Abstract
Aim
Adhesion after pelvic surgery causes infertility, ectopic pregnancy, and ileus or abdominal pain. The materials currently available for clinical use are insufficient. The purpose of this study was to develop an anti‐adhesive material that overcomes the limitations of conventional anti‐adhesive agents.
Methods
The adhesion prevention effects of three methods – a two‐layered sheet composed of gelatin film and gelatin sponge, Seprafilm and INTERCEED – were evaluated in 37 dogs. Anti‐adhesive effects were investigated macroscopically and microscopically in a cauterized uterus adhesion model. Cell growth on the materials in vitro using human peritoneal mesothelial cells, fibroblasts and uterine smooth muscle cells were also evaluated.
Results
The two‐layered gelatin sheet had significantly superior anti‐adhesive effects compared to the conventional materials (Seprafilm and INTERCEED). A single‐cell layer of mature mesothelium formed three weeks after surgery in the gelatin group. Peritoneum regeneration in the Seprafilm and INTERCEED groups was delayed and incomplete in the early phase. Little inflammation around the materials occurred and cell growth was significantly proliferated with the gelatin sheet.
Conclusion
The anti‐adhesive effects of a two‐layered gelatin sheet were superior to conventional agents in a cauterized canine uterus model, demonstrating early regeneration of the peritoneum, little inflammation and material endurance. The newly developed two‐layered gelatin sheet is a useful option as an anti‐adhesive agent for deeply injured and hemorrhagic sites.
Keywords: adhesion, animal experiment, anti‐adhesive agent, dog, two‐layered gelatin sheet
Introduction
Peritoneal adhesions are a serious problem and may develop after pelvic or abdominal surgery. Abdominal surgery induces adhesions at around 90% probability.1, 2, 3 Bowel obstruction is the most common complication resulting from abdominal adhesion.4 Adhesions may involve female reproductive organs leading to infertility, ectopic pregnancy and abdominal pain.1, 2, 3, 5 The prevention of adhesions remains an important issue in abdominal and pelvic surgery. Various kinds of anti‐adhesive agents have been proposed to prevent adhesions after surgery, but controversy over their effectiveness, usability and indication of available agents exists.1, 2
Hyaluronic acid carboxymethyl cellulose membrane (Seprafilm) and oxidized regenerated cellulose (INTERCEED) are now frequently used in gynecological surgery; however, problems remain with the use of these conventional anti‐adhesive agents. Seprafilm has several strengths when used under the proper conditions.6 Studies have shown that in humans, Seprafilm reduces the severity of adhesions but it does not reduce incidence and has no effect on the prevention of pelvic adhesions in women.7, 8
INTERCEED has been reported as having superior efficacy to Seprafilm in pelvic surgery, but also has some limitations.8 Blood infiltration renders the product completely ineffective for preventing adhesions.1, 9, 10 INTERCEED provokes a significant leucocyte response and an inflammatory response may enhance adhesion.11 To avoid the limitations of the conventional anti‐adhesive materials, we devised a new anti‐adhesive agent, a two‐layered gelatin sheet composed of thermally cross‐linked gelatin film and sponge. The two‐layered gelatin sheet was evaluated for its anti‐adhesive effects after pelvic surgery in dogs.
Methods
Animal protocol
The Nara Medical University Experimentation Committee and the Doshisha University Animal Experimentation Committee approved the animal experiments in this study. All surgical procedures and anesthesia were performed in accordance with the Animal Care Guidelines of Nara Medical University. Thirty seven female beagles (two years old, weighing 9.9 ± 0.9 [mean ± standard deviation {SD}] kg) were purchased from Shimizu Laboratory Animal Supply Co. Ltd. (Kyoto, Japan). Before the study commenced, the dogs were housed in the laboratory at least for a week. During the experimental period, all dogs were housed separately and maintained under standard conditions with a light–dark cycle of 12:12 h, mean temperature of 23°C and mean humidity of 50%. Standard laboratory dog food and water were freely available. On the day of the experiment, all dogs underwent a health check.
