Abstract
Background: Gastric cancer is one of the most common cancers in the world. There are many genomic and molecular factors that cause gastric cancer to occur. Also, many markers that associate with tumor invasiveness have been known.
E-cadherin is a calcium- mediated cell adhesion molecule. In some studies, abnormal expression of E-cadherin has been seen in gastric carcinoma. However, in the studies done there has been some conflicting information about abnormal expression of this marker in a variety of gastric carcinoma and also about the expression of this marker and its correlation with various clinicopathologic factors of tumor.
Subjects and Methods: A case control study was performed on total or partial gastrectomy tissue samples obtained from 70 patients with gastric cancer and adjacent non-neoplastic tissues. The immunohistochemistry was used to assess E-cadherin expression. The correlation between abnormal E-cadherin expression and tumor histopathology was evaluated in all patients.
Results: Among 70 patients who were analyzed, 48.6% showed abnormal E-cadherin expression. A significant correlation was seen between abnormal E-cadherin expression and tumor stage, grade, lymph node metastasis, tumor phenotype, tumor type, depth of invasion and age.
Conclusion: Abnormal E-cadherin expression is a common phenomenon in gastric cancer. Because there was a strong correlation between abnormal E-cadherin expression and tumor stage, tumor grade, depth of invasion and regional lymph node involvement, this marker may be used as a predictive factor for tumor invasiveness in gastric cancer.
Key Words: Gastric cancer, E-cadherin, Clinicopathologic, Immunohistochemistry, Stage
Introduction
Gastric cancer is the fourth most common cancer and the second leading cause of death in both sexes in the world.1,2 Gastric cancer is the most common gastrointestinal cancer in Iranian men and the third most common cancer in Iranian women (after breast and colon).3,4 According to the Ministry of Health, gastric cancer is the most common fatal gastrointestinal tract malignancy and the most common cause of death in Iran.5, 6, 7Since the incidence of this cancer in Iran is twice its incidence in the world, it requires special attention.4,5 In general, initial gastric cancers are often adenocarcinomas, which based on pathologic classification are divided into two types of intestinal and diffuse.8
Cadherin is a group of cell adhesion molecules with glycoprotein nature that are located in the membrane of the cell. These molecules are located in combination with other cell adhesion molecules (such as Catenins) and regulate various biological important processes such as cell migration, differentiation, proliferation and cell death (apoptosis).Two types of Cadherins that have been most studied are E-cadherin (epithelial type) and N-Cadherin (neural type).9 Epithelial type is a calcium-mediated cell adhesion molecule and its abnormal (low) expression has associated with advanced stages and more aggressive behavior of some cancers including large bowel, lung and prostate.10 Also, in various studies, there has been a relationship between the level of E-cadherin expression and the sensitivity of tumor cells to chemotherapy.11,12 This marker is detectable by IHC staining and exists in the normal gastric tissue. In some studies, abnormal expression of E-cadherin is found in gastric carcinoma.13 However, in current studies, there are some conflicting information about abnormal expression of this marker in a variety of gastric carcinoma and also about its correlation with various clinicopathologic factors of tumor.
Due to direct correlation between the risk of metastatic disease that is directly related to patient prognosis, and clinicopathologic factors14increasing the prevalence of gastric cancer in different communities, including our country, Iran, lack of appropriate treatment response , shortcomings and contradictions that exist in many previous studies as well as insufficient studies in this field, we decided to investigate the expression of E-cadherin in all different kinds of gastric cancer and its correlation with each of mentioned parameters. It is hoped that this study can be a step towards better recognition of gastric cancer behavior and it can also be effective in the way of intervention and follow-up of these patients.
MATERIALS AND METHODS
We reviewed the tissue obtained from gastrectomy samples in 70 patients. We obtained two different tissue samples from each patient. One, from the area with malignant cells and one from the area with normal cells. This study was an analytic case-control design and was done on two groups. Group one included tissue samples with cancerous cells and group two included tissue samples with normal cells. It aimed to investigate the correlation between abnormal expressions of E-cadherin and clinicopathologic features of tumor in gastric cancer, on paraffin blocks available at Archives of Pathology in Imam Khomeini Hospital in Sari, Iran. There are 70 samples in both groups.
Sample size was calculated through the formula Pokak. The clinicopathologic parameters that were evaluated in this study included age, gender, tumor type, tumor location, tumor size, tumor phenotype, histological grade, lymph node metastasis (N), distant metastasis (M) and stage.
