Abstract
Objective
To study the relationship between acute air pollution exposure and cardiovascular events during labour/delivery.
Methods
The Consortium on Safe Labor (2002–2008), an observational US cohort with 223 502 singleton deliveries provided electronic medical records. Air pollution exposure was estimated by modified Community Multiscale Air Quality models. Cardiovascular events (cardiac failure/arrest, stroke, myocardial infarcts and other events) were recorded in the hospital discharge records for 687 pregnancies (0.3%). Logistic regression with generalised estimating equations estimated the relationship between cardiovascular events and daily air pollutant levels for delivery day and the 7 days preceding delivery.
Results
Increased odds of cardiovascular events were observed for each IQR increase in exposure to nitric oxides at 5 and 6 days prior to delivery (OR=1.17, 99% CI 1.04 to 1.30 and OR=1.15, 1.03 to 1.28, respectively). High exposure to toxic air pollution species such as ethylbenzene (OR=1.50, 1.08 to 2.09), m-xylene (OR=1.54, 1.11 to 2.13), o-xylene (OR=1.51, 1.09 to 2.09), p-xylene (OR=1.43, 1.03 to 1.99) and toluene (OR=1.42, 1.02 to 1.97) at 5 days prior to delivery were also associated with cardiovascular events. Decreased odds of events were observed with exposure to ozone.
Conclusions
Air pollution in the days prior to delivery, especially nitrogen oxides and some toxic air pollution species, was associated with increased risk of cardiovascular events during the labour/delivery admission.
INTRODUCTION
Short-term and long-term ambient air pollution exposures account for 20 000–30 000 deaths/year in the general population of the USA and Western Europe.1 Increased risk of non-fatal cardiovascular or cerebrovascular events is seen with exposure to particulate matter with aerodynamic diameter <10 μm (PM10) and <2.5 μm (PM2.5), nitrogen oxide (NOx), ozone, carbon monoxide (CO) and sulfur dioxide (SO2).2–11 Some subpopulations may be particularly sensitive to air pollution exposure, such as the elderly, diabetics and persons with obesity or existing cardiac disease.12,13
Given the physiological changes in pregnancy, including increased oxygen requirements and cardiac output, elevation in plasma volume, changes in vascular resistance and blood pressure, hypercoagulability and higher risk of acute myocardial infarcts,14,15 we hypothesise that air pollution may be associated with increased risk for maternal cardiovascular events during or immediately after labour and delivery.
In this large, multisite study of pregnant women in the USA, we aimed to study the relationship between acute and/or recent air pollution exposure and maternal cardiovascular events during labour and delivery.
METHODS
Study population
The Consortium on Safe Labor (2002–2008) was an observational cohort including 228 562 deliveries (233 736 newborns) at ≥23 weeks’ gestation from 12 centres across the USA.16 Maternal demographic characteristics; medical, reproductive and prenatal history; labour and delivery summaries and discharge diagnoses in International Classification of Diseases, V.9 codes, were extracted from the antenatal and labour and delivery electronic medical records. The institutional review boards of all participating institutions approved the study. No informed consent was required from the subjects and no identifying information remains in the records. Multifetal pregnancies (n=5050) and pregnancies missing exposure information (n=10) were excluded, rendering 223 502 singleton pregnancies available for study.
Exposure assessment
Air pollutant exposures, both criteria pollutants and air toxics, were estimated using a modified version of the 3D multipollutant regional air quality model, the Community Multiscale Air Quality Model, developed by the US Environmental Protection Agency.17 Meteorology inputs obtained from the Weather Research and Forecasting model and the pollutant emissions from the National Emission Inventories were used for the model simulations.18 This model estimated air pollution exposure for each hour at each location based on local reported air pollution emissions and accounted for the effects of weather, complex mixtures and chemical reactions between pollutants on the exposure.17 The 15 hospital referral regions, ranging in size from 415 to 312, 644 km2, were used as a proxy for residence and local mobility.18 Maternal air pollution exposure was estimated based on the hourly air pollutant concentration predictions weighted by population density within the hospital referral region and discounting the places where women were unlikely to live and work. Inverse distance weighting, which places more weight on monitors close to the area of interest, was used to correct for measurement error between modelled and observed exposures of ozone, CO, NOx, SO2, PM10 and PM2.5, with air pollutant measured retrieved from the US Environmental Protection Agency Air Quality System.18 Only modelled ambient concentrations were obtained for major PM2.5 constituents (elemental carbon, organic compounds, ammonium, sulfate and nitrate) and the unspeciated portion of total PM2.5 (dust particles) as well as for our exploratory investigation of air toxics, as these compounds were rarely observed by air quality monitors. The performance of the final model was quite good and there was a significant improvement in the performance over four other exposure strategies evaluated.18
Outcome assessment
Maternal cardiovascular events during the delivery admission were obtained from discharge records. Most discharge diagnoses (76.7%) indicated events occurring as complications of obstetrical procedures or anaesthesia/sedation in labour and delivery or as postpartum complications. The delivery time was used as a proxy for the event time. Our primary outcome was ‘any cardiovascular event’ consisting of diagnoses of ischaemic heart disease, stroke, heart failure, cardiac arrest/failure and other/unspecified cardiovascular events (see online supplementary table S1).
