Abstract
The benefit of adjuvant trastuzumab with chemotherapy in breast cancer is well-established; however, its impact on outcomes for older women with small, node-negative, human epidermal growth factor receptor 2-positive disease is less clear and unlikely to be addressed in prospective studies. This retrospective, sequential cohort study suggests adjuvant trastuzumab with chemotherapy results in excellent breast cancer outcomes with few cardiac events in this population.
Introduction
The benefit of adjuvant trastuzumab with chemotherapy is well established for women with higher risk human epidermal growth factor receptor 2-positive (HER2+) breast cancer. However, its role in older patients with smaller, node-negative tumors is less clear. We conducted a retrospective, sequential cohort study of this population to describe the impact of trastuzumab on breast cancer outcomes and cardiac safety.
Patients and Methods
Women ≤ 55 years with ≥ 2 cm, node-negative, HER2+ breast cancer were identified and electronic medical records reviewed. A no-trastuzumab cohort of 116 women diagnosed between January 1, 1999 and May 14, 2004 and a trastuzumab cohort of 128 women diagnosed between May 16, 2006 and December 31, 2010 were identified. Overall survival and distant relapse-free survival were estimated by Kaplan-Meier methods.
Results
The median ages of the trastuzumab and no-trastuzumab cohorts were 62 and 64 years, respectively. More patients in the trastuzumab cohort had grade III (P = .001), lymphovascular invasion (P = .001), or estrogen receptor-negative (P < .001) cancers. The majority of the trastuzumab cohort received chemotherapy versus one-half of the no-trastuzumab cohort (98% vs. 53%; P < .0001). The median follow-up was 4 versus 9 years in the trastuzumab versus no-trastuzumab cohorts; therefore, outcomes at 4 years are reported. Despite the higher-risk tumor features in the trastuzumab group, the 4-year overall survival was 99% in both cohorts; the distant relapse-free survival was 99% versus 97% in the trastuzumab versus no-trastuzumab cohorts. Four (3.1 %; 95% confidence interval, 1.0%–7.8%) women in the trastuzumab cohort and 1 in the no-trastuzumab cohort developed symptomatic heart failure. There were no cardiac-related deaths in either arm.
Conclusion
Following adjuvant trastuzumab with chemotherapy, selected older women with small, node-negative, HER2+ breast cancers have excellent disease control. The rate of cardiac events is low.
Keywords: Breast cancer, Cardiac outcomes, Chemotherapy, HER2 positive, Trastuzumab
Introduction
In breast cancer, gene amplification or protein overexpression of human epidermal growth factor receptor 2 (HER2) is associated with an aggressive biologic phenotype, which confers a significant risk of distant recurrence, even in the setting of lymph node-negative disease.1 However, this risk has been largely ameliorated by the successful development of the anti-HER2 monoclonal antibody trastuzumab.2 Specifically, when administered in combination with adjuvant chemotherapy in multiple adequately powered, prospectively conducted randomized studies, trastuzumab dramatically improved disease-specific outcomes for women with HER2-positive (HER2+) disease.3–6 In these pivotal studies, however, older women and those with lower-risk, node-negative disease, were underrepresented. Subsequent studies have since indicated that both populations appear to derive benefit from adjuvant trastuzumab with chemotherapy,7–9 although the benefits in older women may be offset by increased treatment-related hospital admission rates7 and higher rates of trastuzumab-mediated cardiotoxicity.10–13
We have previously reported 2 retrospective, single-institution studies demonstrating the potential benefit of adjuvant trastuzumab-based therapy in women with small, node-negative breast cancers.9,14 Other investigators have since demonstrated consistent results.15,16 However, 65% of new breast cancer diagnoses occur in patients over 55 years of age,17 and the risk of cardiotoxicity from trastuzumab increases above 50 years of age.18,19 Furthermore, patients (including those with larger tumors and node-positive disease) in the meta-analysis of the pivotal III trials of adjuvant trastuzumab had a median age of 49 years.2 Hence, the risks and benefits of trastuzumab with chemotherapy in older women with node-negative HER2+ disease have not been fully defined. Although there is no single definition of “older” patients, we focused on women aged ≥ 55 years and conducted a retrospective, single-institution, sequential cohort study of patients with small, node-negative, HER2+ breast cancer who did or did not receive adjuvant trastuzumab.
