Abstract
Background
Hereditary angioedema (HAE) due to C1‐inhibitor deficiency (C1‐INH‐HAE) is a rare, potentially fatal, bradykinin‐mediated disease. Icatibant is a bradykinin B2 receptor antagonist originally approved in 2008 in the European Union and 2011 in the United States as an acute therapy option for HAE attacks in adults.
Objective
To compare demographics, disease characteristics and treatment outcomes of icatibant‐treated HAE attacks in patients with C1‐INH‐HAE enrolled in the Icatibant Outcome Survey across six European countries: Austria, France, Germany, Italy, Spain and the UK.
Methods
The Icatibant Outcome Survey [IOS; Shire, Zug, Switzerland (NCT01034969)] is an international observational study monitoring the safety and effectiveness of icatibant. Descriptive, retrospective analyses compared IOS country data derived during July 2009–April 2015.
Results
Overall, 481 patients with C1‐INH‐HAE provided demographic data. A significant difference across countries in age at onset (P = 0.003) and baseline attack frequency (P < 0.001) was found although no significant differences were found with respect to gender (majority female; P = 0.109), age at diagnosis (P = 0.182) or delay in diagnosis (P = 0.059). Icatibant was used to treat 1893 attacks in 325 patients with majority self‐administration in all countries. Overall, significant differences (all P < 0.001) were found across countries in time to treatment [median 1.8 h; median range: 0.0 (Germany–Austria) to 4.4 (France) h], time to resolution [median 6.5 h; median range: 3 (Germany–Austria) to 12 (France) h] and attack duration [median 10.5 h; median range: 3.1 (Germany–Austria) to 18.5 (France) h].
Conclusion
These data form the first European cross‐country comparison of disease characteristics and icatibant use in patients with C1‐INH‐HAE who are enrolled in IOS. International variation in icatibant practice and treatment outcomes across the six European countries assessed highlight the need to further investigate the range of country‐specific parameters driving regional variations in icatibant use.
Introduction
Hereditary angioedema due to C1‐inhibitor deficiency (C1‐INH‐HAE) is a rare autosomal dominant disease characterized by recurrent episodes of swelling that can vary in severity, frequency and anatomic location.1 Swelling most commonly involves cutaneous or submucosal tissues of the extremities, face, gastrointestinal tract and upper respiratory airways. Attacks can range in severity from mild to life‐threatening, particularly if laryngeal tissues are involved.1, 2
The dynamic and unpredictable nature of attacks combined with an estimated prevalence of 1 in 50 000 people worldwide provides challenges to the identification and management of this rare disease, particularly among health care professionals (HCPs) unfamiliar with C1‐INH‐HAE.
Icatibant is a bradykinin B2 receptor antagonist originally approved in 2008 in the European Union and 2011 in the United States as an acute therapy option for HAE attacks in adults. The EU indication was updated in 2011 to allow patient self‐administration following appropriate training from HCPs. The safety and efficacy of icatibant in adults with C1‐INH‐HAE have been demonstrated in three phase 3 clinical trials3, 4 and two open‐label extension studies.5, 6
The Icatibant Outcome Survey (IOS) is an international, prospective, observational study (NCT01034969) established in 2009 to monitor the safety and effectiveness of icatibant in a real‐world setting. Recent analyses of IOS data support the clinical trial findings7, 8 and helped delineate real‐world treatment with icatibant across registry countries, particularly with respect to the increasing adoption of patient self‐administration.9 However, the rarity of C1‐INH‐HAE has thus far limited IOS analyses of aggregated total patient data and precluded regional analyses and comparisons of both C1‐INH‐HAE characteristics and icatibant treatment patterns. A steadily increasing enrolment in IOS of patients with C1‐INH‐HAE across IOS European countries presents an ideal opportunity to assess regional demographic data to better characterize the clinical picture of C1‐INH‐HAE. Moreover, a regional evaluation of icatibant treatment outcomes and physician practice patterns across EU member nations may help guide clinical decision‐making by providing real‐world evidence of the ways in which icatibant is utilized for the treatment of acute C1‐INH‐HAE attacks.
The purpose of this analysis was to compare demographics, disease characteristics and treatment outcomes of icatibant‐treated attacks in patients with C1‐INH‐HAE enrolled in IOS across six European countries: Austria, France, Germany, Italy, Spain and the UK.
Methods
Patients with a diagnosis of C1‐INH‐HAE clinically confirmed by laboratory tests (C1‐INH concentration and function), who were receiving or were a candidate for icatibant treatment, were included in this analysis. Data for this analysis were collected via physician‐completed electronic forms at routine patient visits between July 2009 and April 2015. IOS is conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Informed consent was obtained from all participants. The design of IOS has been previously described.8
Analyses
Data were derived from an initial visit at IOS inclusion (baseline) and follow‐up assessments. HAE attack characteristics included the baseline annual frequency and severity of icatibant‐treated attacks. The frequency of attacks was based on patient recall, and attack severity was evaluated according to symptom score attributed to attack interference with daily activities as follows: 0 = very mild interference with daily activities; 1 = mild interference with daily activities; 2 = moderate interference with daily activities and no other countermeasures required; 3 = severe interference with daily activities and with or without other countermeasures; and 4 = very severe interference with daily activities and with other countermeasures required. The method of icatibant administration (either self‐administered or by HCP) was recorded. Icatibant treatment outcomes included time to administration, time to resolution and duration of attack. Treatment outcome data were only derived from attacks with complete data for all three outcomes.
Retrospective descriptive analyses were performed with patients grouped according to IOS country. Gender comparison used the Chi‐square test while comparisons of age at IOS enrolment, age at diagnosis, age at first symptoms and delay in diagnosis used the Kruskal–Wallis test. Treatment outcome data (time to resolution, duration of attack) were analysed using a mixed model analysis of repeated measures, including time to treatment and country as covariates. Analysis of icatibant‐treated attack severity was performed on attack severity data that were grouped into severe/very severe vs. very mild/mild/moderate using a generalized linear mixed analysis for repeated measures. Statistical testing was considered exploratory in this observational study, and no adjustment for multiplicity was performed. Data collected from German and Austrian patients were combined based on the small number of Austrian patients and the similarities in both populations and medical practice. Statistical significance was set at (α = 0.05).
Results
Demographics
A total of 481 patients with C1‐INH‐HAE were included in the demographic analysis (Table 1). The majority of patients were female (61.3%) with a similar ratio of male to female patients across all countries (P = 0.109). HAE type I was the predominant form of HAE in all countries. Of these patients, 325 reported 1893 icatibant‐treated attacks. Since IOS entry, these 325 icatibant‐treated patients were followed for a mean (SD) of 3.3 (1.4) years (<1 year, 8.6%; 1–2 years, 14.5%; 2–3 years, 15.4%; 3–4 years, 23.4%; 4–5 years, 29.2%; and ≥5 years, 8.9%). Follow‐up times (patient‐years) per country were 106.4 (Italy, n = 37), 124.2 (UK, n = 39), 196.0 (Germany–Austria, n = 65), 229.0 (Spain, n = 68) and 407.1 years (France, n = 116) and thus overall, these 325 patients were followed for a total of 1062.6 icatibant‐treated patient‐years.
Table 1.
Patient demographics at baseline
| Characteristic | France | Germany–Austria | Spain | Italy | UK | Overall (N = 481) | Overall comparison P value |
|---|---|---|---|---|---|---|---|
| No. of patients | 194 | 95 | 81 | 60 | 51 | 481 | |
| No. of attacks | 443 | 538 | 305 | 272 | 335 | 1893 | |
| Sex, n (%) | 0.109 | ||||||
| Female | 123 (63.4) | 59 (62.1) | 44 (54.3) | 38 (63.3) | 31 (60.8) | 295 (61.3) | |
| Male | 71 (36.6) | 36 (37.9) | 37 (45.7) | 22 (36.7) | 20 (39.2) | 186 (38.7) | |
| HAE diagnosis, n (%) | |||||||
| HAE type I | 186 (95.9) | 85 (89.5) | 79 (97.5) | 54 (90.0) | 48 (94.1) | 452 (94.0) | |
| HAE type II | 8 (4.1) | 10 (10.5) | 2 (2.5) | 6 (10.0) | 3 (5.9) | 29 (6.0) | |
| Age at IOS enrolment, years | 0.042 | ||||||
| n (missing) | 193 (1) | 95 (0) | 81 (0) | 60 (0) | 51 (0) | 480 (1) | |
| Median (IQR) | 38.0 (27.7–52.0)a | 46.6 (30.8–55.3)a , b | 39.5 (30.3–51.2) | 39.8 (28.5–47.4) | 36.6 (25.6–47.4)b | 39.6 (28.3–52.4) | |
| Age at first symptoms, years | 0.003 | ||||||
| n (missing) | 148 (46) | 88 (7) | 78 (3) | 56 (4) | 37 (14) | 407 (74) | |
| Median (IQR) | 15.0 (8.0–20.0)c , d | 12.0 (6.0–19.0) | 14.5 (4.0–20.0)e | 9.5 (3.5–15.0)c , e | 10.0 (5.0–14.0)d | 13.0 (5.0–19.0) | |
| Age at diagnosis, years | 0.182 | ||||||
| n (missing) | 167 (27) | 93 (2) | 80 (1) | 54 (6) | 48 (3) | 442 (39) | |
| Median (IQR) | 20.3 (13.4–33.6) | 26.6 (14.0–36.3) | 22.3 (12.9–35.3) | 20.6 (14.2–32.6) | 18.3 (12.3–30.5) | 21.1 (13.5–34.3) | |
| Delay in diagnosis, years | 0.059 | ||||||
| n (missing) | 144 (50) | 88 (7) | 77 (4) | 51 (9) | 37 (14) | 397 (84) | |
| Median (IQR) | 3.7 (0.1–14.2)c | 5.3 (0.3–19.1)f | 8.0 (0.8–18.5) | 10.5 (2.5–21.2)c , f , g | 3.9 (0.0–19.5)g | 6.1 (0.3–17.5) | |
P = 0.020 for France vs. Germany–Austria.
P = 0.020 for Germany–Austria vs. UK.
P < 0.001 for France vs. Italy.
P = 0.002 for France vs. UK.
P = 0.030 for Italy vs. Spain.
P = 0.020 for Germany–Austria vs. Italy.
P = 0.040 for Italy vs. UK.
IQR, interquartile range.
Patients with HAE in different countries were different in age at first symptoms, but not age at diagnosis or delay in diagnosis
We found a wide range and significant difference in the age of HAE onset in the six countries compared [median: 13.0; median range: 9.5 (Italy) to 15.0 (France) years; P = 0.003]. At the time of first symptoms, patients with HAE in Italy were significantly younger than patients with HAE in France (P < 0.001), Germany–Austria (P = 0.027) and Spain (P = 0.034). In contrast, we did not find significant differences (P = 0.182) in the age at diagnosis [median: 21.1 years; median range: 18.3 (UK) to 26.6 (Germany–Austria) years] or delay in diagnosis [median: 6.1 years; median range: 3.7 (France) to 10.5 (Italy) years; P = 0.059].
Baseline attack frequencies and icatibant‐treated attack severity were markedly different in patients with HAE from different countries
Wide interpatient variability in the median number of attacks per year at baseline across all countries was observed, ranging from 3.6 attacks/year in France to 18.0 attacks/year in Germany–Austria (P < 0.001; Fig. 1). Patient‐reported icatibant‐treated attack severity also varied significantly overall between countries (P < 0.001; Fig. 2), with France reporting the highest rate of severe/very severe attacks (86.9%) and Germany–Austria reporting the lowest (40.9%). Significant differences in rates of severe/very severe vs. very mild/mild/moderate attacks were found in pairwise comparisons among all countries (P ≤ 0.003), except for comparison between Germany–Austria and Spain (P = 0.140).
Figure 1.
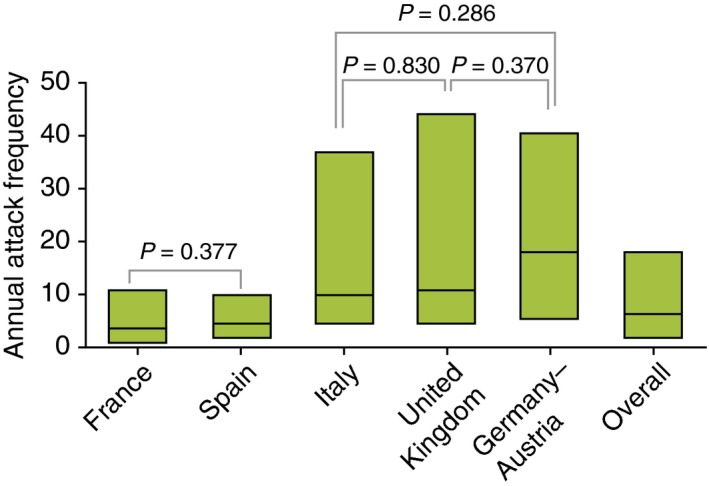
Baseline annual attack frequency. n indicates number of patients: France (n = 177), Spain (n = 80), Italy (n = 55), UK (n = 45), Germany–Austria (n = 90), overall (n = 447). Boxes indicate 25% interquartile range (IQR), median and 75% IQR. Overall comparison: P < 0.001. All pairwise country comparisons: P < 0.001, except for France vs. Spain (P = 0.377), Germany–Austria vs. Italy (P = 0.286), Germany–Austria vs. UK (P = 0.37) and Italy vs. UK (P = 0.83).
Figure 2.
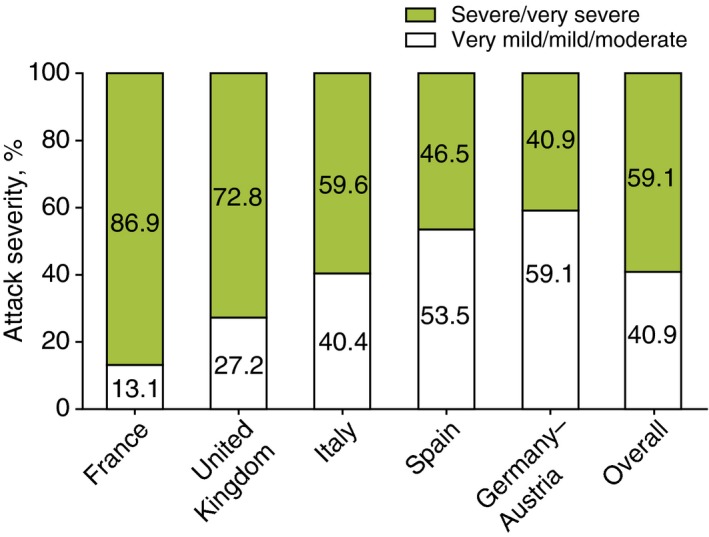
Attack severity (grouped). n indicates number of icatibant‐treated attacks: France (n = 275), UK (n = 335), Italy (n = 270), Spain (n = 258), Germany–Austria (n = 504), overall (n = 1642). Overall comparison: P < 0.001. All pairwise country comparisons: P < 0.001, except for Germany–Austria vs. Spain (P = 0.140). Very mild = very mild interference with daily activities; mild = mild interference with daily activities; moderate = moderate interference with daily activities and no other countermeasures required; severe = severe interference with daily activities and with or without other countermeasures; very severe = very severe interference with daily activities and other countermeasures required.
Attack locations were similar in patients with HAE
Overall, the majority of attacks (90.1%) affected a single anatomical site (ranging from 85.3% in Spain to 94.9% in Germany–Austria). Overall, abdominal attacks predominated (57.8%), followed by attacks localized to the skin (41.7%) and larynx (6.6%). The distribution of attack locations is described in Table 2.
Table 2.
Attack location
| Characteristic | France | Germany–Austria | Spain | Italy | UK | Overall |
|---|---|---|---|---|---|---|
| No. of attacks, n (missing) | 425 (18) | 530 (8) | 292 (13) | 272 (0) | 335 (0) | 1854 (39) |
| Affected sites, n (%) | ||||||
| Single | 385 (90.6) | 503 (94.9) | 249 (85.3) | 236 (86.8) | 298 (89.0) | 1671 (90.1) |
| Multiple | 40 (9.4) | 27 (5.1) | 43 (14.7) | 36 (13.2) | 37 (11.0) | 183 (9.9) |
| Any affected site, n (%) | ||||||
| Abdomen | 274 (64.5) | 298 (56.2) | 170 (58.2) | 156 (57.4) | 174 (51.9) | 1072 (57.8) |
| Skin | 137 (32.2) | 193 (36.4) | 149 (51.0) | 116 (42.6) | 179 (53.4) | 774 (41.7) |
| Larynx | 39 (9.2) | 28 (5.3) | 11 (3.8) | 32 (11.8) | 12 (3.6) | 122 (6.6) |
| Other | 16 (3.8) | 38 (7.2) | 7 (2.4) | 5 (1.8) | 7 (2.1) | 73 (3.9) |
Rates of long‐term prophylaxis, icatibant self‐administration and delays and outcomes of treatment were different across countries
Rates of long‐term prophylaxis use varied among countries, ranging from 11.8% of patients in Germany to 55.4% of patients in the UK. Overall, the predominant prophylaxis medication used were androgens (56.4%) (Table 3). The majority of patients in all countries self‐administered icatibant, but there were considerable differences in the rates of self‐injecting patients, ranging from 70.9% in Spain to 94.6% in the UK (overall P < 0.001; Fig. 3). Overall, the rate of patients self‐administering icatibant increased from 44.5% (2009) to 88.1% (2014) (Table 4). Overall, delays in icatibant administration were significantly different [median: 1.8 h; median range: 0.0 (Germany–Austria) to 4.4 (France) h; P < 0.001; Fig. 4], as were the median times to attack resolution [median: 6.5 h; median range: 3 (Germany–Austria) to 12 (France) h; P < 0.001; Fig. 4] and the duration of attacks [median: 10.5 h; median range: 3.1 (Germany–Austria) to 18.5 (France) h; P < 0.001; Fig. 4]. A significant relationship was found across all countries between time to treatment and both time to resolution and attack duration (P < 0.001 in both cases). However, no significant relationship was found, across all countries, between time to treatment and attack severity (P = 0.657).
Table 3.
Use of long‐term prophylaxis
| Characteristic | France | Germany–Austria | Spain | Italy | UK | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LTP | LTP | LTP | LTP | LTP | LTP | |||||||
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| No. of patients, n (%) | 47 (34.3) | 90 (65.7) | 8 (11.8) | 60 (88.2) | 35 (41.7) | 49 (58.3) | 13 (34.2) | 25 (65.8) | 25 (55.6) | 20 (44.4) | 128 (34.4) | 244 (65.6) |
| No. of attacks, n | 142 | 301 | 30 | 508 | 163 | 142 | 45 | 227 | 249 | 86 | 629 | 1264 |
| No. of icatibant injections per attack, n (missing) | 139 (3) | 273 (28) | 30 (0) | 501 (7) | 146 (17) | 124 (18) | 45 (0) | 227 (0) | 249 (0) | 86 (0) | 609 (20) | 1211 (53) |
| 1 | 115 (82.7) | 208 (76.2) | 28 (93.3) | 490 (97.8) | 137 (93.8) | 114 (91.9) | 41 (91.1) | 209 (92.1) | 231 (92.8) | 66 (76.7) | 552 (90.6) | 1087 (89.8) |
| 2 | 21 (15.1) | 58 (21.2) | 2 (6.7) | 10 (2.0) | 9 (6.2) | 8 (6.5) | 4 (8.9) | 18 (7.9) | 18 (7.2) | 20 (23.3) | 54 (8.9) | 114 (9.4) |
| 3 | 2 (1.4) | 6 (2.2) | – | 1 (0.2) | – | 2 (1.6) | – | – | – | – | 2 (0.3) | 9 (0.7) |
| 4 | 1 (0.7) | 1 (0.4) | – | – | – | – | – | – | – | – | 1 (0.2) | 1 (0.1) |
| LTP treatments, n | 142 | NA | 30 | NA | 163 | NA | 45 | NA | 249 | NA | 629 | NA |
| Androgens, n % | 78 (54.9) | NA | 16 (53.3) | NA | 58 (35.6) | NA | 29 (64.4) | NA | 174 (69.9) | NA | 355 (56.4) | NA |
| Tranexamic acid | 56 (39.4) | NA | – | NA | 87 (53.4) | NA | 5 (11.1) | NA | 12 (4.8) | NA | 160 (25.4) | NA |
| C1 INH | 3 (2.1) | NA | 14 (46.7) | NA | 15 (9.2) | NA | 8 (17.8) | NA | 14 (5.6) | NA | 54 (8.6) | NA |
| Androgens/tranexamic acid | 3 (2.1) | NA | – | NA | 3 (1.8) | NA | 3 (6.7) | NA | 28 (11.2) | NA | 37 (5.9) | NA |
| C1 INH/tranexamic acid | – | NA | – | NA | – | NA | – | NA | 16 (6.4) | NA | 16 (2.5) | NA |
| C1 INH/androgens | 1 (0.7) | NA | – | NA | – | NA | – | NA | 5 (2.0) | NA | 6 (1.0) | NA |
| C1 INH/androgens/tranexamic acid | 1 (0.7) | NA | – | NA | – | NA | – | NA | – | 1 (0.2) | NA | |
CI INH, C1‐inhibitor; LTP, long‐term prophylaxis.
Figure 3.
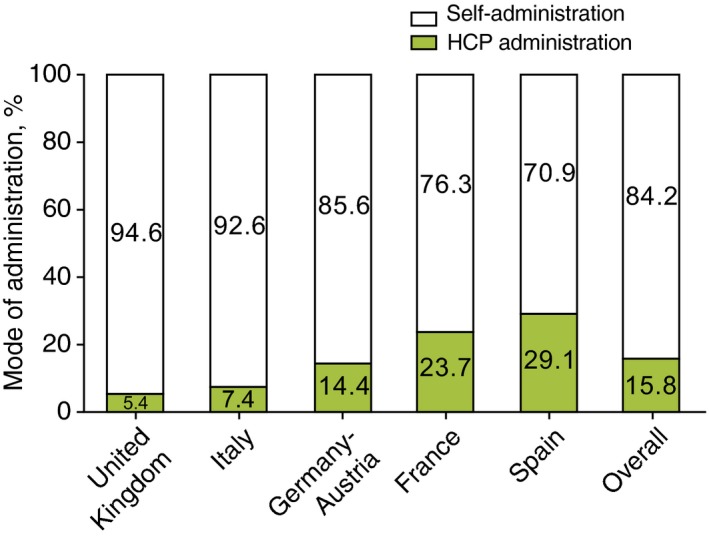
Mode of administration. n indicates number of attacks: UK (n = 335), Italy (n = 272), Germany–Austria (n = 506), France (n = 346), Spain (n = 282), overall (n = 1741). Overall comparison: P < 0.001. All pairwise country comparisons: P < 0.001, except for France vs. Spain (P = 0.130) and Italy vs. UK (P = 0.323).
Table 4.
Mode of icatibant administration by year of attack
| 2008a | 2009 | 2010 | 2011b | 2012 | 2013 | 2014 | 2015a | Overall | ||
|---|---|---|---|---|---|---|---|---|---|---|
| France | Attacks, N | 1 | 26 | 73 | 64 | 68 | 55 | 49 | – | 336 |
| Self, n (%) | – | 17 (65.4) | 44 (60.3) | 53 (82.8) | 62 (91.2) | 50 (90.9) | 30 (61.2) | – | 256 (76.2) | |
| HCP, n (%) | 1 (100.0) | 9 (34.6) | 29 (39.7) | 11 (17.2) | 6 (8.8) | 5 (9.1) | 19 (38.8) | – | 80 (23.8) | |
| Germany–Austria | Attacks, N | 3 | 17 | 32 | 57 | 101 | 188 | 97 | 10 | 505 |
| Self, n (%) | – | 1 (5.9) | 15 (46.9) | 54 (94.7) | 91 (90.1) | 177 (94.1) | 88 (90.7) | 7 (70.0) | 433 (85.7) | |
| HCP, n (%) | 3 (100.0) | 16 (94.1) | 17 (53.1) | 3 (5.3) | 10 (9.9) | 11 (5.9) | 9 (9.3) | 3 (30.0) | 72 (14.3) | |
| Italy | Attacks, N | – | 1 | 26 | 44 | 56 | 88 | 57 | – | 272 |
| Self, n (%) | – | 1 (100.0) | 21 (80.8) | 40 (90.9) | 49 (87.5) | 85 (96.6) | 56 (98.2) | – | 252 (92.6) | |
| HCP, n (%) | – | – | 5 (19.2) | 4 (9.1) | 7 (12.5) | 3 (3.4) | 1 (1.8) | – | 20 (7.4) | |
| Spain | Attacks, N | – | 17 | 23 | 49 | 70 | 80 | 36 | 1 | 276 |
| Self, n (%) | – | 3 (17.6) | 10 (43.5) | 31 (63.3) | 60 (85.7) | 63 (78.8) | 27 (75.0) | 1 (100.0) | 195 (70.7) | |
| HCP, n (%) | – | 14 (82.4) | 13 (56.5) | 18 (36.7) | 10 (14.3) | 17 (21.3) | 9 (25.0) | – | 81 (29.3) | |
| UK | Attacks, N | – | 16 | 68 | 44 | 68 | 57 | 81 | 1 | 335 |
| Self, n (%) | – | 13 (81.3) | 60 (88.2) | 42 (95.5) | 64 (94.1) | 56 (98.2) | 81 (100.0) | 1 (100.0) | 317 (94.6) | |
| HCP, n (%) | – | 3 (18.8) | 8 (11.8) | 2 (4.5) | 4 (5.9) | 1 (1.8) | – | – | 18 (5.4) | |
| Total, by year | Attacks, N | 4 | 77 | 222 | 258 | 363 | 468 | 320 | 12 | 1724 |
| Self, n (%) | 0 (0) | 35 (45.5) | 150 (67.6) | 220 (85.3) | 326 (89.8) | 431 (92.1) | 282 (88.1) | 9 (75.0) | 1453 (84.3) | |
Administration data from 2008 to 2015 (the latter limited due to an April 2015 datacut) were excluded from the statistical analysis.
Icatibant label change in 2011 for EU approval to self‐administer.
HCP, health care provider.
Figure 4.
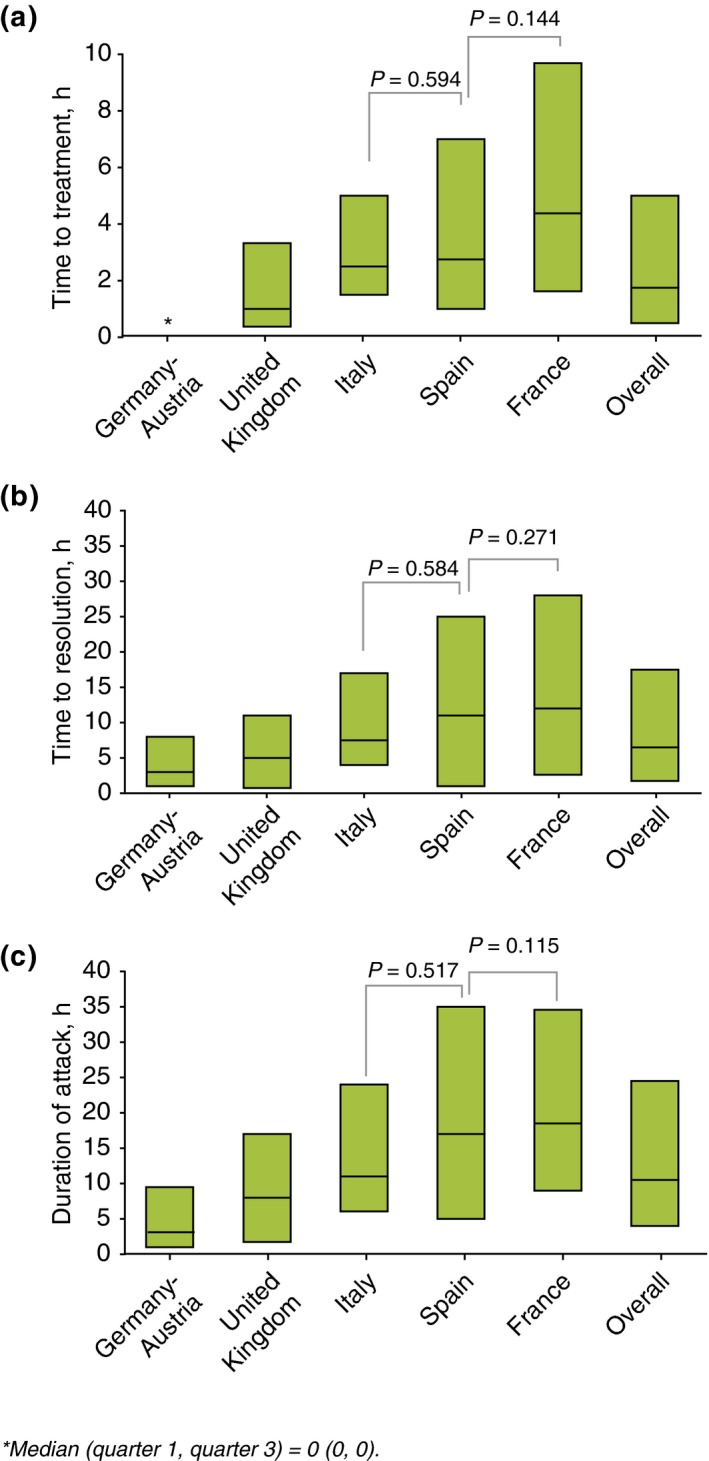
Treatment outcomes. n indicates number of attacks: Germany–Austria (n = 112), UK (n = 171), Italy (n = 181), Spain (n = 67), France (n = 148), overall (n = 679). Boxes indicate 25% interquartile range (IQR), median and 75% IQR. For time to treatment, all comparisons: P < 0.008, except for France vs. Spain (P = 0.144) and Italy vs. Spain (P = 0.594). For time to resolution, all comparisons: P < 0.037, except for France vs. Spain (P = 0.271) and Italy vs. Spain (P = 0.584). For attack duration, all comparisons: P < 0.005, except for France vs. Spain (P = 0.115) and Italy vs. Spain (P = 0.517). Time to events were only calculated for attacks with complete data for time to administration, time to resolution and attack duration.
Treatment outcomes by mode of administration
Treatment outcomes by mode of administration (self administration vs. HCP administration) were derived from complete time to event data for 127 patients reporting 592 self‐treated attacks and 56 patients reporting 79 HCP‐treated attacks (Fig. 5).
Figure 5.
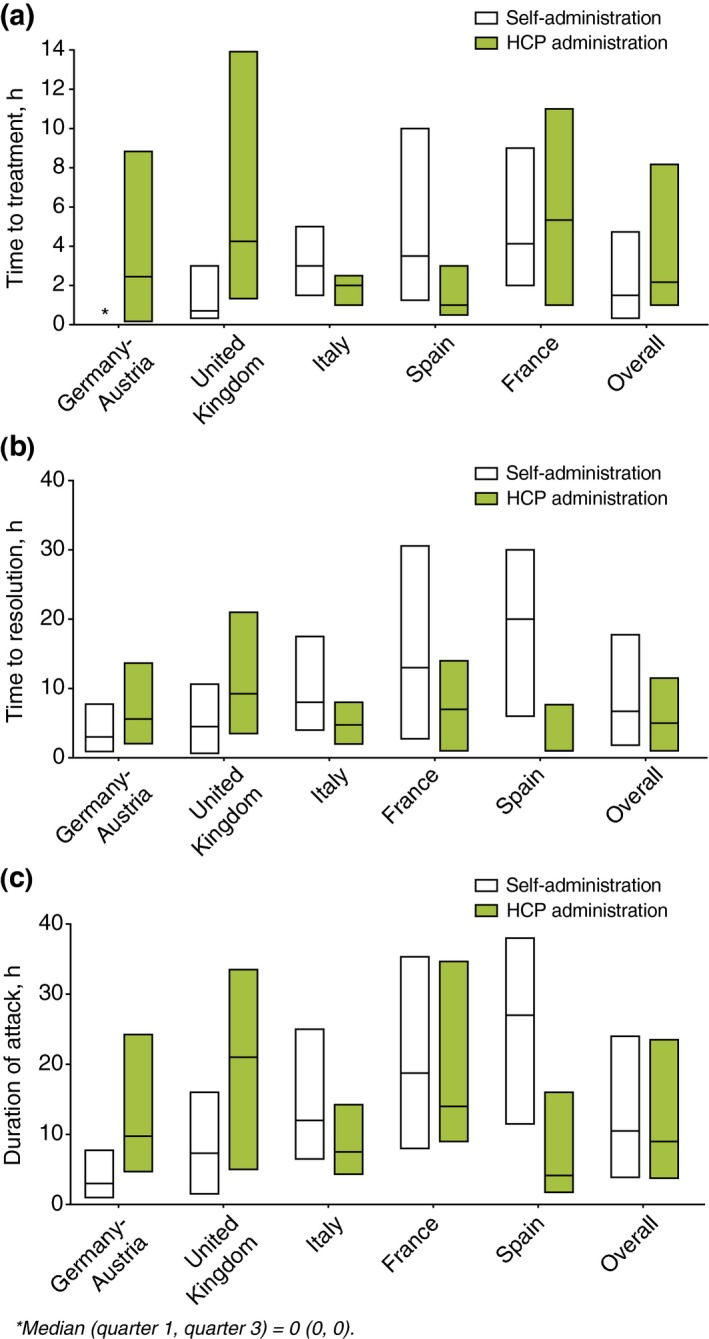
Treatment outcomes by mode of administration. n indicates number of attacks: Germany–Austria [self, n = 100; health care provider (HCP), n = 12], UK (self, n = 160; HCP, n = 11), Italy (self, n = 165; HCP, n = 16), Spain (self, n = 45; HCP, n = 21), France (self, n = 122; HCP, n = 19), overall (self, n = 592; HCP, n = 79); Boxes indicate 25% interquartile range (IQR), median and 75% IQR. For self‐administration time to treatment, all pairwise comparisons: P ≤ 0.047, except for France vs. Spain (P = 0.713) and Italy vs. Spain (P = 0.261). For time to resolution, all pairwise comparisons: P < 0.049, except for France vs. Italy (P = 0.060), France vs. Spain (P = 0.722) and Italy vs. Spain (P = 0.064). For attack duration, all pairwise comparisons: P < 0.001, except for France vs. Spain (P = 0.894) and Italy vs. Spain (P = 0.053). For HCP administration: time to treatment all pairwise comparisons P ≥ 0.051 except for France vs. Spain (P = 0.043) and Italy vs. UK (P = 0.028). For time to resolution, all pairwise comparisons: P ≥ 0.052. For attack duration, all pairwise comparisons: P < 0.028, except for France vs. Germany–Austria (P = 0.216), France vs. UK (P = 0.800), Germany–Austria vs. Italy (P = 0.335), Germany–Austria vs. Spain (P = 0.231), Germany–Austria vs. UK (P = 0.274) and Italy vs. Spain (P = 0.738). Time to events were only calculated for attacks with complete data for time to administration, time to resolution and attack duration.
Discussion
This is the first IOS study to directly compare real‐world icatibant treatment patterns across six European countries for the treatment of acute C1‐INH‐HAE attacks. Patient sex and HAE type were broadly similar across all countries, with a predominance of females as previously described8, 9 and a higher rate of HAE type I vs. HAE type II.10 Delay in timely HAE diagnosis ranged from 3 to 10 years across the countries assessed. When placed in the context of similar diagnostic delay reported in previous IOS data11 and a recent UK audit of HAE,12 these data appear to indicate that, while diagnostic delay is still a challenge, the situation in the assessed European countries may be improving. Increased delay in diagnosis may not necessarily correlate with poorer individual physician performance, as an increase in diagnostic delay may well be expected if, for example, previously undiagnosed older relatives are identified following proband diagnosis and familial follow‐up testing. Several evidence‐based consensus documents have been established in recent years for HAE to raise clinical awareness of the characteristics of C1‐INH‐HAE and the expanding range of treatment options available.10, 13, 14
A wide variation in baseline attack frequency was observed across all regions. The different rates of long‐term prophylaxis use, potential regional variation in attack triggers or trigger avoidance behaviour could contribute to this result. Similarly, self‐reported severity of icatibant‐treated attacks was highly variable. The difficulty of assigning attack severity as a surrogate measure of disease severity has been reported,15 as with no validated measure of attack severity, such is open to patient interpretation of the limitation on activities of daily living.
Overall, treatment outcome data confirm icatibant as an effective acute treatment option for C1‐INH‐HAE in a real‐world setting across all countries assessed. Earlier administration of icatibant resulted in earlier attack resolution, supporting both previous outcome data derived from IOS8, 9 and previous comparison of IOS data with clinical trial data.16
Importantly, treatment outcomes revealed variation in practice, as there were significant differences in time to administration, time to resolution and attack duration between countries. Time to treatment was shortest in Germany–Austria and is reflected in shorter resolution, attack duration and severity. Compared with other countries, patients waited longer to self‐treat in France, perhaps resulting in longer time to resolution, longer attack duration and severity. Interestingly, when time to self‐treatment and self‐reported attack severity from Germany–Austria and France are taken together, it is indeed striking that >85% of French patients and only 40% of German‐Austrian patients deemed their attacks to be severe/very severe. While we demonstrated that time to treatment correlated with time to resolution and attack duration across all countries, however, we could not show such a relationship between time to treatment and attack severity across all countries. These data may reflect differences in national character and their perceptions of pain and disfigurement, or could reflect actual differences in physical characteristics of attacks.
A notably consistent feature across all countries assessed was the majority use of icatibant self‐administration; this has been previously reported.9 These administration data are consistent with phase 3 open‐label extension data that support self‐administration of icatibant in clinical practice.17 Furthermore, a high rate of self‐administration is in keeping with the 2011 indication change to allow icatibant self‐administration and would suggest IOS patient acceptance of icatibant self‐administration. This outcome also may be attributed to the effective adoption among the clinical community of the recent international guideline14 and national consensus documents13, 15, 18 that promote early treatment. The country‐specific differences in self‐administration vs. HCP administration may be due in part to physician advice, the aforementioned availability of icatibant or combinations thereof. Interestingly, in both Spain and Italy, HCPs administered icatibant earlier than patients self‐administered. This may reflect local practices and deserves closer inspection. Overall, despite the difference in time to treatment between self‐administration and HCP administration, time to resolution appears similar between countries. The reasons for this are unclear and warrant further investigation.
The limitations of this analysis are typical of registry‐based studies using data derived from patient recall and treatment outcomes that are, in part, reliant on determination of resolution of an attack that may be open to patient interpretation.
Conclusions
This analysis reveals that there is international variation in icatibant practice and treatment outcomes in patients with C1‐INH‐HAE participating in the IOS registry across six European countries. These preliminary findings form the foundation for ongoing analyses using IOS data that aim to provide insight into possible country‐specific parameters, ranging from the level of the patient through the physician and health care system, that may be driving these regional variations in icatibant use.
Acknowledgements
IOS is funded and supported by Shire International GmbH, Zug, Switzerland. Medical writing support was provided by David Lickorish, PhD, CMPP, of Excel Scientific Solutions and funded by Shire Human Genetic Therapies.
IOS investigators and their staff: Austria: W. Aberer; Italy: F. Arcoleo, M. Bova, M. Cicardi, E. Cillari, V. Montinaro, G. Marone, A. Zanichelli; France: C. Blanchard Delauny, I. Boccon‐Gibod, L. Bouillet, B. Coppere, C. Dzviga, O. Fain, B. Goichot, A. Gompel, S. Guez, P.Y. Jeandel, G. Kanny, D. Launay, H. Maillard, L. Martin, A. Masseau, Y. Ollivier; Germany: M. Maurer, M. Magerl; Spain: M.L. Baeza, T. Caballero, R. Cabañas, M. Guilarte, D. Hernández, C. Hernando de Larramendi, R. Lleonart, T. Lobera, L. Marqués; UK: C. Bangs, M. Buckland, S. Grigoriadou, M. Helbert, H.J. Longhurst, L. Lorenzo.
Conflicts of interest
T. Caballero has received speaker fees from CSL Behring, GSK, MSD, Novartis, Shire; consultancy fees from BioCryst, CSL Behring, Novartis, Shire and Sobi; funding for travel and meeting attendance from CSL Behring, Novartis and Shire; and has participated in clinical trials/registries for BioCryst, CSL Behring, Dyax, Novartis, Pharming and Shire. W. Aberer has acted as a medical advisor and speaker for BioCryst, CSL Behring, Pharming and Shire; and has received funding to attend conferences/other educational events and donations to his departmental fund from and participated in clinical trials for Shire. H.J. Longhurst has received research grant support and/or speaker/consultancy fees from BioCryst, CSL Behring, Dyax, Shire and Sobi. M. Maurer has received research grant support and/or speaker/consultancy fees from BioCryst, CSL Behring, Dyax and Shire. A. Zanichelli has received speaker fees from CSL Behring, Shire and Sobi; consultancy fees from CSL Behring and Shire; and has acted on the medical/advisory boards for CSL Behring and Shire. L. Bouillet has received honoraria from CSL Behring, Pharming and Shire; and her institute has received research funding from CSL Behring and Shire. A. Perrin and I. Andresen are full‐time employees of Shire, Zug, Switzerland.
Funding source
IOS is funded and supported by Shire International GmbH, Zug, Switzerland.
References
- 1. Longhurst H, Cicardi M. Hereditary angioedema. Lancet 2012; 379: 474–481. [DOI] [PubMed] [Google Scholar]
- 2. Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1‐INH deficiency. J Allergy Clin Immunol 2012; 130: 692–697. [DOI] [PubMed] [Google Scholar]
- 3. Cicardi M, Banerji A, Bracho F et al Icatibant, a new bradykinin‐receptor antagonist, in hereditary angioedema. N Engl J Med 2010; 363: 532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lumry WR, Li HH, Levy RJ et al Randomized placebo‐controlled trial of the bradykinin B2 receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST‐3 trial. Ann Allergy Asthma Immunol 2011; 107: 529–537. [DOI] [PubMed] [Google Scholar]
- 5. Malbrán A, Riedl M, Ritchie B et al Repeat treatment of acute hereditary angioedema attacks with open‐label icatibant in the FAST‐1 trial. Clin Exp Immunol 2014; 177: 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baş M, Greve J, Hoffmann TK et al Repeat treatment with icatibant for multiple hereditary angioedema attacks: FAST‐2 open‐label study. Allergy 2013; 68: 1452–1459. [DOI] [PubMed] [Google Scholar]
- 7. Longhurst HJ, Aberer W, Bouillet L et al Analysis of characteristics associated with reinjection of icatibant: results from the Icatibant Outcome Survey. Allergy Asthma Proc 2015; 36: 399–406. [DOI] [PubMed] [Google Scholar]
- 8. Maurer M, Aberer W, Bouillet L et al Hereditary angioedema attacks resolve faster and are shorter after early icatibant treatment. PLoS One 2013; 8: e53773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hernández Fernandez de Rojas D, Ibañez E, Longhurst H et al Treatment of HAE attacks in the Icatibant Outcome Survey: an analysis of icatibant self‐administration versus administration by health care professionals. Int Arch Allergy Immunol 2015; 167: 21–28. [DOI] [PubMed] [Google Scholar]
- 10. Cicardi M, Aberer W, Banerji A et al HAWK under the patronage of EAACI (European Academy of Allergy and Clinical Immunology). Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy 2014; 69: 602–616. [DOI] [PubMed] [Google Scholar]
- 11. Zanichelli A, Magerl M, Longhurst H, Fabien V, Maurer M. Hereditary angioedema with C1 inhibitor deficiency: delay in diagnosis in Europe. Allergy Asthma Clin Immunol 2013; 9: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jolles S, Williams P, Carne E et al A UK national audit of hereditary and acquired angioedema. Clin Exp Immunol 2014; 175: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Betschel S, Badiou J, Binkley K et al Canadian hereditary angioedema guideline. Allergy Asthma Clin Immunol 2014; 10: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Craig T, Aygören‐Pürsün E, Bork K et al WAO guideline for the management of hereditary angioedema. World Allergy Organ J 2012; 5: 182–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zuraw BL, Banerji A, Bernstein JA et al US Hereditary Angioedema Association Medical Advisory Board 2013 recommendations for the management of hereditary angioedema due to C1 inhibitor deficiency. J Allergy Clin Immunol Pract 2013; 1: 458–467. [DOI] [PubMed] [Google Scholar]
- 16. Maurer M, Longhurst HJ, Fabien V, Li HH, Lumry WR. Treatment of hereditary angioedema with icatibant: efficacy in clinical trials versus effectiveness in the real‐world setting. Allergy Asthma Proc 2014; 35: 377–381. [DOI] [PubMed] [Google Scholar]
- 17. Aberer W, Maurer M, Reshef A et al Open‐label, multicenter study of self‐administered icatibant for attacks of hereditary angioedema. Allergy 2014; 69: 305–314. [DOI] [PubMed] [Google Scholar]
- 18. Cicardi M, Bork K, Caballero T et al Evidence‐based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy 2012; 67: 147–157. [DOI] [PubMed] [Google Scholar]


