Abstract
The structures of the polymorphic forms of clathrin light chains were analyzed by two peptide mapping procedures. Comparison of the products of partial digestion by V8 protease showed no common peptides between LCA and LCB from bovine brain. No similarities between clathrin light chains and tropomyosin chains from bovine brain and skeletal muscle were detected with this technique. The peptides produced by complete tryptic digestion of LCA and LCB from bovine brain and bovine adrenal gland were analyzed by reverse phase h.p.l.c. For both LCA and LCB the polypeptides from different tissues showed considerable homology. LCA from brain and adrenal gland shared 10 out of a total of 15 peptides. LCB from brain and adrenal gland shared 10 out of 14 peptides. In contrast, when LCA was compared with the LCB chain from the same tissue very few peptides were shared; 4/23 for brain and 3/21 for adrenal gland. These results strongly indicate that, within a tissue, LCB is not related to LCA by post-translational processing and that each chain is encoded by a separate gene. The data also demonstrate the close homology of the different forms of LCA and LCB expressed in different tissues within the same organism. Thus the polymorphic differences of clathrin light chains within a tissue are greater than those between tissues.
Full text
PDF

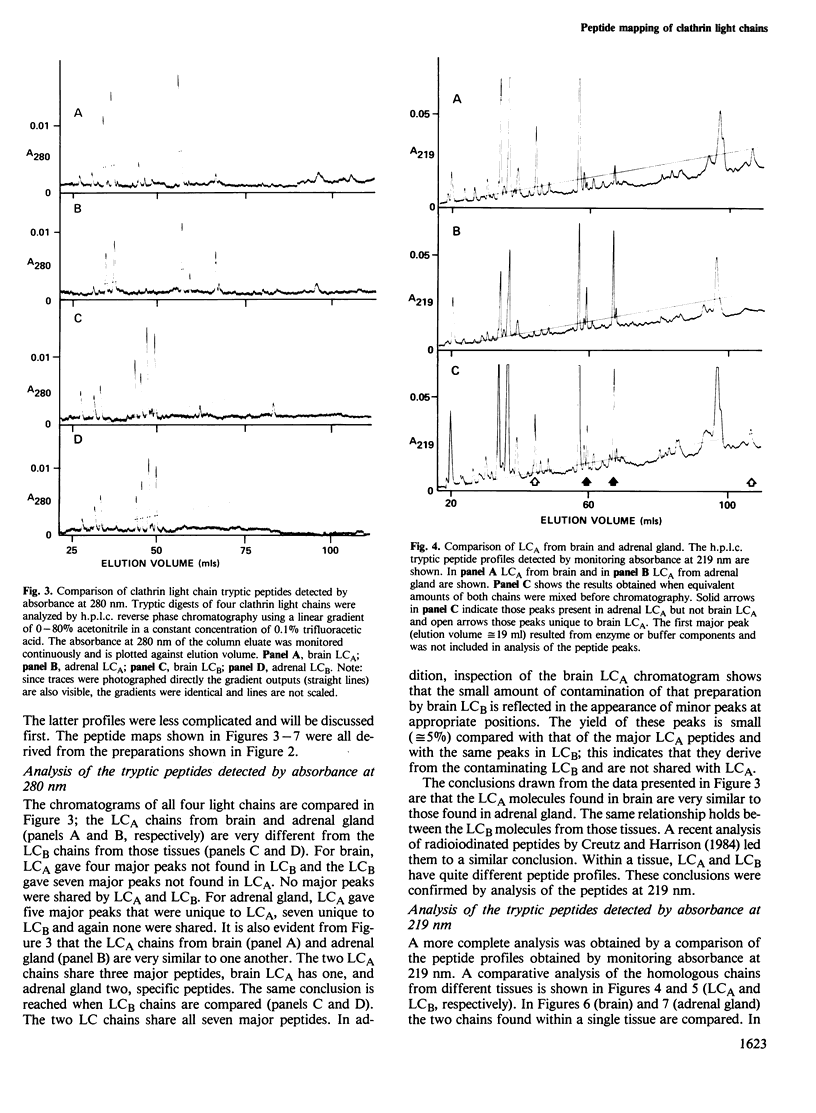




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodsky F. M., Holmes N. J., Parham P. Tropomyosin-like properties of clathrin light chains allow a rapid, high-yield purification. J Cell Biol. 1983 Mar;96(3):911–914. doi: 10.1083/jcb.96.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P. Polymorphism in clathrin light chains from different tissues. J Mol Biol. 1983 Jun 15;167(1):197–204. doi: 10.1016/s0022-2836(83)80041-2. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Creutz C. E., Harrison J. R. Clathrin light chains and secretory vesicle binding proteins are distinct. Nature. 1984 Mar 8;308(5955):208–210. doi: 10.1038/308208a0. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Finch J. T., Pearse B. M. On the structure of coated vesicles. J Mol Biol. 1976 Jun 5;103(4):785–798. doi: 10.1016/0022-2836(76)90209-6. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Pearse B. M. Assembly and packing of clathrin into coats. J Cell Biol. 1981 Dec;91(3 Pt 1):790–797. doi: 10.1083/jcb.91.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine R. E., Blitz A. L. A chemical comparison of tropomyosins from muscle and non-muscle tissues. J Mol Biol. 1975 Jul 5;95(3):447–454. doi: 10.1016/0022-2836(75)90202-8. [DOI] [PubMed] [Google Scholar]