Patients and Methods
Patients treated at Memorial Sloan Kettering Cancer Center (MSKCC) between January 1, 1999 and December 31, 2010 were identified through an institutional database, and electronic medical records were reviewed via an MSKCC institutional review board-approved waiver of informed consent. Women were included in this study if they met the following criteria: age ≥ 55 years, pathologically confirmed invasive breast cancer ≤ 2 cm without lymph node involvement (T1N0), HER2+ disease defined as 3+ by immunohistochemistry and/or gene amplification (≥ 2) by fluorescence in situ hybridization (FISH).
As previously described,9,14 patients were identified as members of the no-trastuzumab or trastuzumab cohorts by use of adjuvant trastuzumab therapy, which was associated with the time of their initial diagnosis and treatment. Specifically, women treated before versus after the reporting of the first 2 pivotal phase III adjuvant trastuzumab trials in May 200520,21 were identified. Given the potential for variable clinical practice patterns in the immediate period after the May 2005 adjuvant trastuzumab clinical trials were reported, women treated between May 15, 2004 and May 15, 2005, who may have been offered delayed trastuzumab, were excluded. Hence, 2 cohorts of women were identified: those diagnosed between January 1, 1999 and May 15, 2004 (no trastuzumab) and those diagnosed between May 15, 2005 and December 31, 2010 (trastuzumab).
In most of the phase III studies, the planned duration of adjuvant trastuzumab was 1 year. However, because some data suggested a comparable benefit for a relatively short course of trastuzumab (≤ 9 weeks) therapy, the duration of trastuzumab administration was not used to define eligibility for the current study.4,22 Only women from the trastuzumab era who did not receive any anti-HER2 therapy were excluded. Other exclusion criteria, applicable to both cohorts, included current bilateral invasive breast cancer, a prior history of invasive breast cancer, or inadequate documentation of locoregional or systemic therapy. Electronic medical records were reviewed, and patient and tumor characteristics were recorded for all eligible patients. In addition, data on adjuvant therapy, including side of radiation, chemotherapy, and hormonal therapy, were collected.
Breast Cancer Outcomes
Breast cancer-specific outcomes were evaluated by administration of trastuzumab versus no administration of trastuzumab. Overall survival (OS) was defined as time from breast cancer diagnosis to date of death. Surviving patients were censored at the time of the most recent direct communication with MSKCC. Distant relapse-free survival (DRFS), distant metastatic disease or death from any cause, and ipsilateral/contralateral invasive breast cancers were defined from date of diagnosis to event as per Standardized Definitions for Efficacy End Points in Adjuvant Breast Cancer Trials (STEEP) criteria.23 Patients who did not have a recurrence event were censored at the date of the most recent assessment by relevant site-specific imaging or physical examination by a an MSKCC physician or nurse practitioner. OS and DRFS were analyzed using the Kaplan-Meier method, and 4-year estimates with 95% confidence intervals (CIs) were evaluated. The 4-year time point was chosen for our primary evaluation because this is the median follow-up for women in the trastuzumab group (the group with the shorter follow-up). No formal hypothesis testing is reported because of the low number of observed events in this low-risk patient population. Patient demographics and disease characteristics were tested by trastuzumab status using the χ2 test or two-sample t test. Given the relatively small numbers of patients who developed ipsilateral and contralateral breast cancer, these events are reported descriptively.
Cardiac Outcomes
Electronic medical records were used to collect baseline cardiac risk factor data from time of breast cancer diagnosis, including smoking history, prior cardiac or cerebrovascular events, hypertension, hyperlipidemia, atrial fibrillation, diabetes mellitus, renal function, and body mass index. The occurrence of any cardiac event, defined as symptomatic heart failure or decline in left ventricular ejection fraction from time of diagnosis to most recent follow-up, was recorded. Attribution of heart failure was determined only if the patient experienced relevant symptoms as outlined by American College of Cardiology Foundation/American Heart Association Practice Guidelines.24 Assessment of left ventricular ejection fraction occurred approximately every 3 months as per clinical practice at our institution at that time. Due to their relative rarity, cardiac outcomes were compared by trastuzumab status using the Fisher exact test or Wilcoxon rank sum test. All analyses were conducted in SAS v 9.4 and R version 3.1.1.
