Abstract
Aims
Dapagliflozin and exenatide reduce body weight by differing mechanisms. Dual therapy with these agents reduces body weight, adipose tissue volume, glycaemia and systolic blood pressure (SBP) over 24 weeks. Here, we examined these effects over 1 year in obese adults without diabetes.
Materials and methods
Obese adults without diabetes (N = 50; aged 18‐70 years; body mass index, 30‐45 kg/m2) were initially randomized to double‐blind oral dapagliflozin 10 mg once daily plus subcutaneous long‐acting exenatide 2 mg once weekly or to placebo. They entered an open‐label extension from 24 to 52 weeks during which all participants received active treatment.
Results
Of the original 25 dapagliflozin + exenatide‐treated and 25 placebo‐treated participants, respectively, 21 (84%) and 17 (68%) entered the open‐label period and 16 (64%) and 17 (68%) completed 52 weeks of treatment. At baseline, mean body weight was 104.6 kg, and 73.5% of participants had prediabetes (impaired fasting glucose or impaired glucose tolerance). Reductions with dapagliflozin + exenatide at 24 weeks were sustained at 52 weeks, respectively, for body weight (−4.5 and −5.7 kg), total adipose tissue volume (−3.8 and −5.3 L), proportion with prediabetes (34.8% and 35.3%), and SBP (−9.8 and −12.0 mm Hg). Effects on body weight, SBP and glycaemia at 52 weeks with placebo → dapagliflozin + exenatide were similar to those observed with continuation of dapagliflozin + exenatide. Nausea and injection‐site reactions were more frequent with dapagliflozin + exenatide than with placebo and diminished over time. Safety and tolerability were similar to that in previous diabetes trials with these agents. No clear difference in adverse event‐related withdrawals between placebo and active treatment periods was observed.
Conclusions
Dapagliflozin + exenatide dual therapy produced sustained reductions in body weight, prediabetes and SBP over 52 weeks and was well tolerated in obese adults without diabetes.
Keywords: dapagliflozin, exenatide, obesity, prediabetes
1. INTRODUCTION
Overweight and obesity are highly prevalent worldwide, affecting 39% and 13% of adults, respectively,1 and are associated with serious health consequences, including type 2 diabetes (T2D), cardiovascular disease, non‐alcoholic fatty liver disease, musculoskeletal disorders and certain cancers.1, 2 Conversely, body weight loss of ≥5% can mitigate cardiometabolic risk associated with overweight and obesity.3 Although intensive lifestyle interventions can achieve substantial reductions in body weight in the context of randomized controlled trials,4 results from real‐life primary care settings have been disappointing.5 Moreover, in response to body weight loss, physiological counter‐regulatory mechanisms, such as compensatory changes in energy intake and expenditure, undermine the long‐term maintenance of body weight loss.6 While pharmacological treatments may augment initial body weight loss and/or maintenance of body weight loss alongside behavioural interventions, there are concerns as to their longer‐term effectiveness and safety.7 Consequently, an unmet need exists for novel body weight loss interventions, including pharmacotherapies, used singly or in combination, that provide durable efficacy and are safe and well tolerated.8
Some glucose‐lowering therapies induce body weight loss, prompting exploration of their use in obese individuals at risk of developing diabetes. Of particular interest are selective sodium‐glucose cotransporter 2 (SGLT2) inhibitors and glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs),9, 10 which cause body weight loss via different mechanisms. SGLT2 inhibitors increase urinary glucose excretion, resulting in urinary caloric loss and minor fluid loss resulting from mild diuresis, but with a compensatory appetite increase,11, 12 whereas GLP‐1RAs reduce appetite and delay gastric emptying.13 The SGLT2 inhibitor dapagliflozin and the GLP‐1RA exenatide consistently reduce body weight in patients with T2D, which is maintained for up to 214, 15, 16 and 6 years,17, 18, 19 respectively.
The differing and possibly complementary mechanisms of action of dapagliflozin and exenatide may be particularly effective when these agents are used in combination to achieve sustained body weight loss and prediabetes reduction.
We previously reported the 24‐week results of a phase 2 randomized placebo‐controlled study comparing dual therapy with oral dapagliflozin 10 mg once daily (DAPA) and subcutaneous exenatide 2 mg once weekly (ExQW) vs placebo (PBO) in obese adults without diabetes (N = 50).20 Those receiving DAPA + ExQW lost weight (−4.5 kg), whereas those receiving PBO did not (−0.4 kg), and 36% vs 0% of participants, respectively, achieved ≥5% body weight loss. Body weight loss with DAPA + ExQW was largely accounted for by reduction in adipose tissue volume as assessed with magnetic resonance imaging (MRI). Improved glycaemic control and reduced blood pressure were also observed with DAPA + ExQW. No unexpected tolerability issues were found with the combination in this small proof‐of‐concept study.20
Participants completing the 24‐week study who had adhered to the protocol were offered a further 28 weeks of treatment with DAPA + ExQW in an open‐label extension of this study. Outcomes evaluated included change in body weight, body composition by MRI, measures of glycaemic control and cardiovascular risk, and safety and tolerability.
2. METHODS
2.1. Study design and participants
The design of this phase 2a study evaluating the efficacy and safety of dual therapy with DAPA + ExQW in obese participants without diabetes (ClinicalTrials.gov identifier: NCT02313220) has been published previously.20 Briefly, this was an investigator‐initiated 24‐week, randomized, parallel‐group, double‐blind, placebo‐controlled trial followed by an optional 28‐week open‐label phase during which all participants received active treatment and had study visits at week 38 and 52 (Figure 1A). It was conducted at a single centre in Sweden from December 2014 to March 2016. Participants were obese (body mass index [BMI], 30‐45 kg/m2), were aged 18 to 70 years and did not have diabetes.
Figure 1.
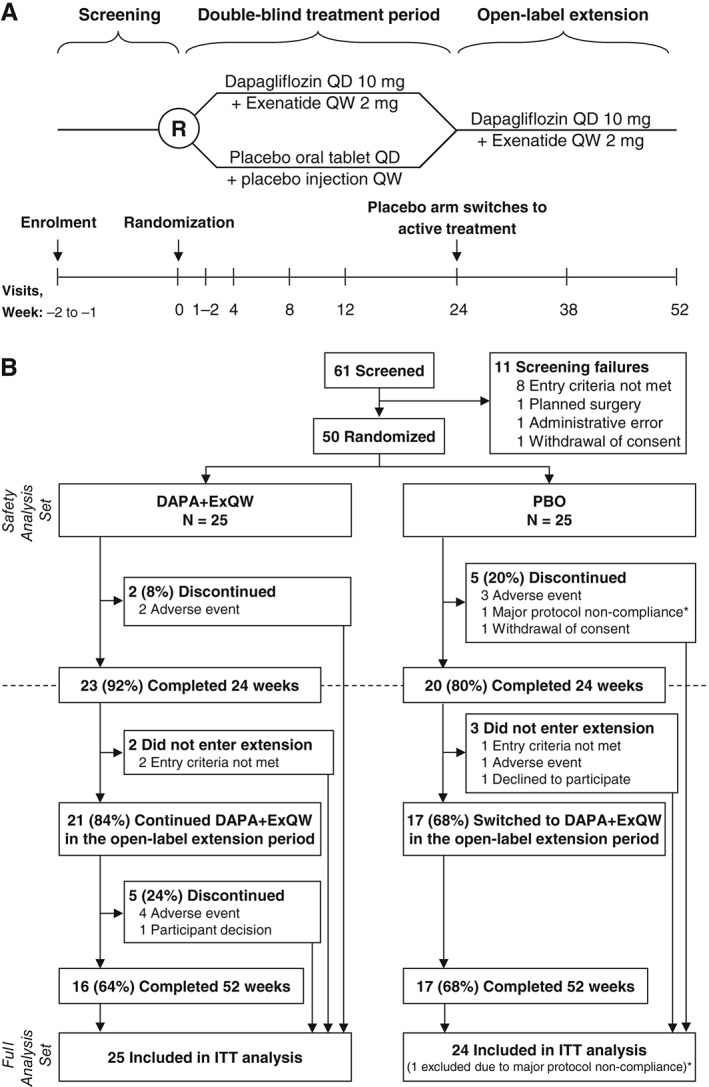
A, Study design and B, CONSORT flow diagram. DAPA + ExQW, dapagliflozin 10 mg once daily plus exenatide 2 mg once weekly; ITT, intention to treat; PBO, placebo; QD, once daily; QW, once weekly. *A profound lifestyle change, including a strict low‐carbohydrate/high‐fat diet that elevated blood ketones. This resulted in withdrawal of this patient during blinded study phase and exclusion of this patient from the full analysis set
2.2. Treatments
Following the 24‐week double‐blind phase, all eligible participants receiving PBO and DAPA + ExQW were offered 28 weeks of open‐label DAPA + ExQW treatment (hereafter referred to as PBO → DAPA + ExQW and continued DAPA + ExQW groups, respectively). Details on randomization procedure, blinding, ethics and informed consent, and guidelines for comedication usage have been published previously.20 Patients received oral and written instructions to follow national guidelines on a balanced normo‐caloric diet, and moderate exercise (eg, walking 30 minutes most days) was recommended. Because the primary purpose of this proof‐of‐concept study was to evaluate pharmacological effects, diet and exercise modification was not strictly reinforced or monitored.
