Abstract
Problem
To characterize the amniotic fluid (AF) inflammatory‐related protein (IRP) network in patients with a sonographic short cervix (SCx) and to determine its relation to early preterm delivery (ePTD).
Method of study
A retrospective cohort study included women with a SCx (≤25 mm; n=223) who had amniocentesis and were classified according to gestational age (GA) at diagnosis and delivery (ePTD <32 weeks of gestation).
Results
(i) In women with a SCx ≤ 22 1/7 weeks, the concentration of most IRPs increased as the cervix shortened; those with ePTD had a higher rate of increase in MIP‐1α, MCP‐1, and IL‐6 concentrations than those delivering later; and (ii) the concentration of most IRPs and the correlation between several IRP pairs were higher in the ePTD group than for those delivering later.
Conclusion
Women with a SCx at 16‐22 1/7 weeks have a unique AF cytokine network that correlates with cervical length at diagnosis and GA at delivery. This network may aid in predicting ePTD.
Keywords: amniocentesis, cervical insufficiency, cytokine, macrophage inflammatory protein, network analysis, preterm birth
1. INTRODUCTION
A sonographic short cervix is a strong predictor of spontaneous preterm delivery.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 The earlier the diagnosis of a short cervix, the more likely the patient will deliver preterm.16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 Compelling evidence supports the view that this condition is syndromic in nature and has multiple etiologies,13, 34, 35, 36, 37 such as a decline in progesterone action,38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 prior cervical surgery,49, 50, 51, 52, 53, 54, 55, 56, 57 intra‐amniotic inflammation and/or infection,58, 59, 60, 61, 62, 63 and genuine cervical insufficiency.35, 64 Patients with a mid‐trimester sonographic short cervix can be offered treatment with vaginal progesterone65, 66, 67, 68, 69, 70, 71, 72, 73 and, for those with a history of preterm birth, a cervical cerclage74, 75, 76, 77, 78, 79, 80 may be placed.
A subset of patients with a mid‐trimester sonographic short cervix (cervical length ≤25 mm) may have microbial invasion of the amniotic fluid (MIAC) with or without inflammation.62, 81, 82 Sterile intra‐amniotic inflammation appears to be more common than MIAC (10% vs 2.2%, respectively) and is associated with an increased risk of spontaneous preterm delivery <34 weeks of gestation.82 The presence of intra‐amniotic inflammation (both microbial and sterile) is associated with adverse pregnancy outcomes35, 83, 84, 85, 86, 87, 88, 89, 90, 91 in patients presenting with preterm labor and intact membranes,92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108 preterm pre‐labor rupture of the membranes (PPROM),109, 110, 111, 112, 113, 114 a sonographic short cervix,60, 62, 82, 115 and cervical insufficiency.58, 59, 64, 116, 117 Thus, we and others have explored the behavior of cytokines and other inflammatory‐related markers in amniotic82, 100, 106, 107, 108, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128 or vaginal129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140 fluid.
Disease states are generally caused by the interaction of a group of correlated molecules (or network) and not by the effect of a single molecule.141, 142, 143, 144, 145, 146, 147 For example, in the context of inflammation, one cytokine can induce the expression of others, which can modulate the immune response and lead to the development of feedback regulatory networks.148 Thus, to understand the pathogenesis of inflammatory‐related diseases, cytokines should ideally be studied as groups of interacting molecules/proteins.149, 150, 151 Indeed, recent evidence suggests that biomarkers derived from network analysis are able to achieve better diagnostic performance than those derived from a single‐molecule approach.61, 152, 153, 154, 155, 156, 157, 158, 159
Women with microbial‐associated or sterile intra‐amniotic inflammation had a more coordinated cytokine network than those without intra‐amniotic inflammation.127 Using a network approach, we were able to demonstrate that microbial and sterile intra‐amniotic inflammation differ in their cytokine networks.127 Therefore, the objective of this study was to examine the amniotic fluid inflammatory‐related protein network in asymptomatic patients with a mid‐trimester sonographic short cervix according to gestational age at the time of diagnosis and delivery.
2. MATERIALS AND METHODS
2.1. Study population
A retrospective cohort study was conducted to include 223 patients with an asymptomatic sonographic short cervix. Amniotic fluid samples of participants were selected from the clinical database and Bank of Biological Materials of Wayne State University, the Detroit Medical Center, and the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) if the patients met the following criteria: (i) singleton gestation, (ii) asymptomatic sonographic cervical length ≤25 mm, (iii) transabdominal amniocentesis performed for molecular microbiological studies, and (iv) available pregnancy outcomes. Exclusion criteria were as follows: (i) rupture of membranes or preterm labor symptoms before amniotic fluid collection, (ii) chromosomal or structural fetal anomaly, or (iii) placenta previa. A subset of patients in this study was included in a prior study,82 which provides a description of the molecular microbiologic findings of the amniotic cavity and amniotic fluid IL‐6 concentrations. All patients provided written informed consent, and the use of biological specimens as well as clinical and ultrasound data for research purposes was approved by the Institutional Review Boards of Wayne State University and NICHD.
Clinical definitions, methods of sonographic assessment of the cervix, sample collection, and the detection of microorganisms as well as amniotic fluid IL‐6 concentrations were previously reported.82 Briefly, spontaneous preterm labor was defined by the presence of regular uterine contractions occurring at least twice every 10 minutes, associated with cervical changes before 37 completed weeks of gestation. Early spontaneous preterm delivery was defined as spontaneous preterm delivery before 32 completed weeks of gestation. Patients classified as having spontaneous preterm delivery included those with spontaneous preterm labor, preterm pre‐labor rupture of the membranes (PPROM), and those whose labor was induced or augmented due to clinical chorioamnionitis.160 Patients who were unavailable for a follow‐up or who had inaccessible delivery data were excluded.
Intra‐amniotic inflammation was characterized by an amniotic fluid IL‐6 concentration ≥2.6 ng/mL.82, 100, 106, 107, 108, 114, 118, 120, 122, 123, 124 Microbial invasion of the amniotic cavity was defined according to the results of amniotic fluid culture and broad‐range polymerase chain reaction (PCR) coupled with electrospray ionization mass spectrometry (PCR/ESI‐MS) analysis.105, 113 Acute histologic chorioamnionitis was diagnosed based on the presence of inflammatory cells in the chorionic plate and/or chorioamniotic membranes, and acute funisitis was diagnosed by the presence of neutrophils in the wall of the umbilical vessels and/or Wharton's jelly, using previously described criteria.86, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170
2.2. Multiplex determination of inflammatory‐related proteins
Amniotic fluid concentrations of 33 inflammatory‐related proteins were determined using a multiplex bead array assay developed by the investigators as previously described in detail (MethodsS1).127
2.3. Statistical analysis
The goal of the statistical analysis was to (i) assess the relation between the risk of preterm delivery and gestational age at amniocentesis, (ii) assess the relation between inflammatory‐related protein concentration and cervical length, (iii) evaluate differences in inflammatory‐related protein concentration between groups (delivery ≤32 or >32 weeks of gestation), and (iv) evaluate differences in the correlation of pairs of proteins between groups and build correlation networks. To accomplish these goals, women were divided into three groups based on their gestational age at amniocentesis with the two cutoff points representing tertiles of the distribution: 16 5/7 to 22 1/7, 22 2/7 to 26 1/7, and 26 2/7 to 31 5/7 weeks of gestation. In addition to creating equal‐sized subgroups of patients, this subclassification of samples is based on (i) previous observations that the populations of patients diagnosed with a short cervix are different, depending upon whether the diagnosis is made earlier as opposed to later in gestation81 and also (ii) the observation that pro‐inflammatory protein concentrations may vary with gestational age at amniocentesis.127
The association between the interval of gestational age at amniocentesis and risk of preterm delivery was tested using a chi‐square test for trends in proportions. To test for differential analyte concentrations between groups within each gestational‐age interval, a linear model was fit to the log (base 2) of the analyte concentration as a function of the group indicator (patient delivered ≤32 or >32 weeks of gestation) while adjusting for gestational age at amniocentesis. Similarly, to test the association between analyte concentration and cervical length, a linear model was fit to the log (base 2) of the analyte concentration as a function of cervical length and the group indicator (patient delivered ≤32 or >32 weeks of gestation) while adjusting for gestational age at amniocentesis. An interaction term was allowed between the group indicator and cervical length to accommodate possible differences in the rate of change of the analyte concentration and cervical length between groups.
Significance test P values for the group (delivery ≤32 or >32 weeks of gestation) coefficient as well as for the cervical length coefficient were adjusted with the Benjamini‐Hochberg method over all 33 analytes to compute q‐values. The significance of differences in concentration was determined based on a q‐value <.1 and a fold change >1.5. For changes in cervical length, the minimum fold change cutoff of 1.5 was defined as more than a 1 cm change in cervical length.
The difference in concentration correlation of each possible pair of analytes (eg, IL‐1α and IL‐6, IL‐1α and IL‐33) between groups was assessed as follows. A linear model was fit to the log‐transformed data of each protein as a function of gestational age using samples of each group separately in each gestational‐age interval. The residuals (actual value−fitted value) were then used to compute the Pearson correlation for each pair of analytes (called partial correlations). To test for differences in partial correlations between groups within each interval, the partial correlations were first converted into an intermediary statistic using Fisher's z transformation. Under the null hypothesis (partial correlations are equal between groups), the standardized differences in z values among groups were assumed to follow a standard normal distribution. Significant differences in partial correlations were inferred based a P value <.01, and the magnitude of correlation differences was at least .2.
A network of differential correlation between groups of women was constructed for each gestational‐age interval by linking/connecting the proteins with significant differences in partial correlations (also referred to as perturbed correlation). For each node (protein) in the network, we calculated the degree (number of perturbed correlations of a given protein) and the average absolute difference in correlations as previously described.127
To identify confounding variables that could impact the analyses described above, we have used chi‐square tests to determine the association of covariates (administration of steroids, antibiotics, and progesterone before amniocentesis) and preterm delivery within each interval. Covariates with a significant association with the outcome were adjusted for in the differential concentration and differential correlation analyses.
3. RESULTS
3.1. Characteristics of the study population
Two hundred and twenty‐three patients who had a sonographic short cervix (≤25 mm) were included in this study. Demographic and clinical characteristics of the study population are displayed in Table 1. The median gestational age at amniocentesis was 24 3/7 weeks. Forty‐four percent (99/223) of patients with a short cervix delivered at term. Most patients did not have intra‐amniotic inflammation (75.3% [168/223]). Among patients with intra‐amniotic inflammation, the frequency of sterile intra‐amniotic inflammation was higher than microbial‐associated intra‐amniotic inflammation (10.3% [23/223] and 2.2% [5/223], respectively) (Table 1). Patients with a sonographic short cervix were classified according to gestational age at delivery, and 28.7% (64/223) delivered ≤32 weeks of gestation while the remaining 71.3% (159/223) delivered >32 weeks of gestation.
Table 1.