- Geisow M. J., Burgoyne R. D. Recruitment of cytosolic proteins to a secretory granule membrane depends on Ca2+-calmodulin. Nature. 1983 Feb 3;301(5899):432–435. doi: 10.1038/301432a0. [DOI] [PubMed] [Google Scholar]
- Keen J. H., Willingham M. C., Pastan I. H. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979 Feb;16(2):303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- Keen J. H., Willingham M. C., Pastan I. Clathrin and coated vesicle proteins Immunological characterization. J Biol Chem. 1981 Mar 10;256(5):2538–2544. [PubMed] [Google Scholar]
- Kirchhausen T., Harrison S. C., Parham P., Brodsky F. M. Location and distribution of the light chains in clathrin trimers. Proc Natl Acad Sci U S A. 1983 May;80(9):2481–2485. doi: 10.1073/pnas.80.9.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T., Harrison S. C. Protein organization in clathrin trimers. Cell. 1981 Mar;23(3):755–761. doi: 10.1016/0092-8674(81)90439-6. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Shapiro L. S., Moskowitz N., Hua E. L., Puszkin S., Schook W. Isolation and preliminary characterization of clathrin-associated proteins. Eur J Biochem. 1982 Jul;125(2):463–470. doi: 10.1111/j.1432-1033.1982.tb06706.x. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. Coated vesicles from pig brain: purification and biochemical characterization. J Mol Biol. 1975 Sep 5;97(1):93–98. doi: 10.1016/s0022-2836(75)80024-6. [DOI] [PubMed] [Google Scholar]
- Pearse B. M. On the structural and functional components of coated vesicles. J Mol Biol. 1978 Dec 25;126(4):803–812. doi: 10.1016/0022-2836(78)90021-9. [DOI] [PubMed] [Google Scholar]
- Ungewickell E. Biochemical and immunological studies on clathrin light chains and their binding sites on clathrin triskelions. EMBO J. 1983;2(8):1401–1408. doi: 10.1002/j.1460-2075.1983.tb01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E., Branton D. Assembly units of clathrin coats. Nature. 1981 Jan 29;289(5796):420–422. doi: 10.1038/289420a0. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Unanue E. R., Branton D. Functional and structural studies on clathrin triskelions and baskets. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):723–731. doi: 10.1101/sqb.1982.046.01.069. [DOI] [PubMed] [Google Scholar]
- Winkler F. K., Stanley K. K. Clathrin heavy chain, light chain interactions. EMBO J. 1983;2(8):1393–1400. doi: 10.1002/j.1460-2075.1983.tb01597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]