Abstract
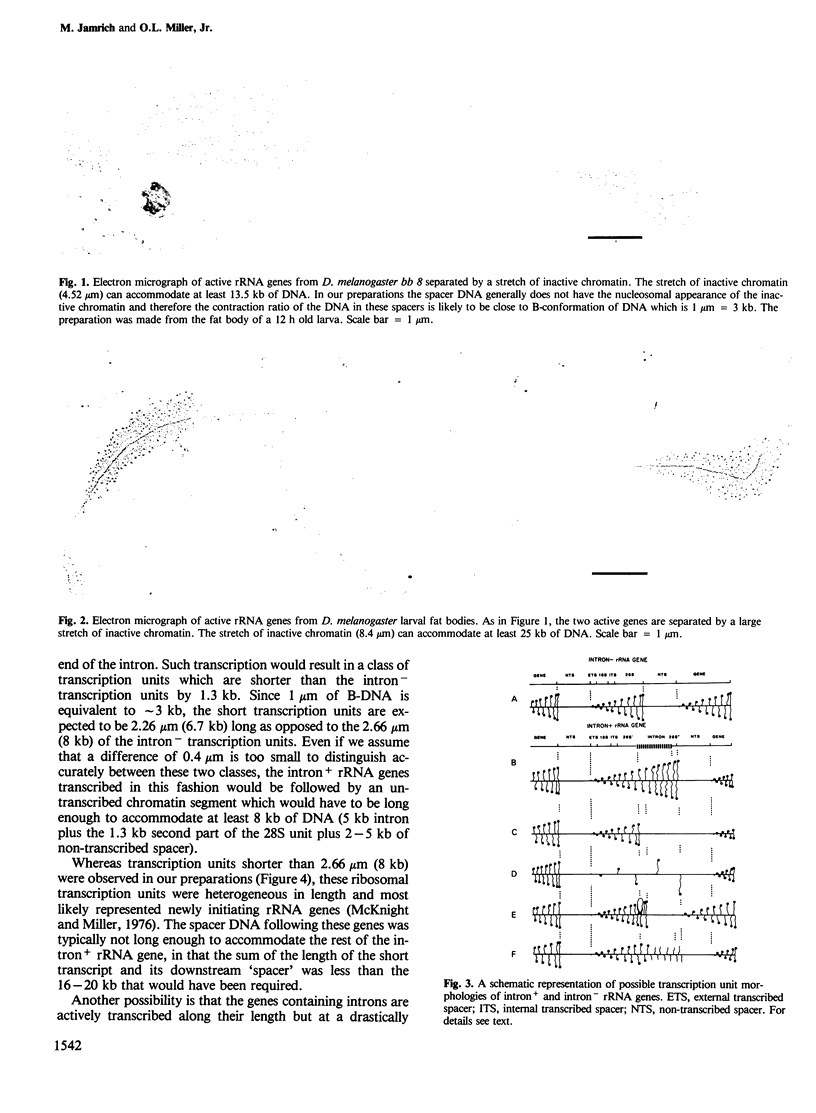
About 50% of the ribosomal transcription units in females of the bobbed 8 mutant of Drosophila melanogaster contain an intervening sequence of 5 kb in the 28S region of the gene. We analysed the transcription of ribosomal genes in this mutant using electron microscopy and found that the majority of the active ribosomal transcription units in larval fat bodies and guts are not long enough to contain the 5-kb intervening sequence; only approximately 1% of active transcription units have a length consistent with the presence of the 5-kb intervening sequence. Transcription units of this length show an interrupted gradient of nascent RNA fibril lengths indicative of processing or degradation during transcription. The position of the discontinuity in RNA length coincides with the position of the intervening sequence. This observation suggests that even though RNA polymerase may infrequently transcribe an entire interrupted gene, the process does not result in a full-length RNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chooi W. Y. The occurrence of long transcription units among the X and Y ribosomal genes of Drosophila melanogaster: transcription of insertion sequences. Chromosoma. 1979 Sep 1;74(1):57–74. doi: 10.1007/BF00344483. [DOI] [PubMed] [Google Scholar]
- Ganguly A. K., Girijavallabhan V. M., Miller G. H., Sarre O. Z. Chemical modification of everninomicins. J Antibiot (Tokyo) 1982 May;35(5):561–570. doi: 10.7164/antibiotics.35.561. [DOI] [PubMed] [Google Scholar]
- Glover D. M., Hogness D. S. A novel arrangement of the 18S and 28S sequences in a repeating unit of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):167–176. doi: 10.1016/0092-8674(77)90212-4. [DOI] [PubMed] [Google Scholar]
- Glätzer K. H. Lengths of transcribed rDNA repeating units in spermatocytes of Drosophila hydei: only genes without an intervening sequence are expressed. Chromosoma. 1979 Nov;75(2):161–175. doi: 10.1007/BF00292205. [DOI] [PubMed] [Google Scholar]
- Grainger R. M., Maizels N. Dictyostelium ribosomal RNA is processed during transcription. Cell. 1980 Jul;20(3):619–623. doi: 10.1016/0092-8674(80)90308-6. [DOI] [PubMed] [Google Scholar]
- Hawley R. S., Tartof K. D. The ribosomal DNA of Drosophila melanogaster is organized differently from that of Drosophila hydei. J Mol Biol. 1983 Jan 25;163(3):499–503. doi: 10.1016/0022-2836(83)90071-2. [DOI] [PubMed] [Google Scholar]