Clinical and demographic characteristics of the study population
| Variable | Overall cohort (N=223) | Gestational age at delivery | P‐valuea | |
|---|---|---|---|---|
| ≤32 wk of gestation (N=64) | >32 wk of gestation (N=159) | |||
| Maternal age (y) | 23.5 (20‐28) | 25 (21‐32.8) | 23 (20‐27) | .03 |
| Pre‐pregnancy body mass index (kg/m2)b | 25.8 (21.5‐32.6) | 29.1 (23.0‐35.4) | 24.7 (21.1‐31.0) | .004 |
| Race, % (n) | ||||
| African American | 91.5% (204) | 93.7% (60) | 90.6% (144) | .64 |
| Hispanic | 3.1% (7) | 3.1% (2) | 3.1% (5) | |
| Caucasian | 2.7% (6) | 1.6% (1) | 3.1% (5) | |
| Asian | 0.9% (2) | 1.6% (1) | 0.6% (1) | |
| Other | 1.8% (4) | 0 | 2.5% (4) | |
| Gestational age at amniocentesis (wk) | 24.4 (21.1‐27.4) | 21.7 (19.9‐24.5) | 25.4 (22.9‐28.4) | <.001 |
| Sonographic cervical length at diagnosis (mm) | 13 (7‐18) | 7 (2.3‐14) | 14 (10‐19) | <.001 |
| Diagnosis | ||||
| No intra‐amniotic inflammation, % (n) | 75.3% (168) | 59.4% (38) | 81.8% (130) | <.001 |
| Microbial invasion of the amniotic cavity, % (n) | 12.1% (27) | 9.4% (6) | 13.2% (21) | .43 |
| Sterile intra‐amniotic inflammation, % (n) | 10.3% (23) | 25.0% (16) | 4.4% (7) | <.001 |
| Microbial‐associated intra‐amniotic inflammation, % (n) | 2.2% (5) | 6.3% (4) | 0.6% (1) | .01 |
| Intra‐amniotic inflammation (ELISA IL‐6 ≥2.6 ng/mL), % (n) | 12.6% (28) | 31.3% (20) | 5.0% (8) | <.001 |
| Treatment | ||||
| Cerclage, % (n/N) | 8.1% (18/221) | 9.4% (6/64) | 7.6% (12/157) | .67 |
| Progesterone supplementation before amniocentesis, % (n/N) | 1.4% (3/214) | 0% | 2.0% (3/152) | .27 |
| Administration of antenatal corticosteroids within 7 d before amniocentesis, % (n) | 11.7% (26) | 12.5% (8) | 11.3% (18) | .80 |
| Antibiotics before amniocentesis, % (n) | 5.8% (13) | 6.3% (4) | 5.7% (9) | .87 |
| Amniotic fluid | ||||
| Amniotic fluid IL‐6 concentration (ng/mL) | 0.65 (0.29‐1.28) | 1.11 (0.31‐4.15) | 0.59 (0.28‐0.99) | .002 |
| Amniotic fluid glucose concentration (mg/dL)c | 30 (25‐35) (N=221) | 31 (24.3‐36) (N=64) | 30 (25‐35) (N=157) | .52 |
| Amniotic fluid white blood cell count (cells/mm3)d | 2 (0‐7.5) (N=218) | 2 (0‐10) (N=63) | 1 (0‐6) (N=155) | .06 |
| Placenta | ||||
| Acute inflammatory lesion of the placenta, % (n/N) | 45.1% (92/204) | 73.3% (44/60) | 33.3% (48/144) | <.001 |
| Acute histologic chorioamnionitis, % (n/N) | 44.1% (90/204) | 73.3% (44/60) | 31.9% (46/144) | <.001 |
| Acute funisitis, % (n/N) | 32.4% (66/204) | 51.7% (31/60) | 24.3% (35/144) | <.001 |
| Delivery | ||||
| Amniocentesis to delivery interval (d) | 68 (36‐95) | 23 (10‐35) | 85 (64‐105) | <.001 |
| Gestational age at delivery (wk) | 35.7 (30.6‐38.7) | 26.6 (22.1‐29.5) | 38.0 (35.1‐39.1) | <.001 |
| Delivery ≥37 wk of gestation, % (n) | 44.4% (99) | 0% | 62.3% (99) | <.001 |
ELISA, enzyme‐linked immunosorbent assay; IL, interleukin.
Data are given as median (interquartile range) and percentage (n).
Acute inflammatory lesion of the placenta is defined as acute histologic chorioamnionitis and/or acute funisitis.
Comparison between patients with the diagnosis of a short cervix who delivered before or after 32 wk of gestation.
Missing data: 18 cases.
Missing data: two cases.
Missing data: five cases.
The samples included in this study were collected before the publication of randomized trials reporting that vaginal progesterone reduces the rate of preterm delivery and neonatal morbidity; therefore, none of our patients received vaginal progesterone. Eight percent (18/221) of patients underwent cerclage. There were no significant differences in the frequencies with which 17‐alpha‐hydroxyprogesterone caproate, antibiotics, and antenatal corticosteroids were administered within 7 days before amniocentesis between women with a short cervix who delivered ≤32 weeks of gestation and those who delivered >32 weeks of gestation (Table 1).
The prevalence of intra‐amniotic inflammation (defined as an amniotic fluid IL‐6 concentration ≥2.6 ng/mL), including both microbial‐associated and sterile intra‐amniotic inflammation, was significantly higher in patients with a sonographic short cervix who delivered ≤32 weeks of gestation than in those who delivered >32 weeks of gestation (P<.05 for all; Table 1). Additionally, the median amniotic fluid IL‐6 concentrations (ng/mL) and the frequency of acute inflammatory lesions of the placenta were significantly higher and more frequent in patients with a short cervix who delivered ≤32 weeks of gestation than in those who delivered >32 weeks of gestation (IL‐6: median [IQR]; 1.11 [0.31‐4.15] vs 0.59 [0.28‐0.99]; P=.002; acute inflammatory lesion of the placenta: 73.3% [44/60] vs 33.3% [48/144]; P<.001; Table 1).
3.2. The risk of preterm delivery according to the presence of a sonographic short cervix and gestational age at diagnosis
Patients with a short cervix who delivered ≤32 weeks of gestation had a significantly lower gestational age at amniocentesis/diagnosis with a short cervix, and they had a shorter sonographic cervical length than those who delivered >32 weeks of gestation (P<.001 for all; Table 1). When patients were stratified according to the tertiles of gestational age at amniocentesis, the rate of delivery ≤32 weeks of gestation was 50% in those with an asymptomatic short cervix identified at 16 5/7 to 22 1/7, 21% in those identified at 22 2/7 to 26 1/7, and 15% in those at 26 2/7‐31 5/7 weeks of gestation (chi‐square test for trend P<.0001).
3.3. Potential effects of confounding variables
We examined whether the administration of steroids, antibiotics, and progesterone before amniocentesis could have had a confounding effect on the differential concentration analysis of proteins. The association of these covariates with the “group indicator” (whether a patient delivered ≤32 or >32 weeks of gestation) for each gestational‐age interval at amniocentesis was assessed and found to be significant only for steroids (P=.014) in the gestational‐age window between 26 2/7 and 31 5/7 weeks at amniocentesis. Therefore, we adjusted for exposure to steroids in the third interval of gestational age at amniocentesis and found little effect on the differential concentration and correlation analysis results, as described below.
3.4. The inflammatory protein concentrations and sonographic cervical length
We tested the association between inflammatory‐related protein concentrations and cervical length within the group of patients in which the diagnosis of a short cervix/gestational age at amniocentesis was made early in pregnancy (16 5/7 to 22 1/7 weeks) while controlling for the gestational age at amniocentesis. This set of patients included an equal number of deliveries ≤32 and >32 weeks of gestation. The concentration of all inflammatory‐related proteins were inversely related to cervical length (ie, the shorter the cervix, the higher the concentration of the proteins; q<.1 for all). Only IP‐10 and IL‐18 did not reach the required minimum of a 1.5‐fold change in abundance per cm change in cervical length to be considered statistically significant. Fold changes for significant proteins ranged from 1.5‐ to 8.5‐fold per cm (IL‐33 and IL‐8, respectively; see Figure 1 and Table 2). For three proteins (MIP‐1α, MCP‐1, IL‐6), the rate of change in abundance with cervical length was higher in patients who delivered ≤32 weeks compared to those who delivered >32 weeks of gestation (Figure 2).
Figure 1.
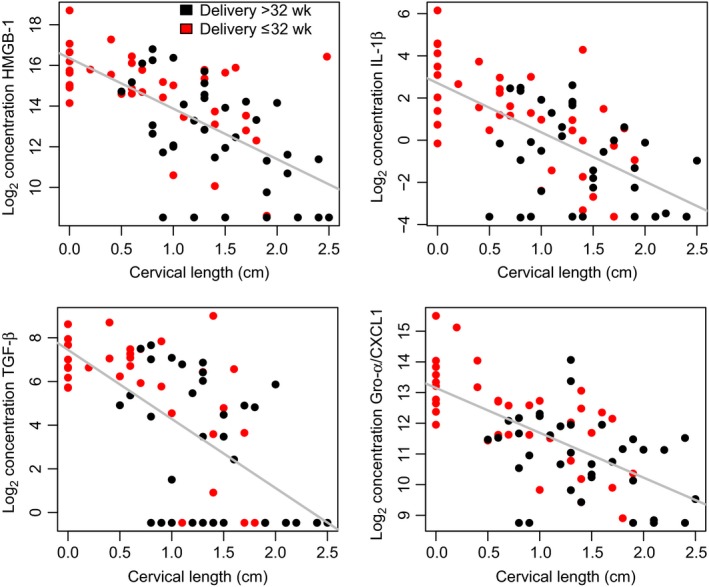
Association between pro‐inflammatory protein concentration and cervical length for patients diagnosed with a short cervix at 16 5/7 to 22 1/7 weeks of gestation. The figure shows the concentration (log2, thereof) as a function of cervical length for 4 of the 33 proteins shown in Table 2. The concentration of these proteins is higher in women with a short cervix regardless of whether they delivered ≤32 weeks of gestation (red) or >32 weeks of gestation (black)
Table 2.
Changes in protein concentration with cervical shortening
| Protein | Overall change with CL | Interaction between group and CL | ||||
|---|---|---|---|---|---|---|
| Fold changea | P | q | Fold changeb | P | q | |
| MCP‐1 | −2.3 | .000 | .000 | −2.4 | .003 | .098 |
| MIP‐1α | −2.7 | .000 | .000 | −2.7 | .006 | .098 |
| IL‐6 | −5.0 | .000 | .000 | −4.6 | .009 | .098 |
| MIP‐1β | −3.7 | .000 | .000 | −3.5 | .014 | .115 |
| MIP‐3α | −5.4 | .000 | .000 | −2.7 | .135 | .735 |
| HMGB‐1 | −5.0 | .000 | .000 | 2.0 | .177 | .735 |
| I‐TAC/CXCL‐11 | −4.5 | .000 | .000 | 2.4 | .190 | .735 |
| Gro‐a/CXCL1 | −2.5 | .000 | .000 | −1.5 | .226 | .735 |
| Calgranulin C | −2.1 | .041 | .043 | −2.5 | .241 | .735 |
| IL‐8 | −8.5 | .000 | .000 | −4.0 | .241 | .735 |
| IL‐1α | −2.7 | .001 | .001 | −2.0 | .273 | .735 |
| IL‐15 | −4.3 | .000 | .000 | 1.9 | .292 | .735 |
| Eotaxin | −4.2 | .000 | .000 | 1.7 | .299 | .735 |
| IL‐12 | −4.4 | .000 | .000 | 1.9 | .330 | .735 |
| RANTES | −2.3 | .004 | .005 | 1.8 | .334 | .735 |
| IL‐1β | −4.9 | .000 | .000 | −1.6 | .392 | .808 |
| IL‐18 | −1.4 | .004 | .005 | 1.2 | .444 | .862 |
| IL‐16 | −2.5 | .000 | .000 | 1.2 | .535 | .897 |
| IL‐10 | −4.0 | .001 | .002 | −1.7 | .558 | .897 |
| GM‐CSF | −3.0 | .001 | .001 | 1.5 | .571 | .897 |
| IFN‐γ | −3.7 | .000 | .000 | 1.3 | .658 | .971 |
| IP‐10 | −1.4 | .042 | .043 | 1.1 | .742 | .971 |
| M‐CSF | −3.0 | .000 | .000 | 1.2 | .749 | .971 |
| IL‐2 | −3.9 | .000 | .000 | 1.1 | .809 | .971 |
| IL‐33 | −1.5 | .013 | .014 | 1.1 | .819 | .971 |
| IL‐7 | −6.2 | .000 | .000 | 1.1 | .846 | .971 |
| IL‐4 | −2.2 | .000 | .000 | 1.1 | .860 | .971 |
| IL‐13 | −6.3 | .000 | .000 | −1.1 | .920 | .971 |
| TGF‐β | −7.8 | .000 | .000 | −1.1 | .940 | .971 |
| TNF‐α | −4.0 | .000 | .000 | 1.0 | .942 | .971 |
| Calgranulin A | −4.8 | .000 | .000 | 1.1 | .949 | .971 |
| Lactoferrin | −2.4 | .000 | .000 | 1.0 | .971 | .971 |
CL, cervical length.
Refers to the change in average protein concentration per cm increase in cervical length. Minus sign denotes decrease in concentration with increasing cervical length (or increase with cervical shortening). Both groups of patients (delivery ≤32 wk and >32 wk of gestation) were included in this analysis.
Refers to the ratio of change in the average protein concentration per cm of cervical length in patients with delivery ≤32 wk than for those who delivered >32 wk. Minus sign denotes that the decrease in protein concentration and cervical length (or increase in concentration with cervical shortening) is greater in patients who delivered ≤32 wk compared to those who delivered >32 wk.
Figure 2.
