Abstract
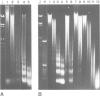
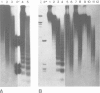
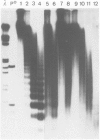
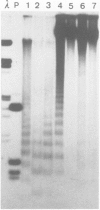
We examined the chromatin structure of a Balbiani ring (secretory protein gene) in the salivary glands of Chironomus larvae in its hyperactive state after stimulation with pilocarpine. For the inactive state of the gene an established tissue culture cell line, not expressing the gene, was used. Electron microscopy showed an RNA polymerase density of approximately 38/microns. Micrococcal nuclease digestion of purified nuclei followed by DNA transfer and hybridization revealed a smear with no recognizable discrete DNA fragments. Without pilocarpine stimulation a faint nucleosomal repeat was superimposed upon the smear, and in tissue culture cells a clear nucleosomal repeat was revealed. The restriction enzyme XbaI, which has a 6-bp recognition sequence, cut the gene in the hyperactive chromatin state, but not in its inactive conformation. The combined results are best explained by the absence of most of the nucleosomes in this hyperactive RNA polymerase II transcribed gene.
Full text
PDF




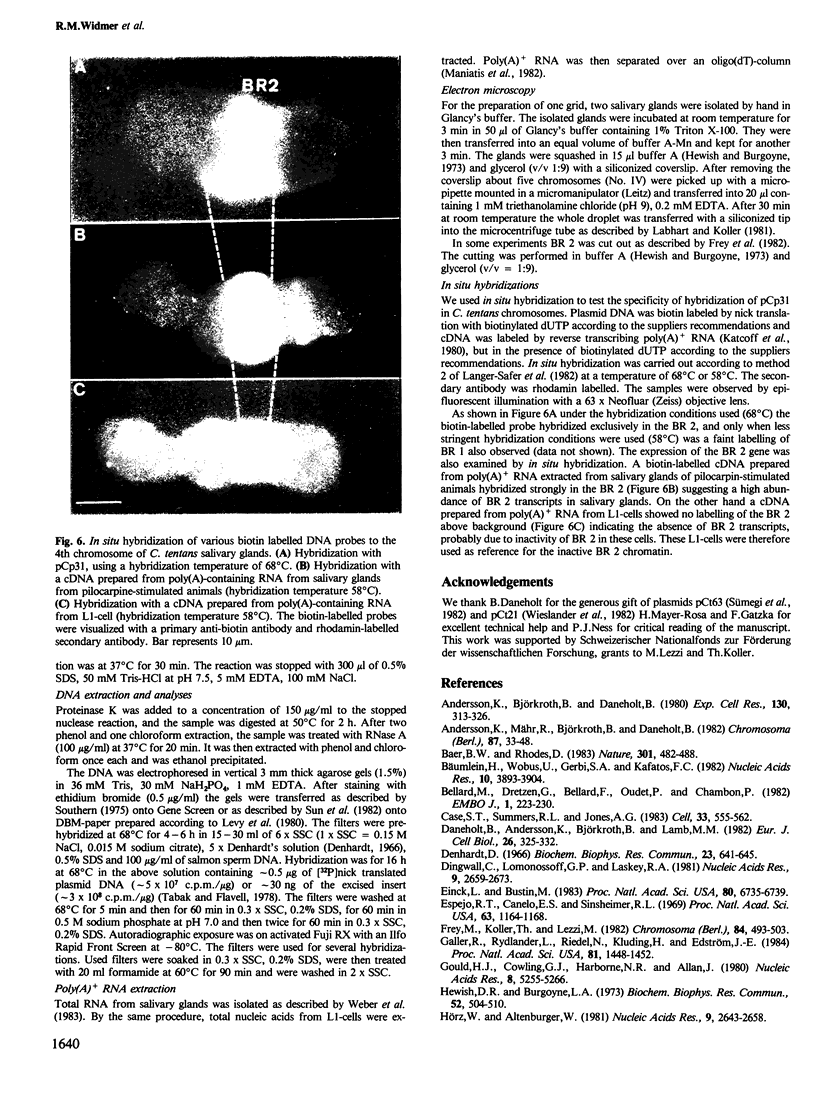

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson K., Mähr R., Björkroth B., Daneholt B. Rapid reformation of the thick chromosome fiber upon completion of RNA synthesis at the Balbiani ring genes in Chironomus tentans. Chromosoma. 1982;87(1):33–48. doi: 10.1007/BF00333508. [DOI] [PubMed] [Google Scholar]
- Baer B. W., Rhodes D. Eukaryotic RNA polymerase II binds to nucleosome cores from transcribed genes. Nature. 1983 Feb 10;301(5900):482–488. doi: 10.1038/301482a0. [DOI] [PubMed] [Google Scholar]
- Bellard M., Dretzen G., Bellard F., Oudet P., Chambon P. Disruption of the typical chromatin structure in a 2500 base-pair region at the 5' end of the actively transcribed ovalbumin gene. EMBO J. 1982;1(2):223–230. doi: 10.1002/j.1460-2075.1982.tb01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäumlein H., Wobus U., Gerbi S. A., Kafatos F. C. The basic repeat unit of a Chironomus Balbiani ring gene. Nucleic Acids Res. 1982 Jul 10;10(13):3893–3904. doi: 10.1093/nar/10.13.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case S. T., Summers R. L., Jones A. G. A variant tandemly repeated nucleotide sequence in Balbiani ring 2 of Chironomus tentans. Cell. 1983 Jun;33(2):555–562. doi: 10.1016/0092-8674(83)90436-1. [DOI] [PubMed] [Google Scholar]
- Daneholt B., Andersson K., Björkroth B., Lamb M. M. Visualization of active 75 S RNA genes in the Balbiani rings of Chironomus tentans. Eur J Cell Biol. 1982 Feb;26(2):325–332. [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Lomonossoff G. P., Laskey R. A. High sequence specificity of micrococcal nuclease. Nucleic Acids Res. 1981 Jun 25;9(12):2659–2673. doi: 10.1093/nar/9.12.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einck L., Bustin M. Inhibition of transcription in somatic cells by microinjection of antibodies to chromosomal proteins. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6735–6739. doi: 10.1073/pnas.80.22.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey M., Koller T., Lezzi M. Isolation of DNA from single microsurgically excised bands of polytene chromosomes of Chironomus. Chromosoma. 1982;84(4):493–503. doi: 10.1007/BF00292850. [DOI] [PubMed] [Google Scholar]