Preparation of anti‐adhesive agents
Two‐layered gelatin sheet
Low endotoxin gelatin extracted from porcine skins (type‐I collagen, Medigelatin, Nippi Co. Ltd. Tokyo, Japan) was dissolved in distilled water into concentrations of 1.0 and 4.8 wt%. The 4.8 wt% gelatin solution was cast onto a polystyrene Petri dish (not treated for tissue‐culture; Corning Inc., NY, USA) and dried at room temperature overnight. Using a non‐treated surface can ensure better detachment of the gelatin sheet. The gelatin film was processed and then cross‐linked by ultraviolet light for 2 min. The 1.0 wt% gelatin solution was cast onto gelatin film in a Petri dish and frozen (MDF‐U538, SANYO, Osaka, Japan) at −80°C for 30 min. It was then freeze‐dried for 24 h in a vacuum freeze‐dryer (DRZ350WA, Advantec, Tokyo, Japan) after which the 1.0 wt% gelatin solution turned into sponge, over the gelatin film.
The two‐layered gelatin sheet composed of the gelatin film (10 μm thickness) and a sponge layer (1 mm thickness) was taken from the Petri dish and cross‐linked dehydrothermally in a vacuum oven (DP41, Yamato Scientific Co., Ltd., Tokyo Japan) at 140°C for 3 h. The sheet was sterilized using ethylene‐oxide gas (0.43 g/L at 40°C for 4 h) for animal experiments.
Seprafilm
Seprafilm (Genzyme Co., Cambridge, MA, USA), widely used as an anti‐adhesive material, is composed of two anionic polysaccharides, sodium hyaluronic acid (HA) and carboxymethyl‐cellulose (CMC).
INTERCEED
INTERCEED (TC7) (Ethicon, Irvine, CA, USA), made of oxidized regenerated cellulose, is commonly used in gynecological surgery. INTERCEED is an absorbable knitted fabric with a mesh structure.
Surgical procedure
A team of two gynecologists and two surgeons blinded to the experimental assignment performed all procedures. All surgeries were performed under sterile conditions. Under intravenous Pentobarbital anesthesia (40 mg/kg of body weight) was administered intravenously and a 5 cm midline incision was made at the lower abdomen, which revealed normal uterus, ovaries and fallopian tubes. An electric scalpel (Hyfrecator2000, CONMED, Utica, NY, USA) was used to cauterize one side of the uterine horns in a longitudinal direction 40 mm long and over the outer whole circumferential periphery, reaching the muscle layer. Hemostasis was confirmed after astriction.
Twenty eight dogs were randomly divided into four groups: (i) a control, not treated with any anti‐adhesive material, (ii) a two‐layered gelatin sheet group, (iii) a Seprafilm group, and (iv) an INTERCEED group. Besides the evaluation of adhesions nine dogs were taken the same treatment at both sides of the uterine horns only for histological evaluation. Each anti‐adhesive agent was applied onto the cauterized site of the uterine horn (two‐layered gelatin sheet, Seprafilm, or INTERCEED, 50 × 75 mm in size), wrapping the entire site without suturing. The sponge side of the two‐layered gelatin sheet was placed onto the traumatized tissue. After these procedures the wound was closed by layers. All dogs were bred under standard conditions and sacrificed humanely by intravenous pentobarbital (100 mg/kg of body weight) at three or six weeks after surgery.
Macroscopic and microscopic evaluation of adhesion was performed on the 28 dogs sacrificed on day 42, while nine dogs (three dogs in each treated group) were subject only to microscopic evaluation of adhesion three weeks after surgery.
Evaluation of adhesion
During autopsy, adhesion of the cauterized site of the uterus to the other abdominal organs was macroscopically evaluated via a full abdominal midline incision. A score of 1–4 regarding the extent or severity of the adhesion, modified from the Adhesion Score of the Surgical Membrane Study Group, was calculated (Table 1).12 Two doctors (one gynecologist and one surgeon), blinded to the animal and treatment assignments, performed the evaluation.
Table 1.
Adhesion score
| Category and description | Score |
|---|---|
| Extent of site involvement | |
| None | 0 |
| ≤ 25% | 1 |
| ≤ 50% | 2 |
| ≤ 75% | 3 |
| ≤ 100% | 4 |
| Severity | |
| No adhesion | 0 |
| Adhesion falls apart | 1 |
| Adhesion lysed with traction | 2 |
| Adhesion required ≤ 50% sharp dissection | 3 |
| Adhesion required > 50% sharp dissection | 4 |
Adhesion was measured using a score of 0–4 for extent or severity.
