Abstract
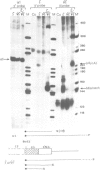
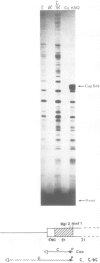
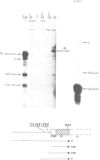
The human embryonic alpha-like globin gene (zeta) and a closely linked pseudogene (psi zeta) are located on chromosome 16. The psi zeta gene has a nonsense mutation in exon 1 but has identical promoter sequence and RNA processing sites to the zeta gene, raising the possibility that both psi zeta and zeta are transcriptionally active. We have studied transcription of the human zeta and psi zeta genes in a number of systems to examine their cell type specificity and enhancer requirement. (i) Cloned zeta and psi zeta genes transfected into human HeLa or monkey Cos7 tissue culture cells show no transcriptional activity. The presence of an SV40 enhancer does not activate the zeta promoter except at low levels when in very close proximity (less than 50 bp from the CCAAT box). (ii) In contrast to other tissue-specific genes tested to date, both zeta and psi zeta gene promoters initiate transcription efficiently when micro-injected into Xenopus oocyte nuclei. We suggest that embryonic-specific factors in the oocyte may permit efficient zeta gene transcription. Furthermore, the zeta promoter sequence from - 111 to + 38 bp is sufficient for transcription in this system.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alan M., Grindlay G. J., Stefani L., Paul J. Epsilon globin gene transcripts originating upstream of the mRNA cap site in K562 cells and normal human embryos. Nucleic Acids Res. 1982 Sep 11;10(17):5133–5147. doi: 10.1093/nar/10.17.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan M., Lanyon W. G., Paul J. Multiple origins of transcription in the 4.5 Kb upstream of the epsilon-globin gene. Cell. 1983 Nov;35(1):187–197. doi: 10.1016/0092-8674(83)90221-0. [DOI] [PubMed] [Google Scholar]
- Asselbergs F. A., Smart J. E., Mathews M. B. Analysis of expression of adenovirus DNA (fragments) by microinjection in Xenopus oocytes. Independent synthesis of minor early region 2 proteins. J Mol Biol. 1983 Jan 15;163(2):209–238. doi: 10.1016/0022-2836(83)90004-9. [DOI] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Carlson D. P., Ross J. Human beta-globin promoter and coding sequences transcribed by RNA polymerase III. Cell. 1983 Oct;34(3):857–864. doi: 10.1016/0092-8674(83)90543-3. [DOI] [PubMed] [Google Scholar]
- Cortese R., Melton D., Tranquilla T., Smith J. D. Cloning of nematode tRNA genes and their expression in the frog oocyte. Nucleic Acids Res. 1978 Dec;5(12):4593–4611. [PMC free article] [PubMed] [Google Scholar]
- Deisseroth A., Nienhuis A., Turner P., Velez R., Anderson W. F., Ruddle F., Lawrence J., Creagan R., Kucherlapati R. Localization of the human alpha-globin structural gene to chromosome 16 in somatic cell hybrids by molecular hybridization assay. Cell. 1977 Sep;12(1):205–218. doi: 10.1016/0092-8674(77)90198-2. [DOI] [PubMed] [Google Scholar]