In order to compare the expression of E-cadherin and intensity of staining in clinicopathologic parameters, the patients were classified according to age, tumor size and lymph node metastasis. The patients' age were divided into two groups (under and above 55 years). Tumor size and lymph node metastasis were divided into three groups (less than 5 cm, 5 to 10 cm and above 10 cm) and four groups (Lack of involvement, 1 to 6 involvement, 7 to 15-involvement, and 15 and more than 15 involvement), respectively.
Patients who had preoperative chemotherapy or radiotherapy were excluded. The patient data were entered in the questionnaire. In order to follow-up patients, their addresses and telephone numbers were used. (All procedures performed in this study were in accordance with the ethical standards of the institution. ethical code: 1393/6/16 )
Tumor blocks or slides that were not suitable for immuno-histochemical staining were omitted from the study.
To continue the study, the needed paraffin blocks were removed from the archive and several slides with hematoxylin – eosin staining were prepared from the areas of tumor and adjacent normal looking tissue.
During the investigation of slides, in addition to detecting tumor, other microscopic parameters such as depth of invasion, neurovascular invasion and differentiation (histological grade) were also evaluated.
Immunohistochemical staining was performed on the samples for assessing the expression of E-cadherin. The staining step followed the routine process. In order to examine the specificity of immune staining, both positive and negative controls were run at the same time in each experiment.15,16 The slides were studied and reported by two expert pathologists who had no clinicopathologic knowledge of the patients' data, E-cadherin expression and intensity of staining.
To improve the accuracy of diagnosis and to determine the staining of cells, multiple microscopic fields in low and high-power (X100-X400) were examined and the findings were reported according to the estimated percentage of the stained tumor cells.
Staining intensity of E-cadherin was reported on a scale with three grades:
Positive staining in less than 10% of tumor cells.:0
Positive staining between 10% to 90% of tumor cells. :+1
Positive staining Over 90% of the tumor cells.:+2
0 to 1+ grade as low staining and +2 grade as high staining were considered for E-cadherin.17
E-cadherin protein was a membranous marker. The obtained results were analyzed by statistical software SPSS (IBM SPSS Statistics 20.0.1). Chi –square, Gama and Fisher's exact tests were used to analyze the correlation between the expression of E-cadherin in gastric cancer and clinicopathologic features. A p-value less than 0.05 was considered as a statistically significant level.
Results
A total of 70 patients (49 men and 21 women) were enrolled and evaluated in this study. The clinicopathologic findings in patients are summarized in Table 1. The mean of patients’ age was 65.87 ± 11.28 years. After immunohistochemical staining, E-cadherin expression and intensity of staining in the two groups (case and control) were compared (Table 2).
Table 1.
Clinicopathologic findings in patients with gastric cancer
| stage |
Lymph
node |
Neurovas
cular involvem ent |
Tumor
grade |
Tumor
shape |
Depth of
invasion |
Tumor
size |
Tumor location |
Tumor
Phenotyp e |
Tumor
type |
Gender | Age |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20% (n=14) Ia-Ib |
40% (n=28) N0 |
48.6 %(n=34) yes |
31.4% (n=22) Well |
95.7% (n=67) Ulcerative |
2.9% (n=2) T1 |
62.9% (n=44) <=5 cm |
17.1% (n=12) cardia |
81.4% (n=57) Intestinal |
94.3% (n=66) Adenocar cinoma |
70 %(n=49) male |
12.9% (n=9) Under55 years |
| 27.1% (n=19) II |
27.1% (n=19)N1 |
51.4% (n=36) no |
30% (n=21) moderate |
4.3%(n=3) Infiltrative |
27.1% (n=19) T2 |
35.7% (n=25) 5-10cm |
4.3% (n=3)fund us |
18.6% (n=13) Diffuse |
5.7% (n=4) Signet ring carcinoma a |
30 % (n=21) Female |
87.1% (n=61) above55 years |
| 52.9% (n=37) IIIa-IIIb |
32.9% (n=23)N2 |
38.6% (n=27)po or |
70% (n=49) T3 |
1.4% (n=1) >10cm |
10% (n=7) body |
||||||
| 17.1% (n=12) antrum |
|||||||||||
| 50% (n=35) Lesser curvature |
|||||||||||
| 1.4% (n=1) Greater curvature |
Table 2.