2.3. Outcomes
Details of outcome measures have been published previously20; however, a brief description is provided below.
2.3.1. Efficacy
Primary and secondary endpoints were change and percent change in body weight (kg) from baseline to 52 weeks and from 24 to 52 weeks. Exploratory efficacy endpoints included proportions of participants achieving ≥5% and ≥10% body weight loss; changes in waist circumference and waist‐to‐hip ratio; MRI‐assessed changes (continued DAPA + ExQW group only) in total adipose and lean tissue volumes, abdominal visceral and subcutaneous adipose tissue volumes, and liver fat percent (defined as liver fat × 100 ÷ [liver fat + liver water]); changes in glycated haemoglobin (HbA1c) and fasting plasma glucose (FPG); changes in oral glucose tolerance test (OGTT)‐derived measures (continued DAPA + ExQW group only), including 2‐hour plasma glucose (2‐hour PG), proportions with impaired fasting glucose (IFG; defined as FPG ≥ 5.6 mmol/L measured just before the OGTT), or impaired glucose tolerance (IGT; defined as a PG value ≥ 7.8 mmol/L measured 120 minutes after initiation of OGTT) or any IFG/IGT (prediabetes); changes in seated systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate; and changes in fasting serum lipids.
2.3.2. Safety
Reports of adverse events (AEs) were collected and coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 18.0E. Furthermore, AEs of special interest for dapagliflozin and exenatide treatment (urinary tract infections [UTIs], genital infections, events related to volume reduction, changes in renal function and gastrointestinal symptomatology) were captured using prespecified lists of relevant MedDRA‐preferred terms. Changes in laboratory parameters of interest were also measured.
2.4. Statistical methods
Primary, secondary and exploratory efficacy endpoints were analysed with an intention‐to‐treat (ITT) approach, using all available data comprising the full analysis set. Analyses of safety employed the safety analysis set. For definitions of analysis sets, see File S1 (Supplementary Statistical Methods). For efficacy endpoints, adjusted mean changes from 0 to 24 weeks, 24 to 52 weeks and 0 to 52 weeks, and associated 95% confidence intervals (CIs) and P values for comparison between time points, were derived from a mixed model for repeated measures (MMRM) with treatment, week, treatment‐by‐week interaction and sex as categorical fixed covariates and baseline value as a continuous fixed covariate. The same approach was used for analysis of changes in HbA1c, FPG, blood pressure, heart rate and fasting serum lipids. For the open‐label extension phase, no between‐group comparisons were made. During the extension period, MRI and OGTT‐derived measurements were available only for the continuing DAPA + ExQW group; therefore, MMRM did not contain treatment terms.
Paired McNemar tests were used to evaluate proportions of participants with IFG and IGT (continuing DAPA + ExQW group only) after 24 and 52 weeks.
To assess potential bias from differential dropout rates in this delayed‐start study,21 sensitivity analyses were conducted for the primary endpoint. For further details, see File S1 (Sensitivity Analysis Methods; Sensitivity Analysis Results).
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina). CIs and P values were unadjusted for multiple comparisons.
3. RESULTS
3.1. Participants
Of the 50 participants randomized, 23 (92%) and 20 (80%) completed 24 weeks of double‐blind treatment with DAPA + ExQW or PBO, respectively. Of these, 21 participants (84%) continued DAPA + ExQW (2 no longer met study criteria) and 17 (68%) switched from PBO to active treatment (PBO → DAPA + ExQW) (1 had developed diabetes, 1 had skin rash and 1 declined to participate) during the open‐label extension period (Figure 1B). At the end of 52 weeks, 16 participants (64%) in the original DAPA + ExQW group (5 discontinued prematurely, 4 because of AEs and 1 because of participant‐perceived lack of efficacy) and 17 participants (68%) in the original PBO group completed DAPA + ExQW treatment.
Baseline demographic, anthropomorphic, body composition and glycaemic characteristics of the study participants have been published previously20; the mean age of participants was 51.4 years, mean BMI was 35.4 kg/m2 and 60% were female. At baseline, glycaemic variables, vital signs and renal function were balanced across treatment groups; however, participants in the DAPA + ExQW group were older and had greater body weight, body fat measures and duration of obesity (Table S1 in File S1). One participant in each treatment arm, neither of whom entered the study extension, were using concomitant lipid‐lowering agents on entering the 0‐ to 24‐week study. Two participants, both in the PBO → DAPA + ExQW group, received atorvastatin at week 13 and at initiation of the extension, respectively. In the PBO → DAPA + ExQW group, antihypertensives were newly prescribed or increased in 4 and 2 participants during the 0‐ to 24‐ and 24‐ to 52‐week periods, respectively. In the continued DAPA + ExQW group, 3 participants altered use of antihypertensives during the 0‐ to 24‐week period (1 discontinued, 1 discontinued and then restarted, and 1 received a new prescription) and none altered use of antihypertensives thereafter.
3.2. Efficacy
3.2.1. Body weight
Among participants continuing on DAPA + ExQW, body weight loss achieved at 24 weeks (−4.5 kg) was sustained at 52 weeks (−5.7 kg), with some additional body weight loss from 24 to 52 weeks (−1.2 kg) that did not reach statistical significance (Table 1, Figure 2A). Participants who switched from placebo to active treatment (PBO → DAPA + ExQW) at 24 weeks achieved body weight loss at 52 weeks (−4.2 kg) comparable to that with DAPA + ExQW during the first 24 weeks of therapy (−4.5 kg). Similar results were obtained for body weight percentage changes (Figure 2B). When examining individual trajectories of body weight change over time, 76.5% of participants (complete case analysis; 52.0%, ITT analysis) continuing on DAPA + ExQW showed sustained weight loss of any magnitude over 52 weeks, with 3 of these participants achieving a loss of ≥15% of initial body weight (Figure 2C,D). Results of sensitivity analyses are shown in Figure S1 of File S1.
Table 1.
