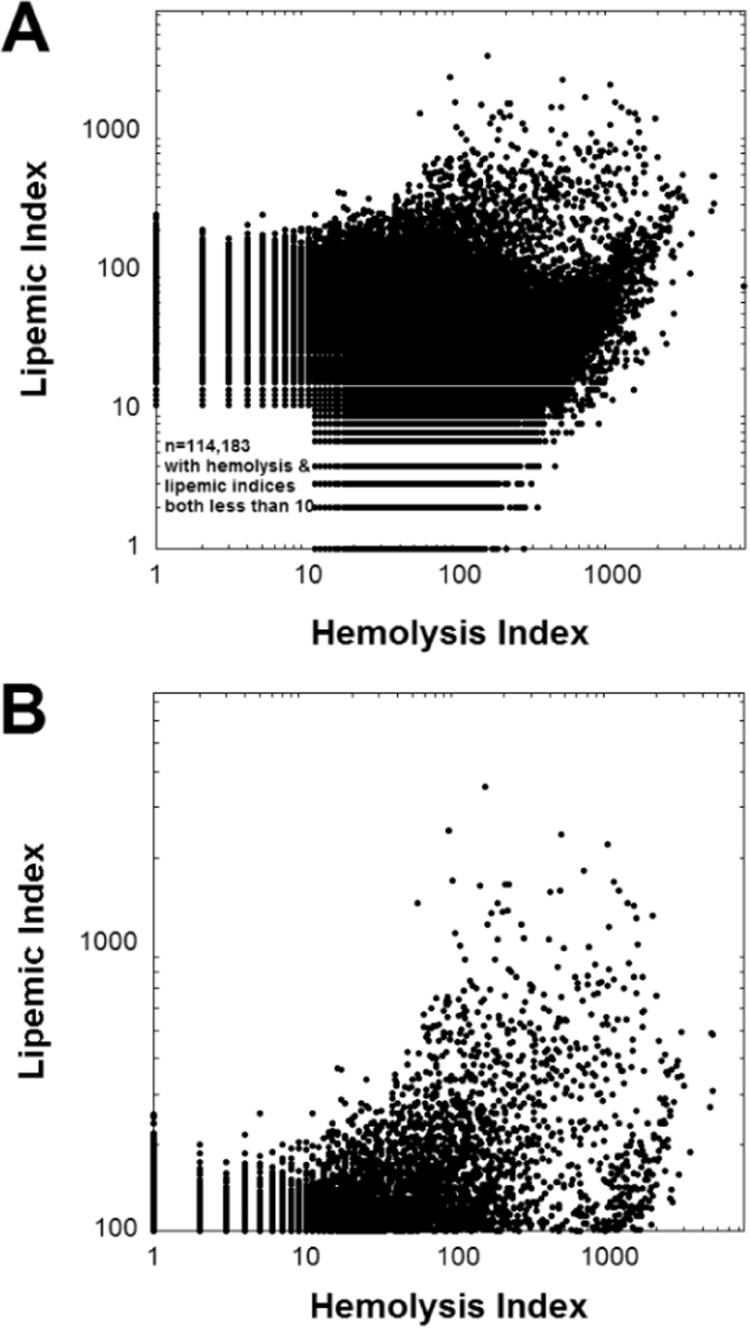
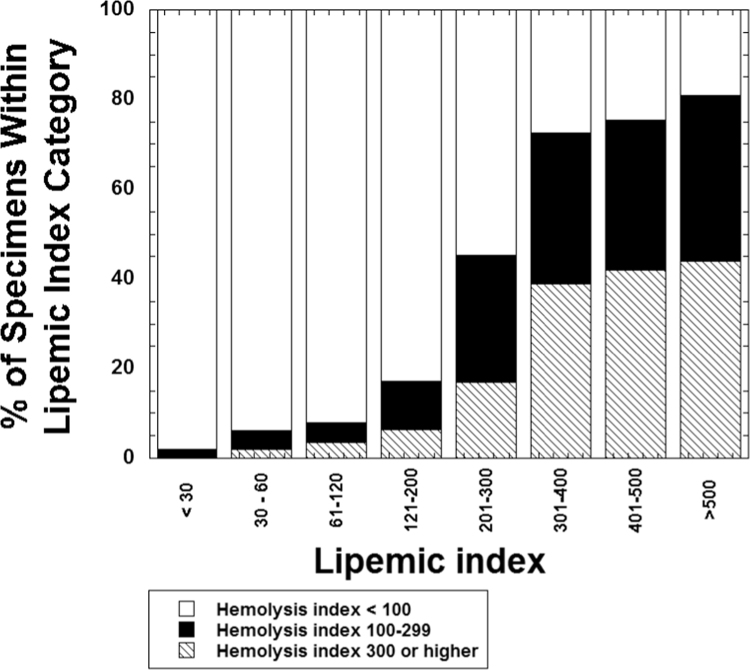
Previous literature has shown that severely lipemic specimens have a higher rate of hemolysis [10], [11]. Fig. 3 plots lipemia versus hemolysis indices, showing the entire range of data (Fig. 3A) and also the subset of data with lipemic indices greater than 100 (Fig. 3B). The overall association is complex, likely reflecting in part the heterogeneity of causes for both lipemia and hemolysis; nevertheless, there is a notable paucity of data points in the upper left portion of the graph (high lipemia, low hemolysis; especially noticeable in Fig. 3B). This relation is also shown in Fig. 4 which demonstrates that the frequency of hemolysis rises with increasing lipemic index. In the samples with lipemic index greater than 500, over 80% have a hemolysis index of 100 or greater. In our clinical laboratory, this level of hemolysis will result in cancellation of hemolysis-sensitive tests such as potassium, aspartate aminotransferase, and lactate dehydrogenase [3], [20]. In contrast, over 95% of specimens with a lipemic index <30 have a hemolysis index of 100 or less.
Fig. 3.
Plots the relationship between hemolysis and lipemic index which is complex but samples with higher lipemic index are more likely to be hemolyzed. The abscissa uses a logarithmic scale. (A) Complete view of data, illustrating that many samples are neither hemolyzed nor lipemic. (B) Data restricted to those specimens with lipemic index >100.
Fig. 4.
The bar graph details the frequency of hemolysis within ranges of lipemic index. Overall, specimens with lipemic indices of 120 or less have much lower rates of hemolysis compared to specimens with lipemic indices of 200 or greater.
3.3. Triglycerides
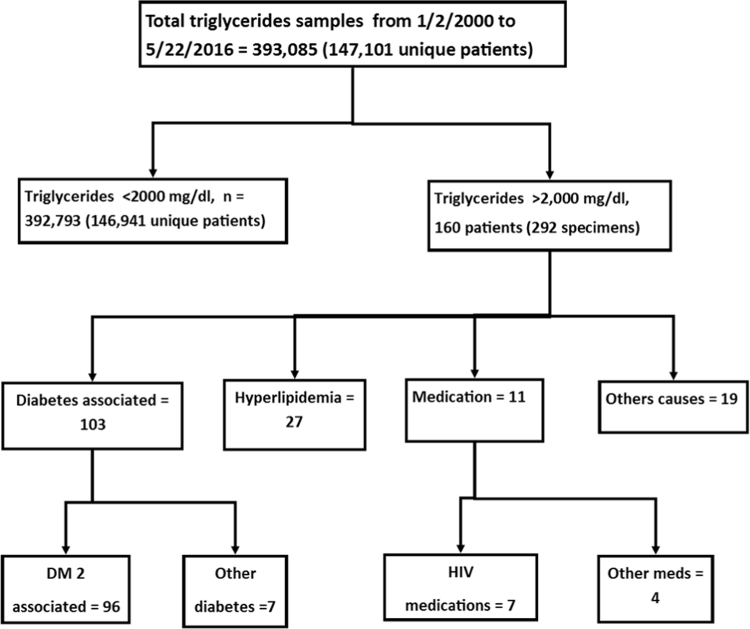