- Galler R., Rydlander L., Riedel N., Kluding H., Edström J. E. Balbiani ring induction in phosphate metabolism. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1448–1452. doi: 10.1073/pnas.81.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould H. J., Cowling G. J., Harborne N. R., Allan J. An examination of models for chromatin transcription. Nucleic Acids Res. 1980 Nov 25;8(22):5255–5266. doi: 10.1093/nar/8.22.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Hörz W., Altenburger W. Sequence specific cleavage of DNA by micrococcal nuclease. Nucleic Acids Res. 1981 Jun 25;9(12):2643–2658. doi: 10.1093/nar/9.12.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo-Kemenes T., Hörz W., Zachau H. G. Chromatin. Annu Rev Biochem. 1982;51:89–121. doi: 10.1146/annurev.bi.51.070182.000513. [DOI] [PubMed] [Google Scholar]
- Jäckle H., Almeida J. C., Galler R., Kluding H., Lehrach H., Edström J. E. Constant and variable parts in the Balbiani ring 2 repeat unit and the translation termination region. EMBO J. 1982;1(7):883–888. doi: 10.1002/j.1460-2075.1982.tb01264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagyozov L. K., Valkanov M. A., Hadjiolov A. A. Transcription of DNA-histone complexes by yeast RNA polymerase B. Nucleic Acids Res. 1978 Jun;5(6):1907–1917. doi: 10.1093/nar/5.6.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katcoff D., Nudel U., Zevin-Sonkin D., Carmon Y., Shani M., Lehrach H., Frischauf A. M., Yaffe D. Construction of recombinant plasmids containing rat muscle actin and myosin light chain DNA sequences. Proc Natl Acad Sci U S A. 1980 Feb;77(2):960–964. doi: 10.1073/pnas.77.2.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labhart P., Koller T. Electron microscope specimen preparation of rat liver chromatin by a modified Miller spreading technique. Eur J Cell Biol. 1981 Jun;24(2):309–316. [PubMed] [Google Scholar]
- Labhart P., Koller T. Structure of the active nucleolar chromatin of Xenopus laevis Oocytes. Cell. 1982 Feb;28(2):279–292. doi: 10.1016/0092-8674(82)90346-4. [DOI] [PubMed] [Google Scholar]
- Labhart P., Ness P., Banz E., Parish R., Koller T. Model for the structure of the active nucleolar chromatin. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 1):557–564. doi: 10.1101/sqb.1983.047.01.065. [DOI] [PubMed] [Google Scholar]
- Laird C. D., Wilkinson L. E., Foe V. E., Chooi W. Y. Analysis of chromatin-associated fiber arrays. Chromosoma. 1976 Oct 28;58(2):169–190. doi: 10.1007/BF00701357. [DOI] [PubMed] [Google Scholar]
- Lamb M. M., Daneholt B. Characterization of active transcription units in Balbiani rings of Chironomus tentans. Cell. 1979 Aug;17(4):835–848. doi: 10.1016/0092-8674(79)90324-6. [DOI] [PubMed] [Google Scholar]
- Langer-Safer P. R., Levine M., Ward D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A., Frei E., Noll M. Efficient transfer of highly resolved small DNA fragments from polyacrylamide gels to DBM paper. Gene. 1980 Nov;11(3-4):283–290. doi: 10.1016/0378-1119(80)90068-2. [DOI] [PubMed] [Google Scholar]
- Levy A., Noll M. Chromatin fine structure of active and repressed genes. Nature. 1981 Jan 15;289(5794):198–203. doi: 10.1038/289198a0. [DOI] [PubMed] [Google Scholar]
- Lilley D. M. Active chromatin structure. Cell Biol Int Rep. 1978 Jan;2(1):1–10. doi: 10.1016/0309-1651(78)90078-4. [DOI] [PubMed] [Google Scholar]