Findings for efficacy endpoints after 24 and 52 weeks (full analysis set)
| Dapagliflozin 10 mg QD + exenatide 2 mg QW (n = 25) | Placebo/dapagliflozin 10 mg QD + exenatide 2 mg QW (n = 24) | |||||
|---|---|---|---|---|---|---|
| Weeks 0 to 24 | Weeks 24 to 52 | Weeks 0 to 52 | Weeks 0 to 24 | Weeks 24 to 52 | Weeks 0 to 52 | |
| Primary endpoint | ||||||
| Body weight, adjusted mean change (95% CI),a kg | −4.48 (−6.09, −2.88)*** | −1.21 (−3.19, 0.78) | −5.69 (−8.63, −2.75)*** | −0.34 (−2.02, 1.33) | −3.80 (−5.85, −1.75)*** | −4.15 (−7.19, −1.10)** |
| Secondary endpoint | ||||||
| Body weight, adjusted mean percent change (95% CI),a % | −4.47 (−6.05, −2.90)*** | −1.19 (−3.00, 0.63) | −5.66 (−8.43, −2.89)*** | −0.27 (−1.91, 1.37) | −3.80 (−5.68, −1.92)*** | −4.07 (−6.94, −1.21)** |
| Exploratory endpoints | ||||||
| Body weight, proportion with ≥5% reduction, n (%) | 9/23 (39.1) | NA | 10/17 (58.8) | 0/20 (0) | NA | 7/17 (41.2) |
| Missing, n | 2 | 8 | 4 | 7 | ||
| Body weight, proportion with ≥10% reduction, n (%) | 3/23 (13.0) | NA | 3/17 (17.6) | 0/20 (0) | NA | 4/17 (23.5) |
| Missing, n | 2 | 8 | 4 | 7 | ||
| Waist circumference, adjusted mean change (95% CI),a cm | −5.3 (−7.4, −3.1)*** | −2.0 (−4.7, 0.7) | −7.3 (−10.5, −4.1)*** | −2.5 (−4.8, −0.2)* | −4.2 (−7.0, −1.4)** | −6.7 (−10.0, −3.3)*** |
| Waist‐to‐hip ratio, adjusted mean change (95% CI)a | −0.02 (−0.04, 0.001) | −0.01 (−0.03, 0.01) | −0.03 (−0.05, −0.01)** | −0.01 (−0.03, 0.01) | −0.02 (−0.04, 0.01) | −0.03 (−0.05, −0.01)** |
| MRI body composition | ||||||
| VAAT, adjusted mean change (95% CI),b L | −0.37 (−0.64, −0.09)* | −0.16 (−0.45, 0.13) | −0.53 (−1.00, −0.05)* | NA | NA | NA |
| SAAT,c adjusted mean change (95% CI),b L | −1.24 (−1.83, −0.66)*** | −0.35 (−0.82, 0.12) | −1.59 (−2.55, −0.64)** | NA | NA | NA |
| Total adipose tissue, adjusted mean change (95% CI),b L | −3.80 (−5.85, −1.75)** | −1.51 (−3.34, 0.32) | −5.31 (−8.90, −1.72)** | NA | NA | NA |
| Total lean tissue, adjusted mean change (95% CI),b L | −1.04 (−1.67, −0.40)** | −0.32 (−1.04, 0.40) | −1.36 (−2.19, −0.52)** | NA | NA | NA |
| Liver fat, adjusted mean percent change (95% CI),b % | −1.22 (−2.70, 0.25) | −0.31 (−2.23, 1.60) | −1.54 (−3.24, 0.17) | NA | NA | NA |
| Glycaemic measures | ||||||
| HbA1c, adjusted mean change (95% CI),a mmol/mol | −3.9 (−4.7, −3.0)*** | 0.8 (−0.1, 1.6) | −3.1 (−3.9, −2.3)*** | −1.6 (−2.4, −0.7)*** | −1.6 (−2.5, −0.7)*** | −3.2 (−4.1, −2.3)*** |
| HbA1c, adjusted mean change (95% CI),a % | −0.36 (−0.43, −0.27)*** | 0.07 (−0.01, 0.15) | −0.28 (−0.36, −0.21)*** | −0.15 (−0.22, −0.06)*** | −0.15 (−0.23, −0.06)*** | −0.29 (−0.38, −0.21)*** |
| FPG, adjusted mean change (95% CI),a mmol/L | −0.41 (−0.60, −0.22)*** | 0.10 (−0.10, 0.31) | −0.31 (−0.48, −0.13)** | 0.25 (0.05, 0.45)* | −0.50 (−0.71, −0.29)*** | −0.25 (−0.42, −0.07)** |
| 2‐h PG, adjusted mean change (95% CI),b mmol/L | −1.85 (−2.62, −1.08)*** | −0.37 (−1.02, 0.29) | −2.22 (−2.98, −1.45)*** | NA | NA | NA |
| Proportion with IFG, n/N (%)d | 8/23 (34.8)** | NA | 6/17 (35.3) | NA | NA | NA |
| Missing, n | 2 | 8 | ||||
| Proportion with IGT, n/N (%)d | 4/23 (17.4)* | NA | 2/15 (13.3)* | NA | NA | NA |
| Missing, n | 2 | 10 | ||||
| Proportion with any IFG or IGT (prediabetes), n/N (%) | 8/23 (34.8) | NA | 6/17 (35.3)* | NA | NA | NA |
| Missing, n | 2 | 8 | ||||
| Urinary glucose excretion,e mean (SD), mmol/3 h | 50.5 (31.4) | NA | 45.7 (29.3) | 0.3 (0.8) | NA | NA |
| Vital signs | ||||||
| Diastolic BP, adjusted mean change (95% CI),a mm Hg | 1.6 (−2.8, 6.0) | −0.2 (−4.3, 3.9) | 1.4 (−2.5, 5.2) | 2.4 (−2.4, 7.1) | −1.6 (−5.9, 2.7) | 0.8 (−3.2, 4.7) |
| Systolic BP, adjusted mean change (95% CI),a mm Hg | −9.8 (−13.7, −5.8)*** | −2.2 (−7.9, 3.4) | −12.0 (−17.5, −6.5)*** | −3.1 (−7.2, 1.1) | −4.1 (−9.9, 1.7) | −7.2 (−12.8, −1.5)* |
| Heart rate, adjusted mean change (95% CI),a bpm | 2.7 (−0.0, 5.4) | −0.2 (−5.1, 4.7) | 2.5 (−1.7, 6.8) | 0.5 (−2.4, 3.4) | 5.5 (0.5, 10.6)* | 6.1 (1.8, 10.3)** |
| Serum lipids | ||||||
| Total cholesterol, adjusted mean change (95% CI),a mmol/L | −0.16 (−0.42, 0.09) | −0.13 (−0.43, 0.18) | −0.29 (−0.57, −0.01)* | −0.26 (−0.53, 0.02) | −0.07 (−0.37, 0.24) | −0.33 (−0.60, −0.05)* |
| LDL cholesterol, adjusted mean change (95% CI),a mmol/L | −0.17 (−0.38, 0.05) | −0.17 (−0.44, 0.10) | −0.34 (−0.58, −0.09)** | −0.22 (−0.44, 0.001) | −0.13 (−0.40, 0.14) | −0.35 (−0.59, −0.11)** |
| HDL cholesterol, adjusted mean change (95% CI),a mmol/L | 0.01 (−0.06, 0.09) | −0.02 (−0.13, 0.10) | −0.004 (−0.12, 0.11) | −0.08 (−0.16, 0.002) | 0.10 (−0.01, 0.21) | 0.02 (−0.09, 0.13) |
| Triglycerides, adjusted mean change (95% CI),a mmol/L | −0.10 (−0.29, 0.10) | −0.15 (−0.39, 0.08) | −0.25 (−0.45, −0.05)* | −0.003 (−0.20, 0.20) | −0.10 (−0.33, 0.13) | −0.10 (−0.30, 0.09) |
Abbreviations: BP, blood pressure; bpm, beats per minute; CI, confidence interval; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; HDL, high‐density lipoprotein; IFG, impaired fasting glucose (defined as FPG ≥5.6 mmol/L measured just before an OGTT at the 24‐week visit); IGT, impaired glucose tolerance (defined as a plasma glucose value ≥7.8 mmol/L measured 2 hours after the start of an OGTT at the 24‐week visit); LDL, low‐density lipoprotein; MRI, magnetic resonance imaging; NA, not available; OGTT, oral glucose tolerance test; 2‐h PG, 2‐hour plasma glucose level measured 2 hours after the start of the OGTT; QD, once daily; QW, once weekly; SAAT, subcutaneous abdominal adipose tissue; SD, standard deviation; VAAT, visceral abdominal adipose tissue.
P < .05;
P < .01;
P < .001 for change from Week 0 to Week 24.
Data are expressed as mean changes and 95% CIs derived from a mixed model for repeated measures adjusted for treatment, week, treatment‐by‐week, sex and baseline value.
Data are expressed as mean changes and 95% CIs derived from a mixed model for repeated measures adjusted for week, sex and baseline value.
Defined as the subcutaneous fat positioned between the hip joint and up to the lower pole of the lungs.
P value based on a paired McNemar test.
Values at 24 and 52 weeks derived from urine collected during a 3‐hour OGTT.
Figure 2.