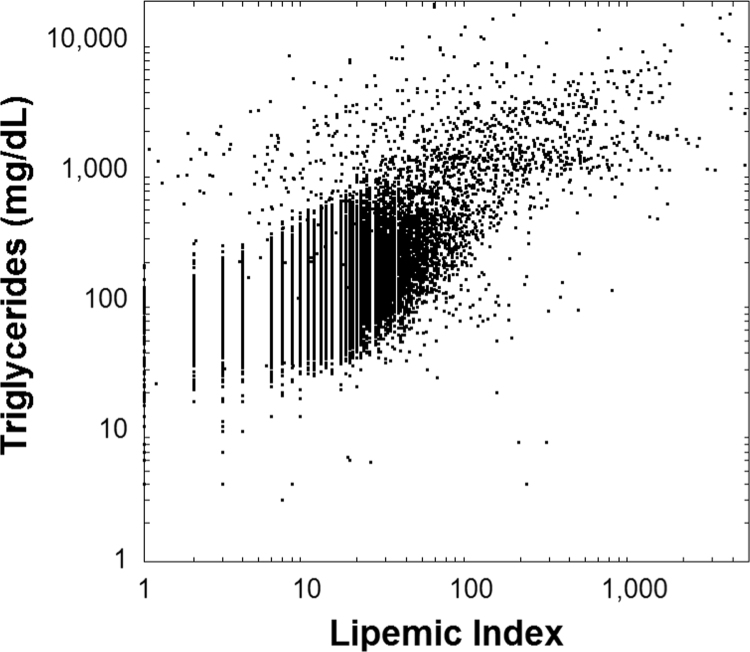
We also analyzed data for specimens on which serum/ plasma triglyceride concentration were ordered by providers (note that in our retrospective analysis triglyceride concentrations were only used from clinical results and not from any measurement for research purposes). Data included 393,085 specimens from 147,101 unique patients in the time period from January 2000 to May 2016. To gain insight into underlying causes of severe hypertriglyceridemia, detailed chart review was performed in all cases with triglycerides >2000 mg/dL which included 292 specimens from 160 patients. The flow diagram for the likely causes of the markedly elevated triglycerides is shown in Fig. 5 with Table 2 summarizing these factors. The most common diagnoses associated with elevated triglycerides were diabetes mellitus (mainly type 2) and one of several Fredrickson subtypes of hyperlipidemia. Unlike the data for lipemic index, intravenous lipid emulsions were an uncommon cause of hypertriglyceridemia concentrations that were ordered by provider. Fig. 6 shows a plot of serum/plasma triglyceride concentrations versus lipemic index. The relationship is complex and shows only a weak linear correlation.
Fig. 5.
Flow chart for the samples with serum/plasma triglycerides greater than 2,000 mg/dL.
Table 2.
Breakdown of likely factors contributing to very elevated serum/plasma triglycerides (>2000 mg/dL).
| Factor | Number of patients |
|---|---|
| Diabetes mellitus | 103 |
| Diabetes type 2 with no other factors identified | 54 |
| Diabetes type 2 with one or more factors | |
| Diabetes type 2 + pancreatitis | 20 |
| Diabetes type 2 + Type I hyperlipidemia | 1 |
| Diabetes type 2 + Type IV hyperlipidemia | 1 |
| Diabetes type 2 + Type IV/V hyperlipidemiaa | 1 |
