SUMMARY
Neural circuits are endowed with several forms of intrinsic and synaptic plasticity that could contribute to adaptive changes in behavior, but circuit complexities have hindered linking specific cellular mechanisms with their behavioral consequences. Eye movements generated by simple brainstem circuits provide a means for relating cellular plasticity to behavioral gain control. Here we show that firing rate potentiation, a form of intrinsic plasticity mediated by reductions in BK-type calcium activated potassium currents in spontaneously firing neurons, is engaged during optokinetic reflex compensation for inner ear dysfunction. Vestibular loss triggers transient increases in postsynaptic excitability, occlusion of firing rate potentiation, and reductions in BK currents in vestibular nucleus neurons. Concurrently, adaptive increases in visually-evoked eye movements rapidly restore oculomotor function in wildtype mice but are profoundly impaired in BK channel null mice. Activity-dependent regulation of intrinsic excitability may be a general mechanism for adaptive control of behavioral output in multisensory circuits.
INTRODUCTION
Neural circuit function depends both on synaptic connectivity and on the concerted action of ion channels that are regulated by neuronal activity. Increasing evidence implicates plasticity of ion channel function in experience-dependent changes in the developing and mature nervous systems (Turrigiano, 2011; Mozzachiodi and Byrne, 2010). Several forms of learning and memory, including associative, trace, delay, and fear conditioning have been associated with changes in intrinsic excitability and associated ionic currents in neurons of the cerebral cortex, hippocampus, or amygdala. Making direct connections between plasticity of intrinsic excitability and cognitive forms of behavioral learning in vivo has been hampered, however, by the complexities of neural circuits in the forebrain, in which neuronal activity is indirectly related to behavioral output.
Eye movements that stabilize images on the retina provide a tractable system for linking cellular mechanisms with behavioral outcomes (Gittis and du Lac, 2006; Kodama and du Lac, 2016). Experience-dependent changes in eye movements occur throughout life, and the role of neuronal firing in eye movement performance and plasticity has been studied intensively over several decades. During self-motion, retinal image motion is minimized via compensatory eye movements mediated by defined neural circuits in the cerebellum and brainstem. Brainstem medial vestibular nucleus (MVN) neurons transform presynaptic signals encoding head movements and image motion into postsynaptic modulations of firing rate which are appropriate for generating adaptive eye movements. Cerebellum-dependent motor learning in the vestibulo-ocular reflex (VOR) produces dramatic changes in the firing responses of MVN neurons (Lisberger, 1988; Lisberger et al., 1994), and restoration of VOR function after damage to the vestibular periphery is thought to involve plastic changes in MVN neuronal excitability (reviewed in: Straka et al., 2005; Beraneck and Idoux, 2012).
An intriguing candidate cellular mechanism of plasticity in the oculomotor system is firing rate potentiation (FRP), in which repeated inhibition of tonically firing neurons results in long-lasting increases in intrinsic excitability via CamKII-dependent reductions in BK calcium-activated potassium currents (Nelson 2003, 2005; Hull 2013). MVN neurons fire spontaneously at high rates in vivo; their firing derives from a combination of strong excitatory drive from spontaneously firing vestibular nerve afferents, synaptic inhibition from cerebellar Purkinje cells and local circuit neurons, and intrinsic pacemaking currents (Lin and Carpenter, 1993; Gittis et al, 2007). We hypothesized that after peripheral vestibular dysfunction, when excitatory synaptic drive to MVN neurons is decreased, ongoing synaptic inhibition could trigger a reduction in BK currents via firing rate potentiation. The subsequent increases in MVN neuronal excitability would amplify remaining inputs and enable intact sensory signals to substitute for the loss of vestibular self-motion information.
To determine whether FRP is critical for oculomotor plasticity, we performed behavioral and electrophysiological analyses in mice subjected to unilateral vestibular deafferentation (UVD), the vestibular equivalent of monocular deprivation. Eye movements, MVN neuronal excitability, and the capacity to induce FRP were assessed in parallel following UVD to establish the temporal relationship of intrinsic and oculomotor plasticity. Our findings demonstrate that vestibular loss induces robust increases in MVN neuronal excitability, occlusion of FRP, and reduction of BK currents, concomitant with rapid visuomotor plasticity (increases in the gain of the optokinetic reflex) which compensates for impaired vestibular function. A critical role for activity-dependent regulation of BK currents in multisensory oculomotor plasticity was confirmed by the normal oculomotor performance but complete absence of optokinetic reflex plasticity in mice with global deletion of BK channels.
RESULTS
VOR and OKR plasticity triggered by unilateral loss of vestibular function
During self-motion, the stability of images on the retina is maintained by two complementary reflexes: the vestibulo-ocular reflex (VOR), which is driven by head movements, and the optokinetic reflex (OKR), which is driven by image motion. In mice, as in other species, these oculomotor reflexes are differentially optimized for fast and slow motion, respectively, and their conjoint operation ensures excellent gaze stability (Stahl, 2004; Faulstich et al., 2004). As demonstrated in Figure 1A, juvenile mice (p21–26) rotated on a turntable (1 Hz ± 5 deg) around a stationary patterned visual stimulus produced eye movements with gains (eye speed/stimulus speed) that approached unity (i.e. perfect compensation). In contrast, the same rotation in darkness evoked a VOR that compensated for about 80% of head motion (gain = 0.8; Fig. 1B). The corresponding OKR, elicited by rotation of a striped drum when mice were stationary, compensated for 20–30% of image motion (OKR gains 0.2 – 0.3; Fig. 1C). Thus, under behaviorally relevant conditions, eye movements are optimized by combining sensory signals originating in both the inner ear and the retina.
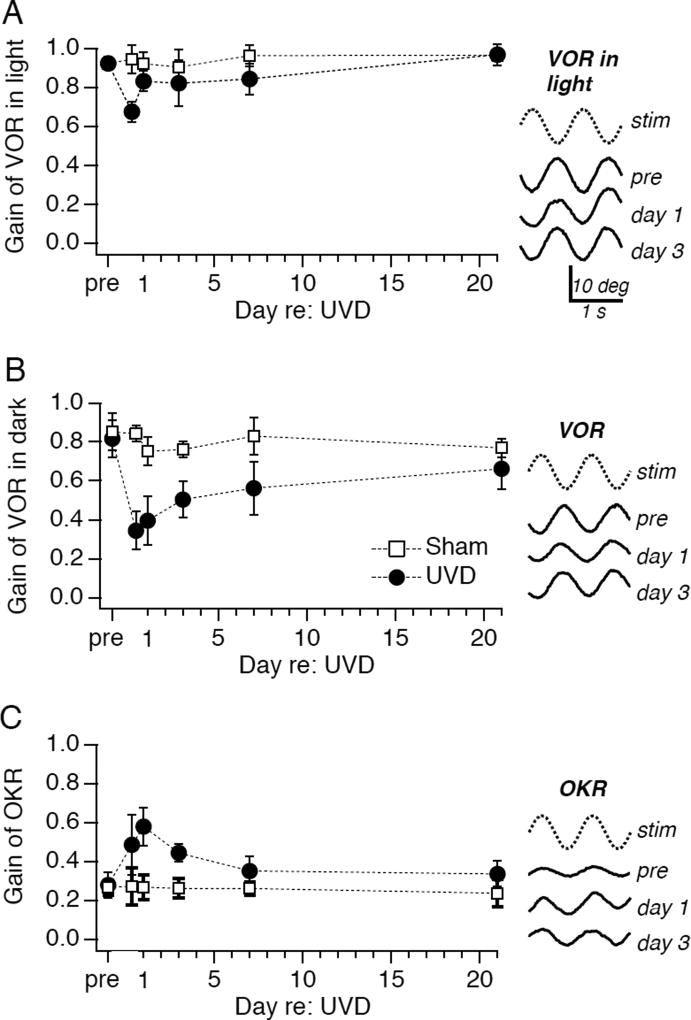
FIGURE 1.