Comparison of the E-cadherin expression and staining intensity in both groups (case & control)
| Staining intensity Groups |
Low | High |
|---|---|---|
| 01+ | 2 + | |
| Case | 20% 28.6% (n=14)(n=20) |
51.4% (n=36) |
| Control | 00 | 100% (n=70) |
By definition (see Materials and Methods) in the case group, 51.4% (n=36) samples showed high staining (2+) and 48.6% (n=34) revealed low staining (0, 1+), but in the control group all of them showed high staining (Figures 1 -3).
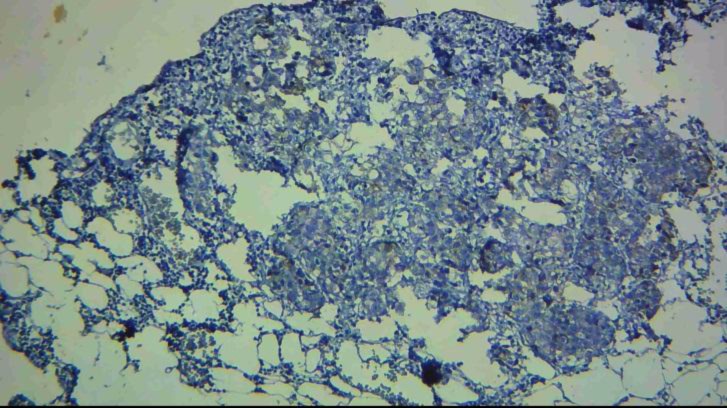
Figure 1.
Low staining in the tumoral cells in gastric carcinomas (intestinal type) with E-cadherin marker in IHC staining (magnification 100X)
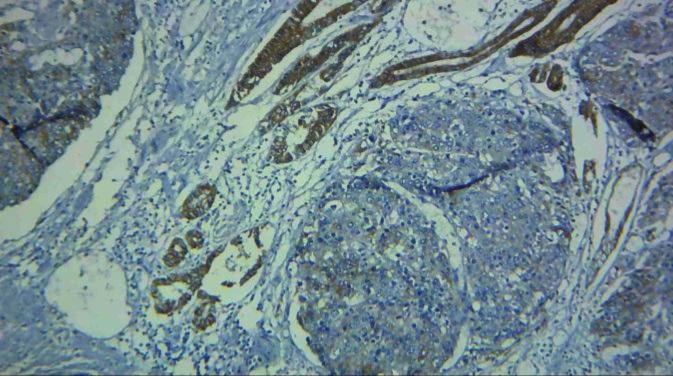
Figure 3.
Low staining in the tumoral cells (right) and high staining in tumor- adjacent normal looking tissue (left) with E-Cadherin marker (magnification 100X)
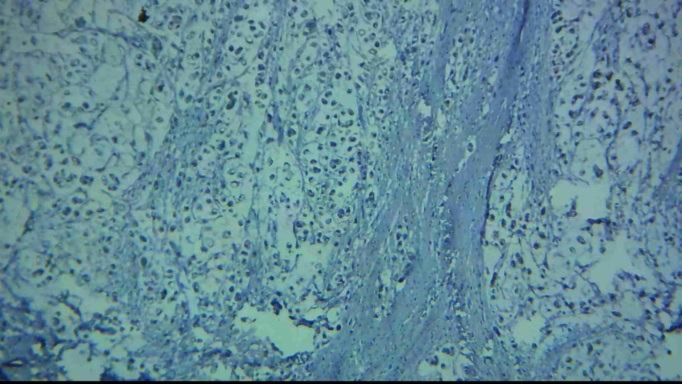
Figure 2.
Low staining in the tumoral cells in gastric carcinomas (signet ring type) with E-cadherin marker in IHC staining (magnification 100X)
By comparing E-cadherin expression with clinicopathologic features in patients with gastric cancer and statistical analysis of data, a significant correlation between the expression of tumor marker and patients’ age, tumor type, tumor phenotype, depth of invasion, histologic grade, number of involved lymph nodes, and the stage of the disease was observed. (The results have been mentioned in Table 4).
Table 4.