Statistical analyses
The hourly air quality estimates were averaged to obtain individual daily estimates of air quality for delivery day (lag 0, calculated as the 24 h preceding the exact date and time of delivery) and for each day of the week preceding the delivery (lags 1–7 days). Logistic regression with generalised estimating equations and robust SEs calculated the odds and 99% CIs of a cardiovascular event, adjusting for site, maternal age, race/ethnicity, insurance status, smoking during pregnancy and pre-pregnancy body mass index (kg/height in m2). We used an autoregressive correlation matrix to account for correlation between repeat pregnancies to the same woman. As the unadjusted and adjusted estimates were similar, only the adjusted models are presented. We estimated the effect of an IQR increase in the concentration of criteria pollutants and PM2.5 constituents (in μg/m3 for PM10, PM2.5 and PM2.5 constituents and in parts per billion (ppb) for SO2, NOx, ozone and CO) and the effects of high concentrations of air toxics (≥75th centile in ppb) on cardiovascular event odds (see online supplementary tables S2 and S3 for concentrations of air pollutants). Air pollutants were first fitted in the model independently, as the air quality model accounted for the biochemical reactions between species and the effects of weather, other natural phenomena and long-term pollutant sources.17 We also performed multipollutant analyses where the effect of PM10, PM2.5 and its constituents, CO, NOx, ozone and SO2 exposure on cardiovascular events was estimated after adjusting for the other pollutants and/or constituent totals (for correlation between species, see online supplementary table S4).
As sensitivity analyses, we first restricted the analysis to caesarean deliveries to account for risk of events associated with operative delivery and second to obese women to assess potential effect modification in this potentially at-risk subgroup.19 We also simulated the potential impact of an unmeasured confounder to assess the robustness of the findings.20 The odds of specific cardiovascular events were also estimated. All analyses were conducted using PROC GENMOD in SAS V.9.3 (SAS Institute Inc., Cary, North Carolina, USA) and R (V.3.1.0).
RESULTS
There were 687 pregnancies (0.3%) with cardiovascular events among 681 women. Six women had events in more than one pregnancy. Five women died during the delivery admission after an event. Women with cardiovascular events were more often non-Hispanic black and obese, were less likely to have private insurance and were more likely to deliver by caesarean (table 1).
Table 1.
Demographics of pregnancies with and without cardiovascular events
| Demographics | Any cardiovascular event (N=687) | No cardiovascular events (N=228 815) |
|---|---|---|
| Pregnancies (n) | ||
| 1 | 642 (93.5) | 203 347 (91.3) |
| 2 | 43 (6.3) | 18 518 (8.3) |
| ≥3 | 2 (0.3) | 950 (0.4) |
| Mean age±SD* | 29.1±6.8 | 27.6±6.2 |
| Insurance type | ||
| Private | 315 (45.9) | 124 642 (55.9) |
| Public/self-pay | 316 (46.0) | 74 549 (33.5) |
| Other | 2 (0.3) | 317 (0.1) |
| Unknown | 54 (7.9) | 23 307 (10.5) |
| Pre-pregnancy BMI | ||
| Underweight (BMI <18.5 kg/m2) | 24 (3.5) | 7975 (3.6) |
| Normal weight (BMI 18.5–24.9 kg/m2) | 200 (29.1) | 78 909 (35.4) |
| Overweight (BMI 25.0–29.9 kg/m2) | 104 (15.1) | 33 393 (15.0) |
| Obese (BMI 30.0–34.9 kg/m2) | 67 (9.8) | 15 718 (7.1) |
| Severely obese (BMI ≥35.0 kg/m2) | 65 (9.5) | 12 097 (5.4) |
| Unknown | 227 (33.0) | 74 723 (33.5) |
| Caesarean delivery | 515 (75.0) | 62 073 (27.9) |
| Nulliparous | 286 (41.6) | 88 949 (39.9) |
| Race/ethnicity | ||
| Non-Hispanic white | 300 (43.7) | 110 200 (49.5) |
| Non-Hispanic black | 204 (29.7) | 50 056 (22.5) |
| Hispanic | 125 (18.2) | 38 941 (17.5) |
| Asian/Pacific islander | 23 (3.4) | 9187 (4.1) |
| Other | 15 (2.2) | 5281 (2.4) |
| Unknown | 20 (2.9) | 9150 (4.1) |
| Cardiovascular events† | ||
| Ischaemic heart disease | 65 (9.5) | |
| Stroke | 71 (10.3) | |
| Heart failure | 88 (12.8) | |
| Cardiac arrest/failure | 323 (47.0) | |
| Unspecified event | 144 (21.0) | |
| Maternal mortality | 5 (0.7) | 14 (0.01) |
All figures are no. (%) unless otherwise specified.