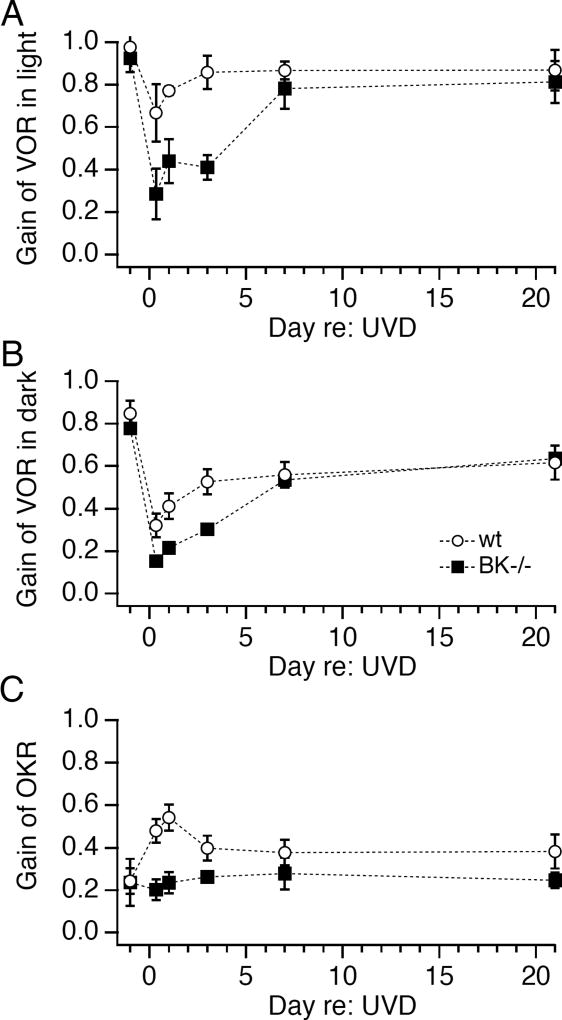
Unilateral vestibular deafferentation induces oculomotor plasticity. Timecourse of effect of unilateral vestibular deafferentation (UVD: filled symbols) or sham operation (open symbols) on (A) the vestibulo-ocular reflex (VOR) in the light, (B) the VOR in the dark, and (C) the optokinetic reflex (OKR). Examples of evoked eye movements prior to, 1 day after, and 3 days after unilateral vestibular deafferentation are shown to the right of each graph. Rotational motion of the animal (A, B) and/or the visually patterned stimulus (C) was 1 Hz, ±5 deg (peak-to-peak). Each point contains data from 6 to 12 mice; error bars represent standard deviations.
Loss of inner ear function (unilateral vestibular deprivation, or UVD: see Methods) triggered dynamic changes in eye movement performance. 8h after UVD, eye movements evoked by rotation of mice in the presence of visual stimuli were reduced significantly (Fig. 1A; gain: 0.68±.05, p<0.001). Remarkably, within 24 hours of UVD, eye movement gain increased rapidly, to 0.83±.05. Within 3 weeks, eye movements evoked by rotation in the light had fully compensated for the loss of vestibular function (Fig. 1A). In control experiments, sham manipulations had no affect on eye movements (Fig. 1A), demonstrating that disruption of vestibular function drives plastic changes in eye movements.
Adaptive changes in both the VOR and OKR contributed to the oculomotor plasticity induced by UVD. When measured in darkness, VOR gain dropped to 42% of pre-operative values 8h after UVD (Fig. 1B; p<0.0001). Compensatory increases in the VOR were evident within 3 days of UVD, and performance continued to improve gradually, with VOR gain reaching 80% of control values within 3 weeks. These effects on VOR performance depended on loss of vestibular function, as evidenced by the relative constancy of VOR gain in sham-operated mice (Fig 1B).
Notably, unilateral vestibular loss triggered rapid adaptive plasticity in the OKR (Fig. 1C). OKR gain increased within 8 h of UVD and was 2-fold higher than control within 24 h (p < 0.0001). Thereafter, OKR gain declined to a steady-state level that did not differ significantly from control. Sham operated mice evaluated in parallel showed no change in OKR gain. These results indicate that in juvenile mice, as in adult mice (Faulstich et al., 2004) and primates (Fetter and Zee, 1988), the oculomotor system exhibits adaptive, multisensory plasticity in which increases in visually-driven eye movements compensate for loss of vestibular function.
Plasticity of intrinsic excitability induced by unilateral vestibular dysfunction
To investigate whether experience-dependent changes in intrinsic neuronal excitability could account for the multisensory plasticity induced by UVD, medial vestibular nucleus (MVN) neurons were targeted for electrophysiological recordings in brainstem slices obtained from mice subjected to UVD and sham manipulations. To control for sampling biases and variability in tissue quality, neurons from both experimental and control mice were recorded by each of three investigators working in parallel. Excitability was assessed 8h, 1d, 3d, 7d, and 21d after UVD. For each time point and condition, recordings were made from an average of 115 neurons (range 64–161). Experiments and analyses were performed blinded to the behavioral manipulation.
UVD alters the proportion of spontaneously firing neurons
In slices from sham-operated mice, 55–70% of MVN neurons recorded with whole cell patch pipettes fired spontaneously, typically at rates between 10 and 15 spikes/s. UVD altered the proportion of spontaneously firing neurons but had no effect on the average rates of neurons that fired spontaneously (Supplemental Fig. 1). The proportion of neurons firing spontaneously was reduced 8h after UVD (p < 0.05), and was unaffected at subsequent time points. Spontaneous firing rates were not affected by UVD at any time point. To control for the possibility that changes in firing rates were masked by intracellular dialysis in the whole-cell recording configuration, extracellular electrodes were used to measure spontaneous firing rates. UVD had no effect on firing rates obtained with extracellular electrodes at any time point (p > 0.25, N > 20 neurons for each condition).
Transient gain increases in intrinsic excitability are triggered by UVD
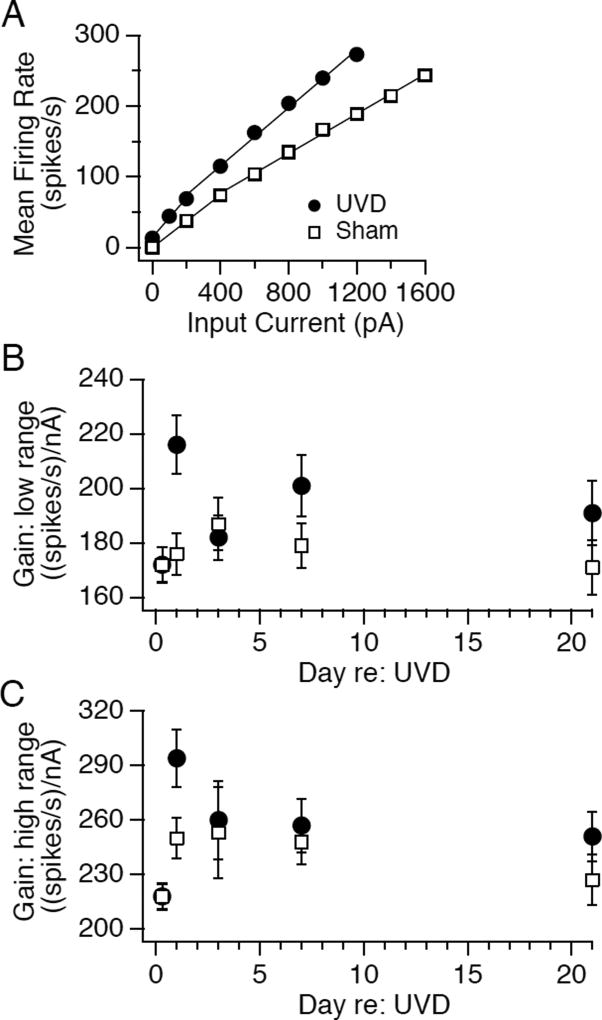
To provide appropriate drive to motoneurons, spike generator mechanisms in MVN neurons must transform synaptic signals into well-calibrated modulations in firing rate. We asked whether UVD altered the sensitivity of spike generation by assessing firing responses to intracellular current injection. When MVN neurons are depolarized, they respond with proportional increases in firing rate up to several hundred Hz (Serafin et al., 1991; Sekirnjak and du Lac, 2006). The gain of intrinsic excitability (quantified as the slope of the firing rate to current relationship) is constant across the firing range or tends to exhibit two values with an inflection at evoked firing rates of 80 Hz (Bagnall et al., 2007). Fig. 2A plots mean firing rates evoked during 1 s of intracellular current injection vs stimulus amplitude for representative MVN neurons recorded 24 h after UVD and sham operations. The gain of the neuron recorded after UVD was higher than the control neuron's gain at firing ranges both below and above 80 Hz. These results are quantified for the population of recorded neurons in Figs. 2B and C. 8 h after UVD, neuronal gains did not differ between groups. In contrast, at the 24h time point, gains in UVD neurons were significantly higher than those in sham neurons (low range: p < 0.05, n=134 UVD, n=146 sham; high range: p < 0.005, n=131 UVD, n=142 sham). Over the next 3 weeks, neurons recorded after UVD had gains that were slightly but not significantly higher than controls (Fig 2B and C).
FIGURE 2.
