Abstract
Aim
To gain insight into the presence of islet cell autoimmunity in an ethnic Asian compared with a white European population.
Methods
For this cross‐sectional study we recruited people with adult‐onset diabetes (age of diagnosis 20–60 years), at tertiary referral centres in Germany (n=1020) and Singapore (n=1088). Glutamic acid decarboxylase and islet antigen 2 antibodies were measured according to Islet Autoantibody Standardization Program protocols.
Results
The prevalence of glutamic acid decarboxylase antibody positivity was 13.9% (95% CI 12.1–16.0; P<0.001) in the white European cohort compared with 6.8% (95% CI 5.5–8.4; P<0.001) in the Asian cohort. Glutamic acid decarboxylase antibody positivity was 11.4% (95% CI 7.7–16.6) in Indian, 6.0% (95% CI 3.6–9.9) in Malay and 5.8% (95% CI 4.3–7.7; P<0.001) in Chinese participants. In the white European participants, the prevalence of islet antigen 2 antibody positivity was 7.8% (95% CI 6.4–9.4) compared with 14.8% (95% CI 12.8–17.0; P<0.001) in the Asian cohort as a whole, and among the three ethnicities in the Asian cohort it was 12.4% (95% CI 8.6–17.7) in Indian, 16.8% (95% CI 12.6–22.2) in Malay and 15.7% (95% CI 13.2–18.6) in Chinese participants. Double antibody positivity was seen in 5.7% (95% CI 4.5–7.1) of white European participants compared with 1.6% (95% CI 1.0–2.5; P<0.01) of Asian participants. In the white European cohort, those who were glutamic acid decarboxylase autoantibody‐positive had a lower BMI than those who were autoantibody‐negative, but this trend was absent in the Asian cohort.
Conclusions
A marked prevalence of islet cell autoimmunity was observed in people with adult‐onset diabetes. While glutamic acid decarboxylase antibodies were more frequent in the European cohort, islet antigen 2 antibody positivity was highest in the three ethnic groups in Singapore, suggesting ethnic‐specific differences in antibody profiles.
What's new?
Data on the prevalence of adult‐onset autoimmune diabetes gathered from population‐based studies in Asia are sparse. We assessed the presence of islet cell antibodies [glutamic acid decarboxylase (GAD) and islet antigen 2 (IA2) antibodies] in three ethnic Asian groups from Singapore as compared with a white European population with diabetes.
In the Asian cohort, IA2 antibodies were most prevalent, across the three ethnicities, whereas GAD antibodies were more frequent in the white European cohort. In the white European cohort, those who were GAD autoantibody‐positive had a lower BMI than those who were GAD autoantibody‐negative, but this trend was absent in the Asian cohort.
What's new?
Data on the prevalence of adult‐onset autoimmune diabetes gathered from population‐based studies in Asia are sparse. We assessed the presence of islet cell antibodies [glutamic acid decarboxylase (GAD) and islet antigen 2 (IA2) antibodies] in three ethnic Asian groups from Singapore as compared with a white European population with diabetes.
In the Asian cohort, IA2 antibodies were most prevalent, across the three ethnicities, whereas GAD antibodies were more frequent in the white European cohort. In the white European cohort, those who were GAD autoantibody‐positive had a lower BMI than those who were GAD autoantibody‐negative, but this trend was absent in the Asian cohort.
Introduction
Diabetes mellitus is prevalent worldwide and has been on the rise in Singapore, with the Asian population becoming both more affluent and obese over the last 25 years 1. A report by the International Diabetes Federation, released in 2015, revealed that Singapore has the second‐highest proportion of people with diabetes among developed nations 1; however, the degree of disease heterogeneity in Asian people with adult‐onset diabetes is not clear, particularly with regard to the presence of islet‐cell autoimmunity 1, 2. Data on the prevalence of adult‐onset autoimmune diabetes gathered from population‐based studies in Asia are sparse 3. Singapore, with its mixed ethnic population, provides a unique environment to look for the existence of islet cell antibodies in three different ethnic groups; namely, Chinese, Malay and Indian people. To enable a trans‐ethnic comparison, we also recruited from another affluent environment: a cohort of white Europeans from the southern part of Germany, which we used, in view of the existing literature, as a reference cohort 4. We determined antibodies against the two major islet cell autoantigens, glutamate decarboxylase (GAD) and islet antigen 2 (IA2), which are considered to be key humoral markers of islet cell autoimmunity.
Research Design and Methods
Participants
The study enrolled participants with adult‐onset diabetes attending tertiary referral centres in Germany and Singapore, with an age of diabetes diagnosis ranging from 20 to 60 years. There was no significant difference between the cohorts in the mean age of disease onset or proportion of men: German cohort (n = 1020) 42.0 ± 1.2 years, 623 men (61.07%), and Asian cohort (n =1088) 43.8 ± 0.4 years, 701 men (64.4%).
Inclusion criteria were diabetes diagnosis according to American Diabetes Association criteria 5, with no insulin treatment. Exclusion criteria included prior insulin therapy, ketosis at diagnosis, pregnancy and presence of any other severe disease. The study was approved by the ethics review committee/institutional review board and written informed consent was obtained from all participants before screening and inclusion in the study, in both Singapore and Germany. Blood samples were taken by venipuncture up to 2 years after the diagnosis of diabetes was made and stored at –80°C until required for assay.
Immunoprecipitation of in vitro translated proteins
The GAD and IA2 antibodies were analysed at Lee Kong Chian School of Medicine [Islet Autoantibody Standardization Program (IASP) 2015 Laboratory ID 1501] by radioligand assay according to the IASP 6, 7, 8. In brief, GAD and the intracellular domain of IA2, IA2ic 9, was transcribed into RNA and translated into protein in vitro using the TNT T7 Quick Coupled System (Promega, Madison, WI, USA), according to the manufacturer's instructions, in the presence of [35S]‐methionine (Perkin Elmer, Waltham, MA, USA). The protein‐bound radiolabel was separated from the unincorporated label through a NAP5 Sephadex G25 DNA Grade column (GE Healthcare, Little Chalfont, UK). A concentration of 20 000/min [35S]‐GAD antibodies in 25 μl assay buffer (TBS/0.05% Tween) was added to 2 μl serum in 96‐deep‐well microtitre plates and incubated for 16 h on ice. The immune complexes were further incubated with a suspension of 50 μl of 50% Protein A Sepharose (SciMed, Singapore) in a 96‐deep‐well plate for 1 h at 4°C with shaking. They were then washed extensively with assay buffer and transferred into a 96‐well scintillation plate, containing 200 μl scintillation fluid (Perkin Elmer), and counts/min were measured in a scintillation counter for radioactivity (TopCount NXT Microplate Scintillation and Luminescence Counter; Perkin Elmer). All samples were measured in duplicate, and 20% of samples were randomly selected and repeatedly tested to confirm their antibody status. GAD and IA2 antibody positivity was determined as the 99.5th percentile of 1192 healthy white European control participants (age range 18–70 years; mean age 39.7 years) and 145 healthy ethnic Asians control participants (age range 20–69 years; mean age 49.1 years). Sera obtained from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) were used as positive controls, as described by The Environmental Determinants of Diabetes in the Young (TEDDY) Study Group 10, 11. Sera with ≥21 arbitrary DK units/ml for GAD antibodies and ≥5 arbitrary DK units/ml for IA2 antibodies were considered to be antibody‐positive.
The sensitivity and specificity of GAD antibodies were 76.0% and 87.8%, and the sensitivity and specificity of IA2 antibody assays were 76.0% and 94.4%, respectively, as evaluated in the 4th Diabetes IASP 2015 (laboratory ID 1501).
Statistical analysis
Statistical analysis was performed using graphpad prism software (version 6). Data were expressed as frequencies (%) ± 95% CI, to one decimal point. Student's t‐tests were used to compare the means between two (ethnic or age‐adjusted) groups as appropriate. Gender groups were analysed using chi‐squared tests. Two‐way anova and Tukey tests were used to determine the differences between the white European and ethnic Asian cohorts. A linear regression model was used to test associations between antibody‐positive frequency and age of onset.
Results
Positivity for GAD antibodies was detected in 13.9% (95% CI 12.1–16.0) of white European participants as compared with 6.8% (95% CI 5.5–8.4; P<0.001) of ethnic Asian participants. Among the three ethnic groups from Singapore, GAD antibody positivity was significantly higher in Indian participants [11.4% (95% CI 7.7–16.6)] as compared with both Chinese (5.8%; 95% CI 4.3–7.7; P<0.001) and Malay participants (6.0%; 95% CI 3.6–9.9; P<0.001; Table 1).
Table 1.
Islet cell antibody prevalence and mean BMI in the three main ethnic Asians ethnic groups (Chinese, Malay, Indian) compared with white European participants
| Mean (± sd) BMI, kg/m2 | GAD antibodies, % (95% CI) | Mean (± sd) BMI, kg/m2 GAD antibody‐positive | IA2 antibody, % (95% CI) | Mean (± sd) BMI, kg/m2 IA2 antibody‐positive | GAD and/or IA2ic antibodies, % (95% CI) | Mean (± sd) BMI, kg/m2 GAD and/or IA2 antibody‐positive | GAD and IA2antibodies, % (95% CI) | Mean (± sd) BMI, kg/m2 GAD and IA2 antibody‐positive | |
|---|---|---|---|---|---|---|---|---|---|
| White European cohort ( n =1020) | 25.2 (± 0.5) | 13.9 (12.1–16.0) | 22.9 (± 0.5) †† | 7.8 (6.4–9.4) | 24.2 (± 0.4) ** | 14.3 (12.4–16.4) | 24.2 (± 0.5) ** | 5.7 (4.5–7.1) | 24.0 (± 0.3) ** |
| Male ( n =623) | 25.3 (± 0.6) | 15.7 (13.2–18.5) | 23.5 (± 0.5) | 8.5 (6.6–10.8) | 25.2 (± 0.3) | 16.1 (13.6–19.0) | 25.3 (± 0.6) | 5.8 (4.3–7.8) | 25.0 (± 0.3) |
| Female ( n =397) | 25.0 (± 0.5) | 11.3 (8.8–14.5) | 21.7 (± 0.7) | 6.7 (4.8–9.3) | 22.5 (± 0.4) | 11.5 (9.0–14.7] | 24.9 (± 0.5) | 5.4 (3.7–7.9) | 22.7 (± 0.4) |
| Ethnic Asian cohort ( n =1088) | 27.7 (± 0.6) | 6.8 (5.5–8.4) † | 28.0 (± 0.5) | 14.8 (12.8 ‐ 17.0) † | 26.1 (± 0.6) | 17.4 (15.2 ‐ 19.6) † | 26.1 (± 1.2) | 1.6 (1.0 ‐ 2.5)* | 26.1 (± 0.6) |
| Men ( n =701) | 27.4 (± 0.5) | 7.2 (5.5–9.3) | 27.6 (± 0.5) | 12.7 (10.4–15.4) | 25.7 (± 0.5) | 19.5 (16.8–22.6) | 27.6 (± 0.5) | 1.9 (1.2–3.2) | 25.7 (± 0.5) |
| Women ( n =387) | 28.2 (± 0.7) | 6.1 (4.2–8.8) | 28.7 (± 0.3) | 18.6 (15.0–22.8) | 26.4 (± 0.7) | 22.0 (18.1–26.4) | 28.6 (± 0.7) | 1.4 (0.7–3.1) | 26.4 (± 0.7) |
| Chinese ( n =655) | 27.0 (± 0.5) | 5.8 (4.3–7.7) | 27.2 (± 0.5) | 15.7 (13.2–18.6) | 25.9 (± 0.5) | 19.4 (16.6–22.4) | 27.0 (± 0.5) | 2.1 (1.3–3.6) | 25.9 (± 0.5) |
| Men ( n =455) | 27.0 (± 0.5) | 5.9 (4.1–8.4) | 27.3 (± 0.4) | 14.9 (12.0–18.4) | 25.7 (± 0.5) | 18.5 (15.3–22.2) | 27.0 (± 0.5) | 2.3 (1.3–4.1) | 25.7 (± 0.5) |
| Women ( n =200) | 27.0 (± 0.5) | 5.5 (3.2–9.2) | 27.1 (± 0.6) | 17.3 (13.0–22.6) | 26.3 (± 0.5) | 21.1 (16.4–26.7) | 27.0 (± 0.5) | 1.7 (0.7 to 4.3) | 26.3 (± 0.5) |
| Malay ( n =232) | 29.9 (± 0.5) | 6.0 (3.6–9.9) | 29.7 (± 0.5) | 16.8 (12.6–22.2) ¶ | 29.3 (± 0.5) | 21.1 (16.4–26.8)§ | 29.8 (± 0.5) | 1.3 (0.4–3.7) § | 29.3 (± 0.5) |
| Men ( n =125) | 29.5 (± 0.6) | 7.2 (3.8–13.1) | 29.0 (± 0.5) | 16.0 (10.6–23.4) | 27.7 (± 0.5) | 21.6 (15.3–29.6) | 29.6 (± 0.6) | 1.6 (0.4 ‐ 5.7) | 27.7 (± 0.5) |
| Women ( n =107) | 30.4 (± 0.6) | 4.7 (2.0–10.5) | 30.6 (± 0.5) | 17.8 (11.7–26.1) | 30.9 (± 0.4) | 20.6 (14.0–29.2) | 30.3 (± 0.6) | 1.9 (0.5–6.6) | 30.9 (± 0.4) |
| Indian ( n =201) | 27.8 (± 0.5) | 11.4 (7.7–16.6) ¶ | 28.0 (± 0.4) | 12.4 (8.6–17.7) ¶ | 28.7 (± 0.5) | 23.4 (18.1–29.7) § | 28.2 (± 0.5) | 0.5 (0.1–2.8) § | 28.7 (± 0.5) |
| Men ( n =121) | 27.1 (± 0.4) | 12.4 (7.7–19.5) | 27.3 (± 0.5) | 9.1 (5.2 to 15.6) | 26.6 (± 0.6) | 20.7 (14.4–28.7) | 27.7 (± 0.4) | 0.8 (0.2–4.5) | 26.6 (± 0.6) |
| Women ( n =80) | 28.9 (± 0.7) | 10.0 (5.2–18.5) | 29.2 (± 0.5) | 17.5 (10.7–27.3) | 30.0 (± 0.7) | 27.5 (18.9–38.1) | 28.9 (± 0.7) | 0 | 0 |
GAD, glutamic acid decarboxylase; IA2, islet antigen 2.
Ratios for GAD antibodies and IA2 antibody positivity were 13.9: 5.7: 6: 11.4 and 7.8: 15.7: 16.8: 12.4 for white European: Chinese: Malay: Indian participants, respectively.*P < 0.01, †P < 0.001 compared with white European cohort. Among ethnic Asians ethnic groups, § P < 0.01, ¶ P < 0.001 in comparison with ethnic Chinese. Among white Europeans, **P < 0.01, †† P < 0.001 compared with white Europeans mean BMI. All P values obtained using the t‐test.
Positivity for IA‐2 antibodies was detected in 7.8% (95% CI 6.4–9.4) of white European participants as compared with 14.8% (95% CI 12.8 ‐17.0; P<0.001) of ethnic Asian participants. Amongst the ethnic groups from Singapore, 15.7% (95% CI 13.2–18.6) of Chinese, 16.8% (95% CI 12.6–22.2; non‐signficant) of Malay and 12.4% (95% CI 8.6–17.7; non‐significant) of Indian participants had similar rates of IA‐2 antibody positivity.
The presence of GAD or IA2 autoantibodies was common both in the white European as well as the ethnic Asians cohort: 14.3% (95% CI 12.4–16.4) vs 18.7% (95% CI 16.5 ‐ 21.0; Table 1). Double antibody positivity was significantly higher in white European participants (5.7%; 95% CI 4.5–7.1) as compared with Asian participants (1.6%; 95% CI 1.0–2.5; P<0.001). Amongst the three ethnic groups from Singapore, 2.1% (95% CI 1.3–3.6) of Chinese, 1.3% (95% CI 0.4–3.7) of Malay and 0.5% (95% CI 0.1–2.8) of Indian participants were double antibody‐positive (Table 1).
White European participants who were GAD antibody‐ and/or IA2 antibody‐positive had a lower mean BMI compared with antibody‐negative participants (24.2 ± 0.5 kg/m2 vs 27.2 ± 0.6 kg/m2; P=0.01); however, the mean BMI of antibody‐positive participants in the Singapore cohort was not different from the antibody‐negative participants (26.1 ± 1.2 kg/m2 vs 27.8 ± 1.1 kg/m2; P=0.35). A significant difference was also found in the mean BMI of GAD antibody‐positive vs IA2 antibody‐positive participants (22.9 kg/m2 vs 24.2 kg/m2) in the white European cohort (P<0.05). Again, no such difference was observed in the Asian cohort (Figures S1–S3).
Antibody titres were also found to be different among the four ethnic groups. The majority of GAD antibody‐positive white Europeans had a GAD antibody titre range of 54.6–148.4 arbitrary NIDDK units/ml 12, whereas 3% had a GAD antibody titre of >406.4 arbitrary NIDDK units/mL (Fig. 1a). GAD antibody‐positive Asian participants had GAD antibody titres of 21.1–54.6 arbitrary NIDDK units/ml (50% of the cohort), 54.6–148.4 arbitrary NIDDK units/mL (40% of the cohort) and >148.4 arbitrary NIDDK units/mL (10% of the cohort; Fig. 1c). The three Asian ethnic groups had similar GAD antibodies titre (Fig. 1e, g and i) and IA2antibody titre distributions (Fig. 1f, h and j).
Figure 1.
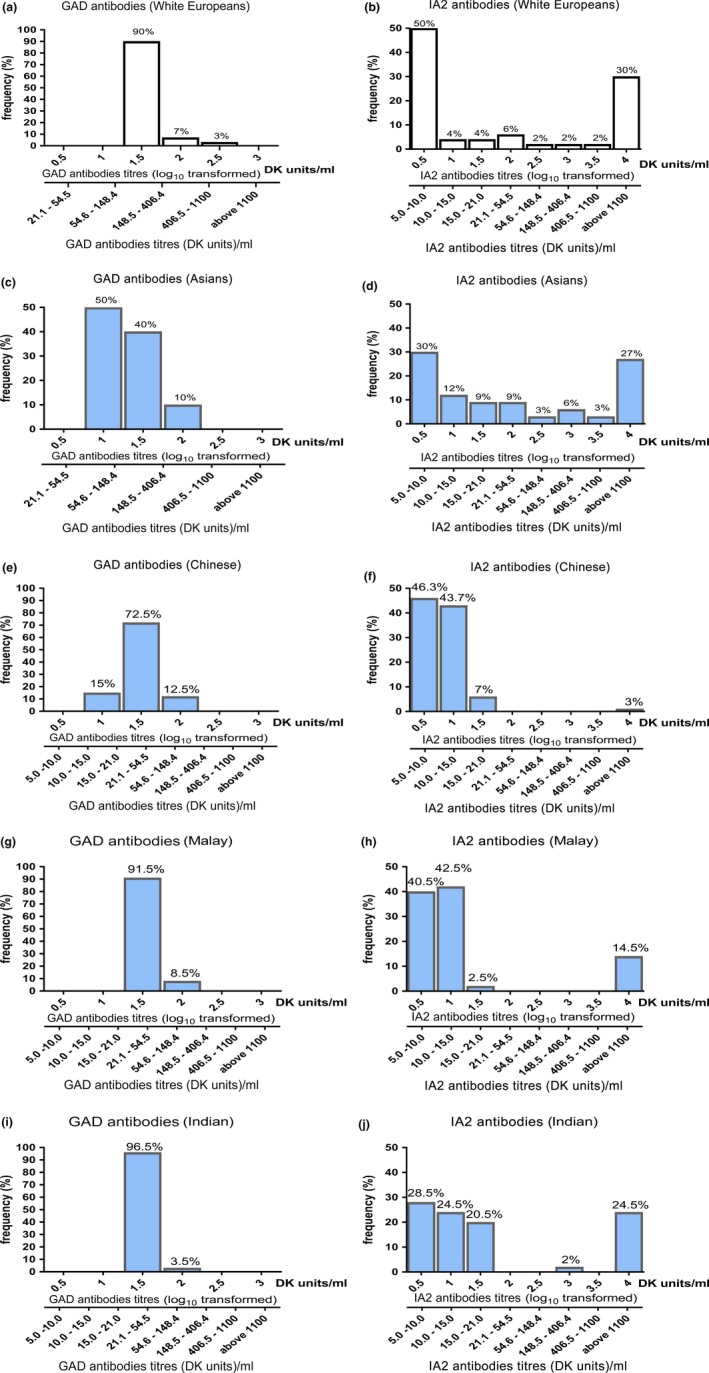
Islet cell antibody prevalence in Asian and white European participants with diabetes. (a) Glutamic acid decarboxylase (GAD) antibodies and (b) islet antigen 2 (IA2) antibody distribution in white European participants. (c) GAD antibodies and (d) IA2 antibody distribution in ethnic Asian participants. Ethnic‐specific GAD antibodies positivity for Chinese (e), Malay (g) and Indian (i) participants; IA2 antibody distribution for Chinese (f), Malay (h) and Indian (j) participants. *P < 0.01, **P < 0.001 compared with white European cohort using the t‐test. DK units, arbitrary National Institute of Diabetes and Digestive and Kidney Diseases units.
There was a different pattern of age of disease onset in relation to antibody positivity and antibody levels in white European compared with Asian participants (Fig. 2). In white European participants, antibody prevalence and antibody titre for both GAD and IA‐2 antibodies was lower after the interval of 30–39 years of disease onset. (Fig. 2a, c and d). Among the Asian participants, such an age‐dependency in prevalence and antibody titre was not observed (Fig. 2b, e and f). In addition, the distribution of antibody titres and antibody positivity among the three ethnic groups was similar (Supporting Information).
Figure 2.
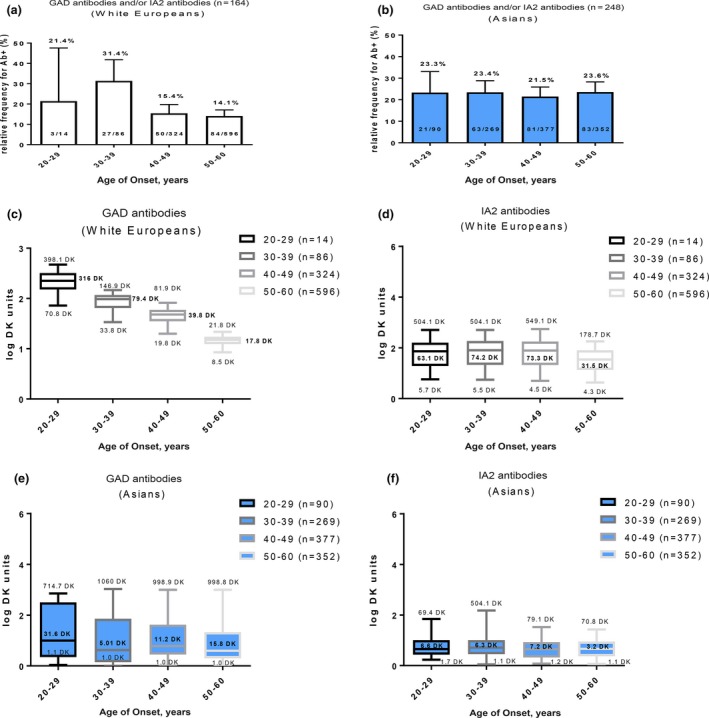
Islet cell antibody prevalence of glutamic acid decarboxylase (GAD) and/or islet antigen 2 (IA2) antibodies in relation to age of disease onset. (a) White European (linear regression slope= ‐0.38±0.03) and (b) ethnic Asian (linear regression slope= ‐0.012±0.05) participants with increasing age of onset. Distribution box plots showing antibody titre distribution in participants with increasing age of onset: (c) GAD antibodies in white European participants (P<0.001) and (d) IA2 antibodies in white European participants (P=0.096; non‐significant) in comparison with (e) GAD antibodies in the Singapore cohort (P=0.534; non‐significant) and (f) IA2 antibodies in the Singapore cohort (P= 0.399; non‐significant). Total population in each age group=100%. P values compared with cohort in 20–29‐year age group using one‐way anova. DK units, arbitrary National Institute of Diabetes and Digestive and Kidney Diseases units.
Discussion
This is the first study to investigate GAD and IA2 antibody positivity and antibody levels in people with non‐insulin‐dependent adult‐onset diabetes from four different ethnic groups. The predominant islet cell antigen in Asian participants with adult‐onset diabetes was IA2, while it was GAD in the white European participants. The prevalence of GAD antibody positivity within the four ethnic groups (Table 1) was consistent with the GAD antibody positivity rate of 7% reported in the UK Prospective Diabetes Study 14, 15 and also with the results from the China LADA Consortium of 5.9% 16.
Age of onset dependency for islet cell antibody positivity had been reported previously, as a characteristic of adult‐onset autoimmune diabetes 16, 17, in various European 13, 15, 16, 17, 18, 19 and US cohorts 20. This association with age of onset was not seen in Asian participants with diabetes.
The presence of single islet cell antibody positivity was a unique feature for the Asian cohorts 21, whereas double antibody positivity was largely confined to white Europeans. White European participants were leaner and had a lower BMI with increasing GAD titre 15, while this trend was not seen in Asian participants 21, 22. Notably, the mean BMI of GAD antibody‐positive participants was lower than that of IA2 antibody‐positive participants within the white European cohort. Again, there was a lack of this association within the various Asian cohorts (Figure S3).
The present study has some limitations. The cohorts analysed were recruited at tertiary referral centres, and therefore more severe clinical cases of the disease spectrum were probably captured, which may have had an impact on the likelihood of autoantibody positivity 13. In addition, participants were not characterized according to their β‐cell (C‐peptide) reserve, unlike in other studies 23, or followed for their long‐term treatment methods 13, 15.
Strengths of the present study include the application of Diabetes IASP methods 6, 7, 8, and the comparison of patient cohorts from similar socio‐economic environments, as determined by income per capita.
To learn more about the clinical impact of islet cell autoimmunity in different ethnic groups, follow‐up studies involving larger cohorts are needed. These studies should take into consideration, in particular, ethnic‐specific differences in antibody patterns and clinical outcomes among people with islet cell autoimmunity in comparison to participants without islet cell antibodies 14, 16, 17, 24, 25, 26.
Funding sources
This study was supported the Ministry of Education Tier 1 (RG 1T1‐10/15), the Joint Singapore‐White European Research Project, the NTU‐NHG Metabolic Diseases Collaboration Grant and the Stratified Medicine Programme Office Invited Research Grant to B.O.B., whos also supported by Deutsche Forschungsgemeinschaft GrK1041, SFB518 and State of Baden‐Württemberg (Centre of Excellence ‘Metabolic Disorders’).
Competing interests
None declared.
Supporting information
Figure S1. Autoantibody (GAD, IA2) titres in (A, B) white European (non‐significant), (C,D) Chinese (P<0.001), (E, F) Malay (P<0.001) and (G,H) Indian (P <0.001) participants with increasing age of onset. P values compared with white European cohort using the t‐test.
Figure S2. Autoantibody (GAD, IA2) titres in (A, B) German (non‐significant), (C, D) Chinese (P <0.001), (E, F) Malay (P <0.001) and (G, H) Indian (P <0.001) participants with increasing BMI. P values compared with white European cohort using the t‐test.
Figure S3. BMI values of islet cell antibody‐negative and islet cell antibody‐positive subgroups. (A) Mean BMI for white European islet cell antibody‐negative participants was 27.2 (95% CI 25.2–29.2). BMI of GAD antibody‐positive Europeans was 22.9 (95% CI 19.3–27.3; P<0.001), for IA2 antibody‐positive participants 24.2 (95% CI 20.7–28.4; P<0.001) and double antibody‐positive participants 24.0 (95% CI 19.9–28.1; P<0.001). (B) Mean BMI for Asians without islet cell antibodies was 27.8 (95% CI 27.4–28.1). BMI of Asian GAD antibody‐positive participants was 28.0 (95% CI 27.0–28.9; P=non‐significant), for IA2 antibody‐positive participants 27.2 (95% CI 26.4–28.0; P=non‐significant), and double antibody‐positive participants 26.1 (95% CI 24.7–28.6; P<0.01). Mean BMI for ethnic Chinese GAD antibody positives was 27.2 (95% CI 25.6–28.9; P=non‐significant), IA2 antibody‐positive participants 26.3 (95% CI 25.3–27.2; P<0.01). Mean BMI for ethnic Malay GAD antibody‐positive participants was 29.8 (95% C.I. 27.4–30.9; P<0.001) and 29.4 (95% CI 27.5–30.5; P<0.001) for IA2 antibody‐positive participants. Mean BMI for ethnic Indian GAD antibody‐positive participants was 28.0 (95% CI 25.5–30.1; P=non‐significant), IA2 antibody‐positive participants had a BMI of 28.7 kg/m2 (95% CI 26.4–30.9; P=non‐significant). P values compared with antibody‐negative participants within each cohort using the t‐test.
Acknowledgements
Plasmid vectors were kindly provided by Professor Åke Lernmark, Lund University, Sweden. The reference sera were kindly provided by the NIDDK Central Repositories (Dr Beena Akolkar, PhD, National Institutes of Health). The authors wish to thank all those who participated in this study.
The physicians involved in recruiting participants in the State of Baden‐Wuerttemberg, Germany–Adult‐Onset Autoimmune Diabetes Mellitus Consortium were (in alphabetical order): R. Blagieva, B. O. Boehm (Chair), J. Boehm, J. Brückel, S. Claudi‐Boehm, T. Haak, M. Haenle, S. Höpfer, G. Jütting, A. Kleger, W. Kratzer, A. Kurkhaus, B. Lippmann‐Grob, B. Manfras, S. Merger, S. Rosinger, S. Schilling, T. Seufferlein, R. Sing and G. Trischler.
Physicians involved in the Adult‐Onset Autoimmune Diabetes Mellitus Singapore Consortium (ADAMS) (see Appendix for the full list).
Figures 2a and b and Figs S1a and b were presented as a poster for the SingHealth Duke‐NUS Scientific Congress 2016.
List of physicians involved in the Adult‐Onset Autoimmune Diabetes Mellitus Singapore Consortium (ADAMS).
From Ulm University Medical Centre, Ulm, Germany:
R. Blagieva
B. O. Boehm (Chair)
J. Boehm
J. Brückel
S. Claudi‐Boehm
T. Haak
M. Haenle
S. Höpfer
G. Jütting
A. Kleger
W. Kratzer
A. Kurkhaus
B. Lippmann‐Grob
B. Manfras
S. Merger
S. Rosinger
S. Schilling
T. Seufferlein
R. Sing S. Tavintharan
G. Trischler
From Khoo Teck Puat Hospital, Singapore:
S. C. Lim
C. F. Sum
From Lee Kong Chian School of Medicine, NTU, Singapore:
Y. Ali
B. O. Boehm (Chair)
S. P. Ng
D. K. Srinivasan
Z. Y. Tam
From National Healthcare Group, Singapore:
E. S. Lee
W. E. Tang
C. M. Khoo
A. C. Thai
From Saw See Hock School of Public Health, Singapore:
K. S. Chia
X. Sim
E. S. Tai
M. P. Toh
From Singapore General Hospital, Singapore:
Y. M. Bee
From Tan Tock Seng Hospital, Singapore:
D. E. K. Chew
R. Dalan
M. K. Leow
Diabet. Med. 32, 1145–1153 (2017)
See Appendix for the full list
References
- 1. Nanditha A, Ma RC, Ramachandran A, Snehalatha C, Chan JC, Chia KS et al Diabetes in Asia and the Pacific: Implications for the Global Epidemic. Diabetes Care 2016; 39: 472–485. [DOI] [PubMed] [Google Scholar]
- 2. Howson JM, Rosinger S, Smyth DJ, Boehm BO. ADBW‐END Study Group, Todd JA. Genetic analysis of adult‐onset autoimmune diabetes. Diabetes 2011; 60: 2645–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guglielmi C, Palermo A, Pozzilli P. Latent autoimmune diabetes in the adults (LADA) in Asia: from pathogenesis and epidemiology to therapy. Diabetes Metab Res Rev 2012; 28(Suppl. 2): 40–46. [DOI] [PubMed] [Google Scholar]
- 4. Naik RG, Brooks‐Worrell BM, Palmer JP. Latent autoimmune diabetes in adults. J Clin Endocrinol Metab 2009; 94: 4635–4644. [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2006; 29(Suppl. 1): S43–48. [PubMed] [Google Scholar]
- 6. Torn C, Mueller PW, Schlosser M, Bonifacio E. Bingley PJ; Participating Laboratories. Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen‐2. Diabetologia 2008; 51: 846–852. [DOI] [PubMed] [Google Scholar]
- 7. Williams AJ, Lampasona V, Schlosser M, Mueller PW, Pittman DL, Winter WE et al Detection of Antibodies Directed to the N‐Terminal Region of GAD Is Dependent on Assay Format and Contributes to Differences in the Specificity of GAD Autoantibody Assays for Type 1 Diabetes. Diabetes 2015; 64: 3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schlosser M, Mueller PW, Achenbach P, Lampasona V, Bingley PJ. Participating Laboratories Diabetes Antibody Standardization Program: First evaluation of assays for autoantibodies to IA‐2beta. Diabetes Care 2011; 34: 2410–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lernmark A. Type 1 diabetes. Clin Chem. 1999; 45(8 Pt 2): 1331–1338. [PubMed] [Google Scholar]
- 10. Bonifacio E, Yu L, Williams AK, Eisenbarth GS, Bingley PJ, Marcovina SM et al Harmonization of glutamic acid decarboxylase and islet antigen‐2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 2010; 95: 3360–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. TEDDY Study Group . The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Ann N Y Acad Sci 2008; 1150: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buzzetti R, Di Pietro S, Giaccari A, Petrone A, Locatelli M, Suraci C et al High titer of autoantibodies to GAD identifies a specific phenotype of adult‐onset autoimmune diabetes. Diabetes Care 2007; 30: 932–938. [DOI] [PubMed] [Google Scholar]
- 13. Kong YH, Kim MS, Lee DY. Comparison of the prevalence of islet autoantibodies according to age and disease duration in patients with type 1 diabetes mellitus. Ann Pediatr Endocrinol Metab 2013; 18: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pozzilli P, Di Mario U. Autoimmune diabetes not requiring insulin at diagnosis (latent autoimmune diabetes of the adult): definition, characterization, and potential prevention. Diabetes Care 2001; 24: 1460–1467. [DOI] [PubMed] [Google Scholar]
- 15. Turner R, Stratton I, Horton V, Manley S, Zimmet P, Mackay IR et al UKPDS 25: autoantibodies to islet‐cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet 1997; 350: 1288–1293. [DOI] [PubMed] [Google Scholar]
- 16. Davis TM, Wright AD, Mehta ZM, Cull CA, Stratton IM, Bottazzo GF et al Islet autoantibodies in clinically diagnosed type 2 diabetes: prevalence and relationship with metabolic control (UKPDS 70). Diabetologia 2005; 48: 695–702. [DOI] [PubMed] [Google Scholar]
- 17. Hawa MI, Kolb H, Schloot N, Beyan H, Paschou SA, Buzzetti R et al Adult‐onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: Action LADA 7. Diabetes Care 2013; 36: 908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mollo A, Hernandez M, Marsal JR, Esquerda A, Rius F, Blanco‐Vaca F et al Latent autoimmune diabetes in adults is perched between type 1 and type 2: evidence from adults in one region of Spain. Diabetes Metab Res Rev 2013; 29: 446–451. [DOI] [PubMed] [Google Scholar]
- 19. Bottazzo GF, Bosi E, Cull CA, Bonifacio E, Locatelli M, Zimmet P et al IA‐2 antibody prevalence and risk assessment of early insulin requirement in participants presenting with type 2 diabetes (UKPDS 71). Diabetologia 2005; 48: 703–708. [DOI] [PubMed] [Google Scholar]
- 20. Zinman B, Kahn SE, Haffner SM, O'Neill MC, Heise MA, Freed MI et al Phenotypic characteristics of GAD antibody‐positive recently diagnosed patients with type 2 diabetes in North America and Europe. Diabetes 2004; 53: 3193–3200. [DOI] [PubMed] [Google Scholar]
- 21. Zhou Z, Xiang Y, Ji L, Jia W, Ning G, Huang G et al Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic‐based cross‐sectional study. Diabetes 2013; 62: 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–163. [DOI] [PubMed] [Google Scholar]
- 23. Xiang Y, Huang G, Shan Z, Pan L, Luo S, Yang L et al Glutamic acid decarboxylase autoantibodies are dominant but insufficient to identify most Chinese with adult‐onset non‐insulin requiring autoimmune diabetes: LADA China study 5. Acta Diabetologica 2015; 52: 1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Torn C, Landin‐Olsson M, Ostman J, Schersten B, Arnqvist H, Blohme G et al Glutamic acid decarboxylase antibodies (GAD antibodies) is the most important factor for prediction of insulin therapy within 3 years in young adult diabetic patients not classified as Type 1 diabetes on clinical grounds. Diabetes Metab Res Rev 2000; 16: 442–447. [DOI] [PubMed] [Google Scholar]
- 25. Merger SR, Leslie RD, Boehm BO. The broad clinical phenotype of Type 1 diabetes at presentation. Diabet Med 2013; 30: 170–178. [DOI] [PubMed] [Google Scholar]
- 26. Johansen OE, Boehm BO, Grill V, Torjesen PA, Bhattacharya S, Patel S et al C‐peptide levels in latent autoimmune diabetes in adults treated with linagliptin versus glimepiride: exploratory results from a 2‐year double‐blind, randomized, controlled study. Diabetes Care 2014; 37: e11–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Autoantibody (GAD, IA2) titres in (A, B) white European (non‐significant), (C,D) Chinese (P<0.001), (E, F) Malay (P<0.001) and (G,H) Indian (P <0.001) participants with increasing age of onset. P values compared with white European cohort using the t‐test.
Figure S2. Autoantibody (GAD, IA2) titres in (A, B) German (non‐significant), (C, D) Chinese (P <0.001), (E, F) Malay (P <0.001) and (G, H) Indian (P <0.001) participants with increasing BMI. P values compared with white European cohort using the t‐test.
Figure S3. BMI values of islet cell antibody‐negative and islet cell antibody‐positive subgroups. (A) Mean BMI for white European islet cell antibody‐negative participants was 27.2 (95% CI 25.2–29.2). BMI of GAD antibody‐positive Europeans was 22.9 (95% CI 19.3–27.3; P<0.001), for IA2 antibody‐positive participants 24.2 (95% CI 20.7–28.4; P<0.001) and double antibody‐positive participants 24.0 (95% CI 19.9–28.1; P<0.001). (B) Mean BMI for Asians without islet cell antibodies was 27.8 (95% CI 27.4–28.1). BMI of Asian GAD antibody‐positive participants was 28.0 (95% CI 27.0–28.9; P=non‐significant), for IA2 antibody‐positive participants 27.2 (95% CI 26.4–28.0; P=non‐significant), and double antibody‐positive participants 26.1 (95% CI 24.7–28.6; P<0.01). Mean BMI for ethnic Chinese GAD antibody positives was 27.2 (95% CI 25.6–28.9; P=non‐significant), IA2 antibody‐positive participants 26.3 (95% CI 25.3–27.2; P<0.01). Mean BMI for ethnic Malay GAD antibody‐positive participants was 29.8 (95% C.I. 27.4–30.9; P<0.001) and 29.4 (95% CI 27.5–30.5; P<0.001) for IA2 antibody‐positive participants. Mean BMI for ethnic Indian GAD antibody‐positive participants was 28.0 (95% CI 25.5–30.1; P=non‐significant), IA2 antibody‐positive participants had a BMI of 28.7 kg/m2 (95% CI 26.4–30.9; P=non‐significant). P values compared with antibody‐negative participants within each cohort using the t‐test.