- Jolly D. J., Thomas C. A., Jr Nuclear RNA transcripts from Drosophila melanogaster ribosomal RNA genes containing introns. Nucleic Acids Res. 1980 Jan 11;8(1):67–84. doi: 10.1093/nar/8.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S. J., Glover D. M. Drosophila melanogaster ribosomal DNA containing type II insertions is variably transcribed in different strains and tissues. J Mol Biol. 1981 Oct 5;151(4):645–662. doi: 10.1016/0022-2836(81)90428-9. [DOI] [PubMed] [Google Scholar]
- Long E. O., Collins M., Kiefer B. I., Dawid I. B. Expression of the ribosomal DNA insertions in bobbed mutants of Drosophila melanogaster. Mol Gen Genet. 1981;182(3):377–384. doi: 10.1007/BF00293925. [DOI] [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Expression of ribosomal DNA insertions in Drosophila melanogaster. Cell. 1979 Dec;18(4):1185–1196. doi: 10.1016/0092-8674(79)90231-9. [DOI] [PubMed] [Google Scholar]
- Long E. O., Rebbert M. L., Dawid I. B. Structure and expression of ribosomal RNA genes of Drosophila melanogaster interrupted by type-2 insertions. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):667–672. doi: 10.1101/sqb.1981.045.01.084. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Ultrastructural patterns of RNA synthesis during early embryogenesis of Drosophila melanogaster. Cell. 1976 Jun;8(2):305–319. doi: 10.1016/0092-8674(76)90014-3. [DOI] [PubMed] [Google Scholar]
- Pellegrini M., Manning J., Davidson N. Sequence arrangement of the rDNA of Drosophila melanogaster. Cell. 1977 Feb;10(2):213–214. doi: 10.1016/0092-8674(77)90215-x. [DOI] [PubMed] [Google Scholar]
- Shermoen A. W., Kiefer B. I. Regulation in rDNA-deficient Drosophila melanogaster. Cell. 1975 Mar;4(3):275–280. doi: 10.1016/0092-8674(75)90176-2. [DOI] [PubMed] [Google Scholar]
- Tartof K. D., Dawid I. G. Similarities and differences in the structure of X and Y chromosome rRNA genes of Drosophila. Nature. 1976 Sep 2;263(5572):27–30. doi: 10.1038/263027a0. [DOI] [PubMed] [Google Scholar]
- Tartof K. D. Redundant genes. Annu Rev Genet. 1975;9:355–385. doi: 10.1146/annurev.ge.09.120175.002035. [DOI] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. The structural organization of ribosomal DNA in Drosophila melanogaster. Cell. 1977 Feb;10(2):193–212. doi: 10.1016/0092-8674(77)90214-8. [DOI] [PubMed] [Google Scholar]
- White R. L., Hogness D. S. R loop mapping of the 18S and 28S sequences in the long and short repeating units of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):177–192. doi: 10.1016/0092-8674(77)90213-6. [DOI] [PubMed] [Google Scholar]