Potentiation of firing responses to intracellular depolarization induced by unilateral vestibular deafferentation. A. Examples of average firing rate evoked during 1 sec of intracellular depolarization as function of current amplitude in a neuron recorded from a deafferented mouse (filled circles) and a neuron recorded from a mouse with sham surgery (open squares). B. Slope of the current to firing rate relationship for evoked firing rates ≤80 spikes/s are plotted vs day after surgery for neurons recorded in mice with unilateral vestibular deafferentation (filled circles) vs sham operation (open squares). The number of neurons recorded at successive time points (8h, 1d, 3d, 7d, 21d) from UVD mice, respectively, were 158, 110, 105, 99 and 64 and for sham mice were 148, 123, 85, 109, and 64. C. Slope of the current to firing rate relationship for evoked firing rates > 80 spikes/s are plotted vs day after surgery for neurons recorded in mice with unilateral vestibular deafferentation (filled circles) vs sham operation (open squares). The number of neurons recorded at successive time points (8h, 1d, 3d, 7d, 21d) from UVD mice, respectively, were 156, 131, 112, 96 and 62 and for sham mice were 140, 142, 91, 105, and 63. All data are displayed as mean +/− SEM.
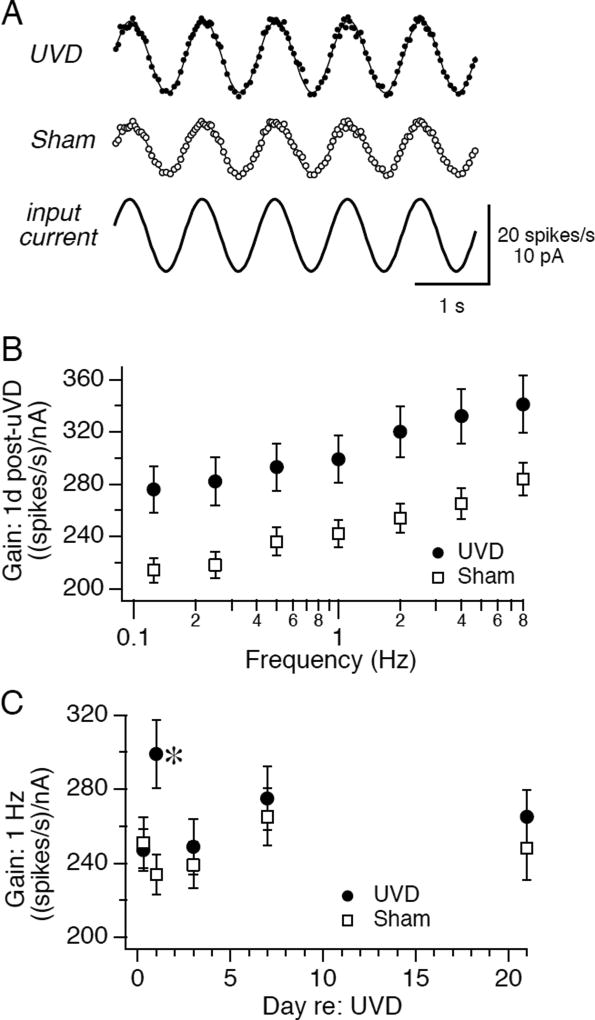
Transient increases in neuronal excitability were also evident in response to temporally modulated stimuli (Fig. 3), which approximate the pattern of vestibular stimulation that a mouse would experience during head rotations. Sinusoidal modulation of input current evoked sinusoidal modulations in firing rate that were larger in neurons recorded 24 h after UVD than in control neurons (Fig. 3A). Sinusoidal gains were on average 25% higher 24 hours after UVD at all frequencies tested (Fig 4B), but did not differ from control at any other time point (Fig. 3C; p < 0.05). Other measures of intrinsic physiological properties were not affected by UVD, including maximum firing rates, spike frequency adaptation, input resistance, action potential width, postinhibitory rebound firing, and action potential afterhyperpolarization (Supplemental Table 1). These data indicate that UVD is accompanied by transient increases in intrinsic excitability that are evident within 24 hours and are restored to control values within 3 days.
FIGURE 3.
Firing responses to sinusoidally modulated inputs are transiently potentiated by unilateral vestibular deafferentation. A. Examples of firing rate responses, plotted as instantaneous firing rate as a function of time, to sinusoidally modulated current (1 Hz, ±30 pA) in exemplar neurons recorded from a deafferented mouse (lesion; gain=303 (spikes/s)/nA) or sham operated mouse (sham; gain =212 (spikes/s)/nA), 24 h after surgery. B. Average firing response gain as a function of sinusoidal stimulation frequency for neurons recorded 24h after surgery from deafferented mice (filled circles: n=53) or sham operated mice (open squares; n=56). C. Average gain recorded in response to 1 Hz intracellular sinusoidal current injection is plotted vs day after surgery for neurons recorded from deafferented mice (filled circles) and sham operated mice (open squares). The number of neurons recorded at successive time points (8h, 1d, 3d, 7d, 21d) from UVD mice, respectively, were 122, 53, 52, 59 and 47 and for sham mice were 108, 56, 53, 68, and 48. All data are displayed as mean +/− SEM.
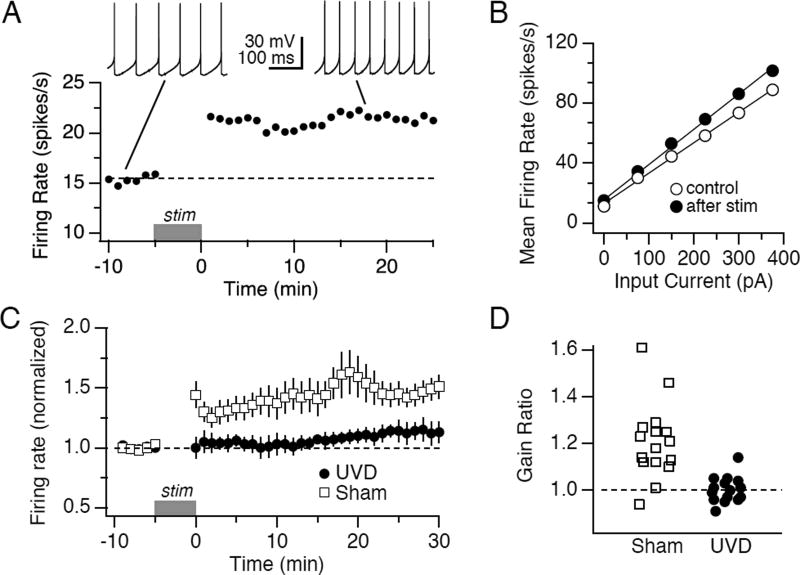
FIGURE 4.
Firing rate potentiation is occluded by unilateral vestibular deafferentation. A. Spontaneous firing rate is plotted as a function of time for a representative control neuron subjected to 5 minutes of periodic intracellular hyperpolarization (see Methods) during the period indicated by the grey bar. Insets show spontaneous firing during the pre-stimulation and post-stimulation periods. B. Average firing rate evoked during 1s of depolarization is plotted as a function of stimulus amplitude for the same neuron shown in A before (open circles) and after (filled circles) 5 min of hyperpolarization. The neuron's firing response gain (slope of the current to firing rate relationship) increased from 202 to 223 (spikes/s)/nA. C. Spontaneous firing rate, normalized to each neuron’s baseline values, is plotted as a function of time prior to and after 5 min of hyperpolarization (grey bar) for populations of neurons recorded 24 hours after UVD (filled circles; n=7) or sham operation (open squares; n=6). D. The firing response gain measured 30 min after the hyperpolarizing induction stimulus, normalized to baseline gain for each neuron, is plotted for the populations of neurons recorded 24h after UVD (n=15) or sham (n=16) operations. Gain was measured for firing rate responses < 80 spikes/s. All data are displayed as mean +/− SEM.
UVD occludes Firing Rate Potentiation
Synaptic inhibition or membrane hyperpolarization of MVN neurons triggers long-lasting increases in intrinsic excitability via a process termed "firing rate potentiation" (Nelson et al., 2003). The downstream mechanisms of firing rate potentiation include reduction of CaMKII activity and decreases in BK type calcium-dependent potassium currents (Nelson et al., 2003; Nelson et al., 2005; Hull et al, 2013). An example of firing rate potentiation in an MVN neuron from a sham-operated animal is shown in Fig 4A, B. The neuron fired spontaneously at 15 spikes/s. Following 5 min of membrane hyperpolarization with current pulses (see Methods), the neuron's spontaneous firing rate increased to 23 spikes/s (Fig. 4A). Concomitantly, firing response gain increased by 13% (Fig 4B). The increases in excitability were sustained beyond the 30 min duration of the recording displayed.
As we hypothesized that firing rate potentiation might be utilized in vivo to mediate behavioral plasticity, we next tested whether it could still be induced in slices from mice subjected to UVD at various points during vestibular compensation. Consistent with this hypothesis, we found that UVD transiently occluded firing rate potentiation. Fig. 4C shows spontaneous firing rates prior to and after 5 min of membrane hyperpolarization for a population of neurons recorded from sham and UVD slices 1 day after surgery. Although sham neurons demonstrated robust firing rate potentiation (146 ± 9%, n=8, p < 0.01), UVD neurons exhibited no change in firing rate (92 ± 7%, n=8). Similarly, as shown in Fig. 4D, firing response gains did not increase after UVD (104 ± 2%, n =15) but did so robustly after sham surgery (126 ± 3%, n=16, p < 0.005). Together, these results demonstrate that 1 day after vestibular deprivation, firing rate potentiation or its constituent cellular mechanisms are occluded.