Missing for 308 pregnancies without cardiovascular events.
The figures add up to more than the number of pregnancies with any cardiovascular event due to having more than one event/pregnancy.
BMI, body mass index.
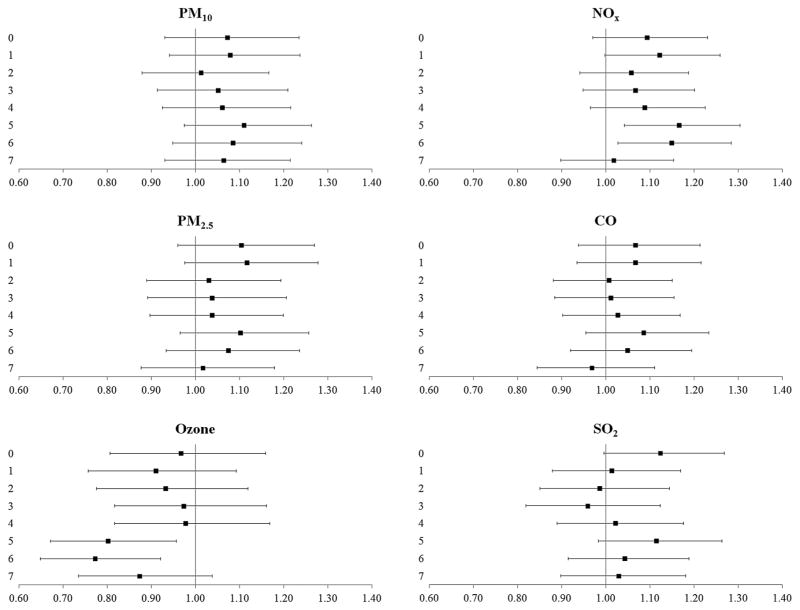
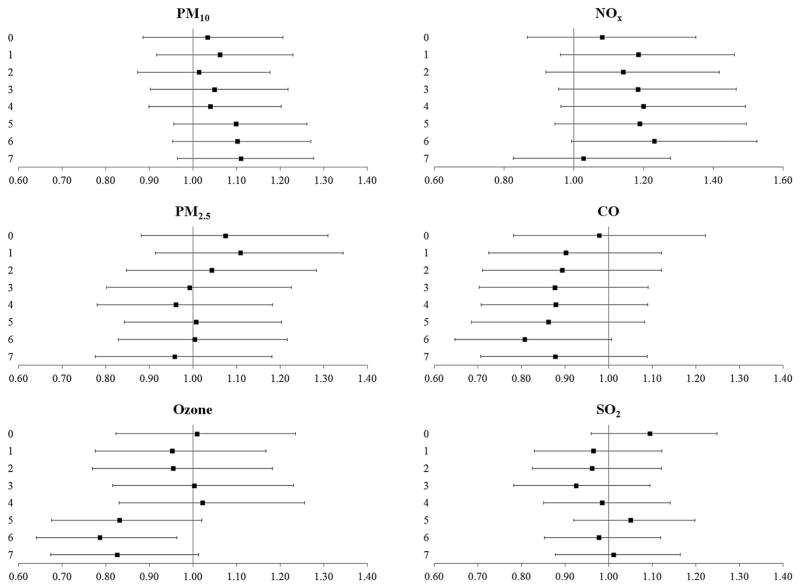
In single-pollutant models, increased odds for cardiovascular events were found with recent (5-day or 6-day lags) exposure to NOx (OR 1.17, 99% CI 1.04 to 1.30 and OR 1.15, 99% CI 1.03 to 1.28 for 5-day and 6-day lags, respectively) (figure 1). These observed odds were attenuated in the multipollutant models (figure 2). Recent exposure to ozone (5-day or 6-day lags) was associated with reduced odds of cardiovascular events in single-pollutant and multipollutant models (figures 1 and 2).
Figure 1.
The odds (99% CIs) of cardiovascular events after exposure to criteria pollutants at delivery day and 1–7 days prior to delivery, single-pollutant models. All results are adjusted for site, maternal age, race/ethnicity, insurance status, smoking during pregnancy and pre-pregnancy body mass index (kg/height in m2). CO, carbon monoxide; NOx, nitrogen oxides; PM10, particulate matter <10 μm in aerodynamic diameter, PM2.5, particulate matter <2.5 μm in aerodynamic diameter, SO2, sulfur dioxide.
Figure 2.