Histological analyses
The cauterized sites of the uterus were microscopically analyzed. These specimens were fixed with 10% buffered formalin, processed for paraffin embedding and stained with hematoxylin and eosin. The examiners were blinded to the materials in the histological evaluation.
Cell growth
Preparation of materials
The two layered gelatin sheets, the Seprafilm, and the INTERCEED were cut into 15 mm diameter circles. The two‐layered gelatin sheets were placed film side up (Gelatin film group of the Figure 4) and sponge side up (Gelatin sponge group of Figure 4). In order to ensure that the materials completely covered the bottom of the wells, two sheets of INTERCEED were stacked to make stitches alternative. The wells in the non‐treated group were not covered with any material.
Figure 4.
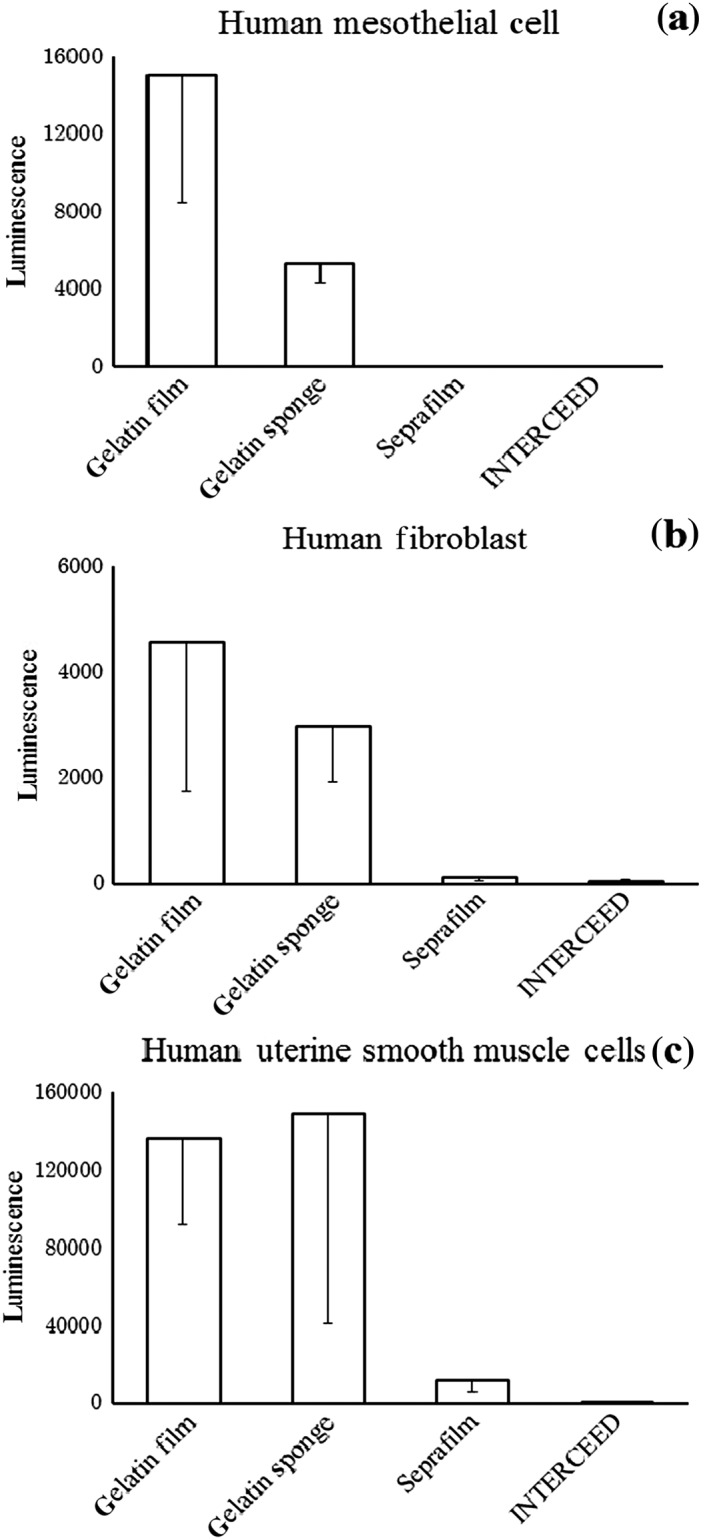
Cell growth on the materials (1 week): (a) human mesothelial cells, (b) humane fibroblasts and (c) human uterine smooth muscle cells. Significantly richer cell growth was observed in the gelatin film and sponge groups, which corresponded to the both surfaces of the two‐layered gelatin sheet, (P < 0.01) than in the Seprafilm or INTERCEED groups.
