Abstract
In this study, we sought to determine the efficacy of tempol on multiple neuropathic endpoints in a diet-induced obese mouse, a model of pre-diabetes, and a high-fat fed low-dose streptozotocin treated mouse, a model of type 2 diabetes. Tempol (4-hydroxy-2,2,6,6-tetramethylpiperdine-1-oxyl) is a low molecular weight, water soluble, membrane permeable, and metal-independent superoxide dismutase mimetic that has been widely used in cellular studies for the removal of intracellular and extracellular superoxide. This in vivo study was designed to be an early intervention. Fourteen weeks post-high-fat diet (6 weeks post-hyperglycemia) control, obese, and diabetic mice were divided into no treatment and treatment groups. The treated mice received tempol by gavage (150 mg/kg in water), while the untreated mice received vehicle. The diet-induced obese and the diabetic mice were maintained on the high-fat diet for the duration of the study, while the control group was maintained on the standard diet. Obesity and diabetes caused slowing of motor and sensory nerve conduction, reduction in intraepidermal nerve fiber density, thermal hypoalgesia, and mechanical allodynia. Treatment with tempol partially or completely protected obese and diabetic mice from these deficits. These studies suggest that tempol or other effective scavengers of reactive oxygen species may be a viable option for treating neural complications associated with obesity or type 2 diabetes.
Keywords: Tempol, diabetic neuropathy, diabetes, sensory nerve fibers
Introduction
Increased oxidative stress has been implicated in the pathology of a variety of metabolic disorders and diseases including obesity and diabetic vascular and neural complications [1–3]. Oxidative stress is a condition resulting from an imbalance between the generation of reactive oxygen species (ROS) and the ability of antioxidant mechanisms to neutralize these compounds. Therefore, increased oxidative stress is the consequence of enhanced ROS production and/or attenuated ROS scavenging capacity, resulting in tissue damage [4]. The most common forms of ROS are superoxide ( ), hydrogen peroxide (H2O2), hydroxyl radical (OH−), and peroxynitrite (ONOO−) [5]. A number of non-enzymatic and enzymatic sources of ROS production particularly , located throughout the cell, including the plasma membrane, cytosol, mitochondria, and peroxisomes, have been identified in obesity and diabetes [5–7].
Because of limitations associated with enzyme therapies, superoxide dismutase mimetics have been developed to treat diseases mediated by radicals [8–11]. Previously, we demonstrated that treating diabetic rats with M40403, a superoxide dismutase mimetic, inhibited the generation of superoxide by aorta and epineurial vessels of the sciatic nerve, the formation of peroxynitrite by epineurial vessels of the sciatic nerve, the reduction in endoneurial blood flow, the slowing of motor nerve conduction velocity, and impairment of endothelium-dependent vasodilation of arterioles that provide circulation to the sciatic nerve [12]. Another superoxide dismutase mimetic that has demonstrated promising results on diabetic complication is 4-hydroxy-2,2,6,6-tetramethylpiperdine-1-oxyl (tempol). Tempol is a low molecular weight, water soluble, membrane permeable, and metal independent superoxide dismutase mimetic that has been widely used for the removal of intracellular and extracellular [6]. Tempol does not scavenge H2O2 but can prevent H2O2-mediated injury by reducing the intracellular concentrations of Fe2+ and hence the formation of hydroxyl radicals [13]. In animal studies of obese and diabetic rodents, tempol has been shown to improve insulin sensitivity, erectile dysfunction, endothelial dysfunction, renal changes, and early retinal damage [6,13,14–20]. However, no information is available of the effect of administration of tempol on neuropathy associated with obesity and diabetes. In these studies, we explored the effect of daily treatment of tempol on neuropathic endpoints in a mouse model of pre-diabetes (diet-induced obesity) and type 2 diabetes [21].
Methods
Unless stated otherwise all chemicals used in these studies were obtained from Sigma Aldrich Chemical Co. (St. Louis, MO).
Animals
C57Bl/6 wild-type mice were purchased from Jackson Laboratories. Mice were housed in a certified animal care facility and food (Harlan Teklad, #7001, Madison, WI) and water were provided ad libitum. Adequate measures were taken to minimize pain or discomfort and all of the experiments were conducted in accordance with international standards on animal welfare and were compliant with all institutional and National Research Council’s guidelines for use of animals (ACURF protocol 1390201).