The odds (99% CIs) of cardiovascular events after exposure to criteria pollutants at delivery day and 1–7 days prior to delivery, multipollutant models. All results are adjusted for site, maternal age, race/ethnicity, insurance status, smoking during pregnancy and pre-pregnancy body mass index (kg/height in m2) and additionally, the model for PM10 is adjusted for ozone, CO, NOx and SO2; model for PM2.5 is adjusted for PM10, ozone, CO, NOx and SO2; model for ozone is adjusted for PM10, CO, NOx and SO2; model for CO is adjusted for PM10, ozone, NOx and SO2; model for NOx is adjusted for PM10, ozone, CO and SO2 and model for SO2 is adjusted for PM10, ozone, CO and NOx. CO, carbon monoxide; NOx, nitrogen oxides; PM10, particulate matter <10 μm in aerodynamic diameter; PM2.5, particulate matter <2.5 μm in aerodynamic diameter; SO2, sulfur dioxide.
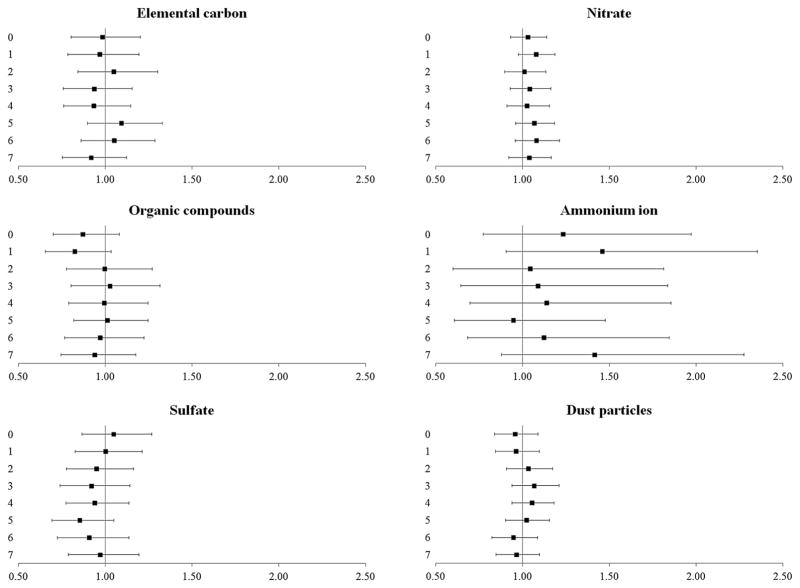
Exposure to PM2.5 constituents was not statistically significantly associated with cardiovascular events, although acute exposure (1-day lag) to ammonium particles was associated with OR 1.46, 99% CI 0.90 to 2.35 for cardiovascular events (figure 3).
Figure 3.
The odds (99% CIs) of cardiovascular events after exposure to PM2.5 constituents at delivery day and 1–7 days prior to delivery. All results are adjusted for site, maternal age, race/ethnicity, insurance status, smoking during pregnancy, pre-pregnancy body mass index (kg/height in m2) and total PM2.5 concentration. PM2.5, particulate matter <2.5 μm in aerodynamic diameter.
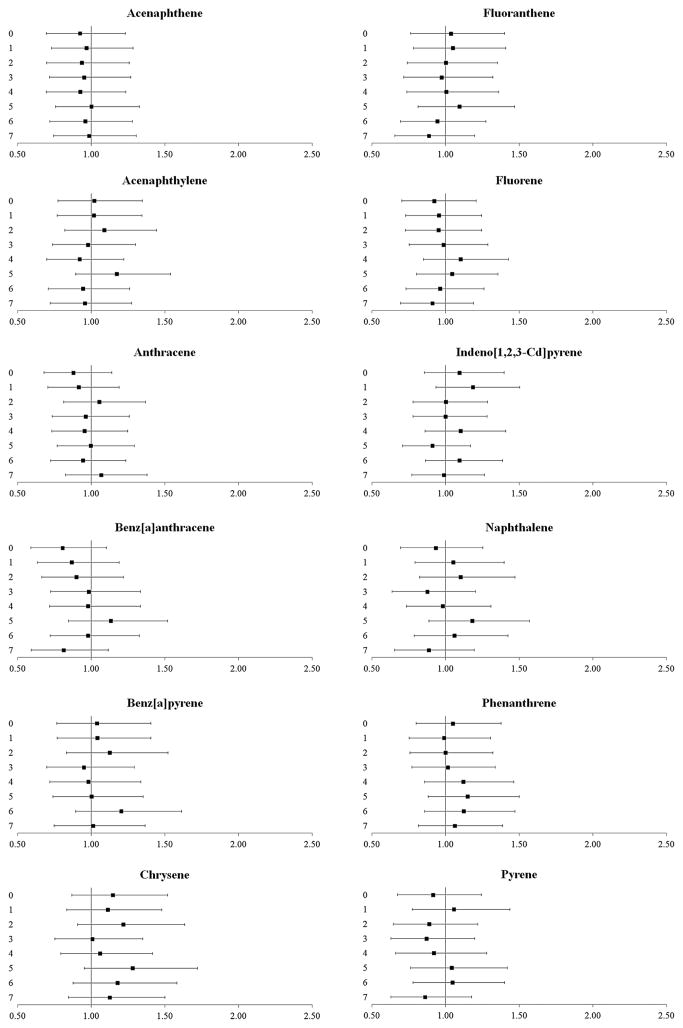
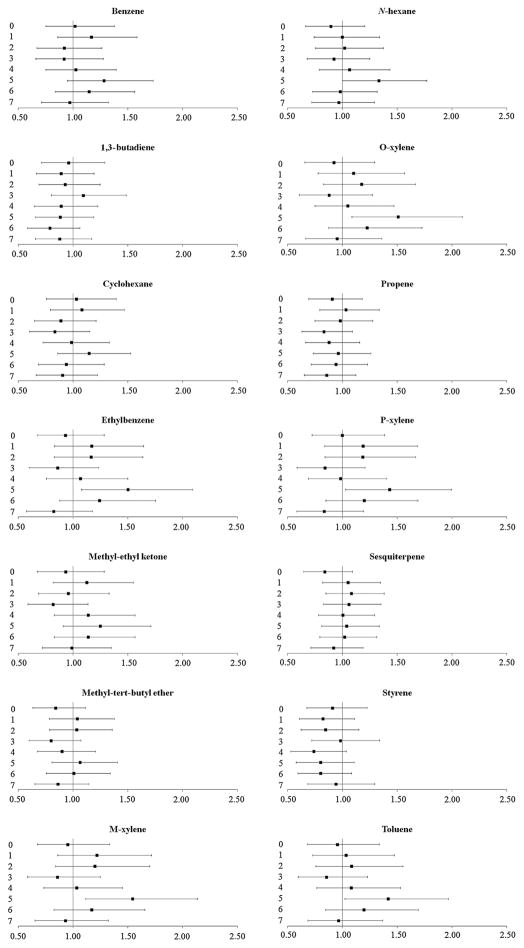
Exposure to polycyclic aromatic hydrocarbons was not associated with cardiovascular events (figure 4). However, recent exposure (5-day lag) to high ambient concentrations of some volatile organic compounds, specifically, exposure to ethylbenzene (OR 1.50, 99% CI 1.08 to 2.09), m-xylene (OR 1.54, 99% CI 1.11 to 2.13), o-xylene (OR 1.51, 99% CI 1.09 to 2.09), p-xylene (OR 1.43, 99% CI 1.03 to 1.99) and toluene (OR 1.42, 99% CI 1.02 to 1.97), was associated with increased odds of cardiovascular events (figure 5).
Figure 4.
The odds (99% CIs) of cardiovascular events after exposure to polycyclic aromatic hydrocarbons at delivery day and 1–7 days prior to delivery. All results are adjusted for site, maternal age, race/ethnicity, insurance status, smoking during pregnancy and pre-pregnancy body mass index (kg/height in m2).
Figure 5.