Cell lines
Human mesothelial cells (MSE‐F, Zen‐Bio Inc., Research Triangle Park, NC, USA) were cultured in mesothelial cell growth medium (MSO‐1, Zen‐Bio). Normal human dermal fibroblasts (NHDF‐AD‐Der fibroblasts FGM‐2, Lonza Japan, Tokyo, Japan) were maintained in Dulbecco's modified Eagle medium (DMEM, Wako Pure Chemical Industries, Ltd., Osaka, Japan) containing 10% fetal bovine serum. Human uterine smooth muscle cells (HUt‐SMC) (PromoCell, Heidelberg, Germany) were maintained in the medium suggested by the vendor with 5% fetal bovine serum, 0.5% epidermal growth factor, basic fibroblast growth factor and insulin (maintenance medium). The cells were cultivated in an incubator at 37°C with 5% CO2 under humid conditions for seven days. Twenty four‐well ultra‐low attachment surface plates for the materials (Corning) and 24‐well culture plates without any coating for the control (Becton, Dickinson and Company Ltd. Franklin Lakes, NJ, USA) were used. The cultured cells were harvested in the form of a single cell suspension. The cell suspension containing 1.0 × 104 cells/well was poured into the wells. After seven days of cultivation, the viable cells in each well were counted using an ATPlite Assay Kit (Perkin Elmer, Inc. Waltham, MA, USA).
Statistical analyses
Anti‐adhesive effects were analyzed using Kruskal–Wallis H and Mann–Whitney U tests with Bonferroni correction. One‐way analysis of variance and Tukey tests were used to analyze cell growth. A P value of < 0.05 was determined to be statistically significant.
Results
Evaluation of the adhesion
Adhesion scores at six weeks after surgery are shown in Figure 1. With regard to the extent of adhesion, the scores (means ± SD, n = 7 in each group) were 3.6 ± 1.0 in the non‐treated, 0.3 ± 0.7 in the gelatin sheet, 2.9 ± 1.4 in the Seprafilm and 2.7 ± 1.3 in the INTERCEED group. The score in the gelatin sheet group was significantly (P < 0.05) lower than the score in the non‐treated group. There were no significant differences in the extent of adhesion between the three treatment groups. The Seprafilm and INTERCEED groups showed no significant anti‐adhesive effects compared to the control group.
Figure 1.
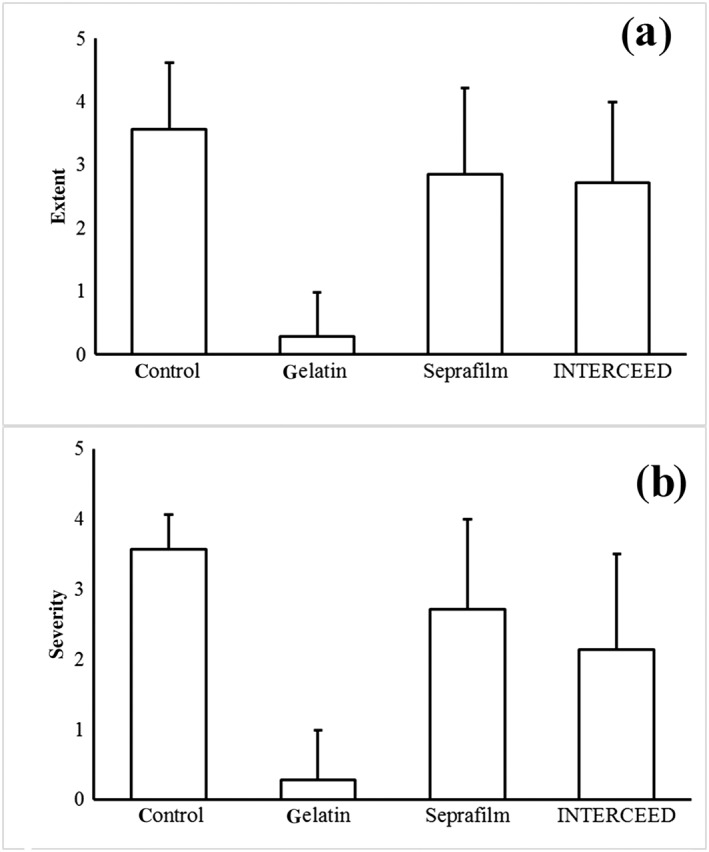
Anti‐adhesive agent scores: (a) the extent and (b) severity of adhesion. The two‐layered gelatin sheet showed a significantly (P < 0.05) lower score than the non‐treated group. There was no significant difference between the gelatin sheet (P < 0.05), Seprafilm or INTERCEED groups.