For the study, C57Bl/6J mice at 12 weeks of age were divided into six groups. After 1 week on a standard diet (Teklad 7001 (protein 25.2 gm%, carbohydrate 39.5 gm% and fat 4.4 gm%), Envigo, Madison, WI) four of the groups were fed a 60 kcal% high-fat diet; containing protein 26.2 gm%, carbohydrate 26.3 gm% and fat 34.9 gm% (D12492; Research Diets, New Brunswick, NJ). The other two groups (designated to be the control and control + tempol groups) remained on the standard diet for the duration of the study period. To create the type 2 diabetic model, two groups of the high-fat fed mice, after 8 weeks on the high-fat diet, were treated with 100 mg/kg streptozotocin, i.p. (EMD Chemicals, San Diego, CA) followed 3 d later if necessary with a second dose of streptozotocin (50 mg/kg). Mice with blood glucose ≥13.8 mM (250 mg/dl) 1 week later were considered diabetic. These two groups (designated to be the diabetic and diabetic + tempol groups) as well as the other two groups of non-diabetic high-fat fed mice (designated to be the obesity and obesity + tempol groups) remained on the high-fat diet for the duration of the study period. After an additional 6 weeks, one group of control mice, high-fat fed (obesity) mice, and diabetic mice were designated as the treatment groups and were treated with tempol by gavage (150 mg/kg in water) daily. Untreated mice received vehicle. The treatment phase was 6 weeks.
Behavioral examinations
Thermal nociceptive response in the hindpaw was measured using the Hargreaves method with instrumentation provided by IITC Life Science; Woodland Hills, CA (model 390G). The mouse was placed in the observation chamber on top of the thermal testing apparatus and allowed to acclimate to the warmed glass surface (30 °C) and surroundings for a period of 15 min. The mobile heat source was maneuvered so that it was under the heel of the hindpaw and then activated, a process that activates a timer and locally warms the glass surface, when the mouse withdrew its paw, the timer, and the heat source was turned off [21]. Following an initial recording, which was discarded, four measurements were made for each hindpaw, with a rest period of 5 min between each set of measurements. The mean of the measurements, reported in seconds, was used as a measure of the thermal nociceptive response latency. Tactile responses were evaluated by quantifying the withdrawal threshold of the hindpaw in response to stimulation with flexible von Frey filaments as previously described [22]. The data were reported in grams. Each of these tests was repeated at least three times with a rest period of 10 min between tests. These tests were performed in a masked manner and completed immediately before the terminal procedures on different days.
Motor and sensory nerve conduction velocity
Mice were anesthetized with Nembutal (75 mg/kg, i.p., Abbott Laboratories, North Chicago, IL). Non-fasting blood glucose levels were determined with the use of glucose oxidase reagent strips (Lifescan Inc., Milpitas, CA). Afterwards, motor and sensory nerve conduction velocities were determined as previously described [21]. Motor nerve conduction velocity (MNCV) was determined noninvasively in the sciatic-posterior tibial conducting system [21]. Sensory nerve conduction velocity (SNCV) was determined using the digital nerve to the second toe [21]. The MNCV and SNCV were reported in meters per second.
Skin intraepidermal nerve fiber density
Footpads were fixed in ice-cold Zamboni’s fixative for 3 h, washed in 100 mM phosphate-buffered saline (PBS) overnight, and then in PBS containing increasing amounts of sucrose i.e. 10%, 15%, and 20%, 3 h in each solution [23]. After washing, the samples were snap frozen in O.C.T. (Sakura Finetek, Torrance, CA) and stored at −80 °C. Three longitudinal 30 μm-thick footpad sections were cut using a Reichert-Jung cryocut 1800 (Leica Microsystems, Nussloch, Germany). Non-specific binding was blocked by 3% goat serum containing 0.5% porcine gelatin and 0.5% Triton X-100 in SuperBlock blocking buffer (Thermo Scientific, Rockford, IL) at room temperature for 2 h. The sections were then incubated overnight with PGP 9.5 antiserum (UltraClone, Isle of Wight, UK) in 1:400 dilution at 4 °C, after which secondary Alexa Fluor 488 conjugated goat anti-rabbit antibody (Invitrogen, Eugene, OR) in 1:1000 dilution was applied at room temperature for 1 h. Sections were then coverslipped with VectaShield mounting medium (Vector Laboratories, Burlingame, CA). Profiles were imaged using a Zeiss LSM710 confocal microscope with a 40× objective and were counted by two individual investigators that were masked to the sample identity. All immunoreactive profiles within the epidermis were counted and normalized to epidermal length. Length of the epidermis was determined by drawing a polyline along the contour of the epidermis and recording its length in mm. The number of intraepidermal nerve fiber profiles was reported per mm length.