Results
Of the 244 eligible patients identified, 116 (48%) and 128 (52%) women were in the no-trastuzumab and trastuzumab cohorts, respectively. The median age of the trastuzumab and notrastuzumab cohorts was 62 years (range, 55–87 years) and 64 years (range, 55–87 years), respectively. The median follow-up was 9 years (range, 0.2–14 years) for the no-trastuzumab cohort and 4.1 years (range, 0.4–8 years) for the trastuzumab cohort. Patient characteristics were well-balanced between the 2 cohorts for age, tumor size, histology, surgery, and left-sided adjuvant radiotherapy (Table 1). However, a higher proportion of women in the trastuzumab cohort had grade III tumors (P = .001) and lymphovascular invasion (P = .001). In addition, 43% of the tumors in the trastuzumab cohort were estrogen receptor-negative, compared with 33% of the tumors in the no-trastuzumab cohort (P < .001). Almost all patients in the trastuzumab cohort (98%) received chemotherapy compared with only one-half of the patients (53%) in the no-trastuzumab cohort (P < .0001).
Table 1.
Baseline Patient and Tumor Characteristics
| No Trastuzumab (n = 116) |
Trastuzumab (n = 128) |
P Value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Median age, years (range) | 64 (55–87) | 62 (55–87) | .13 |
| 55–60 years | 40 (34) | 50 (39) | |
| 60–70 years | 46 (40) | 56 (44) | |
| >70 years | 30 (26) | 22 (17) | |
| Tumor size | |||
| Median, cm (range) | 1.1 (0.1–2.0) | 1.2 (0.25–2.0) | .63 |
| T1a | 29 (25) | 12 (9) | |
| T1b | 23 (20) | 34 (26) | |
| T1c | 64 (55) | 82 (64) | |
| Multifocal | 21 (18) | 19 (15) | .49 |
| Histology | |||
| Ductal | 106 (91) | 120 (94) | |
| Lobular | 5 (4) | 4 (3) | |
| Other, including mixed | 5 (4) | 4 (3) | |
| Grade | .001 | ||
| 1 | 4 (3) | 0 (0) | |
| 2 | 25 (22) | 12 (9) | |
| 3 | 77 (66) | 112 (88) | |
| Unknown | 10 (9) | 4 (3) | |
| Lymphovascular invasion | |||
| Present | 9 (8) | 30 (23) | .001 |
| Absent | 100 (86) | 93 (73) | |
| Unknown | 7 (6) | 5 (4) | |
| Estrogen receptor status | |||
| Positive | 77 (66) | 75 (59) | <.001 |
| Negative | 38 (33) | 53 (41) | |
| Unknown | 1 (1) | 0 (0) | |
| Surgery | |||
| Breast conserving | 78 (67) | 80 (63) | |
| Mastectomy | 38 (33) | 48 (38) | |
| Adjuvant radiation | 79 (68) | 77 (60) | .16 |
| Left-side radiation | 33 (28) | 37 (29) | |
| Adjuvant hormonal therapy | 77 (66) | 69 (54) | .0175 |
| Adjuvant chemotherapy | 61 (53) | 126 (98) | <.0001 |
| Anthracycline/no taxane | 46 (40) | 9 (7) | |
| Taxane/no anthracycline | 0 (0) | 64 (50) | |
| Anthracycline and taxane | 1 (1) | 43 (34) | |
| Other | 14 (12) | 10 (8) |
Grade 2–3, classified as 3.
Note: Percentages may not add to 100 due to rounding.
Breast Cancer Outcomes
Four-year OS was 99% (95% CI, 94%–100%) and 99% (95% CI, 95%–100%) in the no-trastuzumab and trastuzumab cohorts, respectively (Figure 1, Table 2). At 4 years, DRFS was 97% (95% CI, 92%–99%) in the no trastuzumab and 99% (95% CI, 95%–100%) in the trastuzumab cohort (Figure 2, Table 2). Invasive ipsilateral and contralateral breast cancers were similar in both groups at a median of 4 years of follow-up (Table 2).
Figure 1. Overall Survival Per Cohort.