Regarding the severity of adhesion, the scores were 3.6 ± 0.5 in the control, 0.3 ± 0.7 in the gelatin sheet, 2.7 ± 1.3 in the Seprafilm, and 2.1 ± 1.4 in the INTERCEED group. The score in the gelatin sheet group was significantly (P < 0.05) lower than in the control. There were no significant differences in adhesion severity between the three treatment groups. The Seprafilm and INTERCEED groups showed no significant anti‐adhesive effects compared to the control group. There were no mortalities or major morbidities associated with the operation or the application of the anti‐adhesive materials.
Histological analyses
Three weeks after surgery
The histological findings are listed in (Table 2). Autopsies performed three weeks after surgery revealed that the gelatin remained and covered the cauterized site. On the free abdominal cavity side of the gelatin sheet, a single‐cell layer of mature (complete formation of single layer of mesothelium over the whole range of injured sites) mesothelium and submesothelial tissue had formed in all dogs in the two‐layered gelatin sheet group (Fig. 2a). On the opposite side, granulation had grown on the cauterized surface (Fig. 2a). In the Seprafilm group, abundant foamy cells of macrophages ingesting HA/CMC were found around the cauterized surface and little Seprafilm material remained (Fig. 2b). A mesothelial cell layer did not form (Fig. 2b). In the INTERCEED group, a poorly matured (incomplete or scattered formation of mesothelium over the range of injured sites) mesothelial cell layer had formed in all dogs (Fig. 2c). A large number of foamy cells of macrophages ingesting TC7 were found around the cauterized surface, and the INTERCEED was almost absorbed (Fig. 2c).
Table 2.
Histological findings of the site around materials
| Gelatin sheet | Seprafilm | INTERCEED | ||||
|---|---|---|---|---|---|---|
| Weeks | 3 | 6 | 3 | 6 | 3 | 6 |
| Epithelium regeneration | Yes | Yes | No | Yes | Yes | Yes |
| Layer formation of mesothelium | Matured single layer | No | Immature | Poorly matured layer | Matured single layer | |
| Inflammation | Mild | Improved |
Abundant macrophage Rich granulation |
Abundant macrophage Rich granulation |
||
| Remains of material | Fully remained | Little remained | Almost ingested | Ingested | Almost ingested | Ingested |
Figure 2.
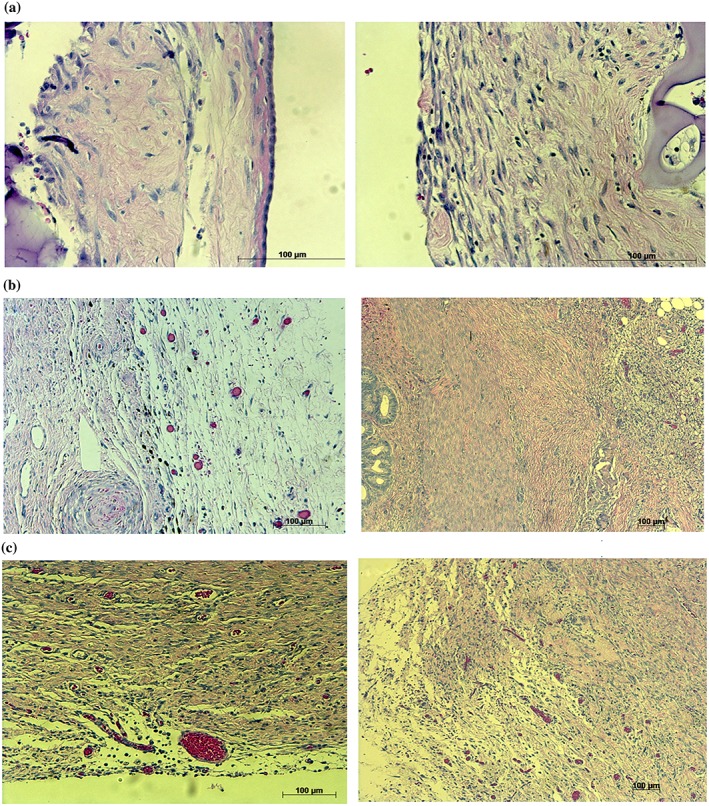
Histological findings three weeks after surgery: (a) the two‐layered gelatin sheet, (b) Seprafilm and (c) INTERCEED. The mature single layered mesothelium was completely regenerated in the two‐layered gelatin sheet group.