The odds (99% CIs) of cardiovascular events after exposure to volatile organic compounds at delivery day and 1–7 days prior to delivery. All results are adjusted for site, maternal age, race/ethnicity, insurance status, smoking during pregnancy and pre-pregnancy body mass index (kg/height in m2).
Stratified analyses and sensitivity analyses
In our stratified analyses by specific events, we observed a similar pattern of increased risk of cardiovascular events as in our main analysis, with some loss of precision due to a smaller number of cases. Specifically, we observed increased odds for cardiac arrest/failure and heart failure with acute and recent air pollution exposures. The results were also similar to those of main analyses when limiting data to deliveries by caesarean and to obese women.
Our sensitivity analysis estimating the robustness of our findings shows that with a small association between the unmeasured confounder and the exposure our findings are generally robust (see online supplementary table S5). However, a confounder with a prevalence of ≤10% among those with events and ≤5% among those without events associating with ≥30% odds of exposure could explain our observed findings. Similarly, a more prevalent unmeasured confounder (prevalence ≥20%) associating with ≥10% odds of exposure could explain our findings if the prevalence of the unmeasured confounder was lower among those without an event. However, we expect a relatively weak correlation between the exposure and subject-level confounders, as, for example, the odds between smoking and NOx was <1% in our data.
DISCUSSION
In this large, contemporary study of labour and delivery, we found an association between increased odds of maternal cardiovascular events and exposure to NOx and several air toxics after adjusting for multiple testing using a 99% CI. We also found elevated associations between recent exposure to SO2, PM2.5 and ammonium ions and cardiovascular events that were non-significant at the 99% CI that suggest potential additional avenues for future research. Pollutants increased risk of cardiovascular events perhaps by initiating a cascade of events related to inflammation, hypertension or other factors that eventually lead to a cardiovascular event. We also found an association between decreased odds of maternal cardiovascular events and exposure to ozone. While prior studies have found increased risk of cardiac events associated with air pollutants in the general population, to our knowledge, ours was the first study to examine the risk of cardiac events among pregnant women at labour/delivery.