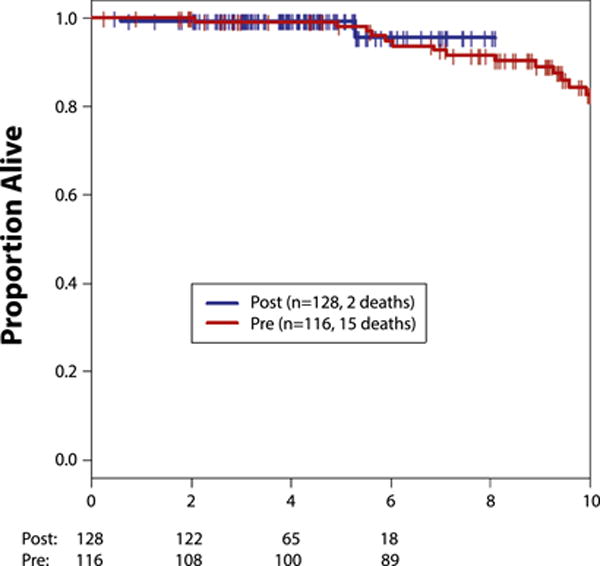
Abbreviations: Post = trastuzumab cohort; Pre = no-trastuzumab cohort.
Table 2.
Outcomes, Per Cohort
| 4-Year Outcomes | No Trastuzumab (n = 116) |
Trastuzumab (n = 128) |
|---|---|---|
| % (95% CI) | % (95% CI) | |
| Distant relapse-free survival | 97 (92–99) | 99 (95–100) |
| Overall survival | 99 (94–100) | 99 (95–100) |
| Ipsilateral invasive breast cancer recurrence | 3 (0.3–5.5) | 2 (0.2–5.5) |
| Contralateral invasive breast cancer | 1 (1) | 0 |
| Second primary invasive cancer (non-breast) | 3 (0.13–6.77) | 2 (−0.4 to 4.4) |
Abbreviation: CI = confidence interval.
Figure 2. Distant Relapse-Free Survival Per Cohort.
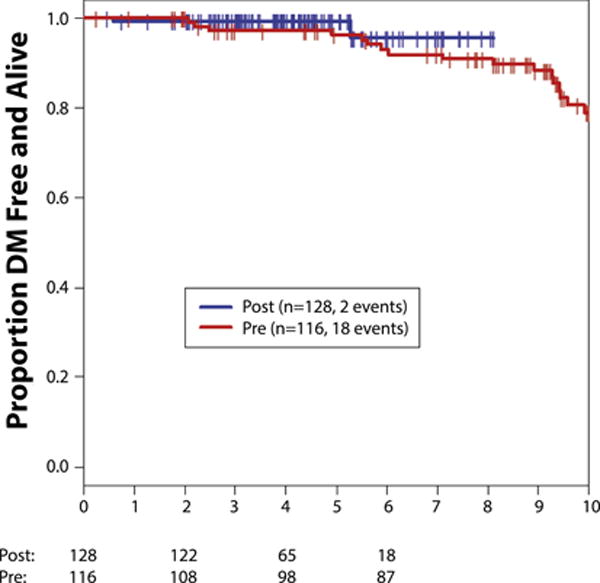
Abbreviations: Post = trastuzumab cohort; Pre = no-trastuzumab cohort.
Cardiac Outcomes
Baseline cardiac risk factors were generally balanced between the 2 cohorts (Table 3). However, more patients in the trastuzumab cohort had diabetes mellitus compared with the no-trastuzumab cohort (16% vs. 7%; P = .03). Available assessments suggested that left ventricular ejection fraction declines were rare in the trastuzumab cohort. At median follow-up of 4 years, 1 patient (1%) in the no-trastuzumab and 4 (3.1%; 95% CI, 1.0%–7.8%) patients in the trastuzumab cohort developed congestive heart failure (CHF).
Table 3.
Cardiac Risk Factors
| No Trastuzumab (n = 116) |
Trastuzumab (n = 128) |
P Value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Baseline LVEF, median | 67% | 68% | |
| BMI, kg/m2 median (range) | 25.5 (16.9–42.7) | 27.3 (18.2–57.5) | .17 |
| Creatinine clearance, mL/min median (range) | 68.4 (32.2–156) | 64.9 (28.4–146.2) | .11 |
| Ever smoker | 52 (45) | 61 (48) | .7 |
| Hypertension | 57 (49) | 57 (45) | .47 |
| Hyperlipidemia | 35 (30) | 54 (42) | .05 |
| Atrial fibrillation | 5 (4) | 4 (3) | .62 |
| Diabetes mellitus | 8 (7) | 20 (16) | .03 |
| Prior myocardial infarct reported | 2 (2) | 3 (2) | .73 |
| Prior cerebrovascular event reported | 3 (3) | 3 (2) | .90 |
| Prior history HF reported | 2 (2) | 3 (2) | .73 |
Abbreviations: BMI = body mass index; HF = heart failure; LVEF = left ventricular ejection fraction.