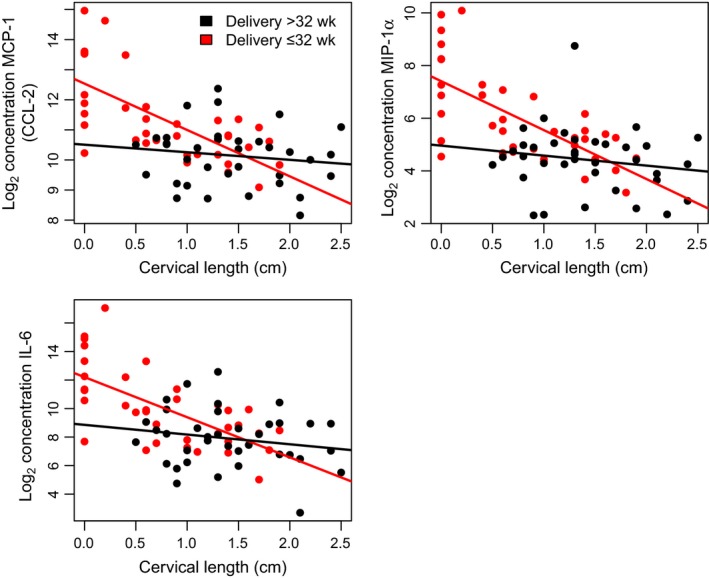
Three proteins that increase in abundance with cervical shortening a higher rate in women who delivered ≤32 weeks of gestation (red) compared to those who delivered >32 weeks of gestation (black). As in Figure 1, only patients diagnosed with a short cervix at 16 5/7 weeks to 22 1/7 weeks of gestation are included in this analysis. Solid lines represent the best linear fit of the log2 protein concentration as a function of gestational age at amniocentesis
3.5. Differences in amniotic fluid inflammatory‐related protein concentrations between patients with a sonographic short cervix who delivered before and after 32 weeks of gestation
The amniotic fluid concentrations of 33 inflammatory‐related proteins were compared between patients with a sonographic short cervix who delivered ≤32 and >32 weeks of gestation. As the amniotic fluid concentration of these analytes has a nonlinear relationship with gestational age and their discriminatory power may vary as a function of duration of pregnancy, we assessed differential concentration within three separate intervals, adjusting for gestational age at amniocentesis.
3.5.1. Amniotic fluid concentration of inflammatory proteins in patients with a short cervix diagnosed between 16 5/7 and 22 1/7 weeks of gestation
All inflammatory proteins but five (IL‐18, IL‐33, IP‐10, MIG, and MIP‐3α) had a significantly higher mean concentrations in patients who delivered ≤32 weeks of gestation than in those who delivered >32 weeks of gestation (fold change >1.5; q<.1). The highest changes of amniotic fluid protein concentration were observed for IL‐8, MIP‐1β, IL‐6, and IL‐10 (5.6‐, 5.1‐, 4.5‐, and 4.4‐fold, respectively; Table 3). Of the 28 inflammatory‐related proteins whose concentrations were significantly different between groups in this interval, 18 would remain significant when the analysis was restricted to patients without intra‐amniotic inflammation (see proteins in boldface, Table 3).
Table 3.
Differential protein concentration analysis for different intervals of gestational age at amniocentesis
| Protein | 16 5/7‐22 1/7 wk | 22 2/7‐26 1/7 wk | 26 2/7‐31 5/7 wk | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FC | P | q | FC | P | q | FC | P | q | |
| Calgranulin A | 3.8 | .0006 | .002 | 1.4 | .0382 | .097 | −1.2 | .4503 | .803 |
| Calgranulin C | 3.3 | .0023 | .004 | 1.3 | .4633 | .493 | 1.4 | .4294 | .803 |
| Eotaxin | 2.4 | .0021 | .004 | 1.3 | .0937 | .155 | 1.1 | .5669 | .813 |
| GM‐CSF | 2.5 | .0023 | .004 | 1.3 | .3937 | .433 | 2.2 | .0686 | .479 |
| Gro‐a/CXCL1 | 2.2 | .0003 | .001 | 1.6 | .0058 | .030 | 1.2 | .3670 | .803 |
| HMGB‐1 | 3.0 | .0008 | .002 | 1.8 | .0064 | .030 | 1.1 | .7594 | .878 |
| I‐TAC/CXCL‐11 | 2.4 | .0062 | .009 | 1.4 | .0484 | .114 | 1.2 | .4836 | .803 |
| IFN‐γ | 2.4 | .0050 | .008 | 1.3 | .0942 | .155 | 1.1 | .7037 | .878 |
| IL‐10 | 4.4 | .0001 | .000 | 1.4 | .0650 | .134 | 1.1 | .6757 | .878 |
| IL‐12 | 2.9 | .0005 | .001 | 1.3 | .1875 | .229 | 1.2 | .5176 | .803 |
| IL‐13 | 1.9 | .0774 | .088 | 1.5 | .0884 | .155 | 1.1 | .7981 | .878 |
| IL‐15 | 1.8 | .0183 | .023 | 1.1 | .5204 | .537 | 1.4 | .1785 | .793 |
| IL‐16 | 2.8 | .0000 | .000 | 1.4 | .0361 | .097 | −1.0 | .9394 | .969 |
| IL‐18 | 1.4 | .0231 | .028 | 1.3 | .0290 | .095 | 1.3 | .0870 | .479 |
| IL‐1α | 2.7 | .0006 | .002 | 1.2 | .5498 | .550 | 2.1 | .0721 | .479 |
| IL‐1β | 3.0 | .0034 | .006 | 1.9 | .0193 | .071 | 1.2 | .5224 | .803 |
| IL‐2 | 2.0 | .0144 | .020 | 1.3 | .1661 | .211 | 1.2 | .3397 | .803 |
| IL‐33 | 1.8 | .3545 | .354 | 1.8 | .1050 | .156 | 1.2 | .6707 | .878 |
| IL‐4 | 1.9 | .0033 | .006 | 1.3 | .0935 | .155 | −1.2 | .2510 | .793 |
| IL‐6 | 4.5 | .0002 | .001 | 4.2 | .0000 | .000 | 1.0 | .9970 | .997 |
| IL‐7 | 3.0 | .0020 | .004 | 1.4 | .1122 | .156 | 1.2 | .4243 | .803 |
| IL‐8 | 5.6 | .0003 | .001 | 5.4 | .0000 | .000 | 2.3 | .0331 | .479 |
| IP‐10 | 1.4 | .0881 | .097 | 1.5 | .1180 | .156 | 1.2 | .4149 | .803 |
| Lactoferrin | 2.8 | .0000 | .000 | 1.4 | .0318 | .095 | 1.6 | .3441 | .803 |
| M‐CSF | 2.8 | .0000 | .000 | 1.3 | .3386 | .385 | 1.4 | .2240 | .793 |
| MCP‐1 | 2.4 | .0000 | .000 | 2.0 | .0027 | .022 | 1.3 | .2644 | .793 |
| MIG | 1.4 | .2105 | .217 | 1.4 | .2975 | .351 | −1.1 | .7902 | .878 |
| MIP‐1α | 2.9 | .0000 | .000 | 1.9 | .0075 | .031 | 1.5 | .0756 | .479 |
| MIP‐1β | 5.1 | .0000 | .000 | 2.6 | .0016 | .018 | 1.4 | .0860 | .479 |
| MIP‐3α | 1.9 | .1479 | .157 | 1.9 | .0046 | .030 | −1.3 | .2384 | .793 |
| RANTES | 1.9 | .0326 | .038 | 1.4 | .1172 | .156 | −1.2 | .5353 | .803 |
| TGF‐β | 2.5 | .0167 | .022 | 1.3 | .1060 | .156 | −1.0 | .9385 | .969 |
| TNF‐α | 2.4 | .0109 | .016 | 1.3 | .0592 | .130 | 1.1 | .7260 | .878 |
FC, fold change. P, nominal P‐values; q, false discovery rate adjusted P‐values.
Negative values represent a decrease in patients who delivered ≤32 wk compared to those who delivered >32 wk. Bolded fold changes are for proteins for which the differential abundance remains significant if only the patients with no intra‐amniotic inflammation are used in the analysis.
3.5.2. Amniotic fluid concentration of inflammatory proteins in patients with a short cervix diagnosed between 22 2/7 and 26 1/7 weeks of gestation
In this gestational‐age window, the differences in amniotic fluid concentrations of inflammatory‐related proteins between groups were of a lesser magnitude than those in the first gestational‐age interval (16 5/7 to 22 1/7 weeks); for nine analytes, the mean amniotic fluid concentration was significantly higher in patients who delivered ≤32 weeks of gestation compared to those who delivered >32 weeks of gestation (fold change >1.5; q<.1). The three analytes with the highest concentration were IL‐8, IL‐6, and MIP‐1β (5.4‐, 4.2‐, and 2.6‐fold, respectively, Table 3).
3.5.3. Amniotic fluid concentration of inflammatory proteins in patients with a short cervix diagnosed between 26 2/7 and 31 5/7 weeks of gestation
Differences in the amniotic fluid concentrations of the inflammatory‐related proteins between groups was less than that of the other two gestational‐age groups. The only protein with a significant difference in median concentration between patients who delivered ≤32 weeks of gestation compared to those who delivered >32 weeks of gestation was IL‐8 (fold change of 2.3 and nominal P value <.05, Table 3). However, this significance was lost when adjusting for the administration of steroids, which was a confounder variable in the analysis of patients in this interval of gestational age (as described above).
3.6. Amniotic fluid cytokine network analysis according to gestational age at diagnosis of a short cervix
As the concentration of pro‐inflammatory proteins changes with gestational age at amniocentesis (see Figure 3, top panel for IL‐10 and MIP‐1β), differences in correlation patterns of pairs of proteins between groups were assessed within three intervals of gestational age, after removing the linear trend with gestational age from log (base 2) protein concentration (Figure 3, bottom panel). The example presented in Figure 3 shows that in the interval of 16 5/7 to 22 1/7 weeks, the partial correlation between MIP‐1β and IL‐10 is .65 in patients who delivered ≤32 weeks and only .05 in patients who delivered >32 weeks (difference in correlation coefficient of .65‐.05=.60, P=.0055, see TableS1).
Figure 3.
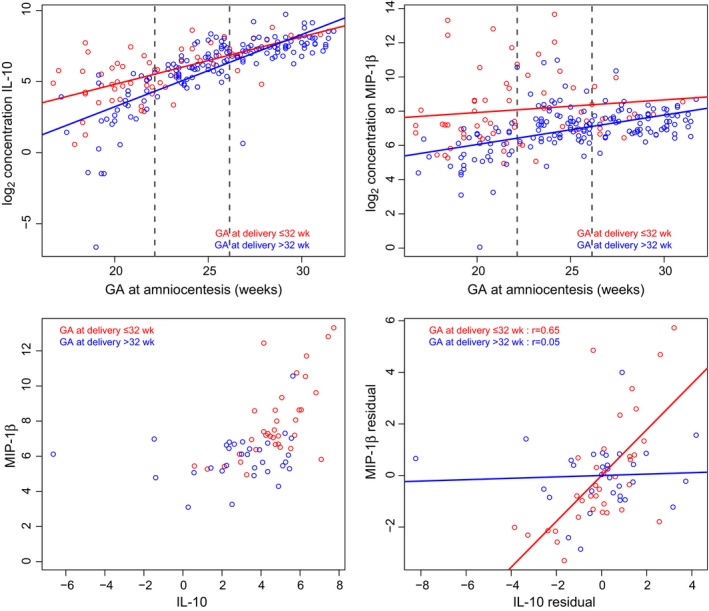
Differential correlation analyses. The figure shows log2 concentration (pg/mL) of IL‐10 (upper left panel) and MIP‐1β (upper right panel) as a function of gestational age at amniocentesis in patients who had a sonographic short cervix and who delivered ≤32 weeks (red) and those >32 weeks (blue) of gestation. Dashed lines denote the two tertiles of the distribution of gestational age at amniocentesis designating patients into three intervals, and the scatterplot of the log2 concentration of IL‐10 and MIP‐1β in the first interval is shown (bottom left panel). A linear model was fit to the log2 concentration of each analyte as a function of gestational age in each group within each gestational‐age interval, and residuals were used to compute partial correlations between analytes (bottom right panel). The partial correlation between the two analytes was significantly increased in patients who delivered ≤32 weeks than those >32 weeks of gestation
Patients with a sonographic short cervix diagnosed between 16 5/7 and 22 1/7 weeks of gestation who delivered ≤32 weeks of gestation had a higher concentration of inflammatory‐related proteins than those who delivered >32 weeks of gestation (22 perturbed proteins). IL‐8, MIP‐1β, and MIP‐1α had the highest number of perturbed correlations (degrees of 6, 5, and 5, respectively), while IL‐8, MIP‐1β, and IL‐6 showed the largest magnitude of change between groups (Figure 4A).