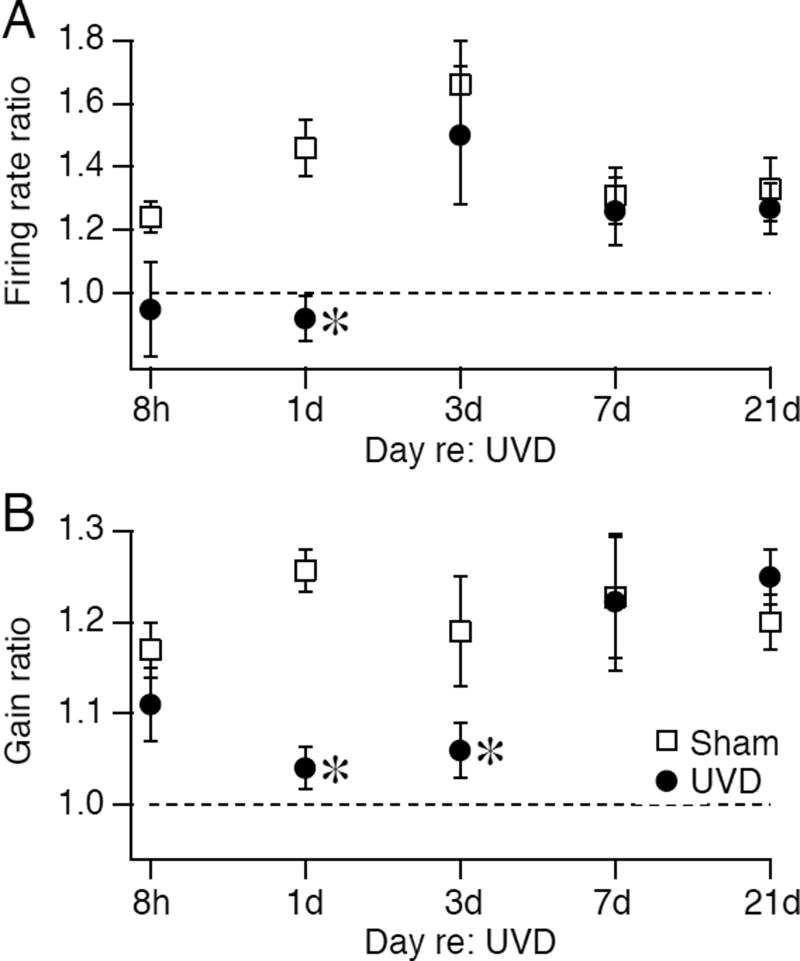
Fig. 5 summarizes the effects of the firing rate potentiation induction protocol on excitability assessed at different time points after UVD. Potentiation of spontaneous firing was occluded 8h and 1d after UVD (p=0.006 and 0.0048, respectively) but not at later times (Fig 5A). Potentiation of firing response gain was occluded 1d and 3d after UVD (p=0.0002 and 0.014, respectively) but was robust at later time points (Fig 5B). These results indicate that loss of vestibular drive to central neurons transiently disrupts the signaling pathways that mediate firing rate potentiation.
FIGURE 5.
Timecourse of effects of unilateral vestibular deafferentation on the induction of firing rate potentiation. The ratio of post-stimulation to baseline values of spontaneous firing (A) or firing response gain (B) to a hyperpolarizing induction stimulus is plotted as a function of time after operation for populations of neurons recorded from mice receiving UVD (filled circles) or sham operation (open squares). The number of neurons recorded at successive time points (8h, 1d, 3d, 7d, 21d) from UVD mice, respectively, were 16, 15, 18, 12 and 11 and for sham mice were 13, 16, 11, 16, and 11. All data are displayed as mean +/− SEM.
Reduced contribution of BK currents to excitability after UVD
The concurrence of increased excitability and occlusion of firing rate potentiation suggests the possibility that UVD triggers firing rate potentiation, with consequent increases in excitability, leading to improved oculomotor performance. It is possible, however, that UVD affects excitability via signaling pathways distinct from those critical for firing rate potentiation. The downstream consequence of the reduced [Ca2+] and CaMKII levels that trigger FRP is a marked reduction of BK currents (Nelson et al., 2005; van Welie and du Lac 2011). To determine whether BK channels are affected by UVD, we assessed the actions of the specific BK channel antagonist iberiotoxin (IBTX) on MVN neuronal excitability, as described previously (Nelson et al., 2005).
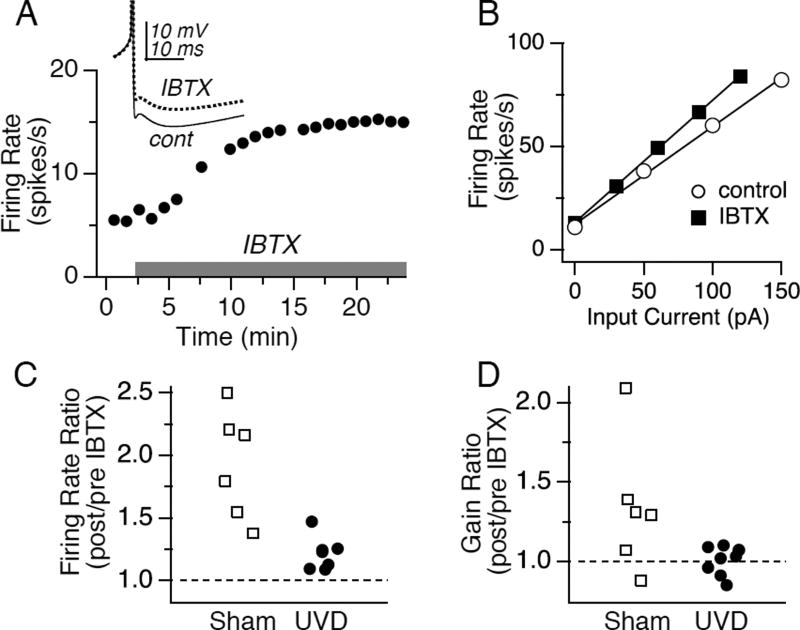
Application of IBTX (150 nM) to control slices resulted in a gradual increase in spontaneous firing rate and firing response gain, as shown in a representative neuron, which more than doubled its firing rate (Fig 6A), increased its gain by 26% (Fig 6B), and decreased the amplitude of the afterhyperpolarization (AHP) by 4.6 mV following BK channel blockade. Notably, IBTX had significantly less effect on excitability in slices obtained 1 day after UVD (Figure 6C, D; n=6, p < 0.05 for firing rate and gain), and the AHP was reduced less by IBTX in UVD vs sham (2.4±0.52 vs 4.3±0.82 mV, respectively, p < 0.05) No differences in the effects of IBTX were observed in sham slices vs lesion slices at other time points (data not shown). These findings support a model in which loss of vestibular function triggers transient increases in excitability via decreases in BK currents.
FIGURE 6.
Pharmacological blockade of BK channels evokes increases in excitability that are occluded by unilateral vestibular deafferentation. A, B. Effect of the specific BK channel blocker iberiotoxin (150 nM, grey bar) on spontaneous firing rate (A) and firing response gain (B) in a representative control neuron. C. Effect of iberiotoxin on spontaneous firing rate, normalized to control levels, for populations of neurons recorded from sham operated and UVD mice. D. Effect of iberiotoxin on gain for the same population of neurons shown in C.
Impaired oculomotor plasticity in BK null mice
The results presented thus far suggest that reduction in BK currents in MVN neurons contributes to oculomotor plasticity after UVD. To test directly whether oculomotor plasticity depends on BK channels, we examined oculomotor performance prior to and after UVD in mice with a genetic deletion of BK channels (Meredith et al., 2004). Eye movements induced by either vestibular or optokinetic stimulation alone or in combination were similar in BK null mice and wildtype littermates (Fig. 7 and Supplemental Fig. 2), indicating that BK channels are not required for normal oculomotor performance.
FIGURE 7.
BK channel null mice exhibit impaired oculomotor plasticity. Time course of effect of unilateral vestibular deafferentation on BK null mice (open symbols) or wildtype littermates (filled symbols) on (A) the vestibulo-ocular reflex (VOR) in the light, (B) the VOR in the dark, and (C) the optokinetic reflex (OKR). Rotational motion of the animal and/or the visually patterned stimulus was 1 Hz, ±5 deg (peak-to-peak). Each point contains data from 5 mice; all data are displayed as mean +/− SD.
In contrast, oculomotor plasticity induced by UVD was impaired in BK null mice (Fig. 7). Eye movements evoked by rotation in the light were significantly smaller 8h after UVD in BK null than in WT control mice (p < 0.001). Oculomotor gains in BK null mice remained depressed to less than half of control values for the subsequent 3 days. Within a week of UVD, eye movements induced by rotation in the light were indistinguishable in BK null and wildtype mice (Fig. 7A).