For the patient who experienced CHF in the no-trastuzumab cohort, the event occurred in the immediate period following definitive breast cancer surgery and prior to any planned adjuvant therapy. In the trastuzumab cohort, 4 (4%) patients developed CHF at 56, 65, 67, and 77 years of age. Events in this cohort occurred at 6 to 35 months after surgery. Of these, 2 patients received an anthracycline-containing chemotherapy, with heart failure events occurring at 6 months and 16 months post-surgery, respectively (Table 4).
Table 4.
Heart Failure Events
| Patient Cohort | Age | Months Post Surgery | Anthracycline | Prior HF | Cardiac Risk Factors |
|---|---|---|---|---|---|
| No Trastuzumab | 80 | 1 | No | Yes | Hypertension Myocardial infarct Coronary artery bypass graft |
| Trastuzumab | 56 | 6 | Yes | No | Prior smoking history |
| Trastuzumab | 65 | 16 | Yes | No | None known |
| Trastuzumab | 67 | 35 | No | Yes | Hypertension Coronary artery disease Prior smoking history |
| Trastuzumab | 77 | 6 | No | Yes | Atrial fibrillation Mitral stenosis |
Abbreviation: HF = heart failure.
An additional 2 (1.6%) patients (56 and 63 years of age) in the trastuzumab cohort had an episode of palpitations/tachycardia without evidence of CHF. Both of these events occurred during adjuvant trastuzumab and led to cessation of therapy. There were no cardiac deaths in either cohort.
Discussion
The development of education and screening mammography programs in the United States have been associated with increased incidence rates for smaller breast cancers.25 Breast cancer incidence increases with age, and the relative proportion of older women is increasing in North America.26,27 The incidence of breast cancer may further increase as a result of the increasing rates of obesity,28,29 which is also associated with an increased risk of hypertension and heart failure.30 Taken together, these observations highlight an increasingly common problem of small, lymph node-negative breast cancers in older women; a portion of these cancers will be HER2+, and a number of these older women may be at increased potential risk of cardiotoxicity with trastuzumab therapy. Evidence that supports the use of adjuvant trastuzumab for women with these tumors has generally been derived from limited retrospective studies or inadequately powered subset analyses from prospective studies.3,31 Thus, our study offers valuable additional information and insights into the safety and efficacy of adjuvant trastuzumab in this population and setting.
In this retrospective study, patients had excellent survival outcomes—the 4-year OS rate was 99% for patients who did and did not receive trastuzumab. The 4-year DRFS rate was 97% (95% CI, 92%–99%) in patients who did not receive trastuzumab and 99% (95% CI, 95%–100%) in patients who did receive trastuzumab, but was not shown to be statistically different. These results are consistent with data from prior cohort/registry studies that predominantly included younger patients.15,32 Of note, patients included in the trastuzumab cohort in this study generally had tumors with poorer prognostic features than those in the no-trastuzumab cohort (higher proportion of grade III, with lymphovascular invasion and estrogen receptor-negative). Almost all patients in the trastuzumab cohort received chemotherapy, compared with one-half of those in the no-trastuzumab group (98% vs. 53%, respectively; P < .0001). Therefore, one possible explanation for our favorable results is that a potentially more-aggressive biologic phenotype in the trastuzumab cohort was successfully treated with adjuvant chemotherapy and/or trastuzumab.
Suggested risk factors for trastuzumab-related cardiotoxicity include age > 50 years, hypertension, and borderline/low left ventricular ejection fraction.18,19 Hence, the second important observation from the current study is that the combination of chemotherapy and trastuzumab in older patients resulted in similar rates of cardiotoxicity as previously reported in younger cohorts. Specifically, the incidence of CHF in the no-trastuzumab arm at 4-year follow-up was 1% compared with 3.1% in the trastuzumab arm, and there were no cardiac-related deaths in either cohort. Collectively, these findings in older patients are consistent with results of the pivotal randomized phase III trials, which generally enrolled younger women.2,19,33–35 For example, the cardiac event rate at 7 years in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 study was 4% for women who had received an anthracycline and trastuzumab.19 Notably, all of these prospective and retrospectively reported results are markedly lower than a retrospective population-based study of patients > 65 years of age (median, 71 years) from the Surveillance, Epidemiology, and End Results (SEER)-Medicare and the Texas Cancer Registry-Medicare databases, which reported congestive heart failure in 29% of patients who had received trastuzumab.10 However, it is likely that treatment toxicities may be overestimated by population-based studies, which rely on databases and billing codes as opposed to audited individual patient data.