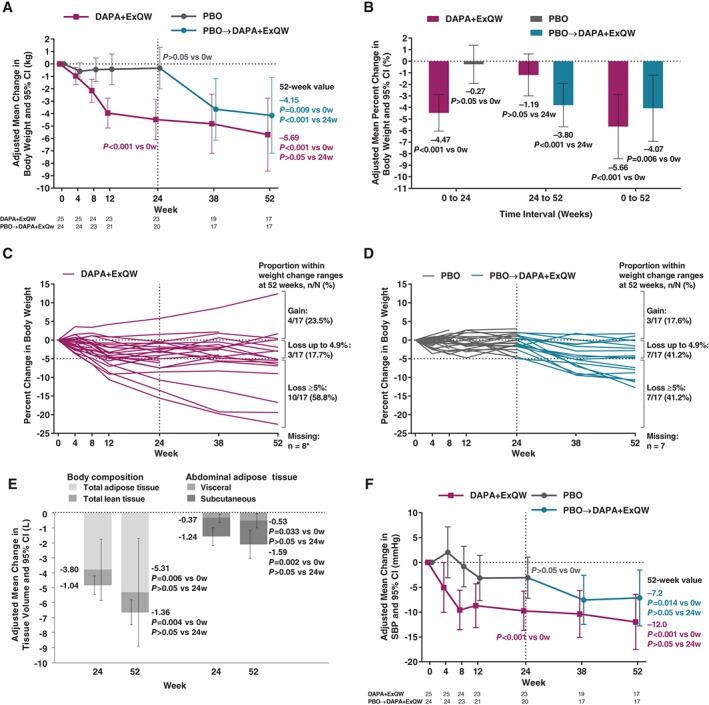
Changes in body weight, body composition, abdominal adipose tissue, and SBP. A, Primary endpoint: adjusted mean change from week 0 in body weight (kg) at 24 and 52 weeks. B, Secondary endpoint: adjusted mean percentage change from week 0 in body weight (%) at 24 and 52 weeks. C, Individual participant trajectories of percent change in body weight over 52 weeks in dapagliflozin/exenatide‐treated participants (*although 9 participants discontinued DAPA + ExQW, 1 of these participants attended the final visit for weight measurement). D, Corresponding trajectories in placebo‐treated participants. E, Adjusted mean change from week 0 in total lean and total adipose tissue volume and in visceral subcutaneous adipose tissue volume at 24 and 52 weeks among participants continuing on DAPA + ExQW throughout the study. F, Adjusted mean change from week 0 in SBP (mm Hg) over 24 and 52 weeks. Analyses in panels A, B, E and F employed mixed models for repeated measures of change or percentage change from baseline adjusted for treatment, week, treatment‐by‐week, sex and baseline value. CI, confidence interval; DAPA + ExQW, dapagliflozin 10 mg once daily plus exenatide 2 mg once weekly; PBO, placebo; SBP, systolic blood pressure; w, week(s)
3.2.2. Waist circumference
Waist circumference was significantly reduced at 52 weeks for both the DAPA + ExQW and PBO → DAPA + ExQW groups (−7.3 and −6.7 cm, respectively). A similar finding was evident for waist‐to‐hip ratio (Table 1).
3.2.3. MRI of body composition
Among participants continuing on DAPA + ExQW, total adipose tissue reduction achieved at 24 weeks (−3.8 L) was sustained at 52 weeks (−5.3 L), with some additional total adipose tissue loss from 24 to 52 weeks (−1.5 L) that did not reach statistical significance (Table 1, Figure 2E). Similar findings were observed for abdominal visceral and subcutaneous adipose tissue volumes (Table 1, Figure 2E). Numeric reductions in liver fat percent units in participants continuing on DAPA + ExQW did not reach statistical significance at either 24 or 52 weeks (Table 1). Mean (Figure S2A in File S1) and individual trajectories of change in liver fat percent units for DAPA + ExQW (Figure S2B in File S1) and PBO (Figure S2C in File S1) are presented in Figure S2 of File S1.
3.2.4. Glycaemic variables
Among participants continuing on DAPA + ExQW, reduction in HbA1c achieved at 24 weeks (−3.9 mmol/mol [−0.36%]) was sustained at 52 weeks (−3.1 mmol/mol [−0.28%]) (Table 1, Figure 3A). In the PBO → DAPA + ExQW group, HbA1c reduction at 52 weeks (−3.2 mmol/mol [−0.29%]) was comparable to that in the continuing DAPA + ExQW group during the first 24 weeks of therapy (−3.9 mmol/mol [−0.36%]). Similar results were obtained for changes in FPG (Table 1, Figure 3B). Of note, among participants receiving PBO from 0 to 24 weeks, FPG significantly rose (+0.25 mmol/L vs baseline) and, upon switch to active treatment, significantly fell at 52 weeks (−0.25 mmol/L vs baseline) (Table 1, Figure 3B).
Figure 3.
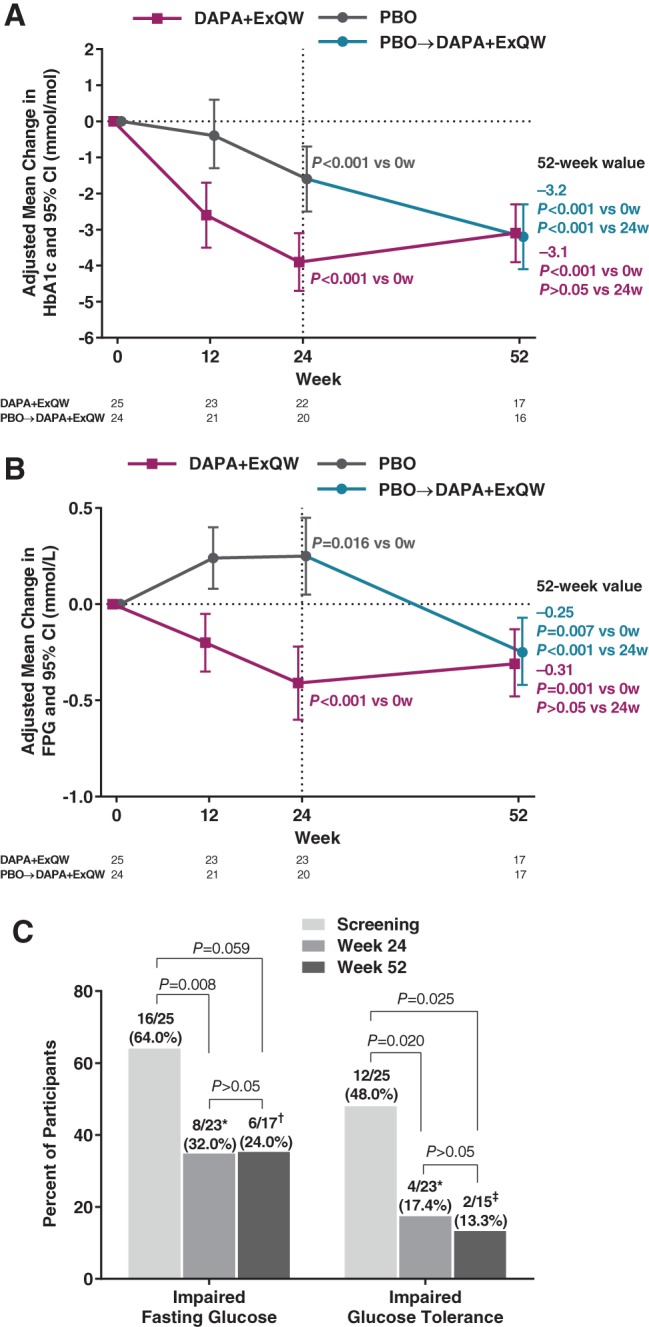
Changes in glycaemic endpoints and prediabetes. A, Adjusted mean change from week 0 in HbA1c (mmol/mol) over 24 and 52 weeks. B, Adjusted mean change from week 0 in FPG (mmol/L) after 24 and 52 weeks. C, Proportion of participants in the original DAPA + ExQW group with impaired fasting glucose or impaired glucose tolerance at screening and after 24 and 52 weeks. Analyses in panels A and B employed mixed models for repeated measures of change or percentage change from baseline adjusted for treatment, week, treatment‐by‐week, sex and baseline value. CI, confidence interval; DAPA + ExQW, dapagliflozin 10 mg once daily plus exenatide 2 mg once weekly; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; PBO, placebo; w, week(s). *Two participants for whom values were missing. †Eight participants for whom values were missing. ‡Ten participants for whom values were missing
As previously reported,20 OGTT‐derived measures showed that 73.5% of participants had evidence of abnormal glucose metabolism at baseline; these parameters improved during treatment with DAPA + ExQW but not with PBO. Among participants continuing on DAPA + ExQW, these reductions in 2‐hour PG (−1.9 mmol/L), IFG (64% → 34.8%), IGT (48% → 17.4%) and prediabetes (68% → 34.8%) at 24 weeks were sustained at 52 weeks (−2.2 mmol/L, 35.3%, 13.3% and 35.3%, respectively) (Table 1, Figure 3C).
3.2.5. Vital signs
In this predominantly normotensive population at baseline (mean baseline SBP, 134 mm Hg),20 participants continuing on DAPA + ExQW showed a significant reduction in SBP at 24 weeks (−9.8 mm Hg), which was sustained at 52 weeks (−12.0 mm Hg) (Table 1, Figure 2F). No changes in DBP were observed for either treatment group. Heart rate was unchanged in the continued DAPA + ExQW group, but increased in the PBO → DAPA + ExQW group from 0 to 52 weeks (Table 1).