| Diabetes type 2 + Type IV and V hyperlipidemiab | 1 |
| Diabetes type 2 + Type V hyperlipidemia | 2 |
| Diabetes type 2 + AIDS | 1 |
| Diabetes type 2 + pancreatitis + alcohol abuse | 1 |
| Diabetes type 2 + alcohol abuse | 2 |
| Diabetes type 2 + hydrochlorothiazide | 5 |
| Diabetes type 2 + estrogen | 3 |
| Diabetes type 2 + propanolol | 1 |
| Diabetes type 2 + propofol | 1 |
| Diabetes type 2 + glucocorticoids | 1 |
| Diabetes type 2 + glucocorticoids + HIV medicationsc | 1 |
| Other diabetes | |
| Diabetes type 1 with no other factors identified | 1 |
| Diabetes type 1 + pancreatitis | 2 |
| Gestational diabetes | 2 |
| Diabetes unknown subtype | 1 |
| Impaired glucose metabolism | 1 |
| Hyperlipidemia | 27 |
| Hyperlipidemia with no other factors identified | 18 |
| Hyperlipidemia with one or more other factors | |
| Hyperlipidemia + hydrochlorothiazide | 3 |
| Hyperlipidemia + risperidone | 1 |
| Hyperlipidemia + estradiol | 1 |
| Hyperlipidemia + steatohepatitis | 1 |
| Hyperlipidemia + acute leukemia | 1 |
| Hyperlipidemia + cholecystectomy | 1 |
| Hypertriglycerides + chronic hepatitis C | 1 |
| Medication(s) | 11 |
| HIV medications | 7 |
| Fat emulsion + glucocorticoids | 1 |
| Propanolol | 1 |
| Glucocorticoids | 1 |
| Tacrolimus + mycophenolate mofetil | 1 |
| Other factors | 19 |
| Alcohol | 6 |
| Alcohol + hydrochlorothiazide | 4 |
| Alcohol + hepatitis C | 1 |
| Alcoholic hepatitis + hydrochlorothiazide | 1 |
| Hydrochlorothiazide + hepatitis C | 1 |
| Type IV/V hyperlipidemiaa | 1 |
| Type IV or Type Vb | 1 |
| Pancreatitis | 1 |
| End stage renal disease + thyroid cancer | 1 |
| Non-fasting specimen collection | 2 |
| Total | 160 |
Patient had lipoprotein analysis showing features intermediate between Type IV and Type V hyperlipidemia.