There are a number of limitations to our study. First, it was retrospective. However, it is unlikely that the risks and benefits of adjuvant trastuzumab-based therapy in this setting will ever be addressed in an adequately powered, prospectively conducted randomized study.36 Given the design of this sequential cohort study, the follow-up in the no-trastuzumab cohort is significantly longer than in the trastuzumab cohort. Therefore, the longer-term toxicity of trastuzumab-based therapy is less well-described here. Although we attempted to minimize bias by creating cohorts defined by year of diagnosis with equivalent follow-up to a milestone time point, there are differences between the cohorts. Specifically, there was a higher proportion of tumors with poorer prognostic features in the trastuzumab cohort (higher proportion grade III, with lymphovascular invasion and estrogen receptor-negative) with resultant greater use of chemotherapy. We excluded patients from the trastuzumab cohort who declined or were not offered anti-HER2 therapy. This was intended to minimize bias in the effects of therapy, but it is probable that those women who were excluded had less-aggressive tumors, poorer performance status, or might have had higher risk of cardiotoxicity. In addition, it is not possible to separate the potential impact of trastuzumab from the chemotherapy administered in the trastuzumab cohort. The chemotherapy administration practices have changed over time, and the majority of patients in the trastuzumab cohort received taxane without anthracycline therapy (50%, compared with 7% anthracycline alone, 34% anthracycline and taxane), whereas the majority of patients in the no-trastuzumab cohort who received chemotherapy had an anthracycline without taxane (40% compared with 0% taxane alone, 1% anthracycline and taxane).
As noted, the definition of “older” patients is not fixed. It has been suggested that risk factors for trastuzumab-related cardiotoxicity include age > 50 years.18,19 In the combined analysis of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 and North Central Cancer Treatment Group (NCCTG) N9831 trials, only 16% of patients were ≥ 60 years of age.6 Similarly, 16% were ≥ 60 years of age in the Herceptin Adjuvant (HERA) trial.21 The Programmes d’Actions Concertées Sein-04 (PACS-04) and Finland Herceptin (FinHer) studies excluded patients ≥ 66 years of age.4,37 Therefore, given this lack of a consistent age cut-point, we adopted a conservative definition of older patients as age ≥ 55 years. However, the current study was biased toward younger patients above that threshold: the median ages of the 2 cohorts were 64 and 62 years, respectively.
The outcome of patients with high-risk early breast cancer that overexpresses HER2 has been improved with sequential advances, including the addition of taxanes to anthracycline-based chemotherapy regimens, the development of dose-dense scheduling, and the addition of trastuzumab.15,18,34 Of note, in the current study, 41% patients in the trastuzumab cohort also received an anthracycline, and cardiotoxicity was consistent with previous reports. However, a more individualized approach to the treatment of older patients with small, node-negative, HER2+ breast cancer is likely needed. One attractive approach is the use of weekly paclitaxel (80 mg/m2 for 12 weeks) with 1 year of trastuzumab.36 This regimen has demonstrated impressive disease control and a manageable side-effect profile in patients unselected for age in a prospective single-arm study.36 In this trial of 406 patients with T1/T2 N0 HER2+ breast cancer, the 3-year invasive disease-free survival was 98.7% (95% CI, 97.6%–99.8%). Of note, the median age of patients on this study was 55 years (range, 24–85 years), and 274 (67.5%) patients ≥ 50 years of age were enrolled. Little cardiac toxicity was demonstrated with this approach,38 consistent with our findings that trastuzumab is safe in older women with HER2+ breast cancer, and this regimen might be particularly attractive for these patients.
Our study adds new and important information to what is known about the risks and benefits of adjuvant trastuzumab in older women with small, node-negative, HER2+ breast cancers. We have demonstrated excellent outcomes in terms of DRFS and OS, and low rates of cardiotoxicity with this therapy. Alternatively, our results also suggest that there may be a cohort of older women with small tumors for whom trastuzumab-based adjuvant therapy is not necessary. It is likely with increased understanding of the genomic diversity within HER2+ disease that it will be possible in the future to further delineate this subset of women for whom treatment can be avoided. However, in the interim, we suggest that trastuzumab-based adjuvant therapy is as appropriate a therapeutic option for selected older women with lower-risk HER2+ breast cancer as it is for younger patients.