VOR gains measured in darkness were more severely affected by UVD in BK null mice than in wildtype littermates (Fig. 7B); 8h after UVD, VOR gain dropped by 80±3% in BK null mice vs 60±1% in controls (p < 0.005, n=5 each). VOR gain remained lower in BK null mice than in controls for 3 days and then gradually increased to attain wildtype values.
Remarkably, BK null mice exhibited no OKR plasticity at any time point (Fig. 7C), whereas control mice exhibited robust increases in OKR gain 24 h after UVD (p < 0.005). These results demonstrate that BK channels are required for adaptive visuomotor plasticity following loss of peripheral vestibular inputs.
DISCUSSION
The data presented here demonstrate a behavioral role for firing rate potentiation, a form of intrinsic plasticity in spontaneously firing neurons in which persistent hyperpolarization triggers increases in intrinsic excitability via reductions in BK-type calcium-activated potassium currents. In mice subjected to unilateral vestibular deprivation, impairments in gaze-stabilizing eye movements are rapidly compensated by adaptive increases in visuomotor drive, as quantified by the gain of the optokinetic reflex. This multisensory behavioral plasticity occurs concomitantly with potentiation of intrinsic excitability in vestibular nucleus neurons. Occlusion of hyperpolarization-induced intrinsic plasticity and reduction of BK currents indicates a mechanistic role for activity-dependent regulation of BK channels via firing rate potentiation. Consistent with this model, mice with genetic ablation of BK channels exhibit profound impairments in eye movement performance and complete loss of optokinetic reflex plasticity following vestibular deprivation. These findings indicate that experience-dependent plasticity of intrinsic excitability via reductions in BK currents contributes to adaptive reweighting of sensory signals that drive eye movements.
Eye movement control systems require computations of self-motion derived from several sensory systems acting in concert, including the vestibular, visual, and proprioceptive systems (Angelaki and Cullen, 2008; Cullen, 2012). Rapid increases in OKR gain during the initial period of impaired vestibular function represent a form of sensory substitution (Brandt et al., 1997; Vibert et al., 1999) in which visual inputs compensate for the loss of vestibular inputs to restore appropriate eye movements. MVN neurons represent a key site of convergence of self-motion signals and are essential for the performance and the adaptive plasticity of smooth eye movements (Broussard and Kassardjian, 2004; Highstein and Holstein, 2006). Head motion signals are transmitted to the MVN from the vestibular nerve, while image motion signals are conveyed via pathways from the pretectal nuclei, the nucleus of the optic tract, the nucleus prepositus hypoglossi, and the cerebellar flocculus (Buttner-Ennever et al., 1996; Cazin et al., 1984). Consistent with our findings of a role for firing rate potentiation in visuomotor plasticity, field potentials evoked in the mouse MVN by vestibular nerve stimulation are potentiated by training-induced increases in the OKR (Shutoh et al, 2006). Because the vestibular nerve does not carry optokinetic signals (Buttner and Waespe, 1981), this result implies that optokinetic training induces an increase in intrinsic excitability of MVN neurons which convey visual motion signals to eye movement motor neurons. The rapid unmasking of latent proprioceptive inputs induced by vestibular deprivation (Sadeghi et al., 2010; Sadeghi et al., 2012) is also consistent with the increases in vestibular nucleus neuronal excitability demonstrated here. Reductions in BK currents may thus serve to enhance throughput of the remaining visual and proprioceptive signals related to self-motion under conditions of compromised vestibular function.
It has long been appreciated that peripheral vestibular dysfunction engenders plastic changes in the excitability of vestibular nucleus neurons (Precht et al, 1966; reviewed in: Darlington et al., 2002), but the underlying cellular mechanisms have been unclear. Our results implicate firing rate potentiation in mediating this plasticity. Vestibular nerve afferents to MVN neurons fire at high baseline rates in intact animals, providing a continuous depolarizing drive onto vestibular nucleus neurons. The loss of this excitatory drive after UVD results in decreased firing rates of vestibular nucleus neurons in vivo (Precht et al., 1966; Smith and Curthoys, 1989), a condition which induces firing rate potentiation in vitro via decreases in intracellular calcium and CaMKII levels and a reduction in BK currents (Nelson et al., 2003; Nelson et al., 2005; Hull et al, 2013). Consistent with a role for this plasticity mechanism in mediating increases in excitability after vestibular loss, we found that UVD generates a reduction in sensitivity to the specific BK channel blocker iberiotoxin and precludes the induction of firing rate potentiation.
Although BK channels are expressed ubiquitously in MVN neurons (Kodama et al, 2012), CaMKII may additionally modulate several other conductances that are differentially expressed across cell types (Smith et al, 2002; Gittis and du Lac, 2007; Kodama et al., 2012). In cerebellar Golgi cells, CamKII-dependent firing rate potentiation results in increases in spontaneous firing rate that do not depend on iberiotoxin-sensitive BK channels and is only partially occluded by blocking paxilline-sensitive BK channels (Hull et al, 2013). SK-type calcium activated potassium conductances exert a predominant influence on excitability gain in MVN neurons (Smith et al, 2002) but do not play a role in firing rate potentiation (Nelson et al, 2003). Peripheral vestibular dysfunction additionally induces dynamic changes in glucocorticoids (Cameron and Dutia, 1999), nitric oxide (Anderson, et al, 1998; Park and Jeong, 2000), and neurotransmitter levels (Yamanaka et al., 2000; Vibert et al., 2000; Johnston et al., 2001; Bergquist et al., 2008) which may enhance or counteract the effects of firing rate potentiation on MVN neuronal excitability, possibly via alterations in sodium channel gating, subthreshold potassium currents, or enzymatic regulation of BK channels (Shao et al, 2009; van Welie and du Lac, 2011). Heterogeneity of physiological factors that modulate spontaneous firing rate and gain differentially across MVN cell types (Shin et al, 2011) could account for differences in our study between the time courses and magnitudes of plasticity in measures of excitability and behavior.
Several cellular and synaptic mechanisms are likely to underlie the multiphasic timecourse of oculomotor plasticity following vestibular loss. The reductions in BK currents and increases in MVN neuronal excitability demonstrated here occur transiently in parallel with initial adaptive increases in OKR gain. Intact gradual recovery of the VOR in BK null mice indicates that longer term vestibular compensation does not depend on firing rate potentiation or modulation of BK channels, consistent with a role for synaptic plasticity in the long term restoration of vestibular function (Dieringer and Precht, 1977; Dieringer and Precht, 1979b; Dieringer and Precht, 1979a). Increases in intrinsic excitability one month after UVD (Beraneck et al, 2003) not apparent in our study could additionally contribute to restoration in VOR gain.
While the results presented here are consistent with decreases in BK currents in MVN neurons contributing to rapid potentiation of the OKR, it is likely that BK channels in other circuit elements are also important for oculomotor plasticity. BK channels are expressed in multiple cell types in oculomotor circuits, including vestibular ganglion cells, MVN neurons, the inferior olive, and cerebellar Purkinje cells (Sausbier et al., 2006). A previous study of BK channel knockout mice demonstrated alterations in cerebellar Purkinje cell excitability and short term synaptic plasticity of their inputs onto cerebellar nucleus neurons (Sausbier et al., 2004). In vivo, BK channel deletions produce irregular firing patterns in Purkinje cells (Cheron et al, 2009) and aberrant responses to climbing fiber activation (Chen et al., 2010). Interestingly, however, measures of oculomotor performance that require the integrity of the cerebellum, including the OKR and long term plasticity in VOR gain after UVD (Faulstich et al., 2006; Beraneck et al., 2008) are unaffected in BK channel null mice, suggesting that the oculomotor plasticity deficits that we observed in BK null mice may primarily reflect perturbed function in MVN neurons. Our findings demonstrate that BK channels are critical for concomitant potentiation of neuronal excitability and OKR gain triggered by vestibular loss; cell type-specific manipulations would clarify specific roles of BK channels in cerebellar-dependent oculomotor learning.
Potentiation of intrinsic excitability occurs during several other forms of behavioral plasticity, including associative conditioning (Woody and Black-Cleworth, 1973; Schreurs et al., 1998; Lorenzetti et al., 2006; Moyer et al., 1996), operant conditioning (Carp and Wolpaw, 1994; Crow and Alkon, 1980; Mozzachiodi et al., 2008), and olfactory discrimination (Saar and Barkai, 2003). Interestingly, reductions in calcium-dependent potassium currents are commonly associated with behaviorally-induced excitability increases (Alkon et al., 1985; Bekisz et al., 2010; Matthews et al., 2008). Bidirectional modulation of BK currents by kinases and phosphatases (Reinhart et al., 1991; van Welie and du Lac, 2011) serves as a potential substrate for their activity-dependent regulation. Although BK channels are expressed ubiquitously in neurons throughout the sensory-motor circuits which signal via modulations in firing rate, they are dispensable for both linear rate coding and for burst firing, making them well suited to serve as a dynamically modifiable gain-control knob (Nelson et al., 2003). Rapid potentiation of intrinsic excitability via reductions in calcium-activated potassium currents may be a common mechanism for increasing throughput in behavioral circuits and for promoting conditions for subsequent synaptic plasticity (Zhang and Linden, 2003; Mozzachiodi and Byrne, 2010).