Figure 4.
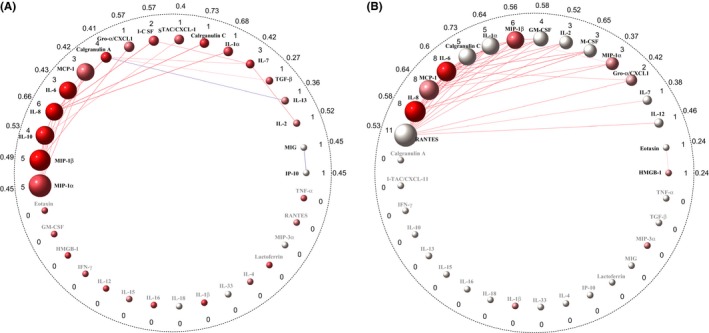
Network of perturbed cytokine concentration correlations between patients who had a sonographic short cervix and who delivered ≤32 weeks of gestation and those who delivered >32 weeks in the first (left panel, A) and second (right panel, B) gestational‐age intervals. Each node (sphere) represents one of the 33 analytes, with a link (line) between two nodes representing a significantly perturbed correlation. The node color represents the direction of concentration change between delivery ≤32 weeks compared to delivery >32 weeks (red=increased, blue=decreased, and white=no change), while the color intensity is proportional to the magnitude of concentration change. The color of links gives the direction of correlation change (red=increased correlation and blue=decreased correlation), while the color intensity is proportional to the magnitude of correlation change. The numbers inside/outside of the dotted black circle represent the node degree/average of the absolute difference in correlations
Patients with a sonographic short cervix diagnosed between 22 2/7 and 26 1/7 weeks of gestation who delivered ≤32 weeks of gestation had higher correlation among 16 amniotic fluid inflammatory‐related proteins than those who delivered >32 weeks of gestation (35 perturbed correlations). RANTES, IL‐8, IL‐6, MCP‐1, and MIP‐1β had the highest number of perturbed correlations (degree of 11, 8, 8, 8, and 6, respectively), whereas IL‐6 and IL‐8 demonstrated the highest magnitude of change in concentration (Figure 4B).
Among patients with a sonographic short cervix diagnosed between 26 2/7 and 31 5/7 weeks of gestation, those who delivered ≤32 weeks of gestation had similar concentrations and concentration correlations of amniotic fluid cytokines to those who delivered >32 weeks of gestation (only 5 perturbed correlations).
4. DISCUSSION
4.1. Principal findings of the study
(i) The earlier the short cervix was diagnosed, the higher the rate of preterm delivery ≤32 weeks of gestation, reaching 50% in the interval of 16 5/7 to 22 1/7 weeks. (ii) In patients with a short cervix diagnosed at 16 5/7 to 22 1/7 weeks, the concentration of most inflammatory‐related proteins increases as the cervix shortens in both delivery groups, yet the rate of increase in MIP‐1α, MCP‐1, and IL‐6 is higher in women who delivered ≤32 weeks compared to those who delivered >32 weeks of gestation. (iii) The concentration of most inflammatory‐related proteins studied herein was higher in patients who delivered ≤32 weeks compared to those who delivered >32 weeks of gestation, yet the magnitude of the difference decreases with increasing gestational age at amniocentesis and diagnosis of a short sonographic cervix. (iv) The concentration correlation of several pairs of inflammatory‐related proteins was overall higher in patients who delivered ≤32 weeks compared to those who delivered >32 weeks of gestation.
4.2. Network of protein correlation perturbations related to preterm delivery in patients diagnosed with a short cervix between 16 and 22 weeks of gestation
Patients with a sonographic short cervix and a subsequent early preterm delivery had more coordinated amniotic fluid cytokine concentrations than those who had late preterm deliveries. IL‐8 and MIP‐1β are the key mediators derived from both differential correlation and concentration analysis. Interestingly, MIP‐1α is the top‐ranked protein obtained from the differential correlation approach despite its small magnitude of change in concentration (fold change=1.56; P=.00016).
The two top‐ranked proteins, IL‐8 and MIP‐1β, obtained from the analysis of both correlation network and differential concentration have been implicated in the pathogenesis of intra‐amniotic inflammation/infection in preterm labor,171, 172, 173 preterm pre‐labor rupture of the membranes,173 and clinical chorioamnionitis.174 Both IL‐8 (neutrophil attractant/activating peptide‐1) and MIP‐1β (CCL4) are chemotactic cytokines that activate neutrophils and other human granulocytes in response to inflammation or infection.175, 176 These chemokines are mainly produced by macrophages, mononuclear cells, and lymphocytes.176, 177 Previous studies demonstrated that amniotic fluid concentrations of IL‐8 and MIP‐1β increase in patients with preterm labor who had intra‐amniotic inflammation/infection and clinical chorioamnionitis at term with proven intra‐amniotic infection, and these chemokines can predict the likelihood of spontaneous preterm delivery.171, 172, 173, 174, 178, 179, 180, 181, 182, 183, 184 In addition, both chemokines are significantly higher in patients with a mid‐trimester short cervix who delivered <34 weeks than in those who delivered ≥34 weeks of gestation,152, 156 and their concentrations correlated with cervical length.61
By contrast, MIP‐1α (or CCL‐3) is the top‐ranked protein identified from correlation analysis but not differential concentration analysis. Interestingly, this cytokine has been implicated in the pathogenesis of intra‐amniotic inflammation/infection in patients with preterm delivery and clinical chorioamnionitis.174 MIP‐1α is produced by macrophages185 and activates human granulocytes in response to inflammation and infection.186, 187, 188, 189, 190, 191, 192, 193, 194 These observations suggest that proteins ranked by network correlation analysis are of value and have biological plausibility in the pathogenesis of intra‐amniotic inflammation/infection in patients with a sonographic short cervix, despite having a small magnitude of change from a simple comparison of mean concentrations.
4.3. Network of protein correlation perturbations related to preterm delivery in patients diagnosed between 22 2/7 and 26 1/7 weeks of gestation
The top‐ranked proteins obtained from differential concentration analysis (IL‐8, IL‐6, and MIP‐1β) are similar to those derived from correlation analysis. Moreover, IL‐8 and MIP‐1β are also the top‐ranked proteins in the network of perturbed inflammatory‐related protein concentrations observed between 16 5/7 and 22 1/7 weeks of gestation. RANTES and MCP‐1, two additional proteins, are key mediators during this interval derived from correlation analysis. MCP‐1 is significantly lower in patients who delivered ≤32 weeks than in those who delivered >32 weeks when a sonographic short cervix was diagnosed between 22 2/7 and 26 1/7 weeks of gestation (fold change=1.97, P=.022). The amniotic fluid concentrations of RANTES were not significantly different (P=.156). Interestingly, RANTES had no perturbed correlations of inflammatory‐related protein concentration in women with a short cervix diagnosed between 16 5/7 and 22 1/7 weeks, but it had the highest number of perturbed correlations in women with a short cervix diagnosed between 22.2 and 26.1 weeks of gestation (n=11), suggesting that gestational age is a factor that may modulate the nature of the cytokine response.
It is well established that amniotic fluid IL‐8, IL‐6, and MIP‐1β are involved in the pathogenesis of intra‐amniotic inflammation/infection in preterm delivery,96, 101, 108, 171, 172, 173, 179, 182, 183, 195, 196, 197 preterm pre‐labor rupture of the membranes,111, 180, 181, 198, 199, 200, 201 clinical chorioamnionitis,174 and cervical insufficiency.202, 203, 204 In patients with a mid‐trimester short cervix, the concentrations of these cytokines/chemokines in the amniotic cavity are correlated with sonographic cervical length61 and gestational age at delivery.152 Additionally, all three cytokines are included in the amniotic fluid inflammatory score model, which can predict gestational age at delivery.152
In the current study, the information obtained from network analysis is of value, because there is evidence that suggests that amniotic fluid MCP‐1 (also known as CCL‐2) and RANTES play a role in intra‐amniotic inflammation/infection in patients with preterm labor,132, 156, 205, 206 preterm pre‐labor rupture of the membranes,132 a sonographic short cervix,61, 156 spontaneous labor at term,207, 208 and histological chorioamnionitis.205
Keeler et al.156 reported that patients with a mid‐trimester sonographic short cervix demonstrated that an elevation of amniotic fluid MCP‐1 concentration (>1320 pg/ml) had a 69% sensitivity, 83% specificity, 36% positive predictive value, and 87% negative predictive value for preterm birth within one week of amniocentesis. Moreover, among the other 25 cytokines, MCP‐1 was the most predictive cytokine of spontaneous preterm delivery in patients with a mid‐trimester short cervix.156 MCP‐1 is capable of recruiting macrophages and other leukocytes into sites of inflammation.209, 210, 211, 212 Similarly, RANTES is a pro‐inflammatory chemokine secreted from T cells, which recruit monocytes, lymphocytes, basophils, and eosinophils in the host response to inflammation/infection.213, 214, 215, 216, 217
Collectively, patients with a sonographic short cervix who subsequently delivered early had a more coordinated cytokine network than those who had a late preterm delivery. The ranked proteins derived from correlation analysis are informative, have known biological properties relevant to parturition, and can be potentially useful in the future for the development of a biomarker pipeline to identify patients with a sonographic short cervix who subsequently deliver early.
4.4. Network of protein correlation perturbations related to preterm delivery in patients diagnosed between 26 2/7 and 31 5/7 weeks of gestation
In this interval, the number of perturbed correlations is small (n=5), and none of the inflammatory‐related protein concentrations differed between patients who delivered early or late preterm. However, we found a connection between the antimicrobial peptide lactoferrin and T‐cell‐associated cytokines IL‐16 and IL‐13. Elevated IL‐16 and lactoferrin amniotic fluid concentrations in women with preterm labor and intra‐amniotic infection/inflammation were previously reported by our group and other investigators.218, 219, 220
4.5. Gestational age at the diagnosis of a sonographic short cervix and the behavior of the cytokine network
It is known that the earlier the diagnosis of a short cervix is made, the greater the degree of intra‐amniotic inflammation and the earlier the delivery.17, 18, 19, 20, 21, 23, 24, 25, 26, 27, 30, 61, 62 Additionally, Moroz and Simhan221 reported a significant association between cervical shortening and maternal systemic inflammation (determined by C‐reactive protein; r2=.44, P=.001) in women with a sonographic short cervix (≤25 mm) diagnosed between 21 and 28 weeks of gestation. However, this association was not observed in patients without a short cervix.221 We present herein for the first time that the earlier the occurrence of a short cervix, the more orchestrated is the inflammatory response associated with it in women who subsequently deliver prior to 32 weeks of gestation. These findings suggest that the risk of early spontaneous preterm delivery in asymptomatic women with a sonographic short cervical length changes as a function of gestational age at diagnosis and is associated with the magnitude and character of the intra‐amniotic inflammatory processes characterized by network analysis.
4.6. Importance of the study of protein networks in the “great obstetrical syndromes”
The improved understanding of the immune response and its soluble mediators (cytokines) coupled with the application of molecular biology has led to substantial gains in the description of the behavior of multiple inflammatory‐related proteins in health and disease. The initial emphasis in the study of inflammatory molecules was on the individual changes in the concentration and expression of these molecules. This is understandable as discoveries of virtually every cytokine occur one at a time. Now, decades later, a more comprehensive, detailed map of the protein inflammatory network is available, as well as an improved understanding of the nature of the protein‐protein interaction and biological function of these molecules. A major advance in the understanding of the biology of the inflammatory response indicates that cytokines are organized in complex, redundant networks; further, there is the realization that global analysis is required to improve insights into the biology and that this approach is superior to a simple catalogue of the changes in the concentrations of individual cytokines. Indeed, the cellular response during inflammation represents the interaction between the input derived from several cytokines that activate different receptors on the cell surface, leading to the generation of several intracellular processes. In addition, each cytokine can attach to different receptors on the cell surface, and this has implications on the type of cellular response, cellular activation, number, and profile of cytokine receptors expressed on the cell membrane.222, 223, 224 Thus, to understand the effect of different cytokines, an integrative model of cytokine activity is needed. There are several reports documenting that information derived from correlation network analysis can (i) improve classification of disease,61, 152, 156 (ii) chart the cytokine interaction in terms of its effect on specific inflammatory cell types (B and T cells, macrophages) in single and multiple cell interactions,223, 224 (iii) identify new interfaces between signaling molecules in uni‐ or multiscale models that incorporate several cell populations,225, 226 (iv) be the basis of hypothesis‐generating studies,227 and (v) identify potential therapeutic targets.228, 229 This has been the case with breast cancer158 and chronic fatigue syndrome.230 The description of the cytokine network presented herein is novel and may assist in identifying the key cytokines involved in the amniotic fluid inflammatory response at different gestational‐age windows that, in turn, may have diagnostic, prognostic, and therapeutic implications.