Clinical Practice Points.
There is little data pertaining to breast cancer outcomes and cardiac toxicity in older patients with small node-negative HER2+ breast cancers receiving adjuvant trastuzumab, as these patients were underrepresented in the original clinical trials.
In a retrospective sequential cohort study, we have demonstrated that these women achieve excellent disease control with trastuzumab and chemotherapy, with few cardiac events.
As this issue is unlikely to be ever addressed in a prospective randomized manner, our study provides valuable additional information which will help to guide clinicians making treatment decisions for this specific group of patients.
Acknowledgments
This study was funded in part by NIH/NCI Cancer Center Support Grant No. P30 CA008748 and presented in poster format at the 2013 San Antonio Breast Cancer Symposium, December 10–14, San Antonio, TX.
Footnotes
Disclosure
Dr McArthur discloses advisory board participation for Celgene, Merck, OBI Pharma, Spectrum Pharmaceuticals, and Syndax Pharmaceuticals, and research support from Bristol-Myers Squibb, MedImmune, LLC/AstraZenica, Eli Lilly, Merck, and ZIOPHARM Oncology. All other authors state that they have no conflicts of interest.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;4:CD006243. doi: 10.1002/14651858.CD006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–83. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27:5685–92. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 5.Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382:1021–8. doi: 10.1016/S0140-6736(13)61094-6. [DOI] [PubMed] [Google Scholar]
- 6.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–73. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman RA, Vaz-Luis I, Barry WT, et al. Patterns of chemotherapy, toxicity, and short-term outcomes for older women receiving adjuvant trastuzumab-based therapy. Breast Cancer Res Treat. 2014;145:491–501. doi: 10.1007/s10549-014-2968-9. [DOI] [PubMed] [Google Scholar]
- 8.Chavez-MacGregor M, Gonzalez-Angulo AM. HER2-neu positivity in patients with small and node-negative breast cancer (pT1a,b,N0,M0): a high risk group? Clin Adv Hematol Oncol. 2009;7:591–8. [PubMed] [Google Scholar]
- 9.McArthur HL, Mahoney KM, Morris PG, et al. Adjuvant trastuzumab with chemotherapy is effective in women with small, node-negative, HER2-positive breast cancer. Cancer. 2011;117:5461–8. doi: 10.1002/cncr.26171. [DOI] [PubMed] [Google Scholar]
- 10.Chavez-MacGregor M, Zhang N, Buchholz TA, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013;31:4222–8. doi: 10.1200/JCO.2013.48.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarantini L, Gori S, Faggiano P, et al. Adjuvant trastuzumab cardiotoxicity in patients over 60 years of age with early breast cancer: a multicenter cohort analysis. Ann Oncol. 2012;23:3058–63. doi: 10.1093/annonc/mds127. [DOI] [PubMed] [Google Scholar]
- 12.Tsai HT, Isaacs C, Fu AZ, et al. Risk of cardiovascular adverse events from trastuzumab (Herceptin((R))) in elderly persons with breast cancer: a population-based study. Breast Cancer Res Treat. 2014;144:163–70. doi: 10.1007/s10549-014-2836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaz-Luis I, Keating NL, Lin NU, Lii H, Winer EP, Freedman RA. Duration and toxicity of adjuvant trastuzumab in older patients with early-stage breast cancer: a population-based study. J Clin Oncol. 2014;32:927–34. doi: 10.1200/JCO.2013.51.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiess AP, McArthur HL, Mahoney K, et al. Adjuvant trastuzumab reduces locoregional recurrence in women who receive breast-conservation therapy for lymph node-negative, human epidermal growth factor receptor 2-positive breast cancer. Cancer. 2012;118:1982–8. doi: 10.1002/cncr.26484. [DOI] [PubMed] [Google Scholar]
- 15.Vaz-Luis I, Ottesen RA, Hughes ME, et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: a multiinstitutional study. J Clin Oncol. 2014;32:2142–50. doi: 10.1200/JCO.2013.53.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodrigues MJ, Peron J, Frenel JS, et al. Benefit of adjuvant trastuzumab-based chemotherapy in T1ab node-negative HER2-overexpressing breast carcinomas: a multicenter retrospective series. Ann Oncol. 2013;24:916–24. doi: 10.1093/annonc/mds536. [DOI] [PubMed] [Google Scholar]