The correlation between E-cadherin expression and clinicopathologic parameters in gastric cancer(No significant correlation)
| Clinicopathologic parameters | E-cadherin-expression | p-value | |||
|---|---|---|---|---|---|
| high | low | ||||
| >90% | 10-90% <10% | ||||
| Gender | male | 53.1%(26) | 24.5%(12) | 22.4%(11) | 0.466 |
| female | 47.6%(10) | 38.1%(8) | 14.3%(3) | ||
| Location | cardia | 41.7%(5) | 41.7%(5) | 16.7%(2) | 0.697 |
| fundus | 33.3%(1) | 33.3%(1) | 33.3%(1) | ||
| body | 42.9%(3) | 28.6%(2) | 28.6%(2) | ||
| antrum | 75%(9) | 16.7%(2) | 8.3%(1) | ||
| Lesser curvature | 51.4%(18) | 25.7%(9) | 22.9%(8) | ||
| Grater curvature | 0 | 1.4%(1) | 0 | ||
| Shape | Ulcerative | 53.7%(36) | 28.4%(19) | 17.9%(12) | 0.061 |
| Infiltrative | 0 | 33.3%(1) | 66.7%(2) | ||
| Size | ≤ 5 | 52.3%(23) | 29.5%(13) | 18.2%(8) | 0.658 |
| 5-10 | 52%(13) | 28%(7) | 20%(5) | ||
| >10 | 0 | 0 | 100%(1) | ||
| Neurovascular invasion | yes | 44.1%(15) | 26.5%(9) | 29.4%(10) | 0.17 |
| no | 58.3%(21) | 30.6%(11) | 11.1%(4) | ||
None of the patients were observed to have distant metastases at the time of initial diagnosis. However, to pursue further follow-up, 5 patients showed distant metastases. No significant correlation was seen between distant metastases and the expression of marker.
A significant correlation between histological grade, the number of involved lymph nodes, the stage of disease and the E-cadherin staining intensity in tumor cells was identified. A Significant difference in E-cadher in staining intensity was observed in stage III compared to stages I and II. The number of involved lymph nodes was directly correlated with decreasing in marker staining intensity. The comparison of clinicopathologic parameters of patients with E-cadherin expression has been listed in Tables 3 and 4.
Table 3.
The correlation between E- cadherin expression and clinicopathologic parameters in gastric cancer (significant correlation)
| Clinicopathologic parameters | E-cadherin expression | p-value | |||
|---|---|---|---|---|---|
| High | low | ||||
| >90 % | 10-90% <10% | ||||
| Age | <55 | 88.9%(8) | 11.1%(1) | 0 | 0.023 |
| ≥ 55 | 45.9%(28) | 31.1%(19) | 23%(14) | ||
| Phenotype | intestinal | 63.2(%36) | 22.8%(13) | 14%(8) | <0.001 |
| diffuse | 0 | 53.8%(7) | 46.2%(6) | ||
| Type | adenocarcinoma | 54.5%(36) | 28.8%(19) | 16.7%(11) | 0.014 |
| Signet ring | 0 | 25%(1) | 75%(3) | ||
| Depth | T1 | 50%(1) | 50%(1) | 0 | 0.013 |
| T2 | 73.7%(14) | 21.1%(4) | 5.3%(1) | ||
| T3 | 42.9%(21) | 30.6%(15) | 26.5%(13) | ||
| Grade | well | 86.4%(19) | 13.6%(3) | 0 | <0.001 |
| moderate | 57.1%(12) | 23.8%(5) | 19%(4) | ||
| poor | 18.5%(5) | 44.4%(12) | 37%(10) | ||
| Lymph node | N0 | 82.1%(23) | 14.3%(4) | 3.6%(1) | <0.001 |
| N1 | 42.1%(8) | 31.6%(6) | 26.3%(5) | ||
| N2 | 21.7%(5) | 43.5%(10) | 34.8%(8) | ||
| Stage | Ia- Ib | 78.6%(11) | 21.4%(3) | 0 | <0.001 |
| II | 78.9%(15) | 10.5%(2) | 10.5%(2) | ||
| IIIa- IIIb | 27% (10) | 40.5%(15) | 32.4%(12) | ||
In our study, tumor-adjacent normal tissues were used as control group, and then E-cadherin expression and intensity of staining in these tissues were evaluated. Since the control group was selected from the adjacent normal tissues, both groups were quite matched for sex and age. In order to analyze and compare E-cadherin expression in the two groups, Chi-square test was used and the results showed significant difference between the groups (p-value <0.001).
Discussion
E-cadherin is a tumor suppressor gene that is located on chromosome 16, and produces a membrane protein.
E-cadherin is a calcium-mediated membrane molecule that plays an important role in adhesion and differentiation of gastric epithelial cells which is a very important protective mechanism against tumor formation.