METHODS
Surgical Procedures
C57Bl/6 mice, aged 20 to 34 days, were implanted with a headpost on the skull under deep isoflurane anesthesia as described previously (Faulstich et al., 2004) and subjected to either peripheral vestibular damage or sham surgery. For induction of unilateral vestibular damage, a small incision was made behind the ear and the tissue overlying the horizontal semicircular canal was dissected. The horizontal semicircular canal was drilled open over a length of about 1 mm and endolymphatic fluid was drained until spontaneous flow ceased. Using a blunt 30 gauge needle attached to a 2 mL syringe, air was flushed through the opened canal to expel remaining endolymphatic fluid and mechanically disrupt the sensory epithelia. A small dental paper tip (size #15; Henry Schein Dental) was introduced into the rostral canal opening and fixed in place with bone wax which was also used to seal up the caudal canal opening. Wounds were closed and sutured with two or three stitches of 6/0 suture. After the return of reflex movements, the animal was returned to its cage until use for eye movement recordings or slice preparation. Surgical disruption of vestibular function was confirmed in each animal by qualitative assessment of postural deficits and quantitative assessment of eye movements. For analyses of the contribution of BK channels to oculomotor plasticity, BK−/− (Kcnma1-/1), mice harboring a global deletion of the BK channel alpha subunit, were maintained on a C57/bl6 background and genotyped as previously described (Meredith et al, 2004); behavioral comparisons were performed at 2–3 months of age in BK−/− mice and wildtype littermates.
Eye movement recordings
The impact of unilateral vestibular damage or sham operation on the gain and phase of the vestibulo-ocular reflex (VOR) and optokinetic reflex (OKR) was determined using infrared video oculography. Experimental procedures have been described in detail previously (Faulstich et al., 2004). In short, mice were implanted with a permanent headpost under deep isoflurane anesthesia. For subsequent awake-behaving eye movement recordings, animals were briefly anesthetized and restrained in a small Plexiglas tube via the headpost. To prevent excessive pupil dilation in the dark, animals were pretreated with 0.5% physostigmine salicylate solution. Animals were placed in the center of a vestibular turntable and oriented such that the horizontal semicircular canals paralleled earth horizontal. An image of the mouse's eye was acquired with a miniature infrared video camera (Elmo 421R, Elmo, USA) and fed into a commercial video eye-tracking system (RK-726I, Iscan). Position of the pupil, pupil diameter and position of a corneal reflection induced by a reference LED that was aligned with the camera axis was recorded at a sample rate of 60Hz. Calibration of the system and conversion of pupil displacement into rotational angle of the eye was performed according to Stahl (Stahl et al., 2000; Stahl, 2002). Stimuli consisted of sinusoidal rotations of either the turntable for VORd (VOR measured in the dark) and VORl (VOR measured in light) or an optokinetic drum displaying vertical black and white striping (stripe width = 5 degrees) for OKR. Stimulus amplitudes were constant at 10 degrees peak-to-peak and stimulus frequencies ranged from Hz to 1.5 Hz.
Electrophysiology
Transverse slices of brainstem were prepared as described previously (Sekirnjak and du Lac, 2002) from unilaterally labyrinthectomized and sham-operated littermates in pairs, with the experimenters blinded to the behavioral condition of the animals. Slices were incubated in carbogenated ACSF at 35°C for 30 minutes, then at room temperature until being placed in a submersion chamber perfused with ACSF at 31–33°C for recordings. ACSF contained (in mM) 124 NaCl, 26 NaHCO3, 5 KCl, 1.3 MgCl2, 2.5 CaCl2, 1 NaH2PO4, and 11 dextrose. Kynurenic acid (Sigma, 2 mM) and picrotoxin (Sigma, 100 microM) were added to the ACSF to block most fast synaptic transmission during electrophysiological recordings. For intracellular recordings, electrodes were filled with a solution containing (in mM) 140 K gluconate, 10 HEPES, 8 NaCl, 0.1 EGTA, 2 MgATP, Na2GTP.
Neurons of the medial vestibular nucleus were visualized under differential interference contrast optics with infrared illumination, and patched in whole-cell, current-clamp mode. A subset of neurons were recorded extracellularly. Membrane potential was sampled at 3 kHz for continuous monitoring of firing rate; 40 kHz sampling was used for all other measurements. Pipette and access resistances were bridge-balanced throughout each recording, and voltage offsets were corrected after removing the electrode from the neuron. A calculated liquid junction potential of −14 mV was subtracted from all membrane potential traces.
Pharmacology
Iberiotoxin (IBTX, Alomone) was applied in the external Ringer's solution (150 nM). Periodic measures were made until the effect of the drug had saturated, assessed as consecutive measurements in which no additional changes were seen.
Data Analysis
Gain and phase of VORd, VORl and OKR were determined from the raw eye movement and stimulus traces as described previously (Faulstich et al., 2004). In short, eye and stimulus position traces acquired during the experiments were transformed into the velocity domain by digital differentiation. Saccades were automatically removed from the traces by a velocity threshold-based algorithm. Cycles exhibiting movement artifacts (blinks or animal motion) were manually edited from the recordings. Gain and phase were determined from sinusoidal fits constrained to the stimulus frequency. A forced least square sine fit was performed to the stimulus and eye velocity traces and gain and phase relation were determined from the sine fits.
Electrophysiology data collection and initial analyses were blinded to the behavioral condition of the animal. Neurons in which action potential height was <30 mV or access resistance exceeded 40 MΩ were excluded from analysis. Neurons recorded for this study resided exclusively in the rostral two-thirds of the medial vestibular nucleus. Firing response gain, defined as the slope of the mean firing rate-current input relationship, was measured by injecting depolarizing current steps of 1 sec over a range of amplitudes up to a point where the neuron could not sustain firing across the step. To fully characterize cells with bilinear firing responses (Bagnall et al., 2007), linear fits to the input-output data were separated into a low firing range (up to 80 spikes/sec) and high firing range (from 80 spikes/sec to the maximum firing rate). The maximum firing rate was calculated from the maximal depolarizing current injection during which the neuron was able to fire stably across the entire 1 second injection. Spike frequency adaptation was measured during 1 second steps, and an “adaptation ratio” was calculated at two firing frequencies for each neuron, 40 and 150 spikes/sec, to represent adaptation in a low and high firing range. Adaptation ratio was defined as the ratio of average evoked firing rate between 50 and 150 ms from current step onset to that evoked between 880 and 980 ms into the step.
Sinusoidally-varying current injections were given to neurons held at approximately 30 spikes/sec baseline firing rate (with direct current injection, if necessary). Current amplitude was adjusted to produce an amplitude of firing rate modulation of approximately 15 spikes/sec. After data collection, a sine fit was made to the plot of interspike intervals in order to calculate the amplitude, gain and phase of firing rate modulation.
Input resistance was measured periodically during each recording and calculated from the change in membrane potential evoked by small (5 to 20 pA) hyperpolarizing current steps, 500 ms in duration, applied to neurons hyperpolarized with direct current to −70 to −80 mV. Action potential shapes were averaged from 5-second traces of membrane potential measured when each neuron was firing 8–12 spikes/sec (held at this rate by direct current injection, if necessary).
Following measurements of intrinsic properties, neurons with stable firing rates greater than 2 spikes/sec were used for firing rate potentiation experiments. Spontaneous firing rate was measured continuously for 5 minutes prior to and 30 minutes following stimulation. Measurements of intrinsic properties were then repeated at 30 minutes following stimulation. Analyses of firing rate potentiation included only those neurons in which baseline firing rate changed by less than 1.5% per min. Neurons were excluded if input resistance fell by more than 15% during the course of the experiment.
Supplementary Material
Highlights.
Optokinetic reflex plasticity is rapidly triggered by vestibular deprivation
Potentiation of intrinsic plasticity in vestibular neurons parallels OKR plasticity
Reductions in BK currents increase excitability after vestibular loss
BK channel null mice are unable to increase OKR gain after vestibular loss
Acknowledgments
Funded by NIH grants EY11027 and EY017106. We thank Richard Aldrich for supporting the generation of the BK−/− mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Conceptualization, A.N. and S.dL.; Methodology, A.N., M.F. and S.dL.; Investigation, A.N., M.F., S.M., K.O, and S.dL.; Resources, A.M., Writing- Original Draft, A.N. and S.dL., Writing-Review and Editing, A.N., A.M., and SdL.; Funding Acquisition, S.dL.