Patient had features consistent with either Type IV or Type V hyperlipidemia
Includes teofovir, raltegravir, abacavir, lamivudine, and efavirez.
Fig. 6.
Plots the relationship between serum/plasma triglyceride concentration and lipemic index. There is a weak linear relationship ([triglycerides] =159.4+2.5 [lipemic index], R2=0.24).
4. Discussion
In our retrospective study, we analyzed a large data set for lipemic index (which is measured on all clinical chemistry specimens in our institutional core laboratory) and serum/plasma triglycerides (data only for specimens for which this testing was ordered for clinical purposes). Published literature has demonstrated assay interferences caused by lipemia [1], [3], [4], [5], [6], [7], [9], [10], [11], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]. Our data from an academic medical center revealed that the most common suspected causes of markedly elevated lipemic index were lipid emulsion (either for parenteral nutrition or as a diluent for poorly water-soluble medications) and diabetes mellitus, consistent with reports in other articles [1], [2], [5], [7], [10], [27], [29], [30], [33], [42], [43]. Although lipid emulsions can be used as antidotes for some poisonings [44], [45], that method is not commonly used at our institution. Intravenous lipid emulsions can interfere with laboratory tests as early as two hours after dosing to more than 20 h after administration [32], [34]. The most likely suspected causes of elevated triglycerides (when ordered for clinical purposes) was diabetes mellitus which accounted for 64% of the total (mostly type 2 diabetes mellitus which accounted for all but 4% of the 64%). Our data show the value in routinely assessing lipemic index, in that serum/plasma triglyceride concentrations were not commonly ordered in patients receiving intravenous lipid emulsions. Without routine measurement of lipemic index for all clinical chemistry specimens, this potential interference would be missed. Similar to previous studies on smaller data sets [18], [46], we find that the relationship between lipemic index and triglycerides is complex with only a weak linear correlation.
Fasting has been recognized as a significant factor in specimen lipemia [12], [13], [46]. For most of the specimens analyzed in our study, the fasting status of the patient was undocumented. It is likely that many of the specimens collected in the emergency department, inpatient units, and even outpatient clinics (especially for collections in the afternoon or evening) were drawn from patients who were not fasting. Although we identified likely medical or iatrogenic causes for lipemia for the vast majority of the markedly lipemic specimens, it is likely that non-fasting status also contributed to elevated lipemia in many cases.
Our study adds to the body of literature that lipemic specimens have a higher rate of hemolysis [18], [46]. In our data set, over 70% of specimens with a lipemic index of greater than 300 are at least mildly hemolyzed. In the severely lipemic specimens (lipemic index greater than 300), approximately 40% are markedly hemolyzed with a hemolysis index exceeding 300. This category of specimens with severe lipemia and hemolysis present a very difficult analytical challenge, as many clinical chemistry analyses may be significantly altered by either or both interferences [1], [3], [4], [5], [38], [39], [47], [48]. Efforts to reduce these type of specimens may benefit patients (including those who may be critically ill) by preventing repeated redraws due to tests that have been canceled by massive hemolysis/lipemia.