Mutation in E-cadherin gene has had different prevalence in various epithelial cancers.18, 19
In this study, 48.6% of cases suffering from gastric cancer showed abnormal expression of E-cadherin in IHC staining. This frequency among cases suffering from gastric cancer was reported 38% in Dr. Anbiaee et al.’s study in Iran,20 49% in Dr. Lazar et al.’s study in Romania,21 and 57% in Dr. Chu et al.’s study in China.17
These differences may be related to the methods of mutation assessment (expression of E-cadherin by gene methylation or IHC).
In this study, a significant correlation was found between abnormal expression of E-cadherin and tumor grade, stage, depth of invasion and lymph node involvement. These parameters show the invasiveness of tumor. Also, in Dr. Anbiaee et al.’s study in Iran, a correlation between E-cadherin mutation and the grade and lymph node involvement was seen, but this marker was not correlated with depth of invasion.20 In Dr. Saad et al.’s study in Egypt, there was a significant correlation between this marker and lymph node involvement and tumor stage, but the depth of invasion and tumor grade were not linked.22In Dr. Chu et al.’s study in China, a correlation between depth of invasion and lymph node involvement was seen, but it was not associated with grade.17
Although there are different results about the correlation between mutation of E-cadherin, histopathology and tumor invasiveness, most studies indicate that this mutation is associated with more aggressive tumors.
So, E-cadherin mutation can be used as a significant prognostic factor, and the rate of invasive cancers can be decreased by detecting the causes of mutation and preventing them.
In this study, such a significant correlation was seen between the expression of abnormal marker and tumor phenotype and type that was similar to Dr. Lazar et al.’s in Romania.21 Also, in Dr. Zhu’s study in China a correlation with tumor type was observed.13In Dr. Micu et al.’s study in Romania23and Dr. Anbiaee et al.’s study in Iran20no significant correlation was found between abnormal expression of marker and tumor type.
Also, in this study a significant correlation was observed between E-cadherin mutation and patients’ age, but this correlation was not seen in other studies.20, 21
In this study, no significant correlation was seen between abnormal expression of marker and sex, tumor location, size, shape and neurovascular invasion. In Dr. Saad et al.’s study in Egypt, a correlation with vascular involvement 22 and in Dr. Chu et al.’s study in China a correlation with neuro- vascular invasion was observed.17
In this study, no significant correlation was found between abnormal expression of the marker and distant metastasis. In Dr. Saad et al.’s22 and also Dr. Chu et al.’s studies,17 there was a significant correlation between abnormal E-cadherin expression and metastasis. This difference is probably due to lack of enough time for patient follow-up.
CONCLUSION
This study showed abnormal E-cadherin expression in 48.6% of cases suffering from gastric cancer. Since abnormal expression of E-cadherin is associated with more aggressive gastric tumors, this marker can be used as a negative prognostic factor.
To achieve more conclusive results, we recommend evaluating the correlation between E-cadherin mutation and survival of patients in future studies. Also, we recommend studying the possible environmental factors that may cause mutation in this gene because it is possible to decrease the rate of invasive gastric cancers by detecting these factors and preventing them.
ACKNOWLEDGMENT
This paper is derived from Dr. Seyedeh Neda Sajadi Saravi’s thesis and supported by a grant from the Research and Technology Department in Mazandaran University of Medical Sciences. The authors would like to express their appreciation to Dr. M. Mehrabianfard for his help with advanced research and drafting.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
References
- 1.Zhao P, Li Y, Lu Y. Aberrant expression of CD133 protein correlates with Ki-67 expression and is a prognostic marker in gastric adenocarcinoma. BMC cancer. 2010;10(1):218. doi: 10.1186/1471-2407-10-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rocco A, Compare D, Nardone G. Cancer stem cell hypothesis and gastric carcinogenesis: Experimental evidence and unsolved questions. World Journal of Gastrointestinal Oncology. 2012;4(3):54. doi: 10.4251/wjgo.v4.i3.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taghavi N, Nasrollahzadeh D, Merat S, et al. Epidemiology of upper gastrointestinal cancers in Iran: a sub site analysis of 761 cases. World Journal of Gastroenterology: WJG. 2007;13(40):5367–7. doi: 10.3748/wjg.v13.i40.5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mousavi S, Alamolhoda A, Gouya M, et al. Implementation of Comprehensive National Cancer Control Program in Iran: an experience in a developing country. Annals of Oncology. 2008;19(2):398–400. doi: 10.1093/annonc/mdm581. [DOI] [PubMed] [Google Scholar]