Analyses in liver and serum
Protein bound 3-nitrotyrosine concentration was measured in liver samples by indirect enzyme-linked immunosorbent assay as described [24] and modified by [25]. Briefly, liver samples were homogenized in 0.05 M sodium phosphate buffer, pH 9.0, and then centrifuged to collect the supernatant, which was used for the analysis. Serum was used for determining levels of free fatty acid, triglyceride and free cholesterol using commercial kits from Roche Diagnostics, Mannheim, Germany; Sigma Aldrich Chemical Co., St. Louis, MO; and BioVision, Mountain View, CA, respectively [24]. Western blot analysis was used to assess 4-hydroxynonenal adducts in mouse sciatic nerve as previously described [23]. Sciatic nerve segments (~20 mg) were placed on ice in 200 μl of radioimmunoprecipitation assay buffer (RIPA) containing 50 mM Tris-HCl, pH 7.2, 150 mM NaCl; 0.1% sodium dodecyl sulfate, 1% NP-40, 5 mM EDTA, 1 mM EGTA, 1% sodium deoxycholate and the protease/phosphatase inhibitors leupeptin (10 μg/ml), pepstatin (1 μg/ml), aprotinin (20 μg/ml), benzamidine (10 mM), phenylmethylsulfonyl fluoride (1 mM), sodium orthovanadate (1 mM), and homogenized. The homogenates were sonicated and centrifuged at 14,000 g for 20 min. All the afore-mentioned steps were performed at 4 °C. The lysates (40 μg protein) were mixed with equal volumes of 2× sample-loading buffer containing 62.5 mM Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate, 5% β-mercaptoethanol, 10% glycerol and 0.025% bromophenol blue, and fractionated in 5–20% SDS-PAGE in an electrophoresis cell (Mini-Protean III; Bio-Rad Laboratories, Richmond, CA). Electrophoresis was conducted at 15 mA constant current for stacking, and at 35 mA for protein separation. Gel contents were electro-transferred (80 V, 2 h) to nitrocellulose membranes using Mini Trans-Blot cell (Bio-Rad Laboratories, Richmond, CA) and western transfer buffer (25 mM Tris-HCl, pH 8.3; 192 mM glycine; and 20% (v/v) methanol). Free binding sites were blocked in 3% (w/v) BSA, all diluted in 20 mM Tris-HCl buffer, pH 7.5, containing 150 mM NaCl and 0.1% Tween 20, for 1 h. After blocking free binding sites, primary antibody to 4-hydroxynonenal adducts (EMD Millipore Corp., Billerica, MA) was applied overnight, at 4 °C. Then the anti-rabbit secondary antibody was applied at room temperature for 1 h. Protein bands detected by the antibodies were visualized with Amersham ECL™ Western Blotting Detection Reagent (Little Chalfont, Buckinghamshire, UK). Afterwards, the membranes were stripped and re-probed with β-actin antibody to confirm equal protein loading. Stripping was conducted in 25 mM glycine–HCl, pH 2.5 buffer containing 2% SDS. The data were quantified by densitometry (Quantity One 4.6.9 software, Bio-Rad Laboratories, Richmond, CA).
Data analysis
The results are presented as mean ± SE. Comparisons between the groups for body weight, blood glucose, MNCV, SNCV, and thermal nociception latency were conducted using a one-way ANOVA and Bonferroni’s test for multiple comparisons (Prism software; GraphPad, San Diego, CA). A p value of less than 0.05 was considered significant.
Results
Data in Table 1 show that all mice at the beginning of the study weighed approximately the same. After 20 weeks of a high-fat diet the untreated and treated diet-induced obese mice weighed significantly more than the control mice. The untreated and treated diabetic mice also weighed significantly more than age-matched control mice but less than the diet-induced obese mice. Blood glucose levels in diet-induced obese mice were similar compared with control mice and this was unchanged by treatment. Untreated diabetic mice were hyperglycemic at the end of the study period compared with control mice (Table 1). Treating diabetic mice with tempol significantly lowered blood glucose levels compared with untreated diabetic mice but blood glucose levels in tempol-treated diabetic mice remained significantly increased compared with control mice and control treated with tempol. Serum triglyceride and free fatty acid levels in untreated and treated diet-induced obese mice and diabetic mice were similar to untreated or treated control mice. Serum cholesterol levels were significantly increased in untreated diet-induced obese mice and diabetic mice compared with control mice and were unchanged by treatment with tempol. Liver nitrotyrosine and sciatic nerve 4-hydroxynonenal levels were determined as a measurement of oxidative stress. Diet-induced obesity and diabetes caused an increase in liver nitrotyrosine and sciatic nerve 4-hydroxynonenal levels compared with control mice (Table 1). Treating diet-induced obese mice and diabetic mice with tempol resulted in lower liver nitrotyrosine and sciatic nerve 4-hydroxynonenal levels compared with untreated obese and diabetic mice, respectively.