- Devine J. M., Tsang A. S., Williams J. G. Differential expression of the members of the discoidin I multigene family during growth and development of Dictyostelium discoideum. Cell. 1982 Apr;28(4):793–800. doi: 10.1016/0092-8674(82)90058-7. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Gilles S. D., Tonegawa S. Expression of cloned immunoglobulin genes introduced into mouse L cells. Nucleic Acids Res. 1983 Nov 25;11(22):7981–7997. doi: 10.1093/nar/11.22.7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
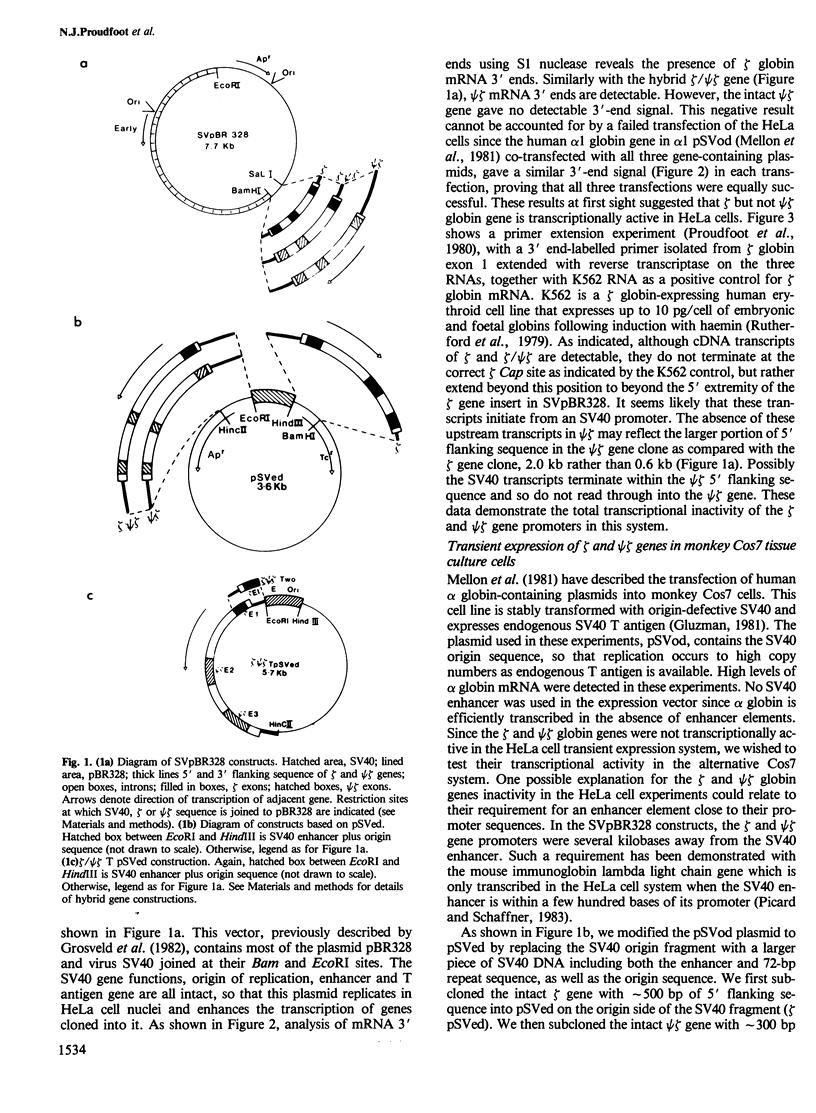
- Grosveld G. C., de Boer E., Shewmaker C. K., Flavell R. A. DNA sequences necessary for transcription of the rabbit beta-globin gene in vivo. Nature. 1982 Jan 14;295(5845):120–126. doi: 10.1038/295120a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Melton D. A. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. doi: 10.1146/annurev.ge.15.120181.001201. [DOI] [PubMed] [Google Scholar]
- HUEHNS E. R., FLYNN F. V., BUTLER E. A., BEAVEN G. H. Two new haemoglobin variants in a very young human embryo. Nature. 1961 Feb 11;189:496–497. doi: 10.1038/189496a0. [DOI] [PubMed] [Google Scholar]
- Hentschel C., Probst E., Birnstiel M. L. Transcriptional fidelity of histone genes injected into Xenopus oocyte nuclei. Nature. 1980 Nov 6;288(5786):100–102. doi: 10.1038/288100a0. [DOI] [PubMed] [Google Scholar]
- Korn L. J. Transcription of Xenopus 5S ribosomal RNA genes. Nature. 1982 Jan 14;295(5845):101–105. doi: 10.1038/295101a0. [DOI] [PubMed] [Google Scholar]
- Kressmann A., Clarkson S. G., Pirrotta V., Birnstiel M. L. Transcription of cloned tRNA gene fragments and subfragments injected into the oocyte nucleus of Xenopus laevis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1176–1180. doi: 10.1073/pnas.75.3.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y. F., Kan Y. W. Versatile cosmid vectors for the isolation, expression, and rescue of gene sequences: studies with the human alpha-globin gene cluster. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5225–5229. doi: 10.1073/pnas.80.17.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer J., Shen C. K., Maniatis T. The chromosomal arrangement of human alpha-like globin genes: sequence homology and alpha-globin gene deletions. Cell. 1980 May;20(1):119–130. doi: 10.1016/0092-8674(80)90240-8. [DOI] [PubMed] [Google Scholar]
- Ley T. J., Nienhuis A. W. A weak upstream promoter gives rise to long human beta-globin RNA molecules. Biochem Biophys Res Commun. 1983 May 16;112(3):1041–1048. doi: 10.1016/0006-291x(83)91723-0. [DOI] [PubMed] [Google Scholar]
- Liebhaber S. A., Goossens M. J., Kan Y. W. Cloning and complete nucleotide sequence of human 5'-alpha-globin gene. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7054–7058. doi: 10.1073/pnas.77.12.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Fritsch E. F., Lauer J., Lawn R. M. The molecular genetics of human hemoglobins. Annu Rev Genet. 1980;14:145–178. doi: 10.1146/annurev.ge.14.120180.001045. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- Mellon P., Parker V., Gluzman Y., Maniatis T. Identification of DNA sequences required for transcription of the human alpha 1-globin gene in a new SV40 host-vector system. Cell. 1981 Dec;27(2 Pt 1):279–288. doi: 10.1016/0092-8674(81)90411-6. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Gurdon J. B. Purified DNAs are transcribed after microinjection into Xenopus oocytes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1502–1506. doi: 10.1073/pnas.74.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Orkin S. H. The 3' untranslated regions of the duplicated human alpha-globin genes are unexpectedly divergent. Cell. 1980 Nov;22(2 Pt 2):371–377. doi: 10.1016/0092-8674(80)90347-5. [DOI] [PubMed] [Google Scholar]