4.7. Strengths and limitations of the study
A major strength of the study is that it is the first to investigate the inflammatory protein network in asymptomatic women at risk for preterm birth because of a sonographic short cervix. Second, the study focuses on amniotic fluid, the biological fluid in which major changes in cytokine activity are observed in the context of a short cervix. Third, the characterization of the protein inflammatory network was not restricted to cytokines, but also included antimicrobial proteins, such as lactoferrin, that have been implicated in premature labor. Fourth, the results of the cytokine network were unknown at the time of patient management and, therefore, could not have biased the clinicians and investigators. As with any other studies, replication of the findings is desirable.
5. CONCLUSIONS
We have characterized the amniotic fluid pro‐inflammatory protein network in women with an asymptomatic short cervix who are at risk for early preterm birth (<32 weeks of gestation) for the first time. Importantly, the shorter the cervical length, the greater are the perturbations in the amniotic fluid inflammatory network and the higher the risk of early preterm delivery. Characterization of the amniotic fluid inflammatory network has implications for the taxonomy of disease for patients with a short cervix and identification of those at risk for early premature birth.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
ACKNOWLEDGMENTS
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with Federal funds from NICHD, NIH under Contract No. HHSN275201300006C.
Tarca AL, Fitzgerald W, Chaemsaithong P, et al. The cytokine network in women with an asymptomatic short cervix and the risk of preterm delivery. Am J Reprod Immunol. 2017;78:e12686 https://doi.org/10.1111/aji.12686
Presented at the 12th World Congress of Perinatal Medicine, Madrid, Spain, November 3‐6, 2015.
Contributor Information
Leonid Margolis, Email: margolil@mail.nih.gov, Email: margolis@helix.nih.gov.
Roberto Romero, Email: prbchiefstaff@med.wayne.edu.
REFERENCES
- 1. Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1990;163:859‐867. [DOI] [PubMed] [Google Scholar]
- 2. Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334:567‐572. [DOI] [PubMed] [Google Scholar]
- 3. Goldenberg RL, Iams JD, Mercer BM, et al. The preterm prediction study: the value of new vs standard risk factors in predicting early and all spontaneous preterm births. NICHD MFMU Network. Am J Public Health. 1998;88:233‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312‐317. [DOI] [PubMed] [Google Scholar]
- 5. Berghella V, Daly SF, Tolosa JE, et al. Prediction of preterm delivery with transvaginal ultrasonography of the cervix in patients with high‐risk pregnancies: does cerclage prevent prematurity? Am J Obstet Gynecol. 1999;181:809‐815. [DOI] [PubMed] [Google Scholar]
- 6. Watson WJ, Stevens D, Welter S, Day D. Observations on the sonographic measurement of cervical length and the risk of premature birth. J Matern Fetal Med. 1999;8:17‐19. [DOI] [PubMed] [Google Scholar]
- 7. Cook CM, Ellwood DA. The cervix as a predictor of preterm delivery in ‘at‐risk’ women. Ultrasound Obstet Gynecol. 2000;15:109‐113. [DOI] [PubMed] [Google Scholar]
- 8. Hassan SS, Romero R, Berry SM, et al. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458‐1467. [DOI] [PubMed] [Google Scholar]
- 9. To MS, Skentou C, Liao AW, Cacho A, Nicolaides KH. Cervical length and funneling at 23 weeks of gestation in the prediction of spontaneous early preterm delivery. Ultrasound Obstet Gynecol. 2001;18:200‐203. [DOI] [PubMed] [Google Scholar]
- 10. Owen J, Yost N, Berghella V, et al. Can shortened midtrimester cervical length predict very early spontaneous preterm birth? Am J Obstet Gynecol. 2004;191:298‐303. [DOI] [PubMed] [Google Scholar]
- 11. Tekesin I, Eberhart LH, Schaefer V, Wallwiener D, Schmidt S. Evaluation and validation of a new risk score (CLEOPATRA score) to predict the probability of premature delivery for patients with threatened preterm labor. Ultrasound Obstet Gynecol. 2005;26:699‐706. [DOI] [PubMed] [Google Scholar]
- 12. DeFranco EA, Lewis DF, Odibo AO. Improving the screening accuracy for preterm labor: is the combination of fetal fibronectin and cervical length in symptomatic patients a useful predictor of preterm birth? A systematic review. Am J Obstet Gynecol. 2013;208:233.e1‐6. [DOI] [PubMed] [Google Scholar]
- 13. Romero R, Yeo L, Miranda J, Hassan SS, Conde‐Agudelo A, Chaiworapongsa T. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med. 2013;41:27‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boots AB, Sanchez‐Ramos L, Bowers DM, Kaunitz AM, Zamora J, Schlattmann P. The short‐term prediction of preterm birth: a systematic review and diagnostic metaanalysis. Am J Obstet Gynecol. 2014;210:54.e1‐10. [DOI] [PubMed] [Google Scholar]
- 15. McIntosh J, Feltovich H, Berghella V, Manuck T. The role of routine cervical length screening in selected high‐ and low‐risk women for preterm birth prevention. Am J Obstet Gynecol. 2016;215:B2‐B7. [DOI] [PubMed] [Google Scholar]
- 16. Kushnir O, Vigil DA, Izquierdo L, Schiff M, Curet LB. Vaginal ultrasonographic assessment of cervical length changes during normal pregnancy. Am J Obstet Gynecol. 1990;162:991‐993. [DOI] [PubMed] [Google Scholar]
- 17. Okitsu O, Mimura T, Nakayama T, Aono T. Early prediction of preterm delivery by transvaginal ultrasonography. Ultrasound Obstet Gynecol. 1992;2:402‐409. [DOI] [PubMed] [Google Scholar]
- 18. Iams JD, Paraskos J, Landon MB, Teteris JN, Johnson FF. Cervical sonography in preterm labor. Obstet Gynecol. 1994;84:40‐46. [PubMed] [Google Scholar]
- 19. Tongsong T, Kamprapanth P, Srisomboon J, Wanapirak C, Piyamongkol W, Sirichotiyakul S. Single transvaginal sonographic measurement of cervical length early in the third trimester as a predictor of preterm delivery. Obstet Gynecol. 1995;86:184‐187. [DOI] [PubMed] [Google Scholar]
- 20. Hasegawa I, Tanaka K, Takahashi K, et al. Transvaginal ultrasonographic cervical assessment for the prediction of preterm delivery. J Matern Fetal Med. 1996;5:305‐309. [DOI] [PubMed] [Google Scholar]
- 21. Rozenberg P, Goffinet F, Malagrida L, et al. Evaluating the risk of preterm delivery: a comparison of fetal fibronectin and transvaginal ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1997;176(1 Pt 1):196‐199. [DOI] [PubMed] [Google Scholar]
- 22. Berghella V, Tolosa JE, Kuhlman K, Weiner S, Bolognese RJ, Wapner RJ. Cervical ultrasonography compared with manual examination as a predictor of preterm delivery. Am J Obstet Gynecol. 1997;177:723–730. [DOI] [PubMed] [Google Scholar]
- 23. Guzman ER, Mellon C, Vintzileos AM, Ananth CV, Walters C, Gipson K. Longitudinal assessment of endocervical canal length between 15 and 24 weeks' gestation in women at risk for pregnancy loss or preterm birth. Obstet Gynecol. 1998;92:31‐37. [DOI] [PubMed] [Google Scholar]
- 24. Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18‐22 weeks' gestation and the risk of preterm delivery. Obstet Gynecol. 1998;92:902‐907. [DOI] [PubMed] [Google Scholar]
- 25. Andrews WW, Copper R, Hauth JC, Goldenberg RL, Neely C, Dubard M. Second‐trimester cervical ultrasound: associations with increased risk for recurrent early spontaneous delivery. Obstet Gynecol. 2000;95:222‐226. [DOI] [PubMed] [Google Scholar]
- 26. Hibbard JU, Tart M, Moawad AH. Cervical length at 16‐22 weeks' gestation and risk for preterm delivery. Obstet Gynecol. 2000;96:972‐978. [DOI] [PubMed] [Google Scholar]
- 27. Owen J, Yost N, Berghella V, et al. Mid‐trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340‐1348. [DOI] [PubMed] [Google Scholar]
- 28. Durnwald CP, Walker H, Lundy JC, Iams JD. Rates of recurrent preterm birth by obstetrical history and cervical length. Am J Obstet Gynecol. 2005;193(3 Pt 2):1170‐1174. [DOI] [PubMed] [Google Scholar]
- 29. Gomez R, Romero R, Medina L, et al. Cervicovaginal fibronectin improves the prediction of preterm delivery based on sonographic cervical length in patients with preterm uterine contractions and intact membranes. Am J Obstet Gynecol. 2005;192:350‐359. [DOI] [PubMed] [Google Scholar]
- 30. Matijevic R, Grgic O, Vasilj O. Is sonographic assessment of cervical length better than digital examination in screening for preterm delivery in a low‐risk population? Acta Obstet Gynecol Scand. 2006;85:1342‐1347. [DOI] [PubMed] [Google Scholar]
- 31. Zhou M, Cool D, Grunwald W, Khamis H, McKenna D. Abstract No.516: Clinical findings in amniotic fluid of women with asymptomatic short cervix in the midtrimester. Am J Obstet Gynecol. 2013;208:S222. [Google Scholar]
- 32. Raiche E, Ouellet A, Berthiaume M, Rousseau E, Pasquier JC. Short and inflamed cervix predicts spontaneous preterm birth (COLIBRI study). J Matern Fetal Neonatal Med. 2014;27:1015‐1019. [DOI] [PubMed] [Google Scholar]
- 33. Melamed N, Pittini A, Hiersch L, et al. Do serial measurements of cervical length improve the prediction of preterm birth in asymptomatic women with twin gestations? Am J Obstet Gynecol. 2016;215:616.e1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Romero R, Gomez R, Mazor M, Ghezzi F, Yoon BH. The preterm labor syndrome In: Elder MG, Romero R, Lamont RF, eds. Preterm Labor. New York, NY: Churchill Livingstone; 1997:29‐49. [Google Scholar]
- 35. Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lockwood CJ. Risk factors for preterm birth and new approaches to its early diagnosis. J Perinat Med. 2015;43:499‐501. [DOI] [PubMed] [Google Scholar]
- 38. Hegele‐Hartung C, Chwalisz K, Beier HM, Elger W. Ripening of the uterine cervix of the guinea‐pig after treatment with the progesterone antagonist onapristone (ZK 98.299): an electron microscopic study. Hum Reprod. 1989;4:369‐377. [DOI] [PubMed] [Google Scholar]
- 39. Wolf JP, Sinosich M, Anderson TL, Ulmann A, Baulieu EE, Hodgen GD. Progesterone antagonist (RU 486) for cervical dilation, labor induction, and delivery in monkeys: effectiveness in combination with oxytocin. Am J Obstet Gynecol. 1989;160:45‐47. [DOI] [PubMed] [Google Scholar]
- 40. Norman J. Antiprogesterones. Br J Hosp Med. 1991;45:372‐375. [PubMed] [Google Scholar]
- 41. Chwalisz K. The use of progesterone antagonists for cervical ripening and as an adjunct to labour and delivery. Hum Reprod. 1994;9(Suppl 1):131‐161. [DOI] [PubMed] [Google Scholar]
- 42. Elliott CL, Brennand JE, Calder AA. The effects of mifepristone on cervical ripening and labor induction in primigravidae. Obstet Gynecol. 1998;92:804‐809. [DOI] [PubMed] [Google Scholar]
- 43. Stenlund PM, Ekman G, Aedo AR, Bygdeman M. Induction of labor with mifepristone – a randomized, double‐blind study versus placebo. Acta Obstet Gynecol Scand. 1999;78:793‐798. [PubMed] [Google Scholar]
- 44. Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69‐79. [DOI] [PubMed] [Google Scholar]
- 45. Timmons B, Akins M, Mahendroo M. Cervical remodeling during pregnancy and parturition. Trends Endocrinol Metab. 2010;21:353‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mahendroo M. Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction. 2012;143:429‐438. [DOI] [PubMed] [Google Scholar]
- 47. Ahn KH, Bae NY, Hong SC, et al. The safety of progestogen in the prevention of preterm birth: meta‐analysis of neonatal mortality. J Perinat Med. 2017;45:11‐20. [DOI] [PubMed] [Google Scholar]
- 48. Areia AL, Vale‐Pereira S, Vaz‐Ambrosio A, et al. Does progesterone administration in preterm labor influence Treg cells? J Perinat Med. 2016;44:605‐611. [DOI] [PubMed] [Google Scholar]
- 49. Moinian M, Andersch B. Does cervix conization increase the risk of complications in subsequent pregnancies? Acta Obstet Gynecol Scand. 1982;61:101‐103. [DOI] [PubMed] [Google Scholar]