3.2.6. Fasting serum lipids
No significant changes in high‐density lipoprotein cholesterol were observed in participants in either treatment group. Low‐density lipoprotein cholesterol decreased significantly from 0 to 52 weeks in both groups, and triglycerides decreased significantly from 0 to 52 weeks in the continued DAPA + ExQW group (Table 1).
3.3. Safety and tolerability
All participants in both groups reported at least 1 AE by week 52 (Table 2). In the continued DAPA + ExQW group, serious AEs occurred in 1 participant during the 0‐ to 24‐week period (injury) and in 2 participants during the 24‐ to 52‐week period (colon adenocarcinoma/gastrointestinal haemorrhage, angioedema; both led to discontinuation). In the PBO → DAPA + ExQW group, a serious AE occurred in 1 participant during the 0‐ to 24‐week period (dyspnoea/fatigue; this also led to discontinuation) and none thereafter. AEs leading to treatment discontinuation occurred in a further 2 participants in the continued DAPA + ExQW group during the 0‐ to 24‐week period (abdominal pain, pruritus) and in 2 participants during the 24‐ to 52‐week period (dizziness/nausea/fatigue, eye allergy). In the PBO → DAPA + ExQW group, a further 2 participants discontinued during the 0‐ to 24‐week period (vasculitis/skin ulcer, malaise) and none thereafter.
Table 2.
Overview of AEs and key laboratory changes occurring from 0 to 24, 24 to 52 and 0 to 52 weeks (safety analysis set)
| Weeks 0 to 24 | Weeks 24 to 52 | Weeks 0 to 52 | ||||
|---|---|---|---|---|---|---|
| DAPA + ExQW (n = 25) | PBO (n = 25) | DAPA + ExQW (n = 25) | PBO → DAPA + ExQW (n = 25) | DAPA + ExQW (n = 25) | PBO → DAPA + ExQW (n = 25) | |
| Any AE | 25 (100.0) | 25 (100.0) | 17 (68.0) | 16 (64.0) | 25 (100.0) | 25 (100.0) |
| Any serious AE a | 1 (4.0) | 1 (4.0) | 2 (8.0) | 0 (0.0) | 3 (12.0) | 1 (4.0) |
| Treatment‐related AEs | 5 (20.0) | 3 (12.0) | 0 (0.0) | 0 (0.0) | 5 (20.0) | 3 (12.0) |
| AEs leading to study discontinuation b | 2 (8.0) | 3 (12.0) | 4 (16.0) | 1 (4.0) | 6 (24.0) | 4 (16.0) |
| Deaths | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Most common AEs occurring in ≥20% in any group | ||||||
| Nasopharyngitis | 9 (36.0) | 4 (16.0) | 0 (0.0) | 0 (0.0) | 9 (36.0) | 4 (16.0) |
| Decreased appetite | 8 (32.0) | 3 (12.0) | 1 (4.0) | 1 (4.0) | 9 (36.0) | 3 (12.0) |
| Dizziness | 5 (20.0) | 3 (12.0) | 3 (12.0) | 1 (4.0) | 7 (28.0) | 4 (16.0) |
| Headache | 8 (32.0) | 4 (16.0) | 2 (8.0) | 1 (4.0) | 8 (32.0) | 4 (16.0) |
| Nausea | 7 (28.0) | 3 (12.0) | 5 (20.0) | 1 (4.0) | 9 (36.0) | 4 (16.0) |
| Pollakiuria | 5 (20.0) | 5 (20.0) | 1 (4.0) | 6 (24.0) | 6 (24.0) | 11 (44.0) |
| Fatigue | 3 (12.0) | 6 (24.0) | 3 (12.0) | 1 (4.0) | 4 (16.0) | 7 (28.0) |
| Injection‐site mass | 7 (28.0) | 5 (20.0) | 0 (0.0) | 1 (4.0) | 7 (28.0) | 6 (24.0) |
| Injection‐site pruritus | 7 (28.0) | 2 (8.0) | 0 (0.0) | 0 (0.0) | 7 (28.0) | 2 (8.0) |
| AEs of special interest | ||||||
| Urinary tract infectionc | 2 (8.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) | 2 (8.0) | 1 (4.0) |
| Acute pyelonephritis | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) | 0 (0.0) |
| Urinary tract infection | 0 (0.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) |
| Fungal urinary tract infection | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) | 0 (0.0) |
| Genital infectionc | 1 (4.0) | 0 (0.0) | 2 (8.0) | 1 (4.0) | 2 (8.0) | 1 (4.0) |
| Fungal | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) | 0 (0.0) | 1 (4.0) |
| Vaginal | 1 (4.0) | 0 (0.0) | 2 (8.0) | 0 (0.0) | 2 (8.0) | 0 (0.0) |
| Volume reductionc | 0 (0.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) |
| Hypotension | 0 (0.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) |
| Renal impairment/failurec | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal symptomsc | 16 (64.0) | 11 (44.0) | 6 (24.0) | 6 (24.0) | 17 (68.0) | 14 (56.0) |
| Nausea | 7 (28.0) | 3 (12.0) | 5 (20.0) | 1 (4.0) | 9 (36.0) | 4 (16.0) |
| Abdominal pain | 4 (16.0) | 2 (8.0) | 0 (0.0) | 2 (8.0) | 4 (16.0) | 4 (16.0) |
| Diarrhoea | 3 (12.0) | 3 (12.0) | 1 (4.0) | 1 (4.0) | 4 (16.0) | 4 (16.0) |
| Abdominal distension | 3 (12.0) | 2 (8.0) | 0 (0.0) | 1 (4.0) | 3 (12.0) | 2 (8.0) |
| Vomiting | 3 (12.0) | 1 (4.0) | 1 (4.0) | 1 (4.0) | 4 (16.0) | 2 (8.0) |
| Gastroesophageal reflux | 3 (12.0) | 1 (4.0) | 0 (0.0) | 1 (4.0) | 3 (12.0) | 2 (8.0) |
| Constipation | 2 (8.0) | 1 (4.0) | 1 (4.0) | 1 (4.0) | 3 (12.0) | 2 (8.0) |
| Dyspepsia | 2 (8.0) | 0 (0.0) | 1 (4.0) | 0 (0.0) | 3 (12.0) | 0 (0.0) |
| Injection‐site reactionsc | 11 (44.0) | 8 (32.0) | 0 (0.0) | 2 (8.0) | 11 (44.0) | 9 (36.0) |
| Injection‐site mass | 7 (28.0) | 5 (20.0) | 0 (0.0) | 1 (4.0) | 7 (28.0) | 6 (24.0) |
| Injection‐site pruritus | 7 (28.0) | 1 (4.0) | 0 (0.0) | 1 (4.0) | 7 (28.0) | 2 (8.0) |
| Injection‐site erythema | 3 (12.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) | 3 (12.0) | 1 (4.0) |
| Injection‐site nodule | 2 (8.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) | 2 (8.0) | 1 (4.0) |
| Injection‐site swelling | 0 (0.0) | 2 (8.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (8.0) |
| Injection‐site pain | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) | 0 (0.0) |
| Injection‐site cyst | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) | 0 (0.0) |
| Injection‐site rash | 0 (0.0) | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) |
| Appetite changes | 10 (40.0) | 5 (20.0) | 2 (8.0) | 2 (8.0) | 11 (44.0) | 5 (20.0) |
| Decreased appetite | 8 (32.0) | 3 (12.0) | 1 (4.0) | 1 (4.0) | 9 (36.0) | 3 (12.0) |
| Increased appetite | 1 (4.0) | 0 (0.0) | 1 (4.0) | 1 (4.0) | 2 (8.0) | 1 (4.0) |
| Hunger | 1 (4.0) | 3 (12.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) | 3 (12.0) |
| Key laboratory changes | ||||||
| eGFR,d mean change (95% CI), mL/min/1.73 m2 | −1.77 (−6.81, 3.27) | 2.95 (−1.20, 7.09) | −0.29 (−5.20, 4.62) | −1.91 (−6.33, 2.52) | 1.34 (−5.39, 8.07) | 0.19 (−3.15, 3.53) |
| Serum creatinine, mean change (95% CI), µmol/L | 1.61 (−1.67, 4.89) | −1.21 (−4.08, 1.66) | 0.38 (−3.47, 4.22) | 0.65 (−2.78, 4.07) | −0.25 (−5.27, 4.77) | −0.06 (−2.47, 2.35) |
| AST, mean change (95% CI), µkat/L | −0.06 (−0.12, 0.01) | −0.09 (−0.16, −0.03) | 0.01 (−0.03, 0.05) | −0.03 (−0.07, 0.01) | −0.04 (−0.10, 0.03) | −0.10 (−0.15, −0.05) |
| ALT, mean change (95% CI), µkat/L | −0.05 (−0.15, 0.06) | −0.13 (−0.31, 0.05) | −0.01 (−0.09, 0.07) | −0.08 (−0.17, 0.02) | −0.05 (−0.14, 0.03) | −0.14 (−0.26, −0.03) |
| Haemoglobin, mean change (95% CI), g/L | 3.4 (−1.9, 8.7) | −1.8 (−5.2, 1.6) | −1.8 (−5.7, 2.1) | 8.3 (5.0, 11.6) | 0.3 (−5.7, 6.3) | 7.8 (3.5, 12.1) |
| hs‐CRP, mean change (95% CI), mg/dL | 0.09 (−0.87, 1.05) | −0.81 (−1.86, 0.24) | 2.13 (−1.41, 5.67) | −0.13 (−0.83, 0.58) | 1.90 (−1.71, 5.51) | −0.75 (−1.74, 0.25) |
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CI, confidence interval; DAPA + ExQW, dapagliflozin 10 mg once daily plus exenatide 2 mg once weekly; eGFR, estimated glomerular filtration rate; hs‐CRP; high‐sensitivity C‐reactive protein; PBO, placebo.