Our findings are generally in accordance with previous studies among non-pregnant adults.2–11 Our results were most robust for NOx, which remained associated with cardiovascular event risk even after accounting for unmeasured confounders and even showed some association after adjusting for other pollutants. However, we note that while performing multipollutant analyses is common practice within environmental epidemiology, the results should be interpreted cautiously, as the interpretation requires holding all other pollutants fixed, which may not be realistic for teasing out the ‘independent’ pollutant effect.
Our observed association between air toxics, especially volatile organic compounds and cardiovascular event risk during labour/delivery is novel and shows that even low ambient levels of hazardous air pollutants can have an adverse effect. Long-term or occupational exposure to n-hexane, styrene and benz[a]pyrene is associated with increased cardiovascular disease mortality,21–23 but the acute effects of gaseous ambient air toxics have not been previously studied in general or pregnant populations. However, our exploratory findings from modelled exposure data merit further investigation.
Air pollution exposure may relate to increased cardiovascular events by altering heart rate variability, fibrinogen, blood viscosity, inflammation and coagulation factors.24–26 Acutely increased risks of cardiovascular events associated with air pollution exposure might be due to alterations in cardiac autonomic tone and increase in arrhythmias,6,24 whereas increased risk after exposure with longer lag times might be due to changes in inflammation and coagulation.25,26 Pregnancy itself poses an increased risk of cardiovascular events due to physiological increases in plasma volume, coagulation and cardiac output.14 Labour and delivery may further exacerbate this risk due to acute increase in cardiac output and blood pressure and due to increased risk of tachycardia and/or hypotension during anaesthesia and caesarean section.14 Despite these plausible biological pathways between air pollutants and cardiac function that might underlie the observed associations, the exact mechanism by which cardiac events in labour/delivery could be related to air pollution exposure is not known. The exposures we studied were complex mixtures and the mechanisms leading to disease or cardiac events are likely to be multifactorial.13
Exposure to ozone was associated with protective effects for cardiovascular events, similar to some previous studies in non-pregnant adults,5,10 but contradicting other.7 The protective effects of ozone in our study might be attributed to its negative association with other pollutants that are more strongly associated with adverse events.
Modelled air pollution exposure data allowed us to obtain air pollution estimates for our full cohort during all time periods of interest. Previous studies have generally relied on monitored air pollution data, which may induce selection bias, as only populations living close to the monitors are included. The model used to estimate air pollution exposure in our study accounts for population density, some spatial distribution and temporal distribution of air pollutants and changes in weather and its effect on the chemical reactions between pollutants.17 We also improved the reliability of our modelled air pollution estimates by accounting for the observed monitor air quality data.18 We also note that if our results were due to a triggering of delivery itself and not cardiac events, we would anticipate the number of deliveries would change in relation to exposure but not the rate of events. In addition, we evaluated the impact of an unmeasured confounder and observed that under many plausible scenarios, our findings remain. As a limitation, our estimates on air pollution exposure were averaged over hospital referral region rather than maternal residence. However, we believe that this approach has provided us with a good estimate of maternal exposure since it accounts for some local mobility over the course of the week preceding delivery. Similar limitations occur in studies using monitored data or land-use regression models on air pollution. Since ambient exposure estimates are often lower than personal exposures, our results can be even considered to be conservative. We did not consider differences in ambient temperatures or non-linear effects that might be important over time,27 which could be explored in studies with more detailed exposure assessment. We also used time of delivery as a proxy for event time. Mistiming of the event could also have affected our exposure lag assessment. As most cardiac events occur around labour and delivery or immediately postpartum,15 and since nearly 80% of all cardiovascular events in our study were linked to labour and delivery using discharge diagnoses codes, we believe the error in outcome and exposure lag timing to be small. We were not able to assess risk for cardiac events outside of the delivery admission in our data. We also performed a large number of statistical tests and adjusted for multiple comparisons by calculating conservative 99% CIs for our OR estimates. Further studies are needed to verify our findings of borderline statistical significance.