Six weeks after surgery
In the gelatin group 42 days after surgery, the cauterized surface was completely covered by mature mesothelium accompanied with a submesothelial layer. Macrophages were scarce, and inflammation of the implanted sites was subsequently improved. Little material remained (Fig. 3a). In the Seprafilm group, the injured surface was covered by poorly matured mesothelium. There were abundant foamy cells ingesting HA/CMC, and the material was almost completely ingested. Greater omentum adhered to the granulation around the Seprafilm (Fig. 3b). In the INTERCEED group, a relatively mature mesothelial layer had formed in all dogs, and there were abundant macrophages ingesting TC7. The material was observed as abundant in phagocytes (Fig. 3c).
Figure 3.
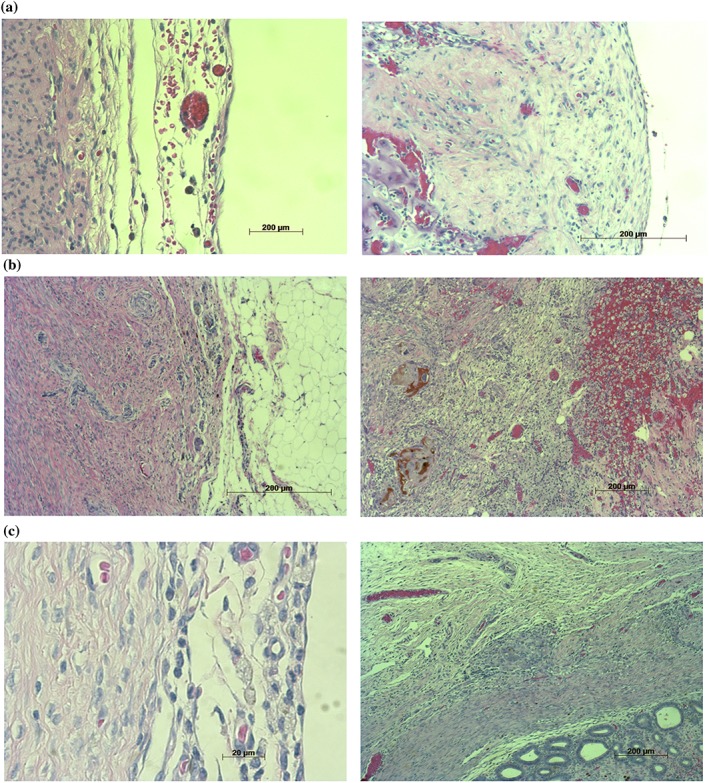
Histological findings six weeks after surgery: (a) the two‐layered gelatin sheet, (b) Seprafilm and (c) INTERCEED. Inflammation of the implanted sites was subsequently improved in the gelatin group compared to the other groups.
Cell growth
In the Seprafilm and INTERCEED groups, fibroblasts or uterine smooth muscle cells did not proliferate and the number of seeded cells decreased in peritoneal mesothelial cells (Fig. 4). On both surfaces of the gelatin sheet (film, sponge) all kinds of cells significantly proliferated (P < 0.01) compared with the Seprafilm and INTERCEED groups.