Serious AEs in the DAPA + ExQW group over 52 weeks were hospitalization because of head trauma/injury, colon adenocarcinoma/gastrointestinal haemorrhage or angioedema.
AEs that led to treatment discontinuation in the DAPA + ExQW group over 52 weeks were abdominal pain, injection‐site pruritus/mass, nausea, dizziness, fatigue and eye allergy.
AEs of urinary tract infections, genital infections, volume reduction, renal impairment/failure, gastrointestinal symptoms, and injection‐site reactions were coded using predefined lists of preferred terms (Medical Dictionary for Regulatory Activities [MedDRA] version 18.0).
Assessed using the Modification of Diet in Renal Disease formula.
Genital infections and UTIs were rare during the 0‐ to 24‐week period, with 2 and 1 participant(s) in the DAPA + ExQW and PBO → DAPA + ExQW groups, respectively, reporting new episodes of genital infections during the 24‐ to 52‐week period. No new UTIs occurred during the 24‐ to 52‐week period. In the continuing DAPA + ExQW group, gastrointestinal symptoms and injection‐site disorders were reported less frequently during the 24‐ to 52‐week period than during the 0‐ to 24‐week period (Table 2).
No participant experienced confirmed hypoglycaemia. One placebo‐treated participant was diagnosed with T2D at the 24‐week visit (according to American Diabetes Association PG and HbA1c criteria).20
No participants receiving DAPA + ExQW experienced AEs of hypotension. No participants in either treatment group reported AEs potentially related to renal impairment or renal failure over 52 weeks, and estimated glomerular filtration rate did not change significantly over time (Table 2). No other clinically significant changes in laboratory assessments were observed in either treatment group over 52 weeks, apart from a small increase in haemoglobin at 52 weeks in the PBO → DAPA + ExQW group (Table 2). No AEs of pancreatitis or pancreatic cancer were reported.
4. DISCUSSION
Achieving long‐term body weight loss to reduce cardiometabolic risk through lifestyle interventions alone is often immensely challenging. A high degree of motivation is required to achieve both initial body weight loss and, especially, to sustain this loss in the face of opposing influences, including physiological counter‐regulatory mechanisms seeking to regain body weight,6, 22 chronic hunger/food craving,23 and ongoing sociocultural pressures (eg, sedentary occupations) that impair consolidation of healthy lifestyles.1 Given the unmet need for novel pharmacotherapies to achieve and sustain body weight loss, and their established efficacy and safety in patients with T2D, the combination of an SGLT2 inhibitor (urinary caloric loss) and a GLP‐1RA (appetite suppression) may be an attractive option to assist obese individuals in reducing body weight as either standalone therapy (eg, when individuals must lose sufficient body weight to initiate an exercise programme)24 or an adjunct to lifestyle intervention.
In this study of obese adults without diabetes who did not undergo a formal lifestyle intervention, a mean body weight loss of −4.5 kg after 24 weeks of double‐blind treatment with DAPA + ExQW was sustained during an additional 28 weeks of open‐label DAPA + ExQW, resulting in a total body weight loss of −5.7 kg, with 40.0% of participants achieving ≥5% reduction in initial body weight at 52 weeks. In addition, participants who switched from PBO to active treatment achieved reductions at 52 weeks similar to those achieved in the continuing DAPA + ExQW group at 24 weeks. MRI assessments indicated that the body weight reduction was largely accounted for by reduction in adipose tissue volume, which similarly involved subcutaneous and visceral depots.
Although making cross‐study comparisons may be inaccurate because of differing populations and study designs, the degree of body weight loss with DAPA + ExQW appeared to be comparable to that achieved at 1 year in obese individuals receiving approved pharmacotherapies for obesity. In a recent meta‐analysis, differences vs placebo in mean changes in body weight at 1 year were −2.63, −3.25, −4.95, −5.24 and −8.80 kg with orlistat, lorcaserin, naltrexone/bupropion, liraglutide and phentermine/topiramate, respectively, and differences vs placebo in proportions achieving ≥5% body weight loss at 1 year were 23.0%, 24.4%, 32.1%, 36.3% and 49.3%, respectively.25 In the current study, placebo‐corrected changes in body weight and the proportion achieving body weight loss ≥5% at 24 weeks were −4.5 kg and 39.1%, respectively. Assuming that values at 52 weeks would be similar to those at 24 weeks had there been a continuing placebo arm, estimated placebo‐corrected changes at 52 weeks and the proportion achieving body weight loss ≥5% would be −5.3 kg and 40.0% (ITT percentage), respectively.
However, the current study did not employ a formal lifestyle intervention in contrast to the studies included in the meta‐analysis by Khera et al25 all of which employed a diet and/or exercise co‐intervention. In addition, dose‐ranging studies with dapagliflozin, indicating that urinary glucose excretion increases at higher doses of dapagliflozin,26 and dose‐ranging studies of liraglutide,27 suggesting greater body weight loss with higher doses of a GLP‐1RA, suggest that increased doses of dapagliflozin and exenatide QW in combination could potentially achieve more substantial body weight loss. Thus, the full potential for weight reduction with this combination therapy is probably greater than described here, and needs to be determined in dose‐ranging studies that optimize the balance between efficacy and safety and in studies that include intensive lifestyle interventions.
DAPA + ExQW improved multiple cardiometabolic risk factors as well as reduced body weight. Reductions in glycaemic measures and SBP at 24 weeks were sustained at 52 weeks; fasting low‐density lipoprotein cholesterol and triglycerides were also reduced by 52 weeks. The reductions in glycaemic measures were similar to, and reductions in SBP were greater than, those achieved with liraglutide at the higher dose approved for obesity (3 mg once daily)28, 29 and compared favourably with other approved therapies for obesity evaluated in participants without diabetes. Thus, reductions in HbA1c (−3.1 mmol/mol [−0.28%]), FPG (−0.31 mmol/L) and SBP (−12.0 mm Hg) at 52 weeks with DAPA + ExQW were greater than average reductions across 8 clinical trials evaluating orlistat 120 mg (−0.4 mmol/mol [−0.04%], −0.14 mmol/L and −4.3 mm Hg, respectively)28, 30, 31, 32, 33, 34, 35, 36; across 2 clinical trials evaluating lorcaserin 10 mg twice daily (–1.3 mmol/mol [−0.12%], −0.04 mmol/L and −1.7 mm Hg, respectively)37, 38; across 3 clinical trials evaluating naltrexone/bupropion 32/360 mg in divided doses twice daily (not reported, −0.16 mmol/L and −0.3 mm Hg, respectively)39, 40, 41; and in 1 clinical trial evaluating controlled‐release phentermine/topiramate 15/92 mg once daily (not reported, −0.03 mmol/L and −2.9 mm Hg, respectively).42
Both the SGLT2 inhibitor empagliflozin43, 44 and the GLP1‐RAs liraglutide and semaglutide45, 46 have recently been shown to substantially reduce fatal or nonfatal cardiovascular events in patients with T2D at high cardiovascular risk. In addition to cardiovascular meta‐analyses for dapagliflozin47 and exenatide,48 dedicated outcome trials are ongoing for both of these compounds (DECLARE TIMI‐58 [NCT01730534] and EXSCEL,49 respectively).