We also note that major cardiovascular events during labour and delivery admission are rare, affecting only 308 per 100 000 singleton pregnancies in our large study cohort. However, cardiovascular conditions account for at least 12% of pregnancy-related deaths and their relative contribution to maternal deaths is increasing.28 If air pollution can cause increased maternal cardiovascular events, the attributable risk of cardiovascular events would be approximately 100 events/1 million births based on our observational cohort. Limiting air pollution might prevent cardiovascular events and have an effect on one of the main sources of maternal mortality and morbidity. Globally, the burden of air pollution exposure on cardiovascular event risk is even greater as ambient air pollution levels are low in the USA compared with developing countries.1
In conclusion, we observed an increased risk of cardiovascular events at labour and delivery after exposure to NOx and air toxics in a large contemporary cohort with detailed clinical data. These novel findings should stimulate further work on the vulnerability of pregnant women to air pollution, including investigations of cardiac events prior to delivery as well as confirmatory studies of the delivery admission. Air pollution control could be helpful in preventing acute cardiovascular events at labour and delivery. This analysis provides support for considering pregnant women an at-risk population for adverse effects related to air pollution.
Supplementary Material
Key messages.
What is already known on this subject?
Acute exposure to air pollution is associated with cardiac mortality and morbidity, but these population-level effects have primarily been seen in elderly persons or those with chronic disease.
What might this study add?
Cardiac events at labour and delivery are rare and unstudied with respect to air pollution. We observed cardiac event risk in this obstetric population that was similar in magnitude to other susceptible populations.
How might this impact clinical practice?
This previously unrecognised relationship is another indicator that air pollution exposure is a potentially modifiable factor underlying cardiac event risk. Pregnant women should be considered a vulnerable population for cardiac events after acute air pollutant exposure.
Acknowledgments
This work and the Consortium on Safe Labor (Contract No. HHSN2672006034 25C) and the Air Quality and Reproductive Health study (Contract No. HHSN275200800002I, Task Order No. HHSN27500008) were supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Institutions involved in the Consortium include: Baystate Medical Center, Springfield, Massachusetts, USA; Cedars-Sinai Medical Center Burnes Allen Research Center, Los Angeles, California, USA; Christiana Care Health System, Newark, Delaware, USA; Georgetown University Hospital, MedStar Health, Washington, District of Columbia, USA; Indiana University Clarian Health, Indianapolis, Indiana, USA; Intermountain Healthcare and the University of Utah, Salt Lake City, Utah, USA; Maimonides Medical Center, Brooklyn, New York, USA; MetroHealth Medical Center, Cleveland, Ohio, USA; Summa Health System, Akron City Hospital, Akron, Ohio, USA; The EMMES Corporation, Rockville, Maryland, USA (Data Coordinating Center); University of Illinois at Chicago, Chicago, Illinois, USA; University of Miami, Miami, Florida, USA and University of Texas Health Science Center at Houston, Houston, Texas, USA. The authors acknowledge the Texas A&M Supercomputing Facility (http://sc.tamu.edu) and the Texas Advanced Computing Center (http://www.tacc.utexas.edu/) for providing computing resources for the air quality simulations. This study used the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health, Bethesda, Maryland, USA (http://biowulf.nih.gov).
Funding National Institutes of Health.
Footnotes
Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/heartjnl-2014-307366).
Contributors All authors have made substantial contributions to the conception or design of the work, or the acquisition, analysis or interpretation of data. TM and PM drafted the work and all authors revised it critically for important intellectual content. All authors have approved the final version published. TM and PM are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests None declared.
Ethics approval All institutional review boards of the participating institutions.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Please see http://grants.nih.gov/grants/policy/data_sharing/ for National Institutes of Health data sharing policy.
References
- 1.Ostro B. Outdoor air pollution: assessing the environmental burden of disease at national and local levels. Geneva: World Health Organization; 2004. [Google Scholar]
- 2.Raza A, Bellander T, Bero-Bedada G, et al. Short-term effects of air pollution on out-of-hospital cardiac arrest in Stockholm. Eur Heart J. 2014;35:861–8. doi: 10.1093/eurheartj/eht489. [DOI] [PubMed] [Google Scholar]
- 3.Peters A, Dockery DW, Muller JE, et al. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–15. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 4.Peng RD, Bell ML, Geyh AS, et al. Emergency admissions for cardiovascular and respiratory diseases and the chemical composition of fine particle air pollution. Environ Health Perspect. 2009;117:957–63. doi: 10.1289/ehp.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metzger KB, Tolbert PE, Klein M, et al. Ambient air pollution and cardiovascular emergency department visits. Epidemiology. 2004;15:46–56. doi: 10.1097/01.EDE.0000101748.28283.97. [DOI] [PubMed] [Google Scholar]