- 50. Blomfield PI, Buxton J, Dunn J, Luesley DM. Pregnancy outcome after large loop excision of the cervical transformation zone. Am J Obstet Gynecol. 1993;169:620‐625. [DOI] [PubMed] [Google Scholar]
- 51. Kristensen J, Langhoff‐Roos J, Wittrup M, Bock JE. Cervical conization and preterm delivery/low birth weight. A systematic review of the literature. Acta Obstet Gynecol Scand. 1993;72:640‐644. [DOI] [PubMed] [Google Scholar]
- 52. Raio L, Ghezzi F, Di Naro E, Gomez R, Luscher KP. Duration of pregnancy after carbon dioxide laser conization of the cervix: influence of cone height. Obstet Gynecol. 1997;90:978‐982. [DOI] [PubMed] [Google Scholar]
- 53. Berghella V, Pereira L, Gariepy A, Simonazzi G. Prior cone biopsy: prediction of preterm birth by cervical ultrasound. Am J Obstet Gynecol. 2004;191:1393‐1397. [DOI] [PubMed] [Google Scholar]
- 54. Bruinsma FJ, Quinn MA. The risk of preterm birth following treatment for precancerous changes in the cervix: a systematic review and meta‐analysis. BJOG. 2011;118:1031‐1041. [DOI] [PubMed] [Google Scholar]
- 55. Orzechowski K, Nicholas S, Berghella V. Abstract No. 504: Does cervical conization increase the risk of a sonographic short cervix in the second trimester of pregnancy? Am J Obstet Gynecol. 2013;208:S217. [Google Scholar]
- 56. Miller ES, Grobman WA. The association between cervical excisional procedures, midtrimester cervical length, and preterm birth. Am J Obstet Gynecol. 2014;211:242.e1‐4. [DOI] [PubMed] [Google Scholar]
- 57. Miller ES, Sakowicz A, Grobman WA. The association between cervical dysplasia, a short cervix, and preterm birth. Am J Obstet Gynecol. 2015;213:543.e1‐4. [DOI] [PubMed] [Google Scholar]
- 58. Romero R, Gonzalez R, Sepulveda W, et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167(4 Pt 1):1086‐1091. [DOI] [PubMed] [Google Scholar]
- 59. Mays JK, Figueroa R, Shah J, Khakoo H, Kaminsky S, Tejani N. Amniocentesis for selection before rescue cerclage. Obstet Gynecol. 2000;95:652‐655. [DOI] [PubMed] [Google Scholar]
- 60. Hassan S, Romero R, Hendler I, et al. A sonographic short cervix as the only clinical manifestation of intra‐amniotic infection. J Perinat Med. 2006;34:13‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kiefer DG, Keeler SM, Rust OA, Wayock CP, Vintzileos AM, Hanna N. Is midtrimester short cervix a sign of intraamniotic inflammation? Am J Obstet Gynecol. 2009;200:374.e1‐5. [DOI] [PubMed] [Google Scholar]
- 62. Vaisbuch E, Hassan SS, Mazaki‐Tovi S, et al. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433.e1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Choi J, Park JW, Kim BJ, Choi YJ, Hwang JH, Lee SM. Funisitis is more common in cervical insufficiency than in preterm labor and preterm premature rupture of membranes. J Perinat Med. 2016;44:523‐529. [DOI] [PubMed] [Google Scholar]
- 64. Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198:633.e1‐8. [DOI] [PubMed] [Google Scholar]
- 65. DeFranco EA, O'Brien JM, Adair CD, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double‐blind, placebo‐controlled trial. Ultrasound Obstet Gynecol. 2007;30:697‐705. [DOI] [PubMed] [Google Scholar]
- 66. Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462‐469. [DOI] [PubMed] [Google Scholar]
- 67. O'Brien JM, Defranco EA, Adair CD, et al. Effect of progesterone on cervical shortening in women at risk for preterm birth: secondary analysis from a multinational, randomized, double‐blind, placebo‐controlled trial. Ultrasound Obstet Gynecol. 2009;34:653‐659. [DOI] [PubMed] [Google Scholar]
- 68. Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double‐blind, placebo‐controlled trial. Ultrasound Obstet Gynecol. 2011;38:18‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Romero R, Nicolaides K, Conde‐Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206:124.e1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Conde‐Agudelo A, Romero R, Nicolaides K, et al. Vaginal progesterone vs. cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. Am J Obstet Gynecol. 2013;208:42.e1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Romero R, Yeo L, Chaemsaithong P, Chaiworapongsa T, Hassan SS. Progesterone to prevent spontaneous preterm birth. Semin Fetal Neonatal Med. 2014;19:15‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Conde‐Agudelo A, Romero R. Vaginal progesterone to prevent preterm birth in pregnant women with a sonographic short cervix: clinical and public health implications. Am J Obstet Gynecol. 2016;214:235‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Suhag A, Saccone G, Berghella V. Vaginal progesterone for maintenance tocolysis: a systematic review and metaanalysis of randomized trials. Am J Obstet Gynecol. 2015;213:479‐487. [DOI] [PubMed] [Google Scholar]
- 74. Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol. 2006;194:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Keeler SM, Kiefer D, Rochon M, Quinones JN, Novetsky AP, Rust O. A randomized trial of cerclage vs. 17 alpha‐hydroxyprogesterone caproate for treatment of short cervix. J Perinat Med. 2009;37:473‐479. [DOI] [PubMed] [Google Scholar]
- 76. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high‐risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201:375.e1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Berghella V, Mackeen AD. Cervical length screening with ultrasound‐indicated cerclage compared with history‐indicated cerclage for prevention of preterm birth: a meta‐analysis. Obstet Gynecol. 2011;118:148‐155. [DOI] [PubMed] [Google Scholar]
- 78. Berghella V, Rafael TJ, Szychowski JM, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: a meta‐analysis. Obstet Gynecol. 2011;117:663‐671. [DOI] [PubMed] [Google Scholar]
- 79. Childress KS, Flick A, Dickert E, Gavard J, Bolanos R, Gross G. Abstract No 173: A comparison of cervical cerclage and vaginal pessaries in the prevention of spontaneous preterm birth in women with short cervix. Am J Obstet Gynecol. 2015;212:S101. [Google Scholar]
- 80. Kiefer DG, Peltier MR, Keeler SM, et al. Efficacy of midtrimester short cervix interventions is conditional on intra‐amniotic inflammation. Am J Obstet Gynecol. 2016;214:276.e1‐276.e6. [DOI] [PubMed] [Google Scholar]
- 81. Vaisbuch E, Romero R, Erez O, et al. Clinical significance of early (< 20 weeks) vs. late (20‐24 weeks) detection of sonographic short cervix in asymptomatic women in the mid‐trimester. Ultrasound Obstet Gynecol. 2010;36:471‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra‐amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med. 2014;1‐17. https://doi.org/10.3109/14767058.2014.954243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Romero R, Kadar N, Hobbins JC, Duff GW. Infection and labor: the detection of endotoxin in amniotic fluid. Am J Obstet Gynecol. 1987;157(4 Pt 1):815‐819. [DOI] [PubMed] [Google Scholar]
- 84. Romero R, Roslansky P, Oyarzun E, et al. Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am J Obstet Gynecol. 1988;158:1044‐1049. [DOI] [PubMed] [Google Scholar]
- 85. Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660‐1667. [DOI] [PubMed] [Google Scholar]
- 86. Yoon BH, Romero R, Kim CJ, et al. Amniotic fluid interleukin‐6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960‐970. [DOI] [PubMed] [Google Scholar]
- 87. Hitti J, Tarczy‐Hornoch P, Murphy J, Hillier SL, Aura J, Eschenbach DA. Amniotic fluid infection, cytokines, and adverse outcome among infants at 34 weeks' gestation or less. Obstet Gynecol. 2001;98:1080‐1088. [DOI] [PubMed] [Google Scholar]
- 88. Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):41‐56. [DOI] [PubMed] [Google Scholar]
- 89. Romero R, Erez O, Espinoza J. Intrauterine infection, preterm labor, and cytokines. J Soc Gynecol Investig. 2005;12:463‐465. [DOI] [PubMed] [Google Scholar]
- 90. Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65(12 Pt 2):S194‐S202. [DOI] [PubMed] [Google Scholar]
- 92. Romero R, Mazor M, Wu YK, et al. Infection in the pathogenesis of preterm labor. Semin Perinatol. 1988;12:262‐279. [PubMed] [Google Scholar]
- 93. Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817‐824. [DOI] [PubMed] [Google Scholar]
- 94. Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515‐1528. [DOI] [PubMed] [Google Scholar]
- 95. Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79:351‐357. [DOI] [PubMed] [Google Scholar]
- 96. Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin‐6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol. 1993;30:167‐183. [DOI] [PubMed] [Google Scholar]
- 97. Romero R, Galasso M, Gomez R, et al. A comparative study of the value of amniotic fluid interleukin‐6, white blood cell count and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients with spontaneous labor at term. Annual Meeting of the Society of Perinatal Obstetricians; Las Vegas, NV; 1994:A250. [DOI] [PubMed]
- 98. Greci LS, Gilson GJ, Nevils B, Izquierdo LA, Qualls CR, Curet LB. Is amniotic fluid analysis the key to preterm labor? A model using interleukin‐6 for predicting rapid delivery. Am J Obstet Gynecol. 1998;179:172‐178. [DOI] [PubMed] [Google Scholar]
- 99. Maymon E, Romero R, Chaiworapongsa T, et al. Amniotic fluid matrix metalloproteinase‐8 in preterm labor with intact membranes. Am J Obstet Gynecol. 2001;185:1149‐1155. [DOI] [PubMed] [Google Scholar]
- 100. Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra‐amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130‐1136. [DOI] [PubMed] [Google Scholar]
- 101. Jacobsson B, Mattsby‐Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand. 2003;82:120‐128. [DOI] [PubMed] [Google Scholar]
- 102. Yoon BH, Romero R, Lim JH, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189:919‐924. [DOI] [PubMed] [Google Scholar]
- 103. Friel LA, Romero R, Edwin S, et al. The calcium binding protein, S100B, is increased in the amniotic fluid of women with intra‐amniotic infection/inflammation and preterm labor with intact or ruptured membranes. J Perinat Med. 2007;35:385‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:294.e1‐6. [DOI] [PubMed] [Google Scholar]
- 105. DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture‐based investigation. PLoS One. 2008;3:e3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage‐associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444‐1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Romero R, Miranda J, Chaiworapongsa T, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra‐amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol. 2014;71:330‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra‐amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol. 2014;72:458‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:661‐666. [DOI] [PubMed] [Google Scholar]
- 110. Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997;11:135‐176. [DOI] [PubMed] [Google Scholar]
- 111. Jacobsson B, Mattsby‐Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82:423‐431. [DOI] [PubMed] [Google Scholar]
- 112. Shim SS, Romero R, Hong JS, et al. Clinical significance of intra‐amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1339‐1345. [DOI] [PubMed] [Google Scholar]
- 113. DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre‐labor rupture of membranes. Am J Reprod Immunol. 2010;64:38‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial‐associated intra‐amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015;28:1394‐1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gomez R, Romero R, Nien JK, et al. A short cervix in women with preterm labor and intact membranes: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2005;192:678‐689. [DOI] [PubMed] [Google Scholar]