The mechanisms leading to cardiovascular risk reduction are probably derived through a number of non‐glycaemic effects for both SGLT2 inhibitors50, 51, 52 and GLP‐1RAs.53, 54 In the current study among obese individuals without T2D, effects on SBP and body weight were similar to those with DAPA + ExQW among patients with T2D.55 However, it is unknown whether such effects will translate into reductions in risk for future cardiovascular disease events in obese individuals without T2D or concurrent cardiovascular disease.
Pharmacological therapies for body weight loss have been associated historically with significant safety issues, leading to withdrawal of a number of agents.7 However, for these therapies to be accepted, they must possess a good safety profile when administered over an extended time period with the aim of reducing body weight and associated long‐term cardiometabolic risk. Even among currently approved treatments, careful patient selection is required to ensure safe use.20
The separate safety profiles of dapagliflozin and exenatide have been evaluated in long‐term clinical trials in patients with T2D for up to 4 years56 and 6 years,18 respectively. The principal AEs with dapagliflozin (UTIs and genital infections) and exenatide (gastrointestinal symptoms and injection‐site reactions) tend to diminish with longer‐term exposure.57, 58 No unexpected AEs occurred with DAPA + ExQW dual therapy over 52 weeks of follow‐up in this study. Discontinuations because of AEs with DAPA + ExQW (24.0% at 52 weeks) in the current study were comparable to previous trials with various weight loss drugs.25 Moreover, in patients with T2D, the frequency of AEs was not overrepresented with DAPA + ExQW over 28 weeks compared with each agent administered alone.55
The current study has limitations. It was a small, single‐centre study without monotherapy comparator groups, making the effect size of each individual component difficult to assess. Placebo‐treated participants with more body weight loss during 0 to 24 weeks showed a higher retention rate when entering the open‐label extension, indicating selection bias. However, our primary ITT analysis used MMRM, minimizing bias in delayed‐start clinical trials.21
In this study, in which diet and exercise modification was not mandated or documented, dual therapy with DAPA + ExQW reduced and maintained body weight loss at approximately 5 kg after 1 year. This is similar to the placebo‐corrected effect of currently approved pharmacotherapies for obesity, whereas we found a greater effect of DAPA + ExQW on reduction in glycaemic parameters, prediabetes prevalence and SBP. This suggests a potential role for prevention of T2D and cardiovascular disease in this population. No unexpected safety and tolerability issues were observed with the combination of DAPA + ExQW, and rates of discontinuation because of AEs and study attrition were comparable to those with approved pharmacotherapies for obesity. Further studies evaluating long‐term effects on body weight, glucose metabolism and cardiovascular risk with DAPA + ExQW in conjunction with formal diet and exercise interventions are warranted.
Supporting information
File S1.
Table S1. Demographic and baseline characteristics.
Figure S1. Sensitivity analyses: A, mean change in bodyweight among patients either entering or not entering the open‐label extension period; B, adjusted mean change in bodyweight among patients entering the extension period; and C, adjusted mean change in bodyweight among patients adhering to the protocol throughout the study. CI, confidence interval; DAPA + ExQW, dapagliflozin 10 mg once daily plus exenatide 2 mg once weekly; PBO, placebo.
Figure S2. Change over time in liver fat percent units, defined as liver fat × 100 ÷ (liver fat + liver water), for: A, adjusted mean changes from baseline; B, individual trajectories of change among DAPA + ExQW‐treated participants; and C, individual trajectories of change among PBO‐treated participants. Data for DAPA + ExQW‐treated participants were available at baseline, 24, and 52 weeks; for PBO‐treated patients, data were available only at baseline and at 24 weeks. CI, confidence interval; DAPA + ExQW, dapagliflozin 10 mg once daily plus exenatide 2 mg once weekly; PBO, placebo.
ACKNOWLEDGEMENTS
Lisa Baker and Julian Martins of inScience Communications, Springer Healthcare, and Amy Zannikos, on behalf of inScience Communications, Springer Healthcare, provided medical writing support that was funded by AstraZeneca. Mary Beth DeYoung and Elise Hardy of AstraZeneca critically reviewed the manuscript.
This study was published previously, in part, in abstract form: Eriksson JW, Lundkvist P, Sjöström CD, et al. One year of treatment with dapagliflozin QD + exenatide QW in obese adults without diabetes: results of an open label extension study. Presented at the European Association for the Study of Diabetes Annual Meeting; September 12 to 16, 2016, Munich, Germany.
Conflict of interest
P. L. has received an honorarium from Merck Sharp & Dohme and a travel grant from AstraZeneca. M. J. P. and P. K. have no conflicts of interest to declare. C. D. S. and E. J. are employees of and own stock in AstraZeneca. J. W. E. has received research grants or honoraria from AstraZeneca, Bristol‐Myers Squibb, Merck Sharp & Dohme, Novo Nordisk and Sanofi.
Author contributions
P. L. contributed to the conduct of the study, interpreting and discussing results, writing the manuscript and critically revising all subsequent versions. M. J. P. contributed to the design and conduct of the study, interpreting and discussing results, writing the manuscript and critically revising all subsequent versions. P. K. contributed to the conduct of the study and to critically revising all versions of the manuscript. C. D. S. and E. J. contributed to interpreting and discussing results, writing the manuscript and critically revising all subsequent versions. J. W. E. designed the study, contributed to the conduct of the study, interpreting and discussing results, writing the manuscript and critically revising all versions of the manuscript.
Lundkvist P, Pereira MJ, Katsogiannos P, Sjöström CD, Johnsson E and Eriksson JW. Dapagliflozin once daily plus exenatide once weekly in obese adults without diabetes: Sustained reductions in body weight, glycaemia and blood pressure over 1 year. Diabetes Obes Metab. 2017;19:1276–1288. https://doi.org/10.1111/dom.12954
Funding Information This study was supported by AstraZeneca.
REFERENCES
- 1. World Health Organization . Obesity and overweight: fact sheet no. 311. 2016. http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed February 2, 2016.