Discussion
Anti‐adhesive agents need to act as barriers to prevent direct contact to adjacent organs. They should be made out of material that: remains in the same place, does not disturb peritoneum regeneration, allows hemostasis (because abundant fibrin formation accelerates adhesion at a bleeding site) and can be absorbed after peritoneum regeneration to avoid inflammation
We developed a two‐layered gelatin sheet with the abovementioned properties. This method was the most effective of all methods used in this study for preventing adhesion after uterine surgery, in terms of extent and severity. Seprafilm and INTERCEED tended to prevent adhesion compared with the non‐treated group, but the effects were so unstable that no significant differences between these groups were noted. The anti‐adhesive effects of Seprafilm or INTERCEED for pelvic surgery are controversial.1, 3, 12
It is important to prevent adhesion of the injured organs to the adjacent organs. The peritoneum prevents fibrin attachment, the first step of adhesion. Early regeneration of the peritoneum of the injured site is one of the most important factors for the prevention of adhesion.
In the group treated with the two‐layered gelatin sheet, a mature single‐layered mesothelium was completely regenerated three weeks after surgery. By contrast, in the Seprafilm group, formation of the mesothelium layer was not observed three weeks after surgery. In the INTERCEED group, the mesothelial cell layer formed to a small extent, and most of the injured surface was uncovered with mesothelial cells at three weeks, but a relatively mature mesothelial cell layer covered the injured surface almost completely at six weeks after surgery. We conclude that rapid regeneration of the peritoneum contributed to the subsequent prevention of adhesion.13, 14 A previous study of anti‐adhesion reported rapid regeneration of the peritoneum using INTERCEED.15 We suspect that regeneration of the peritoneum was delayed because the tissue damage was stronger in the cauterized uterus model than in the study by Haney et al.15
To analyze the cause of rapid regeneration of mesothelial cell layer in the two‐layered gelatin group, cell growth on the each material was observed in vitro. Growth of all kinds of cells was proliferated most in the gelatin film and the sponge groups in vitro. Seprafilm and INTERCEED restrained cell growth in vitro and it suggest Seprafilm and INTERCEED disturbed tissue repair and regeneration in vivo study. An important factor for tissue repair is better cell proliferation, which occurred in the group using gelatin film.
The duration that materials remain on the injured site to act as a barrier against adhesion between adjacent tissues is an important factor. In our study, almost all gelatin remained on the site three weeks after surgery, but disappeared at six weeks. Seprafilm and INTERCEED were ingested by macrophages and had almost completely disappeared three weeks after surgery. Postoperative adhesion was initiated within a week.14 In the Seprafilm and INTERCEED groups, the local inflammation was so substantial that regeneration of the peritoneum was incomplete even at three weeks after surgery, indicating that the progress of adhesion continued until peritoneum regeneration was complete. It is one of the most important functions for anti‐adhesive agents to keep the barrier function over a month after the surgery, particularly for injuries of the peritoneum and other organs, which occur in gynecological malignant tumors.
Local hemorrhage is another important factor that needs to be controlled in order to prevent adhesion. Local bleeding, insufficient hemostasis, or rebleeding after surgery attenuates the anti‐adhesive effects of INTERCEED.14 Seprafilm also has no hemostatic effect. Secure hemostasis of the surgical site is key for the anti‐adhesive agents to produce an effect. During our experiment, the gelatin sheet absorbed the flow of blood and coagulation rapidly occurred. The two‐layered gelatin sheet easily attached and remained on the bleeding site, which did not occur in the Seprafilm or INTERCEED groups. This characteristic of the two‐layered gelatin sheet is an advantage as an anti‐adhesive agent. Surgery for a gynecological malignant tumor requires extensive dissection, with wide‐reaching tissue injury. Significant bleeding also often occurs. Our study showed that the two‐layered gelatin sheet had extensive hemostatic effects.16
There were some limitations to this study. The study was conducted using a canine model and only the cauterized uterus was observed. In preliminary study, significant adhesions did not develop by abrasion or small range cauterization of the uterus. Wide range cautery of the uterus is necessary to evaluate the differences in adhesion prevention effects of the materials. Evaluation of the effects in human abdominal surgery is necessary. We have plans to advance this investigation. Moreover, we are developing a new form of gelatin to apply to laparoscopic surgery.
Conclusion
In conclusion, the anti‐adhesive effect of the two‐layered gelatin sheet is superior to conventional agents in a cauterized canine uterus model. Early regeneration of the peritoneum, little inflammation and material endurance were observed. The newly developed two‐layered gelatin sheet is a useful option as an anti‐adhesive agent for deeply injured and hemorrhagic sites.