- 116. Bujold E, Morency AM, Rallu F, et al. Bacteriology of amniotic fluid in women with suspected cervical insufficiency. J Obstet Gynaecol Can. 2008;30:882‐887. [DOI] [PubMed] [Google Scholar]
- 117. Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med. 2010;38:261‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kim KW, Romero R, Park HS, et al. A rapid matrix metalloproteinase‐8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2007;197:292.e1‐5. [DOI] [PubMed] [Google Scholar]
- 119. Kiefer D, Peltier M, Keeler S, et al. Does intra‐amniotic inflammation influence pregnancy outcome after cerclage or progesterone (17OHP‐C) therapy for midtrimester short cervix? Am J Obstet Gynecol. 2009;201(6 Suppl):S61. [Google Scholar]
- 120. Gervasi MT, Romero R, Bracalente G, et al. Midtrimester amniotic fluid concentrations of interleukin‐6 and interferon‐gamma‐inducible protein‐10: evidence for heterogeneity of intra‐amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med. 2012;40:329‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lee SY, Park KH, Jeong EH, Oh KJ, Ryu A, Kim A. Intra‐amniotic infection/inflammation as a risk factor for subsequent ruptured membranes after clinically indicated amniocentesis in preterm labor. J Korean Med Sci. 2013;28:1226‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210:125.e1‐15. [DOI] [PubMed] [Google Scholar]
- 123. Romero R, Kadar N, Miranda J, et al. The diagnostic performance of the mass restricted (MR) score in the identification of microbial invasion of the amniotic cavity or intra‐amniotic inflammation is not superior to amniotic fluid interleukin‐6. J Matern Fetal Neonatal Med. 2014;27:757‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for the determination of amniotic fluid interleukin‐6 and the chemokine CXCL‐10/IP‐10. J Matern Fetal Neonatal Med. 2015;28:1510‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A point of care test for interleukin‐6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra‐amniotic inflammation/infection. J Matern Fetal Neonatal Med. 2016;29:360‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A rapid interleukin‐6 bedside test for the identification of intra‐amniotic inflammation in preterm labor with intact membranes. J Matern Fetal Neonatal Med. 2016;29:349‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Romero R, Grivel JC, Tarca AL, et al. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015;213:836.e1‐836.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kunze M, Klar M, Morfeld CA, et al. Cytokines in noninvasively obtained amniotic fluid as predictors of fetal inflammatory response syndrome. Am J Obstet Gynecol. 2016;215:96.e1‐8. [DOI] [PubMed] [Google Scholar]
- 129. Rizzo G, Capponi A, Vlachopoulou A, Angelini E, Grassi C, Romanini C. Interleukin‐6 concentrations in cervical secretions in the prediction of intrauterine infection in preterm premature rupture of the membranes. Gynecol Obstet Invest. 1998;46:91‐95. [DOI] [PubMed] [Google Scholar]
- 130. Jun JK, Yoon BH, Romero R, et al. Interleukin 6 determinations in cervical fluid have diagnostic and prognostic value in preterm premature rupture of membranes. Am J Obstet Gynecol. 2000;183:868‐873. [DOI] [PubMed] [Google Scholar]
- 131. Jacobsson B, Holst RM, Mattsby‐Baltzer I, Nikolaitchouk N, Wennerholm UB, Hagberg H. Interleukin‐18 in cervical mucus and amniotic fluid: relationship to microbial invasion of the amniotic fluid, intra‐amniotic inflammation and preterm delivery. BJOG. 2003;110:598‐603. [PubMed] [Google Scholar]
- 132. Jacobsson B, Holst RM, Wennerholm UB, Andersson B, Lilja H, Hagberg H. Monocyte chemotactic protein‐1 in cervical and amniotic fluid: relationship to microbial invasion of the amniotic cavity, intra‐amniotic inflammation, and preterm delivery. Am J Obstet Gynecol. 2003;189:1161‐1167. [DOI] [PubMed] [Google Scholar]
- 133. Holst RM, Mattsby‐Baltzer I, Wennerholm UB, Hagberg H, Jacobsson B. Interleukin‐6 and interleukin‐8 in cervical fluid in a population of Swedish women in preterm labor: relationship to microbial invasion of the amniotic fluid, intra‐amniotic inflammation, and preterm delivery. Acta Obstet Gynecol Scand. 2005;84:551‐557. [DOI] [PubMed] [Google Scholar]
- 134. Jacobsson B, Mattsby‐Baltzer I, Hagberg H. Interleukin‐6 and interleukin‐8 in cervical and amniotic fluid: relationship to microbial invasion of the chorioamniotic membranes. BJOG. 2005;112:719‐724. [DOI] [PubMed] [Google Scholar]
- 135. Holst RM, Laurini R, Jacobsson B, et al. Expression of cytokines and chemokines in cervical and amniotic fluid: relationship to histological chorioamnionitis. J Matern Fetal Neonatal Med. 2007;20:885‐893. [DOI] [PubMed] [Google Scholar]
- 136. Chandiramani M, Seed PT, Orsi NM, et al. Limited relationship between cervico‐vaginal fluid cytokine profiles and cervical shortening in women at high risk of spontaneous preterm birth. PLoS One. 2012;7:e52412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Cobo T, Jacobsson B, Kacerovsky M, et al. Systemic and local inflammatory response in women with preterm prelabor rupture of membranes. PLoS One. 2014;9:e85277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Combs CA, Garite TJ, Lapidus JA, et al. Detection of microbial invasion of the amniotic cavity by analysis of cervicovaginal proteins in women with preterm labor and intact membranes. Am J Obstet Gynecol. 2015;212:482.e1‐12. [DOI] [PubMed] [Google Scholar]
- 139. Hadzi‐Lega M, Markova AD, Stefanovic M, Tanturovski M. Correlation of cervical length, fetal fibronectin, phIGFBP‐1, and cytokines in spontaneous preterm birth up to 14 days from sampling. J Perinat Med. 2015;43:545‐551. [DOI] [PubMed] [Google Scholar]
- 140. Kacerovsky M, Musilova I, Jacobsson B, et al. Cervical fluid IL‐6 and IL‐8 levels in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015;28:134‐140. [DOI] [PubMed] [Google Scholar]
- 141. Oltvai ZN, Barabasi AL. Systems biology. Life's complexity pyramid. Science. 2002;298:763‐764. [DOI] [PubMed] [Google Scholar]
- 142. Barabasi AL, Oltvai ZN. Network biology: understanding the cell's functional organization. Nat Rev Genet. 2004;5:101‐113. [DOI] [PubMed] [Google Scholar]
- 143. Klemm K, Bornholdt S. Topology of biological networks and reliability of information processing. Proc Natl Acad Sci USA. 2005;102:18414‐18419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Dong J, Horvath S. Understanding network concepts in modules. BMC Syst Biol. 2007;1:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Schadt EE. Molecular networks as sensors and drivers of common human diseases. Nature. 2009;461:218‐223. [DOI] [PubMed] [Google Scholar]
- 146. Liu R, Wang X, Aihara K, Chen L. Early diagnosis of complex diseases by molecular biomarkers, network biomarkers, and dynamical network biomarkers. Med Res Rev. 2014;34:455‐478. [DOI] [PubMed] [Google Scholar]
- 147. Menche J, Sharma A, Kitsak M, et al. Disease networks. Uncovering disease‐disease relationships through the incomplete interactome. Science. 2015;347:1257601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Orsi NM, Tribe RM, Cytokine networks and the regulation of uterine function in pregnancy and parturition. J Neuroendocrinol. 2008;20:462‐469 [DOI] [PubMed] [Google Scholar]
- 149. Kitano H. Systems biology: a brief overview. Science. 2002;295:1662‐1664. [DOI] [PubMed] [Google Scholar]
- 150. Chen L, Wang R, Zhang X. Biomolecular Networks: Methods and Application in Systems Biology. Hoboken, NJ: John Wiley and Sons; 2009:391. [Google Scholar]
- 151. Liu ZP, Wang Y, Zhang XS, Chen L. Network‐based analysis of complex diseases. IET Syst Biol. 2012;6:22‐33. [DOI] [PubMed] [Google Scholar]
- 152. Kiefer DG, Keeler SM, Rust O, et al. Amniotic fluid inflammatory score is associated with pregnancy outcome in patients with mid trimester short cervix. Am J Obstet Gynecol. 2012;206:68.e1‐6. [DOI] [PubMed] [Google Scholar]
- 153. Bowen JM, Chamley L, Keelan JA, Mitchell MD. Cytokines of the placenta and extra‐placental membranes: roles and regulation during human pregnancy and parturition. Placenta. 2002;23:257‐273. [DOI] [PubMed] [Google Scholar]
- 154. Segal E, Friedman N, Kaminski N, Regev A, Koller D. From signatures to models: understanding cancer using microarrays. Nat Genet. 2005;37(Suppl):S38‐S45. [DOI] [PubMed] [Google Scholar]
- 155. Orsi NM. Cytokine networks in the establishment and maintenance of pregnancy. Hum Fertil (Camb). 2008;11:222‐230. [DOI] [PubMed] [Google Scholar]
- 156. Keeler SM, Kiefer DG, Rust OA, et al. Comprehensive amniotic fluid cytokine profile evaluation in women with a short cervix: which cytokine(s) correlates best with outcome? Am J Obstet Gynecol. 2009;201:276.e1‐6. [DOI] [PubMed] [Google Scholar]
- 157. Slavov N, Dawson KA. Correlation signature of the macroscopic states of the gene regulatory network in cancer. Proc Natl Acad Sci USA. 2009;106:4079‐4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Taylor IW, Linding R, Warde‐Farley D, et al. Dynamic modularity in protein interaction networks predicts breast cancer outcome. Nat Biotechnol. 2009;27:199‐204. [DOI] [PubMed] [Google Scholar]
- 159. Pinheiro MB, Martins‐Filho OA, Mota AP, et al. Severe preeclampsia goes along with a cytokine network disturbance towards a systemic inflammatory state. Cytokine. 2013;62:165‐173. [DOI] [PubMed] [Google Scholar]
- 160. Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis 1982;145:1‐8. [DOI] [PubMed] [Google Scholar]
- 161. Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124‐1129. [DOI] [PubMed] [Google Scholar]
- 162. Park JS, Romero R, Yoon BH, et al. The relationship between amniotic fluid matrix metalloproteinase‐8 and funisitis. Am J Obstet Gynecol. 2001;185:1156‐1161. [DOI] [PubMed] [Google Scholar]
- 163. Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18‐25. [DOI] [PubMed] [Google Scholar]
- 164. Yoon BH, Romero R, Shim JY, Shim SS, Kim CJ, Jun JK. C‐reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med. 2003;14:85‐90. [DOI] [PubMed] [Google Scholar]
- 165. Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation – a workshop report. Placenta. 2005;26(Suppl A):S114‐S117. [DOI] [PubMed] [Google Scholar]
- 166. Lee SE, Romero R, Kim CJ, Shim SS, Yoon BH. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra‐amniotic inflammation. J Matern Fetal Neonatal Med. 2006;19:693‐697. [DOI] [PubMed] [Google Scholar]
- 167. Redline RW. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med. 2006;11:296‐301. [DOI] [PubMed] [Google Scholar]
- 168. Park CW, Lee SM, Park JS, Jun JK, Romero R, Yoon BH. The antenatal identification of funisitis with a rapid MMP‐8 bedside test. J Perinat Med. 2008;36:497‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S29‐S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Redline RW. Classification of placental lesions. Am J Obstet Gynecol. 2015;213(4 Suppl):S21‐S28. [DOI] [PubMed] [Google Scholar]
- 171. Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide‐1/interleukin‐8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165(4 Pt 1):813‐820. [DOI] [PubMed] [Google Scholar]
- 172. Cherouny PH, Pankuch GA, Romero R, et al. Neutrophil attractant/activating peptide‐1/interleukin‐8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol. 1993;169:1299‐1303. [DOI] [PubMed] [Google Scholar]
- 173. Ghezzi F, Gomez R, Romero R, et al. Elevated interleukin‐8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol. 1998;78:5‐10. [DOI] [PubMed] [Google Scholar]