- 5.Mohebbi M, Mahmoodi M, Wolfe R, et al. Geographical spread of gastrointestinal tract cancer incidence in the Caspian Sea region of Iran: spatial analysis of cancer registry data. BMC cancer. 2008;8(1):137. doi: 10.1186/1471-2407-8-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Movahedi M, Afsharfard A, Moradi A, et al. Survival rate of gastric cancer in Iran. Journal of Research in Medical Sciences: the Official Journal of Isfahan University of Medical Sciences. 2009;14(6):367. [PMC free article] [PubMed] [Google Scholar]
- 7.Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran: epidemiology and risk factors. Arch Iran Med. 2009;12(6):576–83. [PubMed] [Google Scholar]
- 8.Jang BI, Li Y, Graham DY, et al. The role of CD44 in the pathogenesis, diagnosis, and therapy of gastric cancer. Gut and liver. 2011;5(4):397–405. doi: 10.5009/gnl.2011.5.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosai J. Rosai and Ackerman's Surgical Pathology. 10e. Elsevier Health Sciences; 2011. [Google Scholar]
- 10.Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5(6):R217–R22. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Li Z, Wang C, et al. E-cadherin decreased human breast cancer cells sensitivity to staurosporine by up-regulating Bcl-2 expression. Archives of Biochemistry and Biophysics. 2009;481(1):116–22. doi: 10.1016/j.abb.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Berezhnaya N, Belova O, Vinnichuk YD, et al. Expression of E-cadherin in drug resistant human breast cancer cells and their sensitivity to lymphokine-activated lymphocytes action. Experimental Oncology. 2009;31(4):242–5. [PubMed] [Google Scholar]
- 13.Zhu Y, Wu J, Ma W, et al. Expression of TGF-β1, Snail, E-cadherin and N-cadherin in Gastric Cancer and Its Significance. Chinese Journal of Clinical Oncology. 2007;4(6):384–9. [Google Scholar]
- 14.Canavese G, Bernardi A, Candelaresi G, et al. Expression of the E-cadherin–catenins complex in sentinel node is related to tumor morphology but not to spread to nonsentinel nodes. Pathol Res Pract. 2007;203(7):517–23. doi: 10.1016/j.prp.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Ramos-VaraJ Technical aspects of immunohistochemistry. Vet Pathol. 2005;42(4):405–26. doi: 10.1354/vp.42-4-405. [DOI] [PubMed] [Google Scholar]
- 16.Ramos-VaraJ , MillerM When Tissue Antigens and Antibodies Get Along Revisiting the Technical Aspects of Immunohistochemistry-The Red,Brown, and Blue Technique. Vet Pathol. 2014;51(1):42–87. doi: 10.1177/0300985813505879. [DOI] [PubMed] [Google Scholar]
- 17.Chu Y-Q, Ye Z-Y, Tao H-Q, et al. Relationship between cell adhesion molecules expression and the biological behavior of gastric carcinoma. World Journal of Gastroenterology: WJG. 2008;14(13):1990. doi: 10.3748/wjg.14.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeVitaJr VT, Rosenberg SA. Two hundred years of cancer research. New England Journal of Medicine. 2012;366(23):2207–14. doi: 10.1056/NEJMra1204479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halpering E, Perez CA, Brady L. Principles and Practice of radiation Oncology. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 20.Anbiaee R, Sheibani KM, Torbati P, et al. Abnormal expression of e-cadherin in gastric adenocarcinoma, and its correlation with tumor histopathology and helicobacter pylori infection. Iranian Red Crescent Medical Journal. 2013;15(3):218. doi: 10.5812/ircmj.4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazăr D, Tăban S, Ardeleanu C, et al. The immunohistochemical expression of E-cadherin in gastric cancer; correlations with clinicopathological factors and patients’ survival. Rom J Morphol Embryol. 2008;49(4):459–67. [PubMed] [Google Scholar]
- 22.Saad AA, Awed NM, Elkerim NNA, et al. Prognostic significance of E-cadherin expression and peripheral blood micrometastasis in gastric carcinoma patients. Annals of Surgical Oncology. 2010;17(11):3059–67. doi: 10.1245/s10434-010-1151-8. [DOI] [PubMed] [Google Scholar]
- 23.Micu G, Staniceanu F, Zurac S, et al. E-cadherin and β-catenin Expression in Gastric Neoplastic and Non-Neoplastic Lesions–Correlations with H. Pylori Infection. Rom J Intern Med. 2010;48(3):271–80. [PubMed] [Google Scholar]