- Lilley D. M., Jacobs M. F., Houghton M. The nature of the interaction of nucleosomes with a eukaryotic RNA polymerase II. Nucleic Acids Res. 1979 Sep 25;7(2):377–399. doi: 10.1093/nar/7.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D., Oudet P., Chambon P. Structure of transcribing chromatin. Prog Nucleic Acid Res Mol Biol. 1980;24:1–55. doi: 10.1016/s0079-6603(08)60670-4. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Bustin M., Miller O. L., Jr Electron microscopic analysis of chromosome metabolism in the Drosophila melanogaster embryo. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):741–754. doi: 10.1101/sqb.1978.042.01.075. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Ultrastructural patterns of RNA synthesis during early embryogenesis of Drosophila melanogaster. Cell. 1976 Jun;8(2):305–319. doi: 10.1016/0092-8674(76)90014-3. [DOI] [PubMed] [Google Scholar]
- Meyer B., Mähr R., Eppenberger H. M., Lezzi M. The activity of Balbiani rings 1 and 2 in salivary glands of Chironomus tentans larvae under different modes of development and after pilocarpine treatment. Dev Biol. 1983 Aug;98(2):265–277. doi: 10.1016/0012-1606(83)90357-3. [DOI] [PubMed] [Google Scholar]
- Mähr R., Meyer B., Daneholt B., Eppenberger H. M. Activation of Balbiani ring genes in Chironomus tentans after a pilocarpine-induced depletion of the secretory products from the salivary gland lumen. Dev Biol. 1980 Dec;80(2):409–418. doi: 10.1016/0012-1606(80)90415-7. [DOI] [PubMed] [Google Scholar]
- Ness P. J., Labhart P., Banz E., Koller T., Parish R. W. Chromatin structure along the ribosomal DNA of Dictyostelium. Regional differences and changes accompanying cell differentiation. J Mol Biol. 1983 May 25;166(3):361–381. doi: 10.1016/s0022-2836(83)80090-4. [DOI] [PubMed] [Google Scholar]
- Olins A. L., Olins D. E., Lezzi M. Ultrastructural studies of Chironomus salivary gland cells in different states of Balbiani ring activity. Eur J Cell Biol. 1982 Jun;27(2):161–169. [PubMed] [Google Scholar]
- Pfeiffer W., Zachau H. G. Accessibility of expressed and non-expressed genes to a restriction nuclease. Nucleic Acids Res. 1980 Oct 24;8(20):4621–4638. doi: 10.1093/nar/8.20.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargan D. R., Butterworth P. H. Eukaryotic ternary transcription complexes. II. An approach to the determination of chromatin conformation at the site of transcription. Nucleic Acids Res. 1982 Aug 11;10(15):4655–4669. doi: 10.1093/nar/10.15.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller H., Gray C., Herrmann K. Nucleotide sequence of an RNA polymerase binding site from the DNA of bacteriophage fd. Proc Natl Acad Sci U S A. 1975 Feb;72(2):737–741. doi: 10.1073/pnas.72.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U. Changes of nucleosome frequency in nucleolar and non-nucleolar chromatin as a function of transcription: an electron microscopic study. Cell. 1978 Mar;13(3):535–549. doi: 10.1016/0092-8674(78)90327-6. [DOI] [PubMed] [Google Scholar]
- Scheer U., Sommerville J., Bustin M. Injected histone antibodies interfere with transcription of lampbrush chromosome loops in oocytes of Pleurodeles. J Cell Sci. 1979 Dec;40:1–20. doi: 10.1242/jcs.40.1.1. [DOI] [PubMed] [Google Scholar]
