Abstract
Even if osteoarthritis pathogenesis is still poorly understood, numerous evidences suggest that osteoblasts dysregulation plays a key role in osteoarthritis pathogenesis. An abnormal expression of OPG and RANKL has been described in osteoarthritis osteoblasts, which is responsible for abnormal bone remodeling and decreased mineralization. Alterations in genes expression are involved in dysregulation of osteoblast function, bone remodeling, and mineralization, leading to osteoarthritis development. Moreover, osteoblasts produce numerous transcription factors, growth factors, and other proteic molecules which are involved in osteoarthritis pathogenesis.
Keywords: bone, osteoarthritis, osteoblast
1. INTRODUCTION
Osteoarthritis is a chronic joint disease characterized by degeneration and loss of cartilage, synovial inflammation, and alteration of peri‐articular bone with osteophytes formation and subchondral bone sclerosis (Davis, Ettinger, Neuhaus, & Hauck, 1988; Valdes & Spector, 2010). These alterations are mediated by cells, such as chondrocytes in the cartilage and osteoclasts, osteoblasts, and osteocytes in the bone. Among these cells, osteoblasts, which are mesenchymal derived cells responsible for bone production and remodeling, regulate skeletal architecture and bone matrix mineralization by producing extracellular matrix proteins, and induce osteoclastogenesis by producing cytokines or by direct cell contact. In osteoarthritis, these cells seem to function differently with a different profile of genes expression. In this review, by considering that osteoblasts dysregulation is involved in numerous bone diseases, we want to focus current knowledge about the role of osteoblasts in osteoarthritis pathogenesis.
1.1. Osteoblast differentiation
Osteoblasts are mononuclear specialized cells derived from pluripotent mesenchymal stem cells (Caplan, 1991; Pittenger et al., 1999; Owen, 1988), which can differentiate via activation of different signaling transcription pathways, into different mesenchymal cells lineages, such as osteoblasts, chondrocytes, fibroblasts, myoblasts, and adipocytes (Friedenstein, Chailakhyan, & Gerasimov, 1987; Yamaguchi, Komori, & Suda, 2000).
Among these signaling transcription pathways, a key role in inducing mesenchymal cell differentiation into osteoblast differentiation at an early stage, is played by osterix (Osx) and Runt‐related transcription factor 2 (Runx‐2). Runx‐2 is encoded by Runx‐2 gene, which is also involved in inducing the expression of bone matrix protein genes, such as osteocalcin, osteopontin, type I collagen, and bone sialoprotein (Ducy, Zhang, Geoffroy, Ridall, & Karsenty, 1997; Komori et al., 1997; Miyoshi et al., 1991; Ogawa et al., 1993; Otto et al., 1997). Runx‐2 down‐regulation has been observed in the late stage of osteoblast maturation, when mature osteoblasts form mature bone (Komori, 2010). At a late stage Osx is responsible for inhibiting osteoblast differentiation (Komori, 2003).
1.2. The role of osteoblasts in bone metabolism
Osteoblasts are involved in the regulation of bone metabolism by synthesizing bone matrix that becomes progressively mineralized. In fact, osteoblasts are responsible for the deposition of calcium phosphate crystals, such as hydroxyapatite, and produce bone matrix constituents, such as type I collagen. Subsequently, bone matrix progresses into the mineralization phase in which osteoblasts play a role in the production of several proteins, such as sialoprotein, osteopontin, and osteocalcin, that are associated with the mineralized matrix in vivo (Maruotti, Corrado, Neve, & Cantatore, 2012; Neve, Corrado, & Cantatore, 2011). Bone matrix, which is constituted by aligned and ordered collagen fibrils complexed with noncollagenous proteins produced by osteoblasts, is subsequently mineralized via osteoblast regulation of calcium and phosphate local concentrations (Boskey, 1996, 1998).
Moreover, osteoblasts are responsible for osteoclast regulation. Osteoblasts express on their membrane or produce as soluble factor, nuclear factor (NF)‐ĸB ligand (RANKL). The interaction of this ligand, as a consequence of matrix metalloproteinases (MMPs) proteolysis, with RANK, a type I transmembrane receptor expressed on osteoclast precursors, induces osteoclast precursor differentiation into osteoclast (Figure 1a). Subsequently, the RANK‐RANKL complex formation induces the trimerization of RANK and the activation of tumor necrosis factor (TNF) receptor‐associated factor 6 (TRAF6). In turn, TRAF6 is involved in the activation of NF‐ĸB and mitogen‐activated protein kinases (MAPKs), such as p38 and Jun N‐terminal kinase (JNK), which are responsible for the activation of transcription factors such as c‐Src, c‐Fos, and microphtalmia transcription factor (MITF) (Kim et al., 1999; Kobayashi et al., 2001; Matsumoto, Sudo, Saito, Osada, & Tsujimoto, 2000).
Figure 1.
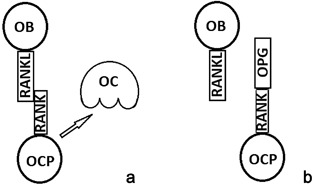
RANKL expressed on osteoblast (OB) mediates a signal for osteoclast (OC) differentiation via binding RANK expressed on osteoclast progenitors (OCP) (a). OPG is a soluble decoy receptor for RANKL, which is involved in the competitive inhibition of RANK/RANKL link, thus avoiding RANK activation and the following osteoclast activation (b)
Moreover, osteoblasts are involved in the regulation of osteoclastogenesis via modulating RANKL/osteoprotegerin (OPG) ratio (Hofbauer & Schoppet, 2004). In fact, osteoblasts synthesize OPG, a soluble decoy receptor for RANKL, which is involved in the competitive inhibition of RANK/RANKL link, thus avoiding RANK activation and the following osteoclast activation (Figure 1b) (Simonet et al., 1997).
Numerous hormones, including parathormon (PTH), vitamin D, calcitonin, oestrogen, serotonin, and leptin, are involved in the regulation of RANKL and OPG expression in osteoblasts (Neve et al., 2011).
1.3. Wnt/β‐catenin signaling pathway
Several osteoblast biological aspects, such as OPG expression, osteoblast proliferation, and differentiation, are regulated by the Wnt/β‐catenin signaling pathway (Bonewald and Johnson, 2008; Glass et al., 2005; Maruotti, Corrado, Neve, & Cantatore, 2013). The Wnt/β‐catenin pathway is characterized by Wnt binding to its coreceptor complex located at the cell surface, which is composed by the low‐density lipoprotein receptor‐related proteins 5 (LRP‐5) or 6, and a member of the frizzled (Fz) family of proteins (Figure 2a,b) (Tamai et al., 2000; Wehrli et al., 2000). Cytosolic β‐catenin is usually phosphorylated by kinases, such as glycogen synthase kinase 3 (GSK3) and casein kinase 1 (CK1), and constitutes a complex together with Axin and the tumor suppressor adenomatous polyposis coli (APC) (Moon, Bowerman, Boutros, & Perrimon, 2002). Axin plays a role in the phosphorylation of β‐catenin by favoring the union of GSK3 to cytosolic β‐catenin. APC is involved in β‐catenin binding to the ubiquitin‐mediated proteolysis pathway. Wnt binding to the coreceptor induces Frizzled activation and the recruitment of cytosolic Disheveled (Dvl) proteins. Subsequently, Dvl inhibits β‐catenin degradation and induces its accumulation and traslocation, turning it into a nuclear transcriptional regulator responsible for the expression of several target genes (Liu et al., 2002; Maruotti et al., 2013; Willert & Jones, 2006; Yu & Malenka, 2003). Wnt signaling is regulated by secreted frizzled‐related protein family (sFRP) and Wnt inhibitory factor (WIF‐1) (Aberle, Bauer, Stappert, Kispert, & Kemler, 1997), which inhibit the interaction of Wnt with its receptor Fz. Moreover, LRP5/6 activity is antagonized by proteins of the Dickoppf (Dkk) family and by sclerostin, a secreted glycoprotein that is mainly expressed by osteocytes (Westendorf, Kahler, & Schroeder, 2004).
Figure 2.
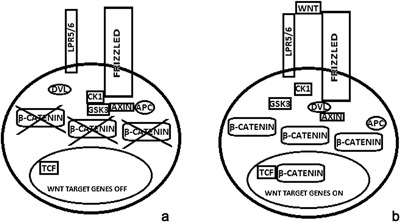
Wnt signaling: at the basal state, GSK3 and CK1 phoshorylate β‐catenin and induce its degradation in the cytosol (a); Wnt binding to LRP‐5/6 and frizzled promotes Dvl‐mediated inactivation of the Axin‐GSK3‐ CK1‐APC complex. Thus, β‐catenin degradation is blocked by avoiding its phosphorylation. Increased β‐catenin levels promote its traslocation into the nucleus, where it forms a complex with T cell factor (TCF) (b). Dvl, dishevelled; GSK3, Glycogen Synthase Kinase 3; CK1, Casein Kinase 1; APC, Adenomatous Polyposis Coli
1.4. Osteoblasts and osteoarthritis
During last years, an increasing amount of evidences has accumulated in the literature about the role of osteoblasts in osteoarthritis pathogenesis (Corrado, Cantatore, Grano, & Colucci, 2005; Dequeker, Mohan, Finkelman, Aerssens, & Baylink, 1993; El Miedany, Mehanna, & El Baddini, 2000; Hilal, Martel‐Pelletier, Pelletier, Ranger, & Lajeunesse, 1998; Lajeunesse & Reboul, 2003).
There is a close correlation between the OPG/RANK/RANKL system and the subchondral bone alteration observed in osteoarthritis. In fact, an altered expression of OPG and RANKL has been seen in osteoarthritis osteoblasts (Kwan Tat, Pelletier, Amiable, et al., 2008; Kwan Tat, Pelletier, Lajeunesse, et al., 2008)
Two different groups of osteoblasts have been found in osteoarthritis, called low osteoarthritis osteoblasts, which are analogous to normal osteoblasts and are characterized by low levels of prostaglandin E2 (PGE2) and interleukin‐6 (IL‐6), and high osteoarthritis osteoblasts, which are characterized by high levels of PGE2 and IL‐6 (Massicotte et al., 2002). Low osteoarthritis osteoblasts are characterized by decrease in OPG expression and by increased RANKL level, while high osteoarthritis osteoblasts are characterized by increased OPG production and by reduced RANKL expression (Tat et al., 2006). Low osteoarthritis subchondral osteoblasts are characterized by significantly increased membranous RANKL levels compared to normal and high osteoarthritis osteoblasts. Moreover, the treatment with osteotropic factors, such as vitamin D3, IL‐1β, TNF‐α, PGE2, IL‐6, but not PTH, and IL‐17, has been correlated with an increased membranous localization of RANKL on low osteoarthritis osteoblasts compared with high osteoarthritis osteoblasts (Tat et al., 2008). This evidence might explain the different metabolic states of subchondral bone osteoblast subpopulations, with low osteoarthritis osteoblasts probably involved in inducing bone resorption, and high osteoarthritis osteoblasts probably involved in favoring bone formation.
1.5. Rate of bone remodeling in osteoarthritis
The rate of bone remodeling is variable across the course of the disease. Early osteoarthritis is characterized by increased remodeling in the subchondral bone, whereas a reduction in bone resorption and an increase in bone formation occur in late osteoarthritis (Findlay & Atkins, 2014). The abnormal bone remodeling and the decreased mineralization are responsible for the altered bone microarchitecture, characterized by increased trabecular number, reduced trabecular spacing, and reduced hardness of the bone (Dall'Ara, Ohman, Baleani, & Viceconti, 2011; Li & Aspden, 1997; Findlay & Atkins, 2014). The extracellular matrix produced by human osteoarthritic subchondral bone osteoblasts is characterized by a reduced amount of minerals and abnormal organization of matrix (Prasadam et al., 2013). The abnormal osteoblast metabolism might also be responsible for the abnormal mineralization of subchondral bone in osteoarthritis. In fact, elevated type I collagen synthesis has been observed in osteoarthritis bone tissue (Mansell & Bailey, 1998). This might be involved in inducing excessive mineralization and subchondral bone sclerosis. In particular, an altered ratio of α1 and α2 chains of type I collagen, with an increased production of the α1 chain, plays a key role in the abnormal mineralization of osteoarthritis bone and may be responsible for the increased levels of transforming growth factor‐β (TGF‐β) in osteoarthritis osteoblasts (Couchourel et al., 2009; Zhen et al., 2013), which is involved in osteophytes production and promotes mineralization via the inhibition of bone morphogenic protein‐2 (BMP‐2) (Neve et al., 2011). Moreover, altered bone remodeling is also involved in osteophytes formation (Findlay & Atkins, 2014). Even if adipokines, including adiponectin, resistin, and visfatin have been associated with the pathogenesis of osteoarthritis, Junker et al. (2016) have recently demonstrated that adipokines do not influence Wnt signaling pathway. Moreover, adiponectin involvement in p38 MAPK signaling activation in osteoblasts has been observed, suggesting that adipokines do not directly influence osteophyte formation but play a proinflammatory role in osteoarthritis (Junker et al., 2016).
1.6. Growth factors effects
Osteoarthritis osteoblasts produce the hepatocyte growth factor (HGF) which plays a key role in cartilage loss. HGF is involved in TGF‐β1 expression and inhibits osteoblast response to BMP‐2 (Abed et al., 2015).
As demonstrated in subchondral bone cell culture, high levels of TGF‐β increase levels of DKK‐2, while TGF‐β and DKK‐2 inhibition corrects the abnormal mineralization (Chan et al., 2011).
Moreover, elevated levels of TGF‐β, insulin growth factor‐I (IGF‐I), and IGF‐II have been observed in cultures of human osteoarthritic subchondral bone osteoblasts and in cortical bone explants from the iliac crest of patients affected by osteoarthritis, suggesting an increased biosynthetic activity of osteoblasts (Dequeker et al., 1993; Massicotte et al., 2006).
1.7. Ephrins role
Ephrin B4 (EphB4) receptor is highly expressed in low subchondral osteoarthritis osteoblasts, while no differences have been seen between normal and high osteoarthritis osteoblasts in EphB4 receptor expression. Activation of EphB4 receptor by Ephrin B2, inhibits the expression of IL‐1β, IL‐6 and RANKL, but not of OPG (Kwan Tat, Pelletier, Amiable, et al., 2008; Kwan Tat, Pelletier, Lajeunesse, et al., 2008), suggesting that activation of EphB4 by ephrin B2 is involved in the altered subchondral bone metabolism in osteoarthritis by reducing resorption factors levels and function.
1.8. Alterations in osteoblast genes expression
The fact that osteoblasts are characterized by an altered function and metabolism in osteoarthritis is also demonstrated by alteration in genes expression which is different from that in either osteoporotic or normal bone. Numerous genes involved in osteoblast function, bone remodeling, and mineralization, such as genes expressing proteins of the Wnt and TGF‐β/BMP signaling pathway, are differently expressed in osteoarthritis (Hopwood, Tsykin, Findlay, & Fazzalari, 2007). Several genes, including soluble Wnts, inhibitors, receptors, co‐receptors, several kinases, and transcription factors, are downregulated in osteoarthritis mesenchimal cells, which include osteoblasts and chondrocytes, during osteogenesis in vitro (Tornero‐Esteban et al., 2015). Several factors involved in Wnt/β‐catenin signaling pathway regulation are dysregulated in osteoarthritis. In fact, reduced levels of Rspo‐2, a Wnt/β‐catenin signaling pathway agonist, and high level of sclerostin, a Wnt/β‐catenin signaling pathway antagonist, have been observed in primary human osteoarthritis osteoblasts cultures (Abed et al., 2011, 2014).
1.9. Increased levels of transcriptional regulators controlling osteoblastogenesis
An increased expression of transcriptional factors for osteoblast differentiation, such as RUNX2 and Osx, has been found in bone samples obtained by osteoarthritis patients than compared to osteoporotic patients, suggesting a more intense osteoblastogenesis in osteoarthritis than in osteoporosis (Dragojevič, Logar, Komadina, & Marc, 2011).
Several transcription factors involved in the regulation of the osteogenic lineage have been found dysregulated in osteoarthritis. In fact, by using primary osteoblast cultures from bone samples of osteoarthritis patients, dysregulated expression of TWIST1, TGFβ1, and SMAD3 mRNA has been observed (Kumarasinghe, Sullivan, Kuliwaba, Fazzalari, & Atkins, 2012).
1.10. Role of systemic and local factors on osteoblasts
Vitamin D3 treatment has been correlated to a significant increase of osteocalcin in osteoarthritic osteoblasts, proportional to the grade of joint damage (Cantatore et al., 2004; Corrado et al., 2005; Gevers & Dequeker, 1987), suggesting that the altered behavior of osteoarthritic osteoblasts may be related to an abnormal response to systemic or local factors (Cantatore et al., 2004). Moreover, several clinical ex/in vivo and in vitro studies have confirmed the presence of elevated alkaline phosphatase activity and increased osteocalcin levels in primary human osteoarthritis subchondral osteoblasts (Cantatore et al., 2004; Hilal et al., 1998, 2001; Mansell, Tarlton, & Bailey, 1997). On the contrary, no difference in osteopontin levels was found between osteoarthritis and normal osteoblasts (Couchourel et al., 2009). Furthermore, osteoarthritis osteoblasts produce high levels of leptin (Mutabaruka, Aoulad Aissa, Delalandre, Lavigne, & Lajeunesse, 2010). High leptin levels in osteoarthritis might play a role in increased levels of bone markers, such as alkaline phosphatase and osteocalcin observed in osteoblasts (Dumond et al., 2003).
1.11. Hypoxia and vascular endothelial growth factor (VEGF) effects on osteoblasts
Alterations of bone vascularization parameters, which may be involved in inducing ischemic episodes associated with hypoxic conditions, have been supposed in osteoarthritis. In fact, hypoxia may stimulate osteoblast production of leptin via hypoxia‐inducible factors (Hif)‐2 regulation, particularly under vitamin D3 stimulation. On the contrary DKK2 is primarily regulated by vitamin D3 rather than hypoxia (Bouvard et al., 2014). Hypoxia also increases the osteoblast production of PGE2, cyclooxygenase 2, angiopoietin‐like 4, type II collagen α1 chain, and insulin‐like growth factor binding protein 1. Moreover, an in vitro study, by using culture of primary osteoblasts isolated from knee bone from patients affected by osteoarthritis, has demonstrated that hypoxia modifies osteoblast phenotype, including the expression of genes that regulate bone matrix, bone remodeling, and bone vasculature (Chang, Jackson, Wardale, & Jones, 2014).
Moreover, osteoblast production of VEGF seems to have a role in osteoarthritis pathogenic mechanisms, as demonstrated by a study on primary human osteoarthritis osteoblast cultures, which showed an increased VEGF expression compared to normal and osteoporotic osteoblasts, both under basal conditions than in the presence of vitamin D3. Moreover, vitamin D3 significantly improved VEGF expression in normal and pathological osteoblasts, suggesting the crucial role of vitamin D3 supplementation in metabolic bone diseases (Corrado, Neve, & Cantatore, 2013).
1.12. Endothelin‐1 (ET‐1) effects
Recently, a role for endothelin‐1 (ET‐1) has been proposed in osteoarthritis pathogenesis (Sin, Tang, Wen, Chung, & Chiu, 2015). In fact, ET‐1 is involved in the degradation of articular cartilage in osteoarthritis, and in osteoblast proliferation and bone formation. ET‐1 is responsible for inducing oncostatin M expression in osteoarthritis osteoblasts by trans‐activating the oncostatin M gene promoter via the transcription factor Ets‐1 (Wu et al., 2016).
1.13. Osteoblast production of proteases
As suggested by in vitro evidence, altered communications between subchondral bone osteoblasts and articular cartilage chondrocytes may be involved in osteoarthritis pathogenesis, by producing abnormal levels of enzymes, such as A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) and MMPs (Prasadam, Crawford, & Xiao, 2012; Sakao et al., 2009; Sanchez et al., 2005).
Moreover, increased levels of MMPs, such as MMP‐13, may also be involved in osteoarthritis cartilage degradation (Sakao et al., 2008). In fact, MMP‐13 is responsible for degrading opticin, a protein typically associated with the extracellular matrix, which plays a role in the structural stability of cartilage. Its cleavage by MMP‐13 may be involved in cartilage degradation (Monfort et al., 2008).
Moreover, lysosomal cathepsins have been reported to play a key role in osteoarthritis pathogenesis and, in particular, cathepsin K has been found highly expressed in osteoarthritis (Logar et al., 2007). Even if cathepsin K is considered as predominantly expressed by osteoclasts, osteoblasts may directly contribute to its production (Mandelin et al., 2006). Nevertheless, the real impact of osteoblast cathepsin K synthesis in osteoarthritis remains to be investigated.
2. CONCLUDING REMARKS
Osteoarthritis pathogenesis is still poorly understood and there are not treatment available to repair degraded cartilage and altered bone. Accumulating evidences underline the important role of osteoblasts in osteoarthritis. In fact, osteoblasts are responsible for inducing altered expression of OPG and RANKL, for altering bone microarchitecture by abnormal bone remodeling and decreased mineralization. Moreover, alterations in gene expression are involved in inducing a dysregulation of osteoblast function, bone remodeling, and mineralization, leading to osteoarthritis development. Numerous transcription factors, growth factors, and other proteic molecules involved in osteoarthritis pathogenesis, are produced by osteoblasts. A full understanding of the osteoarthritis pathogenesis could lead to the development of new therapeutic strategies in this disease.
3. CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.
Maruotti N, Corrado A, Cantatore FP. Osteoblast role in osteoarthritis pathogenesis. J Cell Physiol. 2017;232: 2957–2963. https://doi.org/10.1002/jcp.25969
REFERENCES
- Abed, É , Chan, T. F. , Delalandre, A. , Martel‐Pelletier, J. , Pelletier, J. P. , & Lajeunesse, D. (2011). R‐spondins are newly recognized players in osteoarthritis that regulate Wnt signaling in osteoblasts. Arthritis & Rheumatism, 63, 3865–3875. [DOI] [PubMed] [Google Scholar]
- Abed, É , Couchourel, D. , Delalandre, A. , Duval, N. , Pelletier, J. P. , Martel‐Pelletier, J. , & Lajeunesse, D. (2014). Low sirtuin 1 levels in human osteoarthritis subchondral osteoblasts lead to abnormal sclerostin expression which decreases Wnt/β‐catenin activity. Bone, 59, 28–36. [DOI] [PubMed] [Google Scholar]
- Abed, É , Bouvard, B. , Martineau, X. , Jouzeau, J. Y. , Reboul, P. , & Lajeunesse, D. (2015). Elevated hepatocyte growth factor levels in osteoarthritis osteoblasts contribute to their altered response to bone morphogenetic protein‐2 and reduced mineralization capacity. Bone, 75, 111–119. [DOI] [PubMed] [Google Scholar]
- Aberle, H. , Bauer, A. , Stappert, J. , Kispert, A. , & Kemler, R. (1997). Beta‐catenin is a target for the ubiquitin‐proteasome pathway. EMBO Journal, 16, 3797–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald, L. F. , & Johnson, M. L. (2008). Osteocytes, mechanosensing and Wnt signaling. Bone, 42, 606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey, A. L. (1996). Matrix proteins and mineralization: An overview. Connective Tissue Research, 35, 357–363. [DOI] [PubMed] [Google Scholar]
- Boskey, A. L. (1998). Biomineralization: Conflicts, challenges, and opportunities. J Cell Biochem, Suppl 30‐31, 83–91. [PubMed] [Google Scholar]
- Bouvard, B. , Abed, E. , Yéléhé‐Okouma, M. , Bianchi, A. , Mainard, D. , Netter, P. , … Reboul, P. (2014). Hypoxia and vitamin D differently contribute to leptin and dickkopf‐related protein 2 production in human osteoarthritic subchondral bone osteoblasts. Arthritis Res Ther, 16, 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantatore, F. P. , Corrado, A. , Grano, M. , Quarta, L. , Colucci, S. , & Melillo, N. (2004). Osteocalcin synthesis by human osteoblasts from normal and osteoarthritic bone after vitamin D3 stimulation. Clinical Rheumatology, 23, 490–495. [DOI] [PubMed] [Google Scholar]
- Caplan, A. I. (1991). Mesenchymal stem cells. Journal of Orthopaedic Research, 9, 641–650. [DOI] [PubMed] [Google Scholar]
- Chan, T. F. , Couchourel, D. , Abed, E. , Delalandre, A. , Duval, N. , & Lajeunesse, D. (2011). Elevated DKK‐2 levels contribute to the abnormal phenotype of human osteoarthritic osteoblasts. Journal of Bone and Mineral Research, 26, 1399–1410. [DOI] [PubMed] [Google Scholar]
- Chang, J. , Jackson, S. G. , Wardale, J. , & Jones, S. W. (2014). Hypoxia modulates the phenotype of osteoblasts isolated from knee osteoarthritis patients, leading to undermineralized bone nodule formation. Arthritis Rheumatol, 66, 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrado, A. , Cantatore, F. P. , Grano, M. , & Colucci, S. (2005). Neridronate and human osteoblasts in normal, osteoporotic and osteoarthritic subjects. Clinical Rheumatology, 24, 527–534. [DOI] [PubMed] [Google Scholar]
- Corrado, A. , Neve, A. , & Cantatore, F. P. (2013). Expression of vascular endothelial growth factor in normal, osteoarthritic and osteoporotic osteoblasts. Clinical and Experimental Medicine, 13, 81–84. [DOI] [PubMed] [Google Scholar]
- Couchourel, D. , Aubry, I. , Delalandre, A. , Lavigne, M. , Martel‐Pelletier, J. , Pelletier, J. P. , & Lajeunesse, D. (2009). Altered mineralization of human osteoarthritic osteoblasts is attributable to abnormal type I collagen production. Arthritis & Rheumatism, 60, 1438–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Ara, E. , Ohman, C. , Baleani, M. , & Viceconti, M. (2011). Reduced tissue hardness of trabecular bone is associated with severe osteoarthritis. Journal of Biomechanics, 44, 1593–1598. [DOI] [PubMed] [Google Scholar]
- Davis, M. A. , Ettinger, W. H. , Neuhaus, J. M. , & Hauck, W. W. (1988). Sex differences in osteoarthritis of the knee. The Role of Obesity. American Journal of Epidemiology, 127, 1019–1030. [DOI] [PubMed] [Google Scholar]
- Dequeker, J. , Mohan, S. , Finkelman, R. D. , Aerssens, J. , & Baylink, D. J. (1993). Generalized osteoarthritis associated with increased insulin‐like growth factor types I and II and transforming growth factor beta in cortical bone from the iliac crest. Possible mechanism of increased bone density and protection against osteoporosis. Arthritis & Rheumatism, 36, 1702–1708. [DOI] [PubMed] [Google Scholar]
- Dragojevič, J. , Logar, D. B. , Komadina, R. , & Marc, J. (2011). Osteoblastogenesis and adipogenesis are higher in osteoarthritic than in osteoporotic bone tissue. Archives of Medical Research, 42, 392–397. [DOI] [PubMed] [Google Scholar]
- Ducy, P. , Zhang, R. , Geoffroy, V. , Ridall, A. L. , & Karsenty, G . (1997). Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell, 89, 747–754. [DOI] [PubMed] [Google Scholar]
- Dumond, H. , Presle, N. , Terlain, B. , Mainard, D. , Loeuille, D. , Netter, P. , & Pottie, P. (2003). Evidence for a key role of leptin in osteoarthritis. Arthritis & Rheumatism, 48, 3118–3129. [DOI] [PubMed] [Google Scholar]
- El Miedany, Y. M. , Mehanna, A. N. , & El Baddini, M. A. (2000). Altered bone mineral metabolism in patients with osteoarthritis. Joint Bone Spine, 67, 521–527. [DOI] [PubMed] [Google Scholar]
- Findlay, D. M. , & Atkins, G. J. (2014). Osteoblast‐chondrocyte interactions in osteoarthritis. Current Osteoporosis Reports, 12, 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenstein, A. J. , Chailakhyan, R. K. , & Gerasimov, U. V. (1987). Bone marrow osteogenic stem cells: In vitro cultivation and transplantation in diffusion chambers. Cell and Tissue Kinetics, 20, 263–272. [DOI] [PubMed] [Google Scholar]
- Gevers, G. , & Dequeker, J. (1987). Collagen and non‐collagenous protein content (osteocalcin, sialoprotein, proteoglycan) in the iliac crest bone and serum osteocalcin in women with and without hand osteoarthritis. Collagen and Related Research, 7, 435–442. [DOI] [PubMed] [Google Scholar]
- Glass, D. A., 2nd , Bialek, P. , Ahn, J. D. , Starbuck, M. , Patel, M. S. , Clevers, H. , … Karsenty, G. (2005). Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Developmental Cell, 8, 751–764. [DOI] [PubMed] [Google Scholar]
- Hilal, G. , Martel‐Pelletier, J. , Pelletier, J. P. , Ranger, P. , & Lajeunesse, D. (1998). Osteoblast‐like cells from human subchondral osteoarthritic bone demonstrate an altered phenotype in vitro: Possible role in subchondral bone sclerosis. Arthritis & Rheumatism, 41, 891–899. [DOI] [PubMed] [Google Scholar]
- Hilal, G. , Massicotte, F. , Martel‐Pelletier, J. , Fernandes, J. C. , Pelletier, J. P. , & Lajeunesse, D. (2001). Endogenous prostaglandin E2 and insulin like growth factor 1 can modulate the levels of parathyroid hormone receptor in human osteoarthritic osteoblasts. Journal of Bone and Mineral Research, 16, 713–721. [DOI] [PubMed] [Google Scholar]
- Hofbauer, L. C. , & Schoppet, M. (2004). Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA, 292, 490–495. [DOI] [PubMed] [Google Scholar]
- Hopwood, B. , Tsykin, A. , Findlay, D. M. , & Fazzalari, N. L. (2007). Microarray gene expression profiling of osteoarthritic bone suggests altered bone remodelling, WNT and transforming growth factor‐beta/bone morphogenic protein signaling. Arthritis Research & Therapy, 9, R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker, S. , Frommer, K. W. , Krumbholz, G. , Tsiklauri, L. , Gerstberger, R. , Rehart, S. , … Neumann, E. (2016). Expression of adipokines in osteoarthritis osteophytes and their effect on osteoblasts. Matrix Biol. Pii: S0945‐053X(16), 30195–30190. https://doi.org10.1016/j.matbio.2016.11.005. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Kim, H. H. , Lee, D. E. , Shin, J. N. , Lee, Y. S. , Jeon, Y. M. , Chung, C. H. , … Lee, Z. H. (1999). Receptor activator of NF‐kB recruits multiple TRAF family adaptors and activates c‐jun N‐terminal kinase. FEBS Letters, 443, 297–302. [DOI] [PubMed] [Google Scholar]
- Kobayashi, N. , Kadono, Y. , Naito, A. , Matsumoto, K. , Yamamoto, T. , Tanaka, S. , & Inoue, J. (2001). Segregation of TRAF6‐mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO Journal, 20, 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori, T . (2003). Requisite roles of Runx2 and Cbfb in skeletal development. Journal of Bone Mineral Metabolism, 21, 193 –197. [DOI] [PubMed] [Google Scholar]
- Komori, T . (2010). Regulation of bone development and extracellularmatrix protein genes by RUNX2. Cell and Tissue Research, 339, 189–195. [DOI] [PubMed] [Google Scholar]
- Komori, T. , Yagi, H. , Nomura, S. , Yamaguchi, A. , Sasaki, K. , Deguchi, K. , … Kishimoto, T . (1997). Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell, 89, 755–764. [DOI] [PubMed] [Google Scholar]
- Kumarasinghe, D. D. , Sullivan, T. , Kuliwaba, J. S. , Fazzalari, N. L. , & Atkins, G. J. (2012). Evidence for the dysregulated expression of TWIST1, TGFβ1 and SMAD3 in differentiating osteoblasts from primary hip osteoarthritis patients. Osteoarthritis and Cartilage, 20, 1357–1366. [DOI] [PubMed] [Google Scholar]
- Kwan Tat, S. , Pelletier, J. P. , Amiable, N. , Boileau, C. , Lajeunesse, D. , Duval, N. , & Martel‐Pelletier, J. (2008). Activation of the receptor EphB4 by its specific ligand ephrin B2 in human osteoarthritic subchondral bone osteoblasts. Arthritis & Rheumatism, 58, 3820–3830. [DOI] [PubMed] [Google Scholar]
- Kwan Tat, S. , Pelletier, J. P. , Lajeunesse, D. , Fahmi, H. , Lavigne, M. , & Martel‐Pelletier, J. (2008). The differential expression of osteoprotegerin (OPG) and receptor activator of nuclear factor kappaB ligand (RANKL) in human osteoarthritic subchondral bone osteoblasts is an indicator of the metabolic state of these disease cells. Clinical and Experimental Rheumatology, 26, 295–304. [PMC free article] [PubMed] [Google Scholar]
- Lajeunesse, D. , & Reboul, P. (2003). Subchondral bone in osteoarthritis: A biologic link with articular cartilage leading to abnormal remodeling. Current Opinion in Rheumatology, 15, 628–633. [DOI] [PubMed] [Google Scholar]
- Li, B. , & Aspden, R. M. (1997). Material properties of bone from the femoral neck and calcar femorale of patients with osteoporosis or osteoarthritis. Osteoporosis International, 7, 450–456. [DOI] [PubMed] [Google Scholar]
- Liu, C. , Li, Y. , Semenov, M. , Han, C. , Baeg, G. H. , Tan, Y. , … He, X. (2002). Control of bcatenin phosphorylation/degradation by a dual‐kinase mechanism. Cell, 108, 837–847. [DOI] [PubMed] [Google Scholar]
- Logar, D. B. , Komadina, R. , Prezelj, J. , Ostanek, B. , Trost, Z. , & Marc, J. (2007). Expression of bone resorption genes in osteoarthritis and in osteoporosis. Journal of Bone and Mineral Metabolism, 25, 219–225. [DOI] [PubMed] [Google Scholar]
- Mandelin, J. , Hukkanen, M. , Li, T. F. , Korhonen, M. , Liljeström, M. , Sillat, T. , … Konttinen, Y. T. (2006). Human osteoblasts produce cathepsin K. Bone, 38, 769–777. [DOI] [PubMed] [Google Scholar]
- Mansell, J. P. , & Bailey, A. J. (1998). Abnormal cancellous bone collagen metabolism in osteoarthritis. The Journal of Clinical Investigation, 101, 1596–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell, J. P. , Tarlton, J. F. , & Bailey, A. J. (1997). Biochemical evidence for altered subchondral bone collagen metabolism in osteoarthritis of the hip. British Journal of Rheumatology, 36, 16–19. [DOI] [PubMed] [Google Scholar]
- Maruotti, N. , Corrado, A. , Neve, A. , & Cantatore, F. P. (2012). Bisphosphonates: Effects on osteoblast. European Journal of Clinical Pharmacology, 68, 1013–1018. [DOI] [PubMed] [Google Scholar]
- Maruotti, N. , Corrado, A. , Neve, A. , & Cantatore, F. P. (2013). Systemic effects of Wnt signaling. Journal of Cellular Physiology, 228, 1428–1432. [DOI] [PubMed] [Google Scholar]
- Massicotte, F. , Aubry, I. , Martel‐Pelletier, J. , Pelletier, J. P. , Fernandes, J. , & Lajeunesse, D. (2006). Abnormal insulin‐like growth factor 1 signaling in human osteoarthritic subchondral bone osteoblasts. Arthritis Research & Therapy, 8, R177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massicotte, F. , Lajeunesse, D. , Benderdour, M. , Pelletier, J. P. , Hilal, G. , Duval, N. , & Martel‐Pelletier, J. (2002). Can altered production of interleukin‐1beta, interleukin‐6, transforming growth factor‐beta and prostaglandin E(2) by isolated human subchondral osteoblasts identify two subgroups of osteoarthritic patients? Osteoarthritis and Cartilage, 10, 491–500. [DOI] [PubMed] [Google Scholar]
- Matsumoto, M. , Sudo, T. , Saito, T. , Osada, H. , & Tsujimoto, M. (2000). Involvement of p38 mitogen‐activated protein kinase signaling pathway in osteoclastogenesis mediated by receptor activator of NF‐kB ligand (RANKL). The Journal of Biological Chemistry, 275, 31155–31161. [DOI] [PubMed] [Google Scholar]
- Miyoshi, H. , Shimizu, K. , Kozu, T. , Maseki, N. , Kaneko, Y. , & Ohki, M . (1991). t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene, AML1. Proceedings of the National Academy of Sciences of the United States of America, 88, 10431 –10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfort, J. , Tardif, G. , Roughley, P. , Reboul, P. , Boileau, C. , Bishop, P. N. , … Martel‐Pelletier, J. (2008). Identification of opticin, a member of the small leucine‐rich repeat proteoglycan family, in human articular tissues: A novel target for MMP‐13 in osteoarthritis. Osteoarthritis and Cartilage, 16, 749–755. [DOI] [PubMed] [Google Scholar]
- Moon, R. T. , Bowerman, B. , Boutros, M. , & Perrimon, N. (2002). The promise and perils of Wnt signaling through b‐catenin. Science, 296, 1644–1646. [DOI] [PubMed] [Google Scholar]
- Mutabaruka, M. S. , Aoulad Aissa, M. , Delalandre, A. , Lavigne, M. , & Lajeunesse, D. (2010). Local leptin production in osteoarthritis subchondral osteoblasts may be responsible for their abnormal phenotypic expression. Arthritis Research & Therapy 12, R20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve, A. , Corrado, A. , & Cantatore, F. P. (2011). Osteoblast physiology in normal and pathological conditions. Cell and Tissue Research, 343, 289–302. [DOI] [PubMed] [Google Scholar]
- Ogawa, E. , Inuzuka, M. , Maruyama, M. , Satake, M. , Naito‐Fujimoto, M. , Ito, Y. , & Shigesada, K . (1993). Molecular cloning and characterization of PEBP2 beta, the heterodimeric partner of a novel Drosophila runt‐related DNA binding protein PEBP2 alpha. Virology, 194, 314–331. [DOI] [PubMed] [Google Scholar]
- Otto, F. , Thornell, A. P. , Crompton, T. , Denzel, A. , Gilmour, K. C. , Rosewell, I. R. , … Owen, M. J . (1997). Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell, 89, 765–771. [DOI] [PubMed] [Google Scholar]
- Owen, M. (1988). Marrow stromal stem cells. Journal of Cell Science. Supplement, 10, 63–76. [DOI] [PubMed] [Google Scholar]
- Pittenger, M. F. , Mackay, A. M. , Beck, S. C. , Jaiswal, R. K. , Douglas, R. , Mosca, J. D. , … Marshak, D. R. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284, 143–147. [DOI] [PubMed] [Google Scholar]
- Prasadam, I. , Crawford, R. , & Xiao, Y. (2012). Aggravation of ADAMTS and matrix metalloproteinase production and role of ERK1/2 pathway in the interaction of osteoarthritic subchondral bone osteoblasts and articular cartilage chondrocytes—possible pathogenic role in osteoarthritis. Journal of Rheumatology, 39, 621–634. [DOI] [PubMed] [Google Scholar]
- Prasadam, I. , Farnaghi, S. , Feng, J. Q. , Gu, W. , Perry, S. , Crawford, R. , & Xiao, Y. (2013). Impact of extracellular matrix derived from osteoarthritis subchondral bone osteoblasts on osteocytes: Role of integrinβ1 and focal adhesion kinase signaling cues. Arthritis Research & Therapy, 15, R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakao, K. , Takahashi, K. A. , Mazda, O. , Arai, Y. , Tonomura, H. , Inoue, A. , … Kubo, T. (2008). Enhanced expression of interleukin‐6, matrix metalloproteinase‐13, and receptor activator of NF‐kappaB ligand in cells derived from osteoarthritic subchondral bone. Journal of Orthopaedic Science, 13, 202–210. [DOI] [PubMed] [Google Scholar]
- Sakao, K. , Takahashi, K. A. , Arai, Y. , Saito, M. , Honjo, K. , Hiraoka, N. , … Kubo, T. (2009). Osteoblasts derived from osteophytes produce interleukin‐6, interleukin‐8, and matrix metalloproteinase‐13 in osteoarthritis. Journal of Bone and Mineral Metabolism, 27, 412–423. [DOI] [PubMed] [Google Scholar]
- Sanchez, C. , Deberg, M. A. , Piccardi, N. , Msika, P. , Reginster, J. Y. , & Henrotin, Y. E. (2005). Osteoblasts from the sclerotic subchondral bone downregulate aggrecan but upregulate metalloproteinases expression by chondrocytes. This effect is mimicked by interleukin‐6, ‐1beta and oncostatin M pre‐treated non‐sclerotic osteoblasts. Osteoarthritis and Cartilage, 13, 979–987. [DOI] [PubMed] [Google Scholar]
- Simonet, W. S. , Lacey, D. L. , Dunstan, C. R. , Kelley, M. , Chang, M. S. , Lüthy, R. , … Boyle, W. J. (1997). Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell, 89, 309–319. [DOI] [PubMed] [Google Scholar]
- Sin, A. , Tang, W. , Wen, C. Y. , Chung, S. K. , & Chiu, K. Y. (2015). The emerging role of endothelin‐1 in the pathogenesis of subchondral bone disturbance and osteoarthritis. Osteoarthritis and Cartilage, 23, 516–524. [DOI] [PubMed] [Google Scholar]
- Tamai, K. , Semenov, M. , Kato, Y. , Spokony, R. , Liu, C. , Katsuyama, Y. , … He, X. (2000). LDL‐receptor‐related proteins in Wnt signal transduction. Nature, 407, 530–535. [DOI] [PubMed] [Google Scholar]
- Tat, S. K. , Padrines, M. , Theoleyre, S. , Couillaud‐Battaglia, S. , Heymann, D. , Redini, F. , & Fortun, Y. (2006). OPG/membranous‐RANKL complex is internalized via the clathrin pathway before a lysosomal and a proteasomal degradation. Bone, 39, 706–715. [DOI] [PubMed] [Google Scholar]
- Tat, S. K. , Pelletier, J. P. , Lajeunesse, D. , Fahmi, H. , Duval, N. , & Martel‐Pelletier, J. (2008). Differential modulation of RANKL isoforms by human osteoarthritic subchondral bone osteoblasts: Influence of osteotropic factors. Bone, 43, 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero‐Esteban, P. , Peralta‐Sastre, A. , Herranz, E. , Rodríguez‐Rodríguez, L. , Mucientes, A. , Abásolo, L. , … Lamas, J. R. (2015). Altered expression of wnt signaling pathway components in osteogenesis of mesenchymal stem cells in osteoarthritis patients. PLoS ONE, 10:e0137170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes, A. M. , & Spector, T. D. (2010). The genetic epidemiology of osteoarthritis. Current Opinion in Rheumatology, 22, 139–143. [DOI] [PubMed] [Google Scholar]
- Wehrli, M. , Dougan, S. T. , Caldwell, K. , O'Keefe, L. , Schwartz, S. , Vaizel‐Ohayon, D. , … DiNardo, S. (2000). Arrow encodes an LDL‐receptor‐related protein essential for Wingless signaling. Nature, 407, 527–530. [DOI] [PubMed] [Google Scholar]
- Westendorf, J. J. , Kahler, R. A. , & Schroeder, T. M. (2004). Wnt signaling in osteoblasts and bone diseases. Gen, 341, 19–39. [DOI] [PubMed] [Google Scholar]
- Willert, K. , & Jones, K. A. (2006). Wnt signaling: Is the party in the nucleus? Genes & Development, 20, 1394–1404. [DOI] [PubMed] [Google Scholar]
- Wu, R. , Wang, W. , Huang, G. , Mao, X. , Chen, Y. , Tang, Q. , & Liao, L. (2016). Endothelin‐1 induces oncostatin M expression in osteoarthritis osteoblasts by trans‐activating the oncostatin M gene promoter via Ets‐1. Molecular Medicine Reports, 13, 3559–3566. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, A. , Komori, T. , & Suda, T. (2000). Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocrine Reviews, 21, 393–411. [DOI] [PubMed] [Google Scholar]
- Yu, X. , & Malenka, R. C. (2003). Beta‐catenin is critical for dendritic morphogenesis. Nature Neuroscience, 6, 1169–1177. [DOI] [PubMed] [Google Scholar]
- Zhen, G. , Wen, C. , Jia, X. , Li, Y. , Crane, JL. , Mears, SC. , … Cao, X . (2013). Inhibition of TGF‐β signaling in mesenchymal stemcells of subchondral bone attenuates osteoarthritis. Nature Medicine, 19, 704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]