Table 1.
Effect of diet-induced obesity and type 2 diabetes and treatment with tempol in C57Bl/6J mice on change in body weight, blood glucose, serum triglycerides, free fatty acids, cholesterol, liver nitrotyrosine, and sciatic nerve 4-hydroxynonenal adducts.
| Determination | Control (22) | Control + tempol (11) | Obese (11) | Obese + tempol (11) | Diabetic (22) | Diabetic + tempol (21) |
|---|---|---|---|---|---|---|
| Start weight (g) | 26.6 ± 0.3 | 27.7 ± 0.5 | 26.8 ± 0.5 | 25.7 ± 0.6 | 25.5 ± 0.5 | 26.0 ± 0.4 |
| End weight (g) | 30.9 ± 0.9 | 32.1 ± 1.0 | 50.5 ± 2.5a | 50.8 ± 1.7a | 38.4 ± 1.7a | 40.1 ± 1.7a |
| Blood glucose (mg/dl) | 185 ± 5 | 201 ± 9 | 206 ± 10 | 188 ± 10 | 425 ± 24a | 342 ± 24a,b |
| Serum triglycerides (mg/dl) | 100 ± 4 | 90 ± 5 | 80 ± 8 | 87 ± 8 | 111 ± 18 | 88 ± 14 |
| Serum free fatty acids (mmol/l) | 0.33 ± 0.02 | 0.45 ± 0.05 | 0.42 ± 0.03 | 0.45 ± 0.04 | 0.48 ± 0.05 | 0.37 ± 0.04 |
| Serum cholesterol (mg/ml) | 1.2 ± 0.1 | 1.2 ± 0.2 | 4.7 ± 0.6a | 4.1 ± 0.9a | 4.0 ± 0.3a | 3.4 ± 0.4a |
| Liver nitrotyrosine (pmol/mg protein) | 7.4 ± 0.3 | 6.9 ± 0.5 | 9.3 ± 0.7a | 7.8 ± 0.5 | 8.9 ± 0.4a | 7.8 ± 0.3 |
| Sciatic Nerve 4-Hydroxynonenal adducts (%) | 100 ± 1.7 | 97.3 ± 6.0 | 137.7 ± 6.4a | 80.9 ± 7.7b | 122.7 ± 6.5 | 65.8 ± 6.5b |
Data are presented as the mean ± S.E.M.
p < 0.05 compared with control mice.
p < 0.05 compared with untreated obese or diabetic mice. Parentheses indicate the number of experimental animals.
Data in Figure 1 demonstrated that treating control mice with tempol had no effect on motor or sensory nerve conduction velocity. Motor nerve conduction velocity trended to be slower in diet-induced obese mice but was not significantly different from control mice and this was unchanged with tempol treatment. However, sensory nerve conduction velocity was significantly decreased in diet-induced obese mice compared with control mice and was significantly improved with tempol treatment. In diabetic mice, both motor and sensory nerve conduction velocities were decreased compared with control mice. Treating diabetic mice for 6 weeks with tempol following 6 weeks of untreated hyperglycemia completely prevented the decrease in motor and sensory nerve conduction velocity observed in untreated diabetic mice (Figure 1). Other common endpoints for diabetic peripheral neuropathy are intraepidermal nerve fiber density and mechanical and thermal sensitivity. Data in Figure 2 demonstrate that diet-induced obese mice are hypoalgesic and have significantly decreased intraepidermal nerve fiber profiles compared to control mice. Treating diet-induced obese mice with tempol did not improve thermal nociception but did partially improve intraepidermal nerve fiber density. Untreated diabetic mice have an increased latency to thermal stimulation and intraepidermal nerve fiber density is significantly decreased compared to control mice. Treating diabetic mice with tempol significantly partially improved both these neuropathic endpoints compared with untreated diabetic mice but both also remained significantly impaired compared to control mice. Mechanical allodynia exists in both diet-induced obese mice and to a greater extent in diabetic mice compared with control mice. Treating diabetic mice but not diet