References
- Alkon DL, Sakakibara M, Forman R, Harrigan J, Lederhendler I, Farley J. Reduction of two voltage-dependent K+ currents mediates retention of a learned association. Behav Neural Biol. 1985;44:278–300. doi: 10.1016/s0163-1047(85)90296-1. [DOI] [PubMed] [Google Scholar]
- Anderson TV, Moulton AR, Sansom AJ, Kerr DR, Laverty R, Darlington CL, Smith PF. Evidence for reduced nitric oxide synthase (NOS) activity in the ipsilateral medial vestibular nucleus and bilateral prepositus hypoglossi following unilateral vestibular deafferentation in the guinea pig. Brain Res. 1998;787:311–314. doi: 10.1016/s0006-8993(97)01464-9. [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Annu Rev Neurosci. 2008;31:125–150. doi: 10.1146/annurev.neuro.31.060407.125555. [DOI] [PubMed] [Google Scholar]
- Bagnall MW, Stevens RJ, du Lac S. Transgenic mouse lines subdivide medial vestibular nucleus neurons into discrete, neurochemically distinct populations. J Neurosci. 2007;27:2318–2330. doi: 10.1523/JNEUROSCI.4322-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekisz M, Garkun Y, Wabno J, Hess G, Wrobel A, Kossut M. Increased excitability of cortical neurons induced by associative learning: an ex vivo study. Eur J Neurosci. 2010;32:1715–1725. doi: 10.1111/j.1460-9568.2010.07453.x. [DOI] [PubMed] [Google Scholar]
- Beraneck M, Hachemaoui M, Idoux E, Ris L, Uno A, Godaux E, Vidal PP, Moore LE, Vibert N. Long-term plasticity of ipsilesional medial vestibular nucleus neurons after unilateral labyrinthectomy. J Neurophysiol. 2003;90:184–203. doi: 10.1152/jn.01140.2002. [DOI] [PubMed] [Google Scholar]
- Beraneck M, McKee JL, Aleisa M, Cullen KE. Asymmetric recovery in cerebellar-deficient mice following unilateral labyrinthectomy. J Neurophysiol. 2008;100:945–958. doi: 10.1152/jn.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraneck M, Idoux E. Reconsidering the role of neuronal intrinsic properties and neuromodulation in vestibular homeostasis. Front Neurol. 2012;28:3–25. doi: 10.3389/fneur.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist F, Ludwig M, Dutia MB. Role of the commissural inhibitory system in vestibular compensation in the rat. J Physiol. 2008;586:4441–4452. doi: 10.1113/jphysiol.2008.155291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt T, Strupp M, Arbusow V, Dieringer N. Plasticity of the vestibular system: central compensation and sensory substitution for vestibular deficits. Adv Neurol. 1997;73:297–309. [PubMed] [Google Scholar]
- Broussard DM, Kassardjian CD. Learning in a simple motor system. Learn Mem. 2004;11:127–136. doi: 10.1101/lm.65804. [DOI] [PubMed] [Google Scholar]
- Buttner-Ennever JA, Cohen B, Horn AK, Reisine H. Efferent pathways of the nucleus of the optic tract in monkey and their role in eye movements. J Comp Neurol. 1996;373:90–107. doi: 10.1002/(SICI)1096-9861(19960909)373:1<90::AID-CNE8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Buttner U, Waespe W. Vestibular nerve activity in the alert monkey during vestibular and optokinetic nystagmus. Exp Brain Res. 1981;41:310–315. doi: 10.1007/BF00238888. [DOI] [PubMed] [Google Scholar]
- Cameron SA, Dutia MB. Lesion-induced plasticity in rat vestibular nucleus neurons dependent on glucorticoid receptor activation. J Physiol. 1999;518:151–158. doi: 10.1111/j.1469-7793.1999.0151r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp JS, Wolpaw JR. Motoneuron plasticity underlying operantly conditioned decrease in primate H-reflex. J Neurophysiol. 1994;72:431–442. doi: 10.1152/jn.1994.72.1.431. [DOI] [PubMed] [Google Scholar]
- Cazin L, Lannou J, Precht W. An electrophysiological study of pathways mediating optokinetic responses to the vestibular nucleus in the rat. Exp Brain Res. 1984;54:337–348. doi: 10.1007/BF00236235. [DOI] [PubMed] [Google Scholar]
- Chen X, Kovalchuk Y, Adelsberger H, Henning HA, Sausbier M, Wietzorrek G, Ruth P, Yarom Y, Konnerth A. Disruption of the olivo-cerebellar circuit by Purkinje neuron-specific ablation of BK channels. Proc Natl Acad Sci U S A. 2010;107:12323–12328. doi: 10.1073/pnas.1001745107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ, Alkon DL. Associative behavioral modification in hermissenda: cellular correlates. Science. 1980;209:412–414. doi: 10.1126/science.209.4454.412. [DOI] [PubMed] [Google Scholar]
- Cullen KE. The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci. 2012;35:185–196. doi: 10.1016/j.tins.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington CL, Dutia MB, Smith PF. The contribution of the intrinsic excitability of vestibular nucleus neurons to recovery from vestibular damage. Eur J Neurosci. 2002;15:1719–1727. doi: 10.1046/j.1460-9568.2002.02024.x. [DOI] [PubMed] [Google Scholar]
- Dieringer N, Precht W. Modification of synaptic input following unilateral labyrinthectomy. Nature. 1977;269:431–433. doi: 10.1038/269431a0. [DOI] [PubMed] [Google Scholar]
- Dieringer N, Precht W. Mechanisms of compensation for vestibular deficits in the frog. I. Modification of the excitatory commissural system. Exp Brain Res. 1979a;36:311–328. doi: 10.1007/BF00238914. [DOI] [PubMed] [Google Scholar]
- Dieringer N, Precht W. Mechanisms of compensation for vestibular deficits in the frog. II. Modification of the inhibitory Pathways. Exp Brain Res. 1979b;36:329–357. doi: 10.1007/BF00238915. [DOI] [PubMed] [Google Scholar]
- Faulstich BM, Onori KA, du Lac S. Comparison of plasticity and development of mouse optokinetic and vestibulo-ocular reflexes suggests differential gain control mechanisms. Vision Res. 2004;44:3419–3427. doi: 10.1016/j.visres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Faulstich M, van Alphen AM, Luo C, du Lac S, De Zeeuw CI. Oculomotor plasticity during vestibular compensation does not depend on cerebellar LTD. J Neurophysiol. 2006;96:1187–1195. doi: 10.1152/jn.00045.2006. [DOI] [PubMed] [Google Scholar]
- Fetsch CR, Pouget A, DeAngelis GC, Angelaki DE. Neural correlates of reliability-based cue weighting during multisensory integration. Nat Neurosci. 2011;15:146–154. doi: 10.1038/nn.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, du Lac S. Intrinsic and synaptic plasticity in the vestibular system. Curr Opin Neurobiol. 2006;16:385–390. doi: 10.1016/j.conb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Gittis AH, du Lac S. Firing properties of GABAergic versus non-GABAergic vestibular nucleus neurons conferred by a differential balance of potassium currents. J Neurophysiol. 2007;97:3986–3996. doi: 10.1152/jn.00141.2007. [DOI] [PubMed] [Google Scholar]
- Guilding C, Dutia MB. Early and late changes in vestibular neuronal excitability after deafferentation. Neuroreport. 2005;16:1415–1418. doi: 10.1097/01.wnr.0000176519.42218.a6. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Holstein GR. The anatomy of the vestibular nuclei. Prog Brain Res. 2006;151:157–203. doi: 10.1016/S0079-6123(05)51006-9. [DOI] [PubMed] [Google Scholar]
- Hull CA, Chu Y, Thanawala M, Regehr WG. Hyperpolarization induces a long-term increase in the spontaneous firing rate of cerebellar Golgi cells. J. Neurosci. 2013;33:5895–5902. doi: 10.1523/JNEUROSCI.4052-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AR, Him A, Dutia MB. Differential regulation of GABA(A) and GABA(B) receptors during vestibular compensation. Neuroreport. 2001;12:597–600. doi: 10.1097/00001756-200103050-00033. [DOI] [PubMed] [Google Scholar]
- Kodama T, Guerrero S, Shin M, Moghadam S, Faulstich M, du Lac S. Neuronal classification and marker gene identification via single-cell expression profiling of brainstem vestibular neurons subserving cerebellar learning. J Neurosci. 2012;32:7819–7831. doi: 10.1523/JNEUROSCI.0543-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T, du Lac S. Adaptive acceleration of visually evoked smooth eye movements in mice. J Neurosci. 2016;36:6836–6849. doi: 10.1523/JNEUROSCI.0067-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Carpenter DO. Medial vestibular nucleus neurons are endogenous pacemakers whose discharge in modulated by neurotransmitters. Cell Mol Neurobiol. 1993;13:601–613. doi: 10.1007/BF00711560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG. The neural basis for motor learning in the vestibulo-ocular reflex in monkeys. Trends Neurosci. 1988;11:147–152. doi: 10.1016/0166-2236(88)90140-3. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA, Broussard DM. Neural basis for motor learning in the vestibulo-ocular reflex of primates: I. Changes in the responses of brainstem neurons. J. Neurophysiol. 1994;72:928–953. doi: 10.1152/jn.1994.72.2.928. [DOI] [PubMed] [Google Scholar]