Disclosure
This work was supported, in part, by a grant from the Science and Engineering Institute of Doshisha University. No author has any other financial or personal relationship with people or organizations that could potentially and inappropriately influence our work and conclusions.
Torii, H. , Takagi, T. , Urabe, M. , Tsujimoto, H. , Ozamoto, Y. , Miyamoto, H. , Ikada, Y. , and Hagiwara, A. (2017) Anti‐adhesive effects of a newly developed two‐layered gelatin sheet in dogs. J. Obstet. Gynaecol. Res., 43: 1317–1325. doi: 10.1111/jog.13358.
References
- 1. Ward BC, Panitch A. Abdominal adhesions: Current and novel therapies. J Surg Res 2011; 165: 91–111. [DOI] [PubMed] [Google Scholar]
- 2. Schnüriger B, Barmparas G, Branco BC, Lustenberger T, Inaba K, Demetriades D. Prevention of postoperative peritoneal adhesions: A review of the literature. Am J Surg 2011; 201: 111–121. [DOI] [PubMed] [Google Scholar]
- 3. Kamel RM. Prevention of postoperative peritoneal adhesions. Eur J Obstet Gynecol Reprod Biol 2010; 150: 111–118. [DOI] [PubMed] [Google Scholar]
- 4. Ray NF, Denton WG, Thamer M, Henderson SC, Perry S. Abdominal adhesiolysis: Inpatient care and expenditures in the United States in 1994. J Am Coll Surg 1998; 186: 1–9. [DOI] [PubMed] [Google Scholar]
- 5. ten Broek RPG, Issa Y, van Santbrink EJP et al. Burden of adhesions in abdominal and pelvic surgery: Systematic review and met‐analysis. BMJ 2013; 347: f5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL‐F): A blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril 1996; 66: 904–910. [PubMed] [Google Scholar]
- 7. Vrijland WW, Tseng LNL, Eijkman HJM et al. Fewer intraperitoneal adhesions with use of hyaluronic acid‐carboxymethylcellulose membrane: A randomized clinical trial. Ann Surg 2002; 235: 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Syst Rev 2000: CD000475. [DOI] [PubMed] [Google Scholar]
- 9. Robertson D, Lefebvre G, Leyland N et al. Adhesion prevention in gynaecological surgery. J Obstet Gynaecol Can 2010; 32: 598–608. [DOI] [PubMed] [Google Scholar]
- 10. Wiseman DM, Gravagna P, Bayon Y, Tayot J. Collagen membrane/fleece composite film reduces adhesions in the presence of bleeding in a rabbit uterine horn model. Fertil Steril 2001; 76: 175–180. [DOI] [PubMed] [Google Scholar]
- 11. Haney AF, Doty E. Expanded‐polytetrafluoroethylene but not oxidized regenerated cellulose prevents adhesion formation and reformation in a mouse uterine horn model of surgical injury. Fertil Steril 1993; 60: 550–558. [DOI] [PubMed] [Google Scholar]
- 12. Ouaïssi M, Gaujoux S, Veyrie N et al. Post‐operative adhesions after digestive surgery: Their incidence and prevention: Review of the literature. J Visc Surg 2012; 149: e104–e114. [DOI] [PubMed] [Google Scholar]
- 13. Takagi K, Araki M, Fukuoka H et al. Novel powdered anti‐adhesion material: Preventing postoperative intra‐abdominal adhesions in a rat model. Int J Med Sci 2013; 10: 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boland GM, Weigel RJ. Formation and prevention of postoperative abdominal adhesions. J Surg Res 2006; 132: 3–12. [DOI] [PubMed] [Google Scholar]
- 15. Haney AF, Doty E. Murine peritoneal injury and de novo adhesion formation caused by oxidized‐regenerated cellulose (Interceed [TC7]) but not expanded polytetrafluoroethylene (Gore‐Tex Surgical Membrane). Fertil Steril 1992; 57: 202–208. [DOI] [PubMed] [Google Scholar]
- 16. Takagi T, Tsujimoto H, Torii H, Ozamoto Y, Hagiwara A. Two‐layer sheet of gelatin: A new topical hemostatic agent. Asian J Surg 2016; https://doi.org/10.1016/j.asjsur.2016.09.007 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