- 174. Romero R, Chaemsaithong P, Korzeniewski SJ, et al. Clinical chorioamnionitis at term II: the intra‐amniotic inflammatory response. J Perinat Med. 2016;44:5‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Zeilhofer HU, Schorr W. Role of interleukin‐8 in neutrophil signaling. Curr Opin Hematol. 2000;7:178‐182. [DOI] [PubMed] [Google Scholar]
- 176. Maurer M, von Stebut E. Macrophage inflammatory protein‐1. Int J Biochem Cell Biol. 2004;36:1882‐1886. [DOI] [PubMed] [Google Scholar]
- 177. Mukaida N. Interleukin‐8: an expanding universe beyond neutrophil chemotaxis and activation. Int J Hematol. 2000;72:391‐398. [PubMed] [Google Scholar]
- 178. González‐Bosquet E, Cerqueira MJ, Dominguez C, Gasser I, Bermejo B, Cabero L. Amniotic fluid glucose and cytokines values in the early diagnosis of amniotic infection in patients with preterm labor and intact membranes. J Matern Fetal Med. 1999;8:155–158. [DOI] [PubMed] [Google Scholar]
- 179. Arntzen KJ, Kjollesdal AM, Halgunset J, Vatten L, Austgulen R. TNF, IL‐1, IL‐6, IL‐8 and soluble TNF receptors in relation to chorioamnionitis and premature labor. J Perinat Med. 1998;26:17‐26. [DOI] [PubMed] [Google Scholar]
- 180. Hsu CD, Meaddough E, Aversa K, Copel JA. The role of amniotic fluid L‐selectin, GRO‐alpha, and interleukin‐8 in the pathogenesis of intraamniotic infection. Am J Obstet Gynecol. 1998;178:428‐432. [DOI] [PubMed] [Google Scholar]
- 181. Hsu CD, Meaddough E, Aversa K, et al. Elevated amniotic fluid levels of leukemia inhibitory factor, interleukin 6, and interleukin 8 in intra‐amniotic infection. Am J Obstet Gynecol. 1998;179:1267‐1270. [DOI] [PubMed] [Google Scholar]
- 182. Figueroa R, Garry D, Elimian A, Patel K, Sehgal PB, Tejani N. Evaluation of amniotic fluid cytokines in preterm labor and intact membranes. J Matern Fetal Neonatal Med. 2005;18:241‐247. [DOI] [PubMed] [Google Scholar]
- 183. Witt A, Berger A, Gruber CJ, Petricevic L, Apfalter P, Husslein P. IL‐8 concentrations in maternal serum, amniotic fluid and cord blood in relation to different pathogens within the amniotic cavity. J Perinat Med. 2005;33:22‐26. [DOI] [PubMed] [Google Scholar]
- 184. Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, Svinarich D, Cotton DB. Macrophage inflammatory protein‐1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol. 1994;32:108–113. [DOI] [PubMed] [Google Scholar]
- 185. Sherry B, Tekamp‐Olson P, Gallegos C, et al. Resolution of the two components of macrophage inflammatory protein 1, and cloning and characterization of one of those components, macrophage inflammatory protein 1 beta. J Exp Med. 1988;168:2251‐2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Wolpe SD, Davatelis G, Sherry B, et al. Macrophages secrete a novel heparin‐binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988;167:570‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Saukkonen K, Sande S, Cioffe C, et al. The role of cytokines in the generation of inflammation and tissue damage in experimental gram‐positive meningitis. J Exp Med. 1990;171:439‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Alam R, Forsythe PA, Stafford S, Lett‐Brown MA, Grant JA. Macrophage inflammatory protein‐1 alpha activates basophils and mast cells. J Exp Med. 1992;176:781‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Christman JW, Blackwell TR, Cowan HB, Shepherd VL, Rinaldo JE. Endotoxin induces the expression of macrophage inflammatory protein 1 alpha mRNA by rat alveolar and bone marrow‐derived macrophages. Am J Respir Cell Mol Biol. 1992;7:455‐461. [DOI] [PubMed] [Google Scholar]
- 190. Rot A, Krieger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1 alpha induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489‐1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Schall TJ, Bacon K, Camp RD, Kaspari JW, Goeddel DV. Human macrophage inflammatory protein alpha (MIP‐1 alpha) and MIP‐1 beta chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Wang JM, Sherry B, Fivash MJ, Kelvin DJ, Oppenheim JJ. Human recombinant macrophage inflammatory protein‐1 alpha and ‐beta and monocyte chemotactic and activating factor utilize common and unique receptors on human monocytes. J Immunol. 1993;150:3022‐3029. [PubMed] [Google Scholar]
- 193. Dudley DJ, Hunter C, Mitchell MD, Varner MW. Elevations of amniotic fluid macrophage inflammatory protein‐1 alpha concentrations in women during term and preterm labor. Obstet Gynecol. 1996;87:94‐98. [DOI] [PubMed] [Google Scholar]
- 194. Ramos CD, Canetti C, Souto JT, Silva JS, Hogaboam CM, Ferreira SH, Cunha FQ. MIP‐1alpha[CCL3] acting on the CCR1 receptor mediates neutrophil migration in immune inflammation via sequential release of TNF‐alpha and LTB4. J Leukoc Biol. 2005;78:167–177. [DOI] [PubMed] [Google Scholar]
- 195. Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392‐1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp. 1992;167:205‐220; discussion 20‐3. [DOI] [PubMed] [Google Scholar]
- 197. Marconi C, de Andrade Ramos BR, Peracoli JC, Donders GG, da Silva MG. Amniotic fluid interleukin‐1 beta and interleukin‐6, but not interleukin‐8 correlate with microbial invasion of the amniotic cavity in preterm labor. Am J Reprod Immunol. 2011;65:549‐556. [DOI] [PubMed] [Google Scholar]
- 198. Kacerovsky M, Drahosova M, Hornychova H, et al. Value of amniotic fluid interleukin‐8 for the prediction of histological chorioamnionitis in preterm premature rupture of membranes. Neuro Endocrinol Lett. 2009;30:733‐738. [PubMed] [Google Scholar]
- 199. Cobo T, Kacerovsky M, Holst RM, et al. Intra‐amniotic inflammation predicts microbial invasion of the amniotic cavity but not spontaneous preterm delivery in preterm prelabor membrane rupture. Acta Obstet Gynecol Scand. 2012;91:930‐935. [DOI] [PubMed] [Google Scholar]
- 200. Kacerovsky M, Musilova I, Khatibi A, et al. Intraamniotic inflammatory response to bacteria: analysis of multiple amniotic fluid proteins in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2012;25:2014‐0027. [DOI] [PubMed] [Google Scholar]
- 201. Kacerovsky M, Musilova I, Andrys C, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol. 2014;210:325.e1‐10. [DOI] [PubMed] [Google Scholar]
- 202. Endres LK, Wang EY. Interleukin‐6 and tumor necrosis factor alpha as predictors of success after emergent cerclage. Am J Perinatol. 2004;21:477‐481. [DOI] [PubMed] [Google Scholar]
- 203. Lee KY, Jun HA, Kim HB, Kang SW. Interleukin‐6, but not relaxin, predicts outcome of rescue cerclage in women with cervical incompetence. Am J Obstet Gynecol. 2004;191:784‐789. [DOI] [PubMed] [Google Scholar]
- 204. Jung EY, Park KH, Lee SY, Ryu A, Oh KJ. Non‐invasive prediction of intra‐amniotic infection and/or inflammation in patients with cervical insufficiency or an asymptomatic short cervix (</=15 mm). Arch Gynecol Obstet. 2015;292:579‐587. [DOI] [PubMed] [Google Scholar]
- 205. Esplin MS, Romero R, Chaiworapongsa T, et al. Monocyte chemotactic protein‐1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra‐amniotic infection. J Matern Fetal Neonatal Med. 2005;17:365‐373. [DOI] [PubMed] [Google Scholar]
- 206. Polettini J, Cobo T, Kacerovsky M, et al. Biomarkers of spontaneous preterm birth: a systematic review of studies using multiplex analysis. J Perinat Med. 2017;45:71‐84. [DOI] [PubMed] [Google Scholar]
- 207. Esplin MS, Romero R, Chaiworapongsa T, et al. Amniotic fluid levels of immunoreactive monocyte chemotactic protein‐1 increase during term parturition. J Matern Fetal Neonatal Med. 2003;14:51‐56. [DOI] [PubMed] [Google Scholar]
- 208. Mittal P, Romero R, Tarca AL, et al. Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med. 2010;38:617‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Proost P, Wuyts A, Van Damme J. Human monocyte chemotactic proteins‐2 and ‐3: structural and functional comparison with MCP‐1. J Leukoc Biol. 1996;59:67‐74. [DOI] [PubMed] [Google Scholar]
- 210. Gu L, Rutledge B, Fiorillo J, et al. In vivo properties of monocyte chemoattractant protein‐1. J Leukoc Biol. 1997;62:577‐580. [DOI] [PubMed] [Google Scholar]
- 211. Silva AR, de Assis EF, Caiado LF, Marathe GK, Bozza MT, McIntyre TM, Zimmerman GA, Prescott SM, Bozza PT, Castro‐Faria‐Neto HC. Monocyte chemoattractant protein‐1 and 5‐lipoxygenase products recruit leukocytes in response to platelet‐activating factor‐like lipids in oxidized low‐density lipoprotein. J Immunol. 2002;168:4112‐4120. [DOI] [PubMed] [Google Scholar]
- 212. Muller WA. New mechanisms and pathways for monocyte recruitment. J Exp Med. 2001;194:F47‐F51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669‐671. [DOI] [PubMed] [Google Scholar]
- 214. Alam R, Stafford S, Forsythe P, et al. RANTES is a chemotactic and activating factor for human eosinophils. J Immunol. 1993;150(8 Pt 1):3442‐3448. [PubMed] [Google Scholar]
- 215. Adams DH, Lloyd AR. Chemokines: leucocyte recruitment and activation cytokines. Lancet. 1997;349:490‐495. [DOI] [PubMed] [Google Scholar]
- 216. Campbell EM, Proudfoot AE, Yoshimura T, et al. Recombinant guinea pig and human RANTES activate macrophages but not eosinophils in the guinea pig. J Immunol. 1997;159:1482‐1489. [PubMed] [Google Scholar]
- 217. Appay V, Rowland‐Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83‐87. [DOI] [PubMed] [Google Scholar]
- 218. Athayde N, Romero R, Maymon E, et al. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2000;182(1 Pt 1):135‐141. [DOI] [PubMed] [Google Scholar]
- 219. Pacora P, Maymon E, Gervasi MT, et al. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. Am J Obstet Gynecol. 2000;183:904‐910. [DOI] [PubMed] [Google Scholar]
- 220. Hsu TY, Lin H, Lan KC, et al. High interleukin‐16 concentrations in the early second trimester amniotic fluid: an independent predictive marker for preterm birth. J Matern Fetal Neonatal Med. 2013;26:285‐289. [DOI] [PubMed] [Google Scholar]
- 221. Moroz LA, Simhan HN. Rate of sonographic cervical shortening and biologic pathways of spontaneous preterm birth. Am J Obstet Gynecol. 2014;210:555.e1‐5. [DOI] [PubMed] [Google Scholar]
- 222. Moreau JL, Chastagner P, Tanaka T, et al. Control of the IL‐2 responsiveness of B lymphocytes by IL‐2 and IL‐4. J Immunol. 1995;155:3401‐3408. [PubMed] [Google Scholar]
- 223. Morel BF, Burke MA, Kalagnanam J, McCarthy SA, Tweardy DJ, Morel PA. Making sense of the combined effect of interleukin‐2 and interleukin‐4 on lymphocytes using a mathematical model. Bull Math Biol. 1996;58:569‐594. [DOI] [PubMed] [Google Scholar]
- 224. Burke MA, Morel BF, Oriss TB, Bray J, McCarthy SA, Morel PA. Modeling the proliferative response of T cells to IL‐2 and IL‐4. Cell Immunol. 1997;178:42‐52. [DOI] [PubMed] [Google Scholar]
- 225. Palsson S, Hickling TP, Bradshaw‐Pierce EL, et al. The development of a fully‐integrated immune response model (FIRM) simulator of the immune response through integration of multiple subset models. BMC Syst Biol. 2013;7:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Gong C, Linderman JJ, Kirschner D. Harnessing the heterogeneity of T cell differentiation fate to fine‐tune generation of effector and memory T cells. Front Immunol. 2014;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Kwoh CK, Ng PY. Network analysis approach for biology. Cell Mol Life Sci. 2007;64:1739‐1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Araujo RP, Liotta LA, Petricoin EF. Proteins, drug targets and the mechanisms they control: the simple truth about complex networks. Nat Rev Drug Discovery. 2007;6:871‐880. [DOI] [PubMed] [Google Scholar]
- 229. Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol. 2008;4:682‐690. [DOI] [PubMed] [Google Scholar]
- 230. Broderick G, Fuite J, Kreitz A, Vernon SD, Klimas N, Fletcher MA. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav Immun. 2010;24:1209‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