- Lorenzetti FD, Mozzachiodi R, Baxter DA, Byrne JH. Classical and operant conditioning differentially modify the intrinsic properties of an identified neuron. Nat Neurosci. 2006;9:17–19. doi: 10.1038/nn1593. [DOI] [PubMed] [Google Scholar]
- Matthews EA, Weible AP, Shah S, Disterhoft JF. The BK-mediated fAHP is modulated by learning a hippocampus-dependent task. Proc Natl Acad Sci U S A. 2008;105:15154–15159. doi: 10.1073/pnas.0805855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- Morgan ML, DeAngelis GC, Angelaki DE. Multisensory integration in macaque visual cortex depends on cue reliability. Neuron. 2008;59:662–673. doi: 10.1016/j.neuron.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Jr, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci. 1996;16:5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzachiodi R, Byrne JH. More than synaptic plasticity: role of nonsynaptic plasticity in learning and memory. Trends Neurosci. 2010;33:17–26. doi: 10.1016/j.tins.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzachiodi R, Lorenzetti FD, Baxter DA, Byrne JH. Changes in neuronal excitability serve as a mechanism of long-term memory for operant conditioning. Nat Neurosci. 2008;11:1146–1148. doi: 10.1038/nn.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Gittis AH, du Lac S. Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron. 2005;46:623–631. doi: 10.1016/j.neuron.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Krispel CM, Sekirnjak C, du Lac S. Long-lasting increases in intrinsic excitability triggered by inhibition. Neuron. 2003;40:609–620. doi: 10.1016/s0896-6273(03)00641-x. [DOI] [PubMed] [Google Scholar]
- Park JS, Jeong HS. Effects of nitric oxide on the vestibular functional recovery after unilateral labyrinthectomy. Jpn J Pharmacol. 2000;84:425–430. doi: 10.1254/jjp.84.425. [DOI] [PubMed] [Google Scholar]
- Precht W, Shimazu H, Markham CH. A mechanism of central compensation of vestibular function following hemilabyrinthectomy. J. Neurophysiol. 1966;29:996–1010. doi: 10.1152/jn.1966.29.6.996. [DOI] [PubMed] [Google Scholar]
- Reinhart PH, Chung S, Martin BL, Brautigan DL, Levitan IB. Modulation of calcium-activated potassium channels from rat brain by protein kinase A and phosphatase 2A. J Neurosci. 1991;11:1627–1635. doi: 10.1523/JNEUROSCI.11-06-01627.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar D, Barkai E. Long-term modifications in intrinsic neuronal properties and rule learning in rats. Eur J Neurosci. 2003;17:2727–2734. doi: 10.1046/j.1460-9568.2003.02699.x. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Neural correlates of motor learning in the vestibulo-ocular reflex: dynamic regulation of multimodal integration in the macaque vestibular system. J Neurosci. 2010;30:10158–10168. doi: 10.1523/JNEUROSCI.1368-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Neural correlates of sensory substitution in vestibular pathways following complete vestibular loss. J Neurosci. 2012;32:14685–14695. doi: 10.1523/JNEUROSCI.2493-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, Korth M, Shipston MJ, Knaus HG, Wolfer DP, Pedroarena CM, Storm JF, Ruth P. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci U S A. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausbier U, Sausbier M, Sailer CA, Arntz C, Knaus HG, Neuhuber W, Ruth P. Ca2+ -activated K+ channels of the BK-type in the mouse brain. Histochem Cell Biol. 2006;125:725–741. doi: 10.1007/s00418-005-0124-7. [DOI] [PubMed] [Google Scholar]
- Schreurs BG, Gusev PA, Tomsic D, Alkon DL, Shi T. Intracellular correlates of acquisition and long-term memory of classical conditioning in Purkinje cell dendrites in slices of rabbit cerebellar lobule HVI. J Neurosci. 1998;18:5498–5507. doi: 10.1523/JNEUROSCI.18-14-05498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirnjak C, du Lac S. Intrinsic firing dynamics of vestibular nucleus neurons. J Neurosci. 2002;22:2083–2095. doi: 10.1523/JNEUROSCI.22-06-02083.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirnjak C, du Lac S. Physiological and anatomical properties of mouse medial vestibular nucleus neurons projecting to the oculomotor nucleus. J Neurophysiol. 2006;95:3012–3023. doi: 10.1152/jn.00796.2005. [DOI] [PubMed] [Google Scholar]
- Serafin M, de Waele C, Khateb A, Vidal PP, Muhlethaler M. Medial vestibular nucleus in the guinea-pig. I. Intrinsic membrane properties in brainstem slices. Exp Brain Res. 1991;84:417–425. doi: 10.1007/BF00231464. [DOI] [PubMed] [Google Scholar]
- Shao M, Popratiloff A, Yi J, Lerner A, Hirsch JC, Peusner KD. Adaptation of chicken vestibular nucleus neurons to unilateral vestibular ganglionectomy. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Shin M, Moghadam S, Sekirnjak C, Bagnall MW, Kolkman KE, Jacobs R, Faulstich M, du Lac S. Multiple types of cerebellar target neurons and their circuitry in the vestibulo-ocular reflex. J. Neurosci. 2011;31:10776–10786. doi: 10.1523/JNEUROSCI.0768-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutoh F, Ohki M, Kitazawa H, Itohara S, Nagao S. Memory trace of motor learning shifts transsynaptically from cerebellar cortex to nuclei for consolidation. Neuroscience. 2006;139:767–777. doi: 10.1016/j.neuroscience.2005.12.035. [DOI] [PubMed] [Google Scholar]
- Smith MR, Nelson AB, Du Lac S. Regulation of firing response gain by calcium-dependent mechanisms in vestibular nucleus neurons. J Neurophysiol. 2002;87:2031–2042. doi: 10.1152/jn.00821.2001. [DOI] [PubMed] [Google Scholar]
- Smith PF, Curthoys IS. Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Res Brain Res Rev. 1989;14:155–180. doi: 10.1016/0165-0173(89)90013-1. [DOI] [PubMed] [Google Scholar]
- Stahl JS. Calcium channelopathy mutants and their role in ocular motor research. Ann N Y Acad Sci. 2002;956:64–74. doi: 10.1111/j.1749-6632.2002.tb02809.x. [DOI] [PubMed] [Google Scholar]
- Stahl JS. Using eye movements to assess brain function in mice. Vision Res. 2004;44:3401–3410. doi: 10.1016/j.visres.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Stahl JS, van Alphen AM, De Zeeuw CI. A comparison of video and magnetic search coil recordings of mouse eye movements. J Neurosci Methods. 2000;99:101–110. doi: 10.1016/s0165-0270(00)00218-1. [DOI] [PubMed] [Google Scholar]
- Straka H, Vibert N, Vidal PP, Moore LE, Dutia MB. Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog Neurobiol. 2005;76:349–392. doi: 10.1016/j.pneurobio.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu Rev Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- van Welie I, du Lac S. Bidirectional control of BK channel open probability by CAMKII and PKC in medial vestibular nucleus neurons. J Neurophysiol. 2011;105:1651–1659. doi: 10.1152/jn.00058.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert N, Babalian A, Serafin M, Gasc JP, Muhlethaler M, Vidal PP. Plastic changes underlying vestibular compensation in the guinea-pig persist in isolated, in vitro whole brain preparations. Neuroscience. 1999;93:413–432. doi: 10.1016/s0306-4522(99)00172-4. [DOI] [PubMed] [Google Scholar]
- Vibert N, Beraneck M, Bantikyan A, Vidal PP. Vestibular compensation modifies the sensitivity of vestibular neurones to inhibitory amino acids. Neuroreport. 2000;11:1921–1927. doi: 10.1097/00001756-200006260-00023. [DOI] [PubMed] [Google Scholar]
- Woody CD, Black-Cleworth P. Differences in excitability of cortical neurons as a function of motor projection in conditioned cats. J Neurophysiol. 1973;36:1104–1116. doi: 10.1152/jn.1973.36.6.1104. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Him A, Cameron SA, Dutia MB. Rapid compensatory changes in GABA receptor efficacy in rat vestibular neurones after unilateral labyrinthectomy. J Physiol. 2000;523(Pt 2):413–424. doi: 10.1111/j.1469-7793.2000.t01-1-00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.