The clinical laboratory management of lipemic specimens is not standardized [4], [7], [49]. There is also heterogeneity in analytical methods for measuring lipemia and how manufacturers report lipemic interference in assay package inserts [49]. In vitro study of lipemia is also complicated in that spiking of samples with intralipid may not reproduce lipemia interference from actual patient specimens [7], [26]. There are various maneuvers for reducing the lipemia. The two primary approaches are ultracentrifugation (with subsequent removal of the lipid layer) or addition of reagents to clear the lipids [4], [7]. Both approaches have advantage and disadvantages. Reagents that reduce lipid levels do not require special instrumentation; however, these reagents can themselves interfere or impact some clinical chemistry assays [41], [50]. Ultracentrifugation requires specific equipment that may not be available in all clinical laboratories, but does have the advantage of avoiding adding reagents to the specimen [4], [7]. Thus, reducing frequency of lipemia would be of benefit to the clinical laboratory.
The high frequency of intravenous lipid emulsion as a cause of severe lipemia in our study suggest that education and interventions within the EHR may be helpful in reducing extent of lipemia. For instance, warning alerts within the EHR might prompt physicians and nurses about risk of lipemic interference, especially when samples are drawn close in time to lipid infusions. This may allow the clinical team to time blood draws to reduce risk of severe lipemia. There is ongoing work at our medical center in education and in optimizing EHR alerts on lipemic interference.
5. Conclusions
In conclusion, intravenous lipid infusions and type 2 diabetes were the most common causes of severe lipemia in this study at an academic medical center. Given that iatrogenic factors are the most common cause of severe lipemia, education and intervention may be helpful in reducing frequency of severe lipemia in patient specimens.
Conflict of interest
None of the authors have any conflict to report.
Acknowledgements
None.
Contributor Information
Sandhya Mainali, Email: sandhya-mainali@uiowa.edu.
Scott R. Davis, Email: scott-davis@uiowa.edu.
Matthew D. Krasowski, Email: mkrasows@healthcare.uiowa.edu, matthew-krasowski@uiowa.edu.
References
- 1.Agarwal S., Vargas G., Nordstrom C., Tam E., Buffone G.J., Devaraj S. Effect of interference from hemolysis, icterus and lipemia on routine pediatric clinical chemistry assays. Clin Chim Acta. 2015;438:241–245. doi: 10.1016/j.cca.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Dimeski G. Interference testing. Clin Biochem. Rev. 2008;29(Suppl 1):S43–S48. [PMC free article] [PubMed] [Google Scholar]
- 3.Ji J.Z., Meng Q.H. Evaluation of the interference of hemoglobin, bilirubin, and lipids on Roche Cobas 6000 assays. Clin Chim Acta. 2011;412(17-18):1550–1553. doi: 10.1016/j.cca.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Kroll M., McCudden C. De Gruyter; Berlin: 2013. Endogenous Interferences in Clinical Laboratory Tests. [Google Scholar]
- 5.Lippi G., Plebani M., Favaloro E.J. Interference in coagulation testing: focus on spurious hemolysis, icterus, and lipemia. Semin Thromb Hemost. 2013;39(3):258–266. doi: 10.1055/s-0032-1328972. [DOI] [PubMed] [Google Scholar]