- 17.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: Available at: http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, April 2013. Accessed: August 17, 2016. [Google Scholar]
- 18.Morris PG, Hudis CA. Trastuzumab-related cardiotoxicity following anthracycline-based adjuvant chemotherapy: how worried should we be? J Clin Oncol. 2010;28:3407–10. doi: 10.1200/JCO.2009.26.0125. [DOI] [PubMed] [Google Scholar]
- 19.Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:3792–9. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 21.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 22.Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, et al. Outcome of patients with HER2-positive breast cancer treated with or without adjuvant trastuzumab in the Finland Capecitabine Trial (FinXX) Acta Oncol. 2014;53:186–94. doi: 10.3109/0284186X.2013.820840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudis CA, Barlow WE, Costantino JP, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007;25:2127–32. doi: 10.1200/JCO.2006.10.3523. [DOI] [PubMed] [Google Scholar]
- 24.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 25.Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characteristics among U.S. women. Breast Cancer Res. 2007;9:R28. doi: 10.1186/bcr1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–46. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 27.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–65. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 28.Iyengar NM, Morris PG, Hudis CA, Dannenberg AJ. Obesity, inflammation, and breast cancer. In: Dannenberg AJ, Berger NA, editors. Obesity, inflammation, and cancer. New York: Springer; 2013. pp. 181–217. [Google Scholar]
- 29.Fletcher I. Defining an epidemic: the body mass index in British and US obesity research 1960–2000. Sociol Health Illn. 2014;36:338–53. doi: 10.1111/1467-9566.12050. [DOI] [PubMed] [Google Scholar]
- 30.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of Obesity and the Obesity Paradox on Prevalence and Prognosis in Heart Failure. JACC Heart Fail. 2013;1:93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Q, Yin W, Du Y, Lu J. For or against adjuvant trastuzumab for pT1a-bN0M0 breast cancer patients with HER2-positive tumors: a meta-analysis of published literatures. PLoS One. 2014;9:e83646. doi: 10.1371/journal.pone.0083646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fehrenbacher L, Capra AM, Quesenberry CP, Jr, Fulton R, Shiraz P, Habel LA. Distant invasive breast cancer recurrence risk in human epidermal growth factor receptor 2-positive T1a and T1b node-negative localized breast cancer diagnosed from 2000 to 2006: a cohort from an integrated health care delivery system. J Clin Oncol. 2014;32:2151–8. doi: 10.1200/JCO.2013.52.0858. [DOI] [PubMed] [Google Scholar]
- 33.Morris PG, Iyengar NM, Patil S, et al. Long-term cardiac safety and outcomes of dose-dense doxorubicin and cyclophosphamide followed by paclitaxel and trastuzumab with and without lapatinib in patients with early breast cancer. Cancer. 2013;119:3943–51. doi: 10.1002/cncr.28284. [DOI] [PubMed] [Google Scholar]
- 34.Morris PG, Dickler M, McArthur HL, et al. Dose-dense adjuvant doxorubicin and cyclophosphamide is not associated with frequent short-term changes in left ventricular ejection fraction. J Clin Oncol. 2009;27:6117–23. doi: 10.1200/JCO.2008.20.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dang C, Lin N, Moy B, et al. Dose-dense doxorubicin and cyclophosphamide followed by weekly paclitaxel with trastuzumab and lapatinib in HER2/neu-overexpressed/amplified breast cancer is not feasible because of excessive diarrhea. J Clin Oncol. 2010;28:2982–8. doi: 10.1200/JCO.2009.26.5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372:134–41. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spielmann M, Roche H, Delozier T, et al. Trastuzumab for patients with axillary-node-positive breast cancer: results of the FNCLCC-PACS 04 trial. J Clin Oncol. 2009;27:6129–34. doi: 10.1200/JCO.2009.23.0946. [DOI] [PubMed] [Google Scholar]
- 38.Dang C, Guo H, Najita J, et al. Cardiac outcomes of patients receiving adjuvant weekly paclitaxel and trastuzumab for node-negative, ERBB2-positive breast cancer. JAMA Oncol. 2016;2:29–36. doi: 10.1001/jamaoncol.2015.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]