- Miller T. J., Stephens D. L., Mertz J. E. Kinetics of accumulation and processing of simian virus 40 RNA in Xenopus laevis oocytes injected with simian virus 40 DNA. Mol Cell Biol. 1982 Dec;2(12):1581–1594. doi: 10.1128/mcb.2.12.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Schaffner W. Correct transcription of a cloned mouse immunoglobulin gene in vivo. Proc Natl Acad Sci U S A. 1983 Jan;80(2):417–421. doi: 10.1073/pnas.80.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst E., Kressmann A., Birnstiel M. L. Expression of sea urchin histone genes in the oocyte of Xenopus laevis. J Mol Biol. 1979 Dec 15;135(3):709–732. doi: 10.1016/0022-2836(79)90173-6. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Gil A., Maniatis T. The structure of the human zeta-globin gene and a closely linked, nearly identical pseudogene. Cell. 1982 Dec;31(3 Pt 2):553–563. doi: 10.1016/0092-8674(82)90311-7. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Maniatis T. The structure of a human alpha-globin pseudogene and its relationship to alpha-globin gene duplication. Cell. 1980 Sep;21(2):537–544. doi: 10.1016/0092-8674(80)90491-2. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Shander M. H., Manley J. L., Gefter M. L., Maniatis T. Structure and in vitro transcription of human globin genes. Science. 1980 Sep 19;209(4463):1329–1336. doi: 10.1126/science.6158093. [DOI] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Rusconi S., Schaffner W. Transformation of frog embryos with a rabbit beta-globin gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5051–5055. doi: 10.1073/pnas.78.8.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford T. R., Clegg J. B., Weatherall D. J. K562 human leukaemic cells synthesise embryonic haemoglobin in response to haemin. Nature. 1979 Jul 12;280(5718):164–165. doi: 10.1038/280164a0. [DOI] [PubMed] [Google Scholar]
- Stafford J., Queen C. Cell-type specific expression of a transfected immunoglobulin gene. Nature. 1983 Nov 3;306(5938):77–79. doi: 10.1038/306077a0. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw E., Proudfoot N. J. Transcriptional activity of the human pseudogene psi alpha globin compared with alpha globin, its functional gene counterpart. Nucleic Acids Res. 1983 Nov 25;11(22):7717–7733. doi: 10.1093/nar/11.22.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. P., Gurdon J. B. Post-transcriptional processing of simian virus 40 late transcripts in injected frog oocytes. J Mol Biol. 1983 Jan 5;163(1):1–26. doi: 10.1016/0022-2836(83)90027-x. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Woo S., O'Malley B. W., Gurdon J. B. Expression of a chicken chromosomal ovalbumin gene injected into frog oocyte nuclei. Nature. 1980 Jun 26;285(5767):628–634. doi: 10.1038/285628a0. [DOI] [PubMed] [Google Scholar]