- 6.Salvagno G.L., Lippi G., Gelati M., Guidi G.C. Hemolysis, lipaemia and icterus in specimens for arterial blood gas analysis. Clin Biochem. 2012;45(4-5):372–373. doi: 10.1016/j.clinbiochem.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Nikolac N. Lipemia: causes, interference mechanisms, detection and management. Biochem. Med. (Zagreb) 2014;24(1):57–67. doi: 10.11613/BM.2014.008. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikolac N., Celap I., Filipi P., Hemar M., Kocijancic M., Miler M., Simundic A.M., Smolcic V.S., Vrtaric A. Croatian laboratories have a good knowledge of the proper detection and management of hemolyzed, icteric and lipemic samples. Clin Chem Lab. Med. 2016;54(3):419–425. doi: 10.1515/cclm-2015-0650. [DOI] [PubMed] [Google Scholar]
- 9.Dimeski G., Mollee P., Carter A. Effects of hyperlipidemia on plasma sodium, potassium, and chloride measurements by an indirect ion-selective electrode measuring system. Clin Chem. 2006;52(1):155–156. doi: 10.1373/clinchem.2005.054981. [DOI] [PubMed] [Google Scholar]
- 10.Bashir S., Wiltshire M., Cardigan R., Thomas S. Lipaemic plasma induces haemolysis in resuspended red cell concentrate. Vox Sang. 2013;104(3):218–224. doi: 10.1111/j.1423-0410.2012.01660.x. [DOI] [PubMed] [Google Scholar]
- 11.Dimeski G., Mollee P., Carter A. Increased lipid concentration is associated with increased hemolysis. Clin Chem. 2005;51(12):2425. doi: 10.1373/clinchem.2005.058644. [DOI] [PubMed] [Google Scholar]
- 12.Guidi G.C., Simundic A.M., Salvagno G.L., Aquino J.L., Lima-Oliveira G. To avoid fasting time, more risk than benefits. Clin Chem Lab. Med. 2015;53(10):e261–e264. doi: 10.1515/cclm-2014-1013. [DOI] [PubMed] [Google Scholar]
- 13.Lima-Oliveira G., Salvagno G.L., Lippi G., Gelati M., Montagnana M., Danese E., Picheth G., Guidi G.C. Influence of a regular, standardized meal on clinical chemistry analytes. Ann Lab. Med. 2012;32(4):250–256. doi: 10.3343/alm.2012.32.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boisrame-Helms J., Toti F., Hasselmann M., Meziani F. Lipid emulsions for parenteral nutrition in critical illness. Prog Lipid Res. 2015;60:1–16. doi: 10.1016/j.plipres.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Fell G.L., Nandivada P., Gura K.M., Puder M. Intravenous Lipid Emulsions in Parenteral Nutrition. Adv Nutr. 2015;6(5) doi: 10.3945/an.115.009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fahr A., Liu X. Drug delivery strategies for poorly water-soluble drugs. Expert Opin Drug Deliv. 2007;4(4):403–416. doi: 10.1517/17425247.4.4.403. [DOI] [PubMed] [Google Scholar]
- 17.Zamani G.R., Mohammadi M., Ashrafi M.R., Karimi P., Mahmoudi M., Badv R.S., Tavassoli A.R., Azizi Malamiri R. The effects of classic ketogenic diet on serum lipid profile in children with refractory seizures. Acta Neurol Belg. 2016 doi: 10.1007/s13760-016-0601-x. [DOI] [PubMed] [Google Scholar]
- 18.Twomey P.J., Don-Wauchope A.C., McCullough D. Unreliability of triglyceride measurement to predict turbidity induced interference. J Clin Pathol. 2003;56(11):861–862. doi: 10.1136/jcp.56.11.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermeer H.J., Thomassen E., de Jonge N. Automated processing of serum indices used for interference detection by the laboratory information system. Clin Chem. 2005;51(1) doi: 10.1373/clinchem.2004.036301. [DOI] [PubMed] [Google Scholar]
- 20.Krasowski M.D., Davis S.R., Drees D., Morris C., Kulhavy J., Crone C., Bebber T., Clark I., Nelson D.L., Teul S., Voss D., Aman D., Fahnle J., Blau J.L. Autoverification in a core clinical chemistry laboratory at an academic medical center. J Pathol Inform. 2014;5(1):13. doi: 10.4103/2153-3539.129450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krasowski M.D., Chudzik D., Dolezal A., Steussy B., Gailey M.P., Koch B., Kilborn S.B., Darbro B.W., Rysgaard C.D., Klesney-Tait J.A. Promoting improved utilization of laboratory testing through changes in an electronic medical record: experience at an academic medical center. BMC Med Inform Decis Mak. 2015;15:11. doi: 10.1186/s12911-015-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson L.S., Steussy B., Morris C.S., Krasowski M.D. Vol. 4. Springerplus; 2015. p. 760. (Effect of Specimen Type on Free Immunoglobulin Light Chains Analysis on the Roche Diagnostics Cobas 8000 Analyzer). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krasowski M.D., Wilford J.D., Howard W., Dane S.K., Davis S.R., Karandikar N.J., Blau J.L., Ford B.A. Implementation of Epic Beaker Clinical Pathology at an academic medical center. J Pathol Inform. 2016;7:7. doi: 10.4103/2153-3539.175798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akbas N., Gonzalez G., Devaraj S. Evaluation of the Lipid Interference for Siemens BN ProSpec Cystatin C Assay Using Pediatric Samples. Ann Clin Lab. Sci. 2015;45(5):562–564. [PubMed] [Google Scholar]
- 25.Appierto V., Callari M., Cavadini E., Morelli D., Daidone M.G., Tiberio P. A lipemia-independent NanoDrop(®)-based score to identify hemolysis in plasma and serum samples. Bioanalysis. 2014;6(9):1215–1226. doi: 10.4155/bio.13.344. [DOI] [PubMed] [Google Scholar]
- 26.Bornhorst J.A., Roberts R.F., Roberts W.L. Assay-specific differences in lipemic interference in native and intralipid-supplemented samples. Clin Chem. 2004;50(11):2197–2201. doi: 10.1373/clinchem.2004.040154. [DOI] [PubMed] [Google Scholar]
- 27.Calmarza P., Cordero J. Lipemia interferences in routine clinical biochemical tests. Biochem. Med. (Zagreb) 2011;21(2):160–166. doi: 10.11613/bm.2011.025. [DOI] [PubMed] [Google Scholar]
- 28.Cook S.L., Bruns D.E. Persistent hemolysis in a patient with pancreatitis. Clin Chem. 2012;58(6):974–977. doi: 10.1373/clinchem.2011.167452. [DOI] [PubMed] [Google Scholar]
- 29.Grunbaum A.M., Gilfix B.M., Gosselin S., Blank D.W. Analytical interferences resulting from intravenous lipid emulsion. Clin Toxicol (Phila) 2012;50(9):812–817. doi: 10.3109/15563650.2012.731509. [DOI] [PubMed] [Google Scholar]
- 30.Grunbaum A.M., Gilfix B.M., Hoffman R.S., Lavergne V., Morris M., Miller-Nesbitt A., Gosselin S. Review of the effect of intravenous lipid emulsion on laboratory analyses. Clin Toxicol (Phila) 2016;54(2):92–102. doi: 10.3109/15563650.2015.1115515. [DOI] [PubMed] [Google Scholar]
- 31.Hasanato R., Brearton S., Alshebani M., Bailey L., Aldugashim S., Alothaim A., Tamimi W. Effects of serum indices interference on hormonal results from the Abbott Architect i2000 immunoassay analyser. Br J Biomed Sci. 2015;72(4):151–155. doi: 10.1080/09674845.2015.11665744. [DOI] [PubMed] [Google Scholar]
- 32.Johnson-Arbor K., Salinger L., Luczycki S. Prolonged Laboratory Interference After Administration of Intravenous Lipid Emulsion Therapy. J Med. Toxicol. 2015;11(2):223–226. doi: 10.1007/s13181-014-0438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroll M.H. Evaluating interference caused by lipemia. Clin Chem. 2004;50(11):1968–1969. doi: 10.1373/clinchem.2004.038075. [DOI] [PubMed] [Google Scholar]
- 34.Levine M., Skolnik A.B., Ruha A.M., Bosak A., Menke N., Pizon A.F. Complications following antidotal use of intravenous lipid emulsion therapy. J Med. Toxicol. 2014;10(1):10–14. doi: 10.1007/s13181-013-0356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meany D., Schowinsky J., Clarke W. Effects of hemolysis and lipemia on the COBAS salicylate and acetaminophen assays compared to GDS assays. Clin Biochem. 2008;41(18):1486–1488. doi: 10.1016/j.clinbiochem.2008.09.111. [DOI] [PubMed] [Google Scholar]
- 36.Punja M., Neill S.G., Wong S. Caution with interpreting laboratory results after lipid rescue therapy. Am J Emerg Med. 2013;31(10) doi: 10.1016/j.ajem.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Rosenthal M.A., Katz H.B. An innovative method for determining lipemia interference in blood specimens. Clin Chim Acta. 2011;412(7–8):665–667. doi: 10.1016/j.cca.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 38.Ryder K.W., Glick M.R. Erroneous laboratory results from hemolyzed, icteric, and lipemic specimens. Clin Chem. 1993;39(1):175–176. [PubMed] [Google Scholar]
- 39.Steen G., Klerk A., Laan K., Eppens E.F. Evaluation of the interference due to haemoglobin, bilirubin and lipids on Immulite 2500 assays: a practical approach. Ann Clin Biochem. 2011;48(Pt 2):170–175. doi: 10.1258/acb.2010.010187. [DOI] [PubMed] [Google Scholar]
- 40.Venado A., Wille K., Belott S.C., Diaz-Guzman E. Unexplained hemolysis in patients undergoing ECMO: beware of hypertriglyceridemia. Perfusion. 2015;30(6) doi: 10.1177/0267659114557693. [DOI] [PubMed] [Google Scholar]
- 41.Vermeer H.J., Steen G., Naus A.J., Goevaerts B., Agricola P.T., Schoenmakers C.H. Correction of patient results for Beckman Coulter LX-20 assays affected by interference due to hemoglobin, bilirubin or lipids: a practical approach. Clin Chem Lab. Med. 2007;45(1):114–119. doi: 10.1515/CCLM.2007.004. [DOI] [PubMed] [Google Scholar]
- 42.Bartos M., Knudsen K. Use of intravenous lipid emulsion in the resuscitation of a patient with cardiovascular collapse after a severe overdose of quetiapine. Clin Toxicol (Phila) 2013;51(6):501–504. doi: 10.3109/15563650.2013.803229. [DOI] [PubMed] [Google Scholar]
- 43.Bucklin M.H., Gorodetsky R.M., Wiegand T.J. Prolonged lipemia and pancreatitis due to extended infusion of lipid emulsion in bupropion overdose. Clin Toxicol (Phila) 2013;51(9):896–898. doi: 10.3109/15563650.2013.831436. [DOI] [PubMed] [Google Scholar]
- 44.Cave G., Harvey M., Graudins A. Intravenous lipid emulsion as antidote: a summary of published human experience. Emerg Med. Australas. 2011;23(2):123–141. doi: 10.1111/j.1742-6723.2011.01398.x. [DOI] [PubMed] [Google Scholar]
- 45.Ozcan M.S., Weinberg G. Intravenous lipid emulsion for the treatment of drug toxicity. J Intensive Care Med. 2014;29(2):59–70. doi: 10.1177/0885066612445978. [DOI] [PubMed] [Google Scholar]
- 46.De Haene H., Taes Y., Christophe A., Delanghe J. Comparison of triglyceride concentration with lipemic index in disorders of triglyceride and glycerol metabolism. Clin Chem Lab. Med. 2006;44(2):220–222. doi: 10.1515/CCLM.2006.040. [DOI] [PubMed] [Google Scholar]
- 47.Lippi G., Ippolito L., Fontana R. Prevalence of hemolytic specimens referred for arterial blood gas analysis. Clin Chem Lab. Med. 2011;49(5):931–932. doi: 10.1515/CCLM.2011.136. [DOI] [PubMed] [Google Scholar]
- 48.Monneret D., Mestari F., Atlan G., Corlouer C., Ramani Z., Jaffre J., Dever S., Fressart V., Alkouri R., Lamari F., Devilliers C., Imbert-Bismut F., Bonnefont-Rousselot D. Hemolysis indexes for biochemical tests and immunoassays on Roche analyzers: determination of allowable interference limits according to different calculation methods. Scand J Clin Lab. Invest. 2015;75(2):162–169. doi: 10.3109/00365513.2014.993691. [DOI] [PubMed] [Google Scholar]
- 49.Nikolac N., Simundic A.M., Miksa M., Lima-Oliveira G., Salvagno G.L., Caruso B., Guidi G.C. Heterogeneity of manufacturers' declarations for lipemia interference--an urgent call for standardization. Clin Chim Acta. 2013;426:33–40. doi: 10.1016/j.cca.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Saracevic A., Nikolac N., Simundic A.M. The evaluation and comparison of consecutive high speed centrifugation and LipoClear(R) reagent for lipemia removal. Clin Biochem. 2014;47(4–5) doi: 10.1016/j.clinbiochem.2014.01.001. [DOI] [PubMed] [Google Scholar]