- Smith R. D., Seale R. L., Yu J. Transcribed chromatin exhibits an altered nucleosomal spacing. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5505–5509. doi: 10.1073/pnas.80.18.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spinelli G., Albanese I., Anello L., Ciaccio M., Di Liegro I. Chromatin structure of histone genes in sea urchin sperms and embryos. Nucleic Acids Res. 1982 Dec 20;10(24):7977–7991. doi: 10.1093/nar/10.24.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiak A., Kłopotowski T. Four-stranded DNA structure and DNA base methylation in the mechanism of action of restriction endonucleases. J Theor Biol. 1979 Sep 7;80(1):65–82. doi: 10.1016/0022-5193(79)90180-2. [DOI] [PubMed] [Google Scholar]
- Sun Y. L., Xu Y. Z., Chambon P. A simple and efficient method for the separation and detection of small DNA fragments by electrophoresis in formamide containing agarose gels and Southern blotting to DBM-paper. Nucleic Acids Res. 1982 Oct 11;10(19):5753–5763. doi: 10.1093/nar/10.19.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sümegi J., Wieslander L., Daneholt B. A hierarchic arrangement of the repetitive sequences in the Balbiani ring 2 gene of Chironomus tentans. Cell. 1982 Sep;30(2):579–587. doi: 10.1016/0092-8674(82)90254-9. [DOI] [PubMed] [Google Scholar]
- Tabak H. F., Flavell R. A. A method for the recovery of DNA from agarose gels. Nucleic Acids Res. 1978 Jul;5(7):2321–2332. doi: 10.1093/nar/5.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg M. F., Scheer U., Zentgraf H., Franke W. W. Heterogeneity of spacer lengths in circles of amplified ribosomal DNA of two insect species, Dytiscus marginalis and Acheta domesticus. J Mol Biol. 1976 Dec;108(2):453–470. doi: 10.1016/s0022-2836(76)80130-1. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Chambon P. Studies on the mechanism of transcription of nucleosomal complexes. Eur J Biochem. 1980 Jan;103(2):219–226. doi: 10.1111/j.1432-1033.1980.tb04306.x. [DOI] [PubMed] [Google Scholar]
- Wasylyk B., Chambon P. Transcription by eukaryotic RNA polymerases A and B of chromatin assembled in vitro. Eur J Biochem. 1979 Aug 1;98(2):317–327. doi: 10.1111/j.1432-1033.1979.tb13191.x. [DOI] [PubMed] [Google Scholar]
- Weihe A., von Mickwitz C. U., Grade K., Lindigkeit R. Complexes of DNA with arginine-rich and slightly lysine-rich histones. Transcription and electron microscopy. Biochim Biophys Acta. 1978 Mar 29;518(1):172–176. doi: 10.1016/0005-2787(78)90126-0. [DOI] [PubMed] [Google Scholar]
- Weisbrod S. Active chromatin. Nature. 1982 May 27;297(5864):289–295. doi: 10.1038/297289a0. [DOI] [PubMed] [Google Scholar]
- Wieslander L., Lendahl U. The Balbiani ring 2 gene in Chironomus tentans is built from two types of tandemly arranged major repeat units with a common evolutionary origin. EMBO J. 1983;2(7):1169–1175. doi: 10.1002/j.1460-2075.1983.tb01563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander L., Sümegi J., Daneholt B. Evidence for a common ancestor sequence for the Balbiani ring 1 and Balbiani ring 2 genes in Chironomus tentans. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6956–6960. doi: 10.1073/pnas.79.22.6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Wong Y. C., Elgin S. C. The chromatin structure of specific genes: II. Disruption of chromatin structure during gene activity. Cell. 1979 Apr;16(4):807–814. doi: 10.1016/0092-8674(79)90096-5. [DOI] [PubMed] [Google Scholar]
- Wyss C. Chironomus tentans epithelial cell lines sensitive to ecdysteroids, juvenile hormone, insulin and heat shock. Exp Cell Res. 1982 Jun;139(2):309–319. doi: 10.1016/0014-4827(82)90255-5. [DOI] [PubMed] [Google Scholar]