- 6.Ljungman PL, Berglind N, Holmgren C, et al. Rapid effects of air pollution on ventricular arrhythmias. Eur Heart J. 2008;29:2894–901. doi: 10.1093/eurheartj/ehn463. [DOI] [PubMed] [Google Scholar]
- 7.Hong YC, Lee JT, Kim H, et al. Effects of air pollutants on acute stroke mortality. Environ Health Perspect. 2002;110:187–91. doi: 10.1289/ehp.02110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ensor KB, Raun LH, Persse D. A case-crossover analysis of out-of-hospital cardiac arrest and air pollution. Circulation. 2013;127:1192–9. doi: 10.1161/CIRCULATIONAHA.113.000027. [DOI] [PubMed] [Google Scholar]
- 9.Chan CC, Chuang KJ, Chien LC, et al. Urban air pollution and emergency admissions for cerebrovascular diseases in Taipei, Taiwan. Eur Heart J. 2006;27:1238–44. doi: 10.1093/eurheartj/ehi835. [DOI] [PubMed] [Google Scholar]
- 10.Bhaskaran K, Hajat S, Armstrong B, et al. The effects of hourly differences in air pollution on the risk of myocardial infarction: case crossover analysis of the MINAP database. BMJ. 2011;343:d5531. doi: 10.1136/bmj.d5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turin TC, Kita Y, Rumana N, et al. Ambient air pollutants and acute case-fatality of cerebro-cardiovascular events: Takashima Stroke and AMI Registry, Japan (1988–2004) Cerebrovasc Dis. 2012;34:130–9. doi: 10.1159/000339680. [DOI] [PubMed] [Google Scholar]
- 12.Martinelli N, Olivieri O, Girelli D. Air particulate matter and cardiovascular disease: a narrative review. Eur J Intern Med. 2013;24:295–302. doi: 10.1016/j.ejim.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Routledge HC, Ayres JG, Townend JN. Why cardiologists should be interested in air pollution. Heart. 2003;89:1383–8. doi: 10.1136/heart.89.12.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiol Clin. 2012;30:317–29. doi: 10.1016/j.ccl.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Ladner HE, Danielsen B, Gilbert WM. Acute myocardial infarction in pregnancy and the puerperium: a population-based study. Obstet Gynecol. 2005;105:480–4. doi: 10.1097/01.AOG.0000151998.50852.31. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Troendle J, Reddy UM, et al. Contemporary cesarean delivery practice in the United States. Am J Obstet Gynecol. 2010;203:326e1–10. doi: 10.1016/j.ajog.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foley KM, Roselle SJ, Appel KW, et al. Incremental testing of the Community Multiscale Air Quality (CMAQ) modeling system version 4. 7. Geosci Model Develop. 2010;3:205–26. doi: 10.5194/gmd-10-1703-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen G, Li J, Ying Q, et al. Evaluation of observation-fused regional air quality model results for population air pollution exposure estimation. Sci Total Environ. 2014;485–486:563–74. doi: 10.1016/j.scitotenv.2014.03.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weichenthal S, Hoppin JA, Reeves F. Obesity and the cardiovascular health effects of fine particulate air pollution. Obesity. 2014;22:1580–9. doi: 10.1002/oby.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groenwold RH, Nelson DB, Nichol KL, et al. Sensitivity analyses to estimate the potential impact of unmeasured confounding in causal research. Int J Epidemiol. 2010;39:107–17. doi: 10.1093/ije/dyp332. [DOI] [PubMed] [Google Scholar]
- 21.Burstyn I, Kromhout H, Partanen T, et al. Polycyclic aromatic hydrocarbons and fatal ischemic heart disease. Epidemiology. 2005;16:744–50. doi: 10.1097/01.ede.0000181310.65043.2f. [DOI] [PubMed] [Google Scholar]
- 22.Matanoski GM, Tao X. Case-cohort study of styrene exposure and ischemic heart disease. Res Rep Health Eff Inst. 2002;108:1–29. [PubMed] [Google Scholar]
- 23.Villeneuve PJ, Jerrett M, Su J, et al. A cohort study of intra-urban variations in volatile organic compounds and mortality, Toronto, Canada. Environ Pollut. 2013;183:30–9. doi: 10.1016/j.envpol.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 24.Luttmann-Gibson H, Suh HH, Coull BA, et al. Systemic inflammation, heart rate variability and air pollution in a cohort of senior adults. Occup Environ Med. 2010;67:625–30. doi: 10.1136/oem.2009.050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters A, Doring A, Wichmann HE, et al. Increased plasma viscosity during an air pollution episode: a link to mortality? Lancet. 1997;349:1582–7. doi: 10.1016/S0140-6736(97)01211-7. [DOI] [PubMed] [Google Scholar]
- 26.Wu S, Deng F, Wei H, et al. Chemical constituents of ambient particulate air pollution and biomarkers of inflammation, coagulation and homocysteine in healthy adults: a prospective panel study. Part Fibre Toxicol. 2012;9:49. doi: 10.1186/1743-8977-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg MS, Burnett RT, Steib DM, et al. Associations between ambient air pollution and daily mortality among elderly persons in Montreal, Quebec. Sci Total Environ. 2013;463–464:931–42. doi: 10.1016/j.scitotenv.2013.06.095. [DOI] [PubMed] [Google Scholar]
- 28.Berg CJ, Callaghan WM, Henderson Z, et al. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116:1302–9. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.