Abstract
BACKGROUND
The phenolic composition of grapes is key when making decisions about harvest date and ensuring the quality of grapes. The present study aimed to investigate the relationship between the detailed phenolic composition of grapes and the agronomic parameters and hyperspectral indices, with the latter being measured via field radiometry techniques.
RESULTS
Good correlations were found between phenolic composition (both anthocyanin and flavanol composition) and some hyperspectral indices related to vigor, such as the NDVI (normalized difference vegetation index) and the SAVI (soil adjusted vegetation index). The strongest correlations were observed between the phenolic composition of grape skin at harvest time and variables measured from grapes at veraison time, as well as variables determined from grapevines at harvest time. The potential usefulness of these hyperspectral indices calculated from measurements performed directly on grapes or grapevines for estimating the anthocyanin and flavanol composition of grape skins was indicated by the high coefficients of determination (R 2 = 0.7955 and R 2 = 0.8594, respectively) as obtained by means of principal component regression.
CONCLUSION
According to the results of the present study, hyperspectral indices calculated from measurements performed directly on grapes at veraison time or on grapevines at harvest time may be useful for estimating the anthocyanin and flavanol composition of grape skins. This suggests that field radiometry might provide valuable information for estimating the phenolic composition of grapes, which may prove to be very useful when establishing strategies for harvest planning. © 2017 The Authors. Journal of The Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: phenolic composition, Tempranillo grapes, hyperspectral indices, water status, principal component regression
INTRODUCTION
The organoleptic properties of wines, such as color, astringency or bitterness, are important aspects for determining wine quality. These organoleptic properties are mainly related to phenolic composition,1 and so the phenolic composition of grapes is an important factor for ensuring its quality.
The main phenolic compounds related to these organoleptic properties are flavanols and anthocyanins. First, flavanols are mainly related to astringency and bitterness.2 The structure of the flavanols, which in turn can be associated with their localization in the grape, could elicit different subqualities of astringency. (Epi)catechin (galloylated or not) is the structural unit from which the flavanols located in the seed are made up (procyanidins), whereas flavanols found in grape skin, which hardly show galloylation in their structures, could be derivatives of both (epi)catechin (procyanidins) and (epi)gallochatechin (prodelphinidins).3, 4 The main differences in the organoleptic properties of flavanols are a result of: (i) galloylation (tannic and coarse sensations could be attributed to the galloylated flavanols) and (ii) the presence (or not) of the third hydroxyl group in the B‐ring of the flavanol (prodelphinidins are related to more pleasant sensations than procyanidins, which are more astringent and persistent).5, 6, 7
Second, wine color is mainly a result of anthocyanins, which are extracted from the skin of the grape during winemaking. These pigments are found not only as monoglucoside derivatives, but also as their acetyl, caffeoyl and p‐coumaroyl derivatives.8 Indeed, in Tempranillo cultivar, monoglucosides are the main anthocyanins along with their acetyl and p‐coumaroyl derivatives.9
Accordingly, it can be inferred that the phenolic composition of grapes must be considered when selecting the moment of harvest. Nevertheless, as a consequence of the progressive warming of the northern hemisphere and the alteration of the rainfall patterns resulting from global climate change, the determination of the optimum harvest date is becoming a more and more difficult task. The main consequences of global climate change have been reported to influence grapevine water status.10 Consequently, vegetative growth, canopy microclimate, and fruit growth and metabolism,11 including the synthesis and accumulation of secondary metabolites (e.g. flavanols and anthocyanins), are affected. The overall result is an increase in the gap between the technological maturity (which comes earlier) and the phenolic and aromatic maturity (which comes later), making it difficult to select the most appropriate harvest date.12 For these reasons, it is important to obtain more detailed knowledge for an easy and non‐destructive determination or estimation of the phenolic composition of grapes during maturation, thus making the selection of the harvest date easier. Low‐vigor grapevines were reported to show higher anthocyanin and flavanol levels in grape skins,13 although an influence on flavanol accumulation in seeds has not been demonstrated.14 Remote sensing approaches for vegetation status monitoring can potentially enable estimations of vine water use as a result of its capability for estimating vine biophysical variables such as size and vigor.15 Indeed, field radiometry can be considered as a non‐destructive method for quantifying either leaf, plant or canopy growing variables, with all of them being related to water status.16 Vegetation indices developed from spectral observations in the visible‐near infrared (NIR) regions are highly correlated with several plant stand parameters, such as green leaf area index, chlorophyll content, percentage ground cover of vegetation, etc.11, 17, 18 These non‐destructive techniques also have demonstrated their estimation abilities with respect to determining both technological maturity19 and the extractable polyphenols of grapes.20 However, no studies have correlated such indices with the detailed phenolic composition of grapes. A good correlation between NIR‐imaging technology,21, 22 NIR spectroscopy23 and the phenolic composition of seeds and intact grapes has also been reported when measurements are performed directly on the seeds and grapes, respectively.
Thus, increasing knowledge about the relationship between these indirect techniques and the detailed phenolic composition of grapes would be very useful when establishing strategies for harvest planning. The non‐destructive measurements can give an early and direct estimation not only of grape composition, but also the spatial distribution of vine characteristics (linked in turn to grape composition) using plant‐derived indices. The radiometric indices at the grape‐scale are expected to be directly linked to grape composition, whereas the link with vine‐derived indices should be expected to be indirect (e.g. related with the plant vigor and water status).
The present study aimed to assess the usefulness of hyperspectral indices, plant parameters and soil water status as indicative tools of the phenolic composition of the grape. In particular, the present study focused on an investigation of the correlation between the phenolic composition of Tempranillo grapes and hyperspectral indices relating to (i) plant canopy structure and vigor; (ii) plant pigment contents; and (iii) plant water status. Moreover, the relationship between plant parameters and soil water content and grape phenolic composition was also evaluated.
MATERIALS AND METHODS
Samples
The present study was carried out in a vineyard of 100 hectares of Vitis vinifera cultivar Tempranillo located in Zamora, Spain (coordinates 41.18°N, 5.21°W, 717 m.a.s.l.). Soil texture in the vineyard is mainly sandy loam and loamy sand and the vines are trellised to a vertical shoot position and equispaced at 1.5 m.24 In this vineyard, 15 locations were selected based on different orographic terrain features, such as orientation, altitude and slope.25 In each of these locations, an access tube for a portable capacitance soil moisture sensor was installed (Deviner 2000 probe; Sentek Technologies, Stepney, SA, Australia). Soil moisture measurements were made, each 10 cm down to the lower soil layer until a depth of 100 cm was attained. In addition, two vines next to the soil moisture sensor were monitored through the spectral and biophysical measurements at the level of the grape, leaf and canopy. In total, 300 grapes from each vine were randomly‐selected from all bunches on these two vines. These were then collected at harvest time and submitted to extraction to study the phenolic composition. For each location, phenolic composition was determined in triplicate.
High‐performance liquid chromatography‐diode array detection with mass spectrometry (HPLC‐DAD‐MS) analysis
The phenolic composition of grapes was determined at harvest time similarly as reported previously.25 Skins and seeds were manually separated from whole grapes and the detailed phenolic composition was determined by means of HPLC‐DAD‐MS. Both skin and seeds were extracted in triplicate and each extract was analyzed individually. Compounds were identified by means of their ultraviolet–visible spectra and the results from mass spectrometry. Quantification was performed from the HPLC‐DAD data. Skins were extracted with MeOH:HCl 0.5 N (95:5) and the flavanol and anthocyanin composition was analyzed in accordance with the method described by Quijada‐Morín et al.,26 as well as that of Alcalde‐Eon et al.9 From these analyses, 23 anthocyanin pigments, 19 flavanols, two hydroxybenzoic acids and 11 hydroxycinnamic acids were identified and quantified in the skin of the grapes. Anthocyanins were grouped into six variables depending on the type of anthocyanin derivative, whereas flavanols were grouped into five variables depending on the type of flavanol and on the presence or the absence of galloylation in the structure (Table 1). Grape seeds were extracted with MeOH:H2O (75:25) and analyzed by means of the procedure reported by Ferrer‐Gallego et al.6 As a result, 36 catechins and procyanidins (which were grouped into three groups) (Table 1) and two hydroxybenzoic acids were determined. Thus, individual phenolic composition of the grapes was grouped into 17 clusters depending on the features of the compounds (Table 1).
Table 1.
Variables built from phenolic composition of grapes
| Variable | Meaning |
|---|---|
| Anthoc_monoglc | Sum of anthocyanin monoglucosides |
| Anthoc_acet | Sum of anthocyanin acetylglucosides |
| Anthoc_coum | Sum of anthocyanin coumaroylglucosides |
| Anthoc_caffe | Sum of anthocyanin caffeoylglucosides |
| Anthoc_acyl_T | Total anthocyanin acylglucosides |
| Anthoc_total | Total anthocyanin |
| PC_gal_sk | Sum of galloylated procyanidins form grape skin |
| PC_non_gal_sk | Sum of non‐galloylated procyanidins form grape skin |
| Total_PC_sk | Total of procyanidins form grape skin |
| Total_PD_sk | Total of prodelphinidins form grape skin |
| Total_PAC_sk | Total of proanthocyanidins form grape skin |
| HC_sk | Total of hydroxycinnamic acids form grape skin |
| HB_sk | Total of hydroxybenzoic acids form grape skin |
| PC_gal_sd | Sum of galloylated procyanidins form grape seed |
| PC_non_gal_sd | Sum of non‐galloylated procyanidins form grape seed |
| Total_PC_sd | Total of procyanidins from grape seed |
| HB_sd | Total of hydroxybenzoic acids form grape seed |
Agronomic parameters
Biophysical parameters were controlled in the two vines for each location. They were related to leaf area and chlorophyll content and were determined both at veraison (V; 14 August 2013) and at harvest (H; 24 September 2013) time. The measurements followed the protocol described in Sánchez et al.27 adapted for vineyards.24 The Leaf area (Leaf_total_area), which comprises the extent of the photosynthesizing and transpiring surface of the plants, was measured by a leaf count based on the number of vine shoots per plant and the number of leaves per vine shoot. The area of individual leaves was estimated via a destructive method in which leaves (four leaves from each vine) were scanned and converted into a green surface using ImageJ (NIH, Bethesda, MS, USA).28
At the plant level, the Leaf Area Index (LAI_Plant) is defined as the projected area of leaves per unit of ground area. Because of the plantation frame, this parameter considers the projected area of each vine over the soil as being equal to 1 m2, and is determined by:
| (1) |
Chlorophyll content (Chl) was measured by means of a Minolta SPAD‐502 Chlorophyll Meter (Konica Minolta, Tokyo, Japan). Replicated and averaged measurements in several leaves were taken for each vine.
Hyperspectral indices
Several vegetation indices have also been determined at plant (P) and grape (G) level both at veraison (V) and at harvest (H) time. The basis for the indices calculation was the reflectance measurements taken with a spectroradiometer Ocean Optics USB4000 (Ocean Optics, Dunedin, FL, USA), which has a spectral range of 500–1100 nm and a spectral resolution of 0.21 nm. For plant measurements, the spectroradiometer was mounted on a vertical framing square and held in a nadir orientation at a height of 2.6 m above the vine. Grape measurements were carried out directly on bunches placing the sensor at a height of 10 cm. This instrument is a portable, fiber optic‐based spectrometer fitted in this case to a 14° field of view. This geometry leads to a measured area that is 64 cm in diameter for plants and 2.4 cm for bunches. All of the measurements (two replicates for each vine) were made under sunny conditions, avoiding shadows, between 11.00 h and 14.00 h, and aiming to avoid changes in solar elevation. The reflectance was referred to a calibrated white reflectance panel before each measurement.
The indices were calculated from spectral observations of reflectance in the visible and near‐infrared regions are listed in Table 2.
Table 2.
Hyperspectral indices considered in the present study
| Index | Index ID | Formula |
|---|---|---|
| Normalized difference vegetation index29 | NDVI | NDVI = (R900‐R680)/(R900 + R680) |
| Soil‐adjusted vegetation index30 | SAVI | SAVI = (1 + L) × (R800‐R670)/(R800 + R67O + L) |
| Water index31 | WI | WI = R900/R970 |
| Transformed chlorophyll absorption reflectance index32 | T‐CARI | T‐CARI = 3 × [(R700‐ R670) ‐ 0.2 × (R700‐ R550) × (R700/ R670)] |
| Greenness index18 | Greenness | Greenness = R554/R677 |
| Chlorophyll normalized difference index33 | CNDI | CNDI = (R680‐R430)/(R680 + R430) |
| Carotenoid Chappelle index34 | CARChap | CARChap = R760/R500 |
| Carotenoid reflectance indices35 | CRI550 | CRI550 = R510 −1‐R550 −1 |
| CRI700 | CRI700 = R510 −1‐R700 −1 | |
| Photochemical reflectance index36 | PRI_NORM | PRI = [(R531‐ R570) /(R531+ R570)]([(R800 − R670)/(R800 + R670)0.5] · R700/R670)−1 |
Rλ, reflectance at a given wavelength; L, soil brightness correction factor.
Water status
Regarding the water status of plants, different parameters were calculated at veraison and at harvest time, both at the leaf and plant level. The leaf water content (Leaf_water) was estimated as the average of the difference between the wet and dry weights of four leaves per plant. For dry weight, the sample was dried in an oven at 60 °C for at least 24 h until a constant weight was obtained.37 From this parameter, the leaf water percentage (Perc_Leaf_water) and plant water content (Plant_water) were calculated; the latter by multiplying the leaf water content and the number of leaves per plant. Moreover, leaf water potential was determined both before sunrise (pre‐dawn leaf water potential, Pre_Dawn_Potential) and at midday (midday leaf water potential, Midday_Potential) by means of a Skye SKPM 1044 Scholander‐type pressure chamber (Skye Instruments Ltd, Llandrindod Wells, UK).38
The soil moisture was assessed by means of frequency domain reflectometry using a DIVINER 2000 portable capacitance soil moisture sensor (Sentek Technologies).39, 40 Measurements were conducted, both at veraison and at harvest time, each 10 cm along the access tube installed, from surface to a depth of 100 cm. Three variables were built from these data: soil moisture in the soil column of 0–50 cm (Soil_moist0_50), which is considered the root zone; soil moisture in the soil column of 50–100 cm (Soil_moist50_100), which could be considered the reserve area; and Total soil moisture (Soil_moist_total).
Statistical analysis
Statistical analysis was performed with respect to the data matrix consisting of the averaged values determined for all the previously described variables at each selected location. The phenolic composition of grapes comprised the response variables (17 variables, including total contents, which result from the total sum of compounds from the same family), whereas six variables corresponding to biophysical parameters, 40 related to hyperspectral indices and 14 resulting from water status measurements were used as predicting variables. Principal component analysis (PCA), correlation analyses and principal component regressions (PCR) were performed to assess the relationship between phenolic composition and the rest of the variables. SPSS, version 21 (IBM, Armonk, NY, USA) was used to perform the statistical analysis.
RESULTS AND DISCUSSION
PCA
The relationship between biophysical variables, hyperspectral indices and water‐related variables and those related to phenolic composition of grapes was first assessed by means of PCA. From the projection of the samples on the plane defined by the first and second PCs (Fig. 1a), it can be determined that there are important differences among the selected locations. Moreover, a distribution of samples without any important grouping can be observed. Figure 1(b) shows the loading plot in which only the variables whose scores are more than 0.5 appear (i.e. the variables that contribute the most to the analysis). There is a strong opposition along PC1 between the phenolic composition of the grape skin and some of the hyperspectral indices, the biophysical and the water‐related variables investigated in the present study. Among them, not only the hyperspectral indices determined from plant at harvest (SAVI_PH or NDVI_PH) and at veraison time (CNDI_PV), but also those determined from grapes at veraison time (NDVI_GV or SAVI_GV) showed high negative scores in PC1, in contrast to the high positive scores in this PC shown by the phenolic composition of grape skin. In the same way, the levels of procyanidins in the seed of the grape showed high positive values in PC2, in contrast to the T‐CARI index measured for vines both at veraison and at harvest time.
Figure 1.
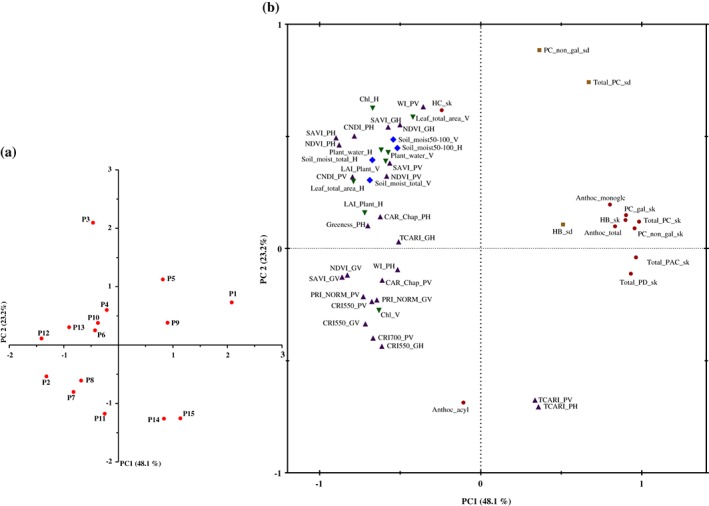
Score plot of the samples (a) and loading of the variables (b) on the plane defined by the first and second principal components.
Study of correlations
The significance of the relationships observed in the PCA was evaluated through the value and significance of Pearson's coefficients (r) with respect to the correlations between all variables. This section is divided into (i) the relationship between radiometric indices measured at the grape scale and the grape components and (ii) the relationships between radiometric indices measured at the vine scale and the grape components.
Relationships at the grape scale
The phenolic composition of grape skin showed significant correlations with several variables employed in the present study. However, no significant correlations were found between any of the variables employed and the phenolic composition of the grape seed. Studies available in the literature have shown that NIR hyperspectral imaging21, 23 can be useful for predictive purposes with respect to flavanols in the seed when measurements are performed directly on seeds. In the present study, hyperspectral measurements were performed directly on the whole grape, which could explain why significant correlations were not found between the hyperspectral indices and the flavanol composition of seeds. With regard to the rest of variables (agronomic parameters and water‐related variables), this lack of correlation may indicate that there is no significant relationship between these parameters and the phenolic composition of seeds.
Regarding anthocyanin composition, significant negative correlations were found mostly with hyperspectral indices determined at veraison time from grape measurements (Table 3). Total anthocyanins comprised the group of compounds that showed more significant correlations with the variables employed in the present study. NDVI and SAVI indices determined for grapes at veraison time are the variables that showed the strongest negative correlation with anthocyanin composition.
Table 3.
Coefficients of correlations between anthocyanin composition and the rest of variables measured in the study
| Anthocyanin | |||
|---|---|---|---|
| Variables | Anthoc_monoglc | Anthoc_acyl_T | Total |
| Plant | |||
| Leaf_total_area_H | −0.519* | ||
| NDVI_PH | −0.648** | −0.644** | |
| SAVI_PH | −0.614* | −0.611* | |
| Greenness_PH | −0.610* | −0.605* | |
| CNDI_PV | −0.686** | −0.665** | |
| Grape | |||
| NDVI_GV | −0.747** | −0.702** | |
| SAVI_GV | −0.767** | −0.728** | |
| WI_GV | −0.555* | ||
| CAR_Chap_GV | −0.517* | ||
| PRI_NORM_GV | −0.576* | −0.574* | |
Variables were measured at harvest (H) and veraison (V) time at the plant (P) and grape (G) scale. Statistical significance is shown at
P < 0.05 and
P < 0.01 respectively. Anthoc_acyl_T included acetyl, coumaroyl and caffeoyl groups (Table 1).
Monoglucoside derivatives showed the highest absolute values of the correlation coefficients with some hyperspectral indices determined at the grape level (i.e. NDVI_GV and SAVI_GV) (Table 3). However, when anthocyanins are grouped depending upon the anthocyanidin moiety, only the malvidin, petunidin and delphinidin derivatives showed significant correlations (r < −0.6, P < 0.01) with NDVI and SAVI indices determined from grapes at veraison time (data not shown).
It is worth noting that the anthocyanin composition of grapes determined at harvest time also showed a good correlation with variables measured at the grape level at veraison time (Table 3). This could mean that information about grape phenolic composition may be inferred from the status of grapes at veraison time.
As indicated above, the flavanol composition of grape seed did not show any significant correlation with the variables studied. It has been reported previously that winemakers prefer procedures leading to an increase in the content of flavanols from skins and to a lower extraction of flavanols from seeds.41 Thus, the possible relationships allowing an estimation of the flavanol composition of grape skin are of interest. Significant correlations were found between the flavanol composition of the grape skins and many variables (Table 4). Among the hyperspectral indices, NDVI and SAVI are again the variables that best correlate with phenolic composition, showing significant correlations with the flavanol composition of grape skins when they are measured from vines at harvest time (NDVI_PH and SAVI_PH) and from grapes at veraison time (NDVI_GV and SAVI_GV). Thus, it appears that the status of grapes at veraison time is key also for the flavanol composition of grapes skins.
Table 4.
Coefficients of correlations between flavanol composition of grape skin and the rest of variables measured in the present study
| Flavanols | |||
|---|---|---|---|
| Variable | Total_PC_sk | Total_PD_sk | Total_PAC_sk |
| Soil | |||
| Soil_moist_total_H | −0.560* | −0.531* | −0.553* |
| Soil_moist50‐100_H | −0.540* | −0.531* | |
| Soil_moist_total_V | −0.571* | −0.538* | −0.563* |
| Soil_moist50‐100_V | −0.534* | −0.525* | |
| Plant | |||
| Plant_water_V | −0.524* | −0.527* | −0.532* |
| LAI_Plant_H | −0.623* | −0.595* | −0.617* |
| Leaf_total_area_H | −0.672** | −0.706** | −0.698** |
| Chl_H | −0.562* | −0.641* | −0.609* |
| NDVI_PH | −0.791** | −0.825** | −0.818** |
| SAVI_PH | −0.785** | −0.815** | −0.810** |
| WI_PH | −0.527* | ||
| CAR_Chap_PH | −0.668** | −0.598* | −0.643** |
| CNDI_PH | −0.687** | −0.731** | −0.718** |
| Greenness_PH | −0.720** | −0.665** | −0.703** |
| PRI_NORM_PH | −0.698** | −0.778** | −0.746** |
| NDVI_PV | −0.582* | −0.528* | |
| SAVI_PV | −0.533* | ||
| TCARI_PV | −0.549* | ||
| CNDI_PV | −0.661** | −0.750** | −0.713** |
| Grape | |||
| NDVI_GV | −0.815** | −0.769** | −0.803** |
| SAVI_GV | −0.848** | −0.810** | −0.841** |
Variables were measured at harvest (H) and veraison (V) time at the plant (P) and grape (G) scale. Statistical significance is shown at
P < 0.05 and
P < 0.01 respectively.
Relationships at the vine scale
Hyperspectral indices NDVI and SAVI determined for vines at harvest time are the variables that showed the strongest negative correlation with anthocyanin composition (Table 3). These indices could be related to vine vigor because healthy and dense vegetation shows large values of NDVI, and consequently of SAVI, whereas both are null or negative for soil and water.30, 42 Thus, in view of the observed correlations, it appears that the higher vigor of vines, the lower amounts of anthocyanins in the skin of their grapes. This is in accordance with the negative correlations observed between greenness index measured at the plant level (Greenness_PH) and leaf area (Leaf_total_area_H) measured at harvest time and total anthocyanin content (Table 3). However, this inverse relationship between anthocyanins and vigor, although frequent in the related literature, may be controversial. Bonilla et al.43 reported that, when there is sufficient and moderate light resching to the bunches, the limiting factor in anthocyanin synthesis is temperature. Accordingly, several studies have reported that high sunlight exposures may lead to high berry temperatures and a reduction of anthocyanin accumulation.44, 45, 46 Then, vigorous vines (i.e. those sowed with high NDVI or SAVI) create more shading effects in the bunch area, protecting grapes from high temperatures, and leading to higher anthocyanin and color contents. This positive effect of bunch shading on anthocyanins was demonstrated by Bonilla et al.,43 who found a positive correlation between NDVI and anthocyanins. However, similar to the present study, other studies have reported a negative correlation between this index and color and the anthocyanin composition of grapes.47 This suggests that vigor influence on anthocyanin accumulation essentially depends on environmental factors and especially on maximal temperatures. Furthermore, Ledderhof et al.47 also found a negative correlation between NDVI and total phenols in grapes, which suggests that not only anthocyanins, but also other phenolic compounds of grapes could be related to this index. This is confirmed in the present study, as discussed below, because this index, amongst others, showed a significant negative correlation with the flavanol composition of grape skin.
In the case of acyl‐derived anthocyanins, only a significant correlation was found with the PRI_NORM index (Table 3), which can be considered as an indicator of vine stress because it is used for estimating the photosynthetic light‐use efficiency.36 This correlation is mostly a result of the correlation found between this index and acetyl‐derived anthocyanins (r = −0.576, P < 0.05), which may indicate that the synthesis of acetyl derivatives in the skin of the grape could be favored by stress situations of the vine.
NDVI and SAVI indices correlate with both procyanidins and prodelphinidins content except when they are measured from vines at veraison time (Table 4). In this case, these indices showed significant correlations only with the level of prodelphinidins in the skin of the grape. This was also observed for other variables such as TCARI_PV and the soil moisture measured in the deep zone at harvest and at veraison time (Soil_moist50‐100_H and Soil_moist50‐100_V). By contrast, water index determined from vines at harvest time (WI_PH) showed a significant correlation only with procyanidin content (Table 4). Important differences on the properties of procyanidins and prodelphinidins in relation to their sensory characteristics have been reported: pleasant oral sensations such as smoothness or velvety appear to be associated more with gallocatechins and prodelphinidins, whereas sensations attributed to catechins and procyanidins are more related to the high intensity of astringency and persistence.7 Thus, the fact that some variables correlate only with one or another type of these families of flavanols can be considered as highly encouraging because it could help to implement quasi‐selective strategies to favor/diminish the presence of certain types of flavanols.
For the flavanol composition of grapes skins, the status of vines at harvest time is more related to the phenolic composition of the grapes than the status at veraison, as occurred with the grapes. Flavanol composition also shows a strong negative correlation with variables related to chlorophyll and photosynthetic activity of vine, such as CNDI, greenness or total leaf area. Thus, as well as in the case of anthocyanins, vine vigor appears to be negatively correlated to flavanol content of grape skin.
PCR
Taking into account the existence of important correlations mentioned above, the influence of these hyperspectral or agronomic variables on the phenolic composition of the grapes was assessed by means of PCR. In this technique, a linear regression is performed after a reduction of the number of variables through a PCA. PCA was carried out using a dataset built from the variables that showed a significant correlation with the phenolic composition (Tables 3 and 4). Then, PC1 obtained from that analysis was used as independent variable and the corresponding phenolic composition as the dependent variable.
First, PCR was carried out using total anthocyanin content as dependent variable and, as independent variable, the PC1 obtained in the PCA performed using the variables that showed a significant correlation with total anthocyanin content (Table 3). In this case, PC1 explained 62.2% of the variability. All of the variables involved in this PC showed positive values for the scores, with NDVI, SAVI and CNDI measured for plants at harvest time and SAVI and NDIV measured for grapes at veraison time showing the highest scores (Table 5) and being the most important variables in this PC because of their high scores. When this PC was used as independent variable in the linear regression of the total anthocyanin content, a model was built with a coefficient of determination of 0.7955, thus indicating that PC1 can explain approximately 80% of the variability of the anthocyanin composition. This linear regression was significant (P < 0.001) and the slope of the regression showed a negative value significantly different from zero (P < 0.001). The negative sign of the slope again indicated the inverse relationship among the hyperspectral and agronomic parameters involved in the PC1 and the anthocyanin composition. From these results, it could be determined that the most adequate variables for building models aiming to estimate anthocyanin composition may be NDVI and SAVI measured from plants at harvest time and from grapes at veraison time. Moreover, the parameters measured from the vine appear to be most important for estimating the anthocyanin composition of grapes. This could be explained by the relationship between theses indices and plant vigor, which, as indicated before, could have an important effect on the anthocyanin composition of grapes. Thus, these results highlight the importance of vigor for anthocyanin composition. The involvement of variables measured at veraison time in this model is also noteworthy because it may indicate that the information obtained from grapes at veraison time through non‐destructive methods, such as the determination of these indices, is very valuable for explaining the anthocyanin composition of grapes.
Table 5.
Results of PCR carried out using total anthocyanin content and the corresponding significantly correlated variables
| Variable | Score in PC1 |
|---|---|
| NDVI_PH | 0.956 |
| SAVI_PH | 0.951 |
| CNDI_PH | 0.890 |
| SAVI_GV | 0.849 |
| NDVI_GV | 0.834 |
| GREENNESS_PH | 0.808 |
| Leaf_total_area_H | 0.805 |
| CARChap_GV | 0.659 |
| PRI_NORM_GV | 0.516 |
| WI_GV | 0.429 |
| Regresion results | |
| Constant | 5.0492 |
| Slope | −0.8411 |
| R2 | 0.7955 |
Variables were measured at harvest (H) and veraison (V) time at plant (P) and at grape (G) scale.
Similarly, the flavanol composition of grape skin was selected as dependent variable and PCR was performed using, as a predicting variable, PC1 obtained in the PCA carried out using the variables showing a significant correlation with flavanol total content in grape skin (Table 4). PC1 obtained explained 66.6% of the variability and all of the variables involved in PCA showed positive scores in this PC. The most influent variables in this PC were SAVI, NDVI and CNDI measured at harvest time from grapevines and NDVI and SAVI measured from grapes at veraison time (Table 6). Thus, as in the case of anthocyanin composition, it appears that NDVI and SAVI measured from grapevines and harvest time and from grapes at veraison time may be the most useful indices for the development of predicting models of flavanol composition of grape skin. Moreover, in this case, other variables different than hyperspectral indices, such as variables related to water status (Soil_moist_total_H) and the photosynthetic activity of plant (LAI_Plant_H, Leaf_total_area_H) also showed high scores in PC1, thus indicating that other variables different from hyperspectral indices could be useful for estimating flavanol composition. These variables could also be associated with vine vigor, again indicating that vigor is a very important factor to flavanol composition of grape skin. When this PC1 was used as independent variable for estimating flavanol composition, a significant regression (P < 0.001) with a coefficient of determination of 0.8594 was obtained, which means that PC1 explained approximately 86% of the variability of flavanol composition. The slope showed a negative value that was significantly different from zero (P < 0.001), which indicated, when taking into account the positive scores in PC1, the inverse relationship among predicting variables involved in the PC1 and the flavanol composition.
Table 6.
Results of PCR carried out using total flavanol content of grape skin and the corresponding significantly correlated variables
| Variable | Score in PC1 |
|---|---|
| SAVI_PH | 0.957 |
| NDVI_PH | 0.951 |
| CNDI_PH | 0.930 |
| NDVI_GV | 0.863 |
| SAVI_GV | 0.838 |
| Soil_moist_total_H | 0.832 |
| LAI_Plant_H | 0.816 |
| Leaf_total_area_H | 0.815 |
| Soil_moist50_100_H | 0.812 |
| Plant_water_V | 0.809 |
| Soil_moist50_100_V | 0.803 |
| CNDI_PV | 0.794 |
| Chl_H | 0.783 |
| GREENNESS_PH | 0.778 |
| PRI_NORM_PH | 0.723 |
| Soil_moist_total_V | 0.713 |
| CARChap_PH | 0.706 |
| NDVI_PV | 0.700 |
| Regresion results | |
| Constant | 1.7233 |
| Slope | −0.5032 |
| R 2 | 0.8594 |
Variables were measured at harvest (H) and veraison (V) time at plant (P) and grape (G) scale.
In general, the indices measured for grapes are more important when they are measured at veraison time, whereas those measured for vines are more important at harvest time. Because of the importance of flavanols for some organoleptic properties of wines and the problems related to their determination in winery (these compounds are usually estimated through nonspecific spectrophotometric measurements), these hyperspectral indices could be considered as an important non‐destructive tool for monitoring the vigor of vines and grapes, and therefore for estimating the flavanol composition of grapes. Nonetheless, a comprehensive study should be made to develop broader models that could be applied independently of the grape variety or the production area.
CONCLUSIONS
The results obtained from the present study suggest that field radiometry might provide valuable information for estimating the phenolic composition of grapes. Hyperspectral indices such as NDVI and SAVI show strong negative correlations with both the anthocyanin and flavanol composition of the grape skin. Moreover, water status and photosynthetic activity of grapevine appear to have an important influence on the content of flavanols in grape skin because of the negative correlation with variables related to those aspects. However, the flavanol content of grape skins did not show any significant correlations with the variables investigated in the present study. The hyperspectral indices calculated from measurements on grapes at veraison time showed the strongest correlations, whereas, in the case of vines, the more important correlations are observed when these indices are measured at harvest time. These variables have shown their usefulness for building models that may be used to estimate the phenolic content of grape skin. According to the results obtained, it is feasible to use hyperspectral indices calculated from measurements performed directly on grapes at veraison time or on grapevines at harvest time for estimating the anthocyanin and flavanol composition of grape skins at harvest.
Supporting information
Table S1 Maximum, minimum and mean values of the variables related to grape phenolic composition.
Table S2. Maximum minimum, median and mean values of the variables related to the agronomic parameters and to water status.
ACKNOWLEDGEMENTS
We thank the Spanish MINECO (Projects ref. AGL2014‐58486‐C2‐1‐R and ESP2015‐67549‐C3‐3‐R, co‐financed with FEDER), the Castilla y León Region Government (Project ref. SA007U16, co‐financed with FEDER) and the European Community Seventh Framework Programme (FP7‐Space‐2013‐1, Project E‐GEM‐ID 607126) for financial support. We also thank Dehesa La Granja winery for their collaboration during this study and Dr. J. M. Hernández‐Hierro for his advice regarding the statistical analysis.
REFERENCES
- 1. Moreno‐Arribas MV and Polo MC, Wine Chemistry and Biochemistry. Springer, New York, NY: (2009). [Google Scholar]
- 2. Gawel R, Red wine astringency: a review. Aust J Grape Wine Res 4:74–95 (1998). [Google Scholar]
- 3. Escribano‐Bailón MT, Guerra MT, Rivas‐Gonzalo JC and Santos‐Buelga C, Proanthocyanidins in skins from different grape varieties. Z Lebensm Unters For 200:221–224 (1995). [Google Scholar]
- 4. Kennedy JA, Hayasaka Y, Vidal S, Waters EJ and Jones GP, Composition of grape skin proanthocyanidins at different stages of berry development. J Agric Food Chem 49:5348–5355 (2001). [DOI] [PubMed] [Google Scholar]
- 5. Bindon KA, Smith PA, Holt H and Kennedy JA, Interaction between grape‐derived proanthocyanidins and cell wall material. 2. Implications for vinification. J Agric Food Chem 58:10736–10746 (2010). [DOI] [PubMed] [Google Scholar]
- 6. Ferrer‐Gallego R, García‐Marino M, Hernández‐Hierro JM, Rivas‐Gonzalo JC and Escribano‐Bailón MT, Statistical correlation between flavanolic composition, colour and sensorial parameters in grape seed during ripening. Anal Chim Acta 660:22–28 (2010). [DOI] [PubMed] [Google Scholar]
- 7. Ferrer‐Gallego R, Quijada‐Morín N, Bras NF, Gomes P, de Freitas V, Rivas‐Gonzalo JC et al, Characterization of sensory properties of flavanols – a molecular dynamic approach. Chem Senses 40:381–390 (2015). [DOI] [PubMed] [Google Scholar]
- 8. Alcalde‐Eon C, Escribano-Bailón MT, Santos‐Buelga C and Rivas‐Gonzalo JC, Changes in the detailed pigment composition of red wine during maturity and ageing – a comprehensive study. Anal Chim Acta 563:238–254 (2006). [Google Scholar]
- 9. Alcalde‐Eon C, García‐Estévez I, Ferreras‐Charro R, Rivas‐Gonzalo JC, Ferrer‐Gallego R and Escribano‐Bailón MT, Adding oenological tannin vs. overripe grapes: effect on the phenolic composition of red wines. J Food Compost Anal 34:99–113 (2014). [Google Scholar]
- 10. Mira de Orduña R, Climate change associated effects on grape and wine quality and production. Food Res Int 43:1844–1855 (2010). [Google Scholar]
- 11. Serrano L, González‐Flor C and Gorchs G, Assessment of grape yield and composition using the reflectance based water index in Mediterranean rainfed vineyards. Remote Sens Environ 118:249–258 (2012). [Google Scholar]
- 12. Mozell MR and Thack L, The impact of climate change on the global wine industry: challenges & solutions. Wine Econ Policy 3:81–89 (2014). [Google Scholar]
- 13. Cortell JM, Halbleib M, Gallagher AV, Righetti TL and Kennedy JA, Influence of vine vigor on grape (Vitis vinifera L. cv. Pinot Noir) anthocyanins. 1. Anthocyanin concentration and composition in fruit. J Agric Food Chem 55:6575–6584 (2007). [DOI] [PubMed] [Google Scholar]
- 14. Cortell JM, Halbleib M, Gallagher AV, Righetti TL and Kennedy JA, Influence of vine vigor on grape (Vitis vinifera L. cv. Pinot noir) and wine proanthocyanidins. J Agric Food Chem 53:5798–5808 (2005). [DOI] [PubMed] [Google Scholar]
- 15. Hall A, Lamb DW, Holzapfel B and Louis J, Optical remote sensing applications in viticulture – a review. Aust J Grape Wine Res 8:36–47 (2002). [Google Scholar]
- 16. Serrano L, González‐Flor C and Gorchs G, Assessing vineyard water status using the reflectance based water index. Agric Ecosyst Environ 139:490–499 (2010). [Google Scholar]
- 17. Wiegand CL, Richardson AJ, Escobar DE and Gerbermann AH, Vegetation indexes in crop assessments. Remote Sens Environ 35:105–119 (1991). [Google Scholar]
- 18. Zarco‐Tejada PJ, Berjón A, López‐Lozano R, Miller JR, Martín P, Cachorro V et al, Assessing vineyard condition with hyperspectral indices: leaf and canopy reflectance simulation in a row‐structured discontinuous canopy. Remote Sens Environ 99:271–287 (2005). [Google Scholar]
- 19. Nogales‐Bueno J, Hernández‐Hierro JM, Rodríguez‐Pulido FJ and Heredia FJ, Determination of technological maturity of grapes and total phenolic compounds of grape skins in red and white cultivars during ripening by near infrared hyperspectral image: a preliminary approach. Food Chem 152:586–591 (2014). [DOI] [PubMed] [Google Scholar]
- 20. Nogales‐Bueno J, Baca‐Bocanegra B, Rodríguez‐Pulido FJ, Heredia FJ and Hernández‐Hierro JM, Use of near infrared hyperspectral tools for the screening of extractable polyphenols in red grape skins. Food Chem 172:559–564 (2015). [DOI] [PubMed] [Google Scholar]
- 21. Rodríguez‐Pulido FJ, Hernández‐Hierro JM, Nogales‐Bueno J, Gordillo B, González‐Miret ML and Heredia FJ, A novel method for evaluating flavanols in grape seeds by near infrared hyperspectral imaging. Talanta 122:145–150 (2014). [DOI] [PubMed] [Google Scholar]
- 22. Hernández‐Hierro JM, Nogales‐Bueno J, Rodríguez‐Pulido FJ and Heredia FJ, Feasibility study on the use of near‐infrared hyperspectral imaging for the screening of anthocyanins in intact grapes during ripening. J Agric Food Chem 61:9804–9809 (2013). [DOI] [PubMed] [Google Scholar]
- 23. Ferrer‐Gallego R, Hernández‐Hierro JM, Rivas‐Gonzalo JC and Escribano‐Bailón MT, Feasibility study on the use of near infrared spectroscopy to determine flavanols in grape seeds. Talanta 82:1778–1783 (2010). [DOI] [PubMed] [Google Scholar]
- 24. Sánchez N, Martínez‐Fernández J, Aparicio J and Herrero‐Jiménez CM, Field radiometry for vineyard status monitoring under Mediterranean conditions, in IEEE International Geoscience and Remote Sensing Symposium (IGARSS) IEEE International, Québec, pp. 2094–2097 (2014).
- 25. García‐Estévez I, Andrés‐García P, Alcalde‐Eon C, Giacosa S, Rolle L, Rivas‐Gonzalo JC et al, Relationship between agronomic parameters, phenolic composition of grape skin, and texture properties of Vitis vinifera L. cv. Tempranillo. J Agric Food Chem 63:7663–7669 (2015). [DOI] [PubMed] [Google Scholar]
- 26. Quijada‐Morín N, Hernández‐Hierro JM, Rivas‐Gonzalo JC and Escribano-Bailón MT, Extractability of low molecular mass flavanols and flavonols from red grape skins. Relationship to cell wall composition at different ripeness stages. J Agric Food Chem 63:7654–7662 (2015). [DOI] [PubMed] [Google Scholar]
- 27. Sánchez N, Martínez‐Fernández J, González‐Piqueras J, González‐Dugo MP, Baroncini‐Turrichia G, Torres E, Calera A et al, Water balance at plot scale for soil moisture estimation using vegetation parameters. Agr Forest Meteorol 166:1–9 (2012). [Google Scholar]
- 28. Schneider CA, Rasband WS and Eliceiri KW, NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rouse JW, Haas RH, Shell JA, Deering DW and Harlan JC, Monitoring the Vernal Advancement of Retrogradation of Natural Vegetation. Final Report, Type III, NASA/GSFC, Greenbelt, MD (1974). [Google Scholar]
- 30. Huete AR, A soil‐adjusted vegetation index (SAVI). Remote Sens Environ 25:295–309 (1988). [Google Scholar]
- 31. Peñuelas J, Piñól J, Ogaya R and Filella I, Estimation of plant water concentration by the reflectance water index WI (R900/R970). Int J Remote Sens 18:2869–2875 (1997). [Google Scholar]
- 32. Haboudane D, Miller JR, Tremblay N, Zarco‐Tejada PJ and Dextraze L, Integrated narrow‐band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens Environ 81:416–426 (2002). [Google Scholar]
- 33. Peñuelas J, Baret F and Filella I, Semiempirical indexes to assess carotenoids chlorophyll‐A ratio from leaf spectral reflectance. Photosynthetica 31:221–230 (1995). [Google Scholar]
- 34. Chappelle EW, Kim MS and McMurtrey JE, Ratio analysis of reflectance spectra (RARS) – an algorithm for the remote estimation of the concentrations of chlorophyll‐A, chlorophyll‐B, and carotenoids in soybean leaves. Remote Sens Environ 39:239–247 (1992). [Google Scholar]
- 35. Gitelson AA, Zur Y, Chivkunova OB and Merzlyak MN, Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem Photobiol 75:272–281 (2002). [DOI] [PubMed] [Google Scholar]
- 36. Zarco‐Tejada PJ, González-Dugo V, Williams LE, Suárez L, Berni JAJ, Goldhamer D et al, A PRI‐based water stress index combining structural and chlorophyll effects: assessment using diurnal narrow‐band airborne imagery and the CWSI thermal index. Remote Sens Environ 138:38–50 (2013). [Google Scholar]
- 37. Gilabert MA, Gandía S and Melia J, Analyses of spectral biophysical relationships for a corn canopy. Remote Sens Environ 55:11–20 (1996). [Google Scholar]
- 38. Williams LE and Araujo FJ, Correlations among predawn leaf, midday leaf, and midday stem water potential and their correlations with other measures of soil and plant water status in Vitis vinifera . J Am Soc Hortic Sci 127:448–454 (2002). [Google Scholar]
- 39. Groves SJ and Rose SC, Calibration equations for Diviner 2000 capacitance measurements of volumetric soil water content of six soils. Soil Use Manage 20:96–97 (2004). [Google Scholar]
- 40. Burgess PJ, Reinhard BR and Pasturel P, Compatible measurements of volumetric soil water content using a neutron probe and Diviner 2000 after field calibration. Soil Use Manage 22:401–404 (2006). [Google Scholar]
- 41. Kennedy JA, Grape and wine phenolics: observations and recent findings. Cienc Investig Agrar 35:107–120 (2008). [Google Scholar]
- 42. Kogan FN, Application of vegetation index and brightness temperature for drought detection. Adv Space Res 15:91–100 (1995).11539265 [Google Scholar]
- 43. Bonilla I, Martínez de Toda F and Martínez‐Casasnovas JA, Vine vigor, yield and grape quality assessment by airborne remote sensing over three years: analysis of unexpected relationships in cv. Tempranillo. Span J Agric ReS 13:1–8 (2015). [Google Scholar]
- 44. Kliewer WM, Lider LA and Schultz HB, Influence of artificial shading of vineyards on the concentration of sugar and organic acid in grapes. Am J Enol Vitic 18:78–86 (1967). [Google Scholar]
- 45. Haselgrove L, Botting D, van Heeswijck R, Hoj P, Dry P, Ford C et al, Canopy microclimate and berry composition: the effect of bunch exposure on the phenolic composition of Vitis vinifera L cv. Shiraz grape berries. Aust J Grape Wine Res 6:141–149 (2000). [Google Scholar]
- 46. Spayd SE, Tarara JM, Mee DL and Ferguson JC, Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. Am J Enol Vitic 53:171–182 (2002). [Google Scholar]
- 47. Ledderhof D, Brown R, Reynolds A and Jollineau M, Using remote sensing to understand Pinot noir vineyard variability in Ontario. Can J Plant Sci 96:89–108 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Maximum, minimum and mean values of the variables related to grape phenolic composition.
Table S2. Maximum minimum, median and mean values of the variables related to the agronomic parameters and to water status.