- 2. International Diabetes Federation . IDF Diabetes Altas. 7th ed; 2015. http://www.diabetesatlas.org/. Accessed February 15, 2016. [Google Scholar]
- 3. Diabetes Prevention Program Research Group . Long‐term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15‐year follow‐up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3:866‐875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johns DJ, Hartmann‐Boyce J, Jebb SA, Aveyard P. Diet or exercise interventions vs combined behavioral weight management programs: a systematic review and meta‐analysis of direct comparisons. J Acad Nutr Diet. 2014;114:1557‐1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hartmann‐Boyce J, Johns DJ, Jebb SA, Summerbell C, Aveyard P. Behavioural weight management programmes for adults assessed by trials conducted in everyday contexts: systematic review and meta‐analysis. Obes Rev. 2014;15:920‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhurandhar EJ, Kaiser KA, Dawson JA, Alcorn AS, Keating KD, Allison DB. Predicting adult weight change in the real world: a systematic review and meta‐analysis accounting for compensatory changes in energy intake or expenditure. Int J Obes. 2015;39:1181‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krentz AJ, Fujioka K, Hompesch M. Evolution of pharmacological obesity treatments: focus on adverse side‐effect profiles. Diabetes Obes Metab. 2016;18:558‐570. [DOI] [PubMed] [Google Scholar]
- 8. Halpern B, Oliveira ES, Faria AM, et al. Combinations of drugs in the treatment of obesity. Pharmaceuticals. 2010;3:2398‐2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bays HE, Weinstein R, Law G, Canovatchel W. Canagliflozin: effects in overweight and obese subjects without diabetes mellitus. Obesity. 2014;22:1042‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang F, Tong Y, Su N, et al. Weight loss effect of glucagon‐like peptide‐1 mimetics on obese/overweight adults without diabetes: a systematic review and meta‐analysis of randomized controlled trials. J Diabetes. 2015;7:329‐339. [DOI] [PubMed] [Google Scholar]
- 11. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy balance after sodium‐glucose cotransporter 2 inhibition. Diabetes Care. 2015;38:1730‐1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon‐like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 2014;221:T1‐T16. [DOI] [PubMed] [Google Scholar]
- 14. Bailey CJ, Morales Villegas EC, Woo V, Tang W, Ptaszynska A, List JF. Efficacy and safety of dapagliflozin monotherapy in people with type 2 diabetes: a randomized double‐blind placebo‐controlled 102‐week trial. Diabet Med. 2015;32:531‐541. [DOI] [PubMed] [Google Scholar]
- 15. Bolinder J, Ljunggren O, Johansson L, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014;16:159‐169. [DOI] [PubMed] [Google Scholar]
- 16. Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16:124‐136. [DOI] [PubMed] [Google Scholar]
- 17. Diamant M, Van Gaal L, Guerci B, et al. Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION‐3): 3‐year results of an open‐label randomised trial. Lancet Diabetes Endocrinol. 2014;2:464‐473. [DOI] [PubMed] [Google Scholar]
- 18. Henry RR, Klein EJ, Han J, Iqbal N. Efficacy and tolerability of exenatide once weekly over 6 years in patients with type 2 diabetes: an uncontrolled open‐label extension of the DURATION‐1 study. Diabetes Technol Ther. 2016;18:677‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wysham CH, MacConell LA, Maggs DG, Zhou M, Griffin PS, Trautmann ME. Five‐year efficacy and safety data of exenatide once weekly: long‐term results from the DURATION‐1 randomized clinical trial. Mayo Clin Proc. 2015;90:356‐365. [DOI] [PubMed] [Google Scholar]
- 20. Lundkvist P, Sjöström CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin once‐daily and exenatide once‐weekly dual therapy: a 24‐week randomized, placebo‐controlled, phase II study examining effects on body weight and prediabetes in obese adults without diabetes. Diabetes Obes Metab. 2017;19:49‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu‐Seifert H, Andersen SW, Lipkovich I, Holdridge KC, Siemers E. A novel approach to delayed‐start analyses for demonstrating disease‐modifying effects in Alzheimer's disease. PLoS ONE. 2015;10:e0119632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621‐628. [DOI] [PubMed] [Google Scholar]
- 23. Boswell RG, Kober H. Food cue reactivity and craving predict eating and weight gain: a meta‐analytic review. Obes Rev. 2016;17:159‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342‐362. [DOI] [PubMed] [Google Scholar]
- 25. Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta‐analysis. JAMA. 2016;315:2424‐2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kasichayanula S, Liu X, Lacreta F, Griffen SC, Boulton DW. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium‐glucose co‐transporter type 2. Clin Pharmacokinet. 2014;53:17‐27. [DOI] [PubMed] [Google Scholar]
- 27. Astrup A, Rossner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double‐blind, placebo‐controlled study. Lancet. 2009;374:1606‐1616. [DOI] [PubMed] [Google Scholar]
- 28. Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once‐daily human GLP‐1 analog, liraglutide. Int J Obes. 2012;36:843‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pi‐Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11‐22. [DOI] [PubMed] [Google Scholar]
- 30. Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155‐161. [DOI] [PubMed] [Google Scholar]
- 31. Krempf M, Louvet JP, Allanic H, Miloradovich T, Joubert JM, Attali JR. Weight reduction and long‐term maintenance after 18 months treatment with orlistat for obesity. Int J Obes Relat Metab Disord. 2003;27:591‐597. [DOI] [PubMed] [Google Scholar]
- 32. Broom I, Wilding J, Stott P, Myers N. Randomised trial of the effect of orlistat on body weight and cardiovascular disease risk profile in obese patients: UK Multimorbidity Study. Int J Clin Pract. 2002;56:494‐499. [PubMed] [Google Scholar]
- 33. Rossner S, Sjöström L, Noack R, Meinders AE, Noseda G. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. European Orlistat Obesity Study Group. Obes Res. 2000;8:49‐61. [DOI] [PubMed] [Google Scholar]
- 34. Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long‐term treatment of obesity in primary care settings. Arch Fam Med. 2000;9:160‐167. [DOI] [PubMed] [Google Scholar]
- 35. Davidson MH, Hauptman J, DiGirolamo M, et al. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999;281:235‐242. [DOI] [PubMed] [Google Scholar]
- 36. Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo‐controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352:167‐172. [DOI] [PubMed] [Google Scholar]
- 37. Fidler MC, Sanchez M, Raether B, et al. A one‐year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067‐3077. [DOI] [PubMed] [Google Scholar]
- 38. Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo‐controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245‐256. [DOI] [PubMed] [Google Scholar]
- 39. Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity‐related risk factors (COR‐II). Obesity. 2013;21:935‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR‐BMOD trial. Obesity. 2011;19:110‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR‐I): a multicentre, randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2010;376:595‐605. [DOI] [PubMed] [Google Scholar]
- 42. Allison DB, Gadde KM, Garvey WT, et al. Controlled‐release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity. 2012;20:330‐342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 44. Wu JH, Foote C, Blomster J, et al. Effects of sodium‐glucose cotransporter‐2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2016;4:411‐419. [DOI] [PubMed] [Google Scholar]
- 45. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 47. Sonesson C, Johansson PA, Johnsson E, Gause‐Nilsson I. Cardiovascular effects of dapagliflozin in patients with type 2 diabetes and different risk categories: a meta‐analysis. Cardiovasc Diabetol. 2016;15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Monami M, Dicembrini I, Nardini C, Fiordelli I, Mannucci E. Effects of glucagon‐like peptide‐1 receptor agonists on cardiovascular risk: a meta‐analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16:38‐47. [DOI] [PubMed] [Google Scholar]
- 49. Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103‐110. [DOI] [PubMed] [Google Scholar]
- 50. Rajasekeran H, Lytvyn Y, Cherney DZ. Sodium‐glucose cotransporter 2 inhibition and cardiovascular risk reduction in patients with type 2 diabetes: the emerging role of natriuresis. Kidney Int. 2016;89:524‐526. [DOI] [PubMed] [Google Scholar]
- 51. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA‐REG OUTCOME Study? A unifying hypothesis. Diabetes Care. 2016;39:1115‐1122. [DOI] [PubMed] [Google Scholar]
- 52. Gilbert RE. SGLT2 inhibitors: beta blockers for the kidney? Lancet Diabetes Endocrinol. 2016;4:814. [DOI] [PubMed] [Google Scholar]
- 53. Irace C, De Luca S, Shehaj E, et al. Exenatide improves endothelial function assessed by flow mediated dilation technique in subjects with type 2 diabetes: results from an observational research. Diab Vasc Dis Res. 2013;10:72‐77. [DOI] [PubMed] [Google Scholar]
- 54. Mendis B, Simpson E, MacDonald I, Mansell P. Investigation of the haemodynamic effects of exenatide in healthy male subjects. Br J Clin Pharmacol. 2012;74:437‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION‐8): a 28 week, multicentre, double‐blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:1004‐1016. [DOI] [PubMed] [Google Scholar]
- 56. Del Prato S, Nauck M, Durán‐Garcia S, et al. Long‐term glycaemic response and tolerability of dapagliflozin versus a sulphonylurea as add‐on therapy to metformin in patients with type 2 diabetes: 4‐year data. Diabetes Obes Metab. 2015;17:581‐590. [DOI] [PubMed] [Google Scholar]
- 57. Ptaszynska A, Johnsson KM, Parikh SJ, de Bruin TW, Apanovitch AM, List JF. Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events. Drug Saf. 2014;37:815‐829. [DOI] [PubMed] [Google Scholar]
- 58. Horowitz M, Aroda VR, Han J, Hardy E, Rayner C. Upper and/or lower GI adverse events with long‐ vs short‐acting GLP‐1 receptor agonists: incidence, co‐incidence, effects on HbA1c and weight [abstract 15]. Diabetologia. 2015;58 (suppl):S7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1.
Table S1. Demographic and baseline characteristics.
Figure S1. Sensitivity analyses: A, mean change in bodyweight among patients either entering or not entering the open‐label extension period; B, adjusted mean change in bodyweight among patients entering the extension period; and C, adjusted mean change in bodyweight among patients adhering to the protocol throughout the study. CI, confidence interval; DAPA + ExQW, dapagliflozin 10 mg once daily plus exenatide 2 mg once weekly; PBO, placebo.
Figure S2. Change over time in liver fat percent units, defined as liver fat × 100 ÷ (liver fat + liver water), for: A, adjusted mean changes from baseline; B, individual trajectories of change among DAPA + ExQW‐treated participants; and C, individual trajectories of change among PBO‐treated participants. Data for DAPA + ExQW‐treated participants were available at baseline, 24, and 52 weeks; for PBO‐treated patients, data were available only at baseline and at 24 weeks. CI, confidence interval; DAPA + ExQW, dapagliflozin 10 mg once daily plus exenatide 2 mg once weekly; PBO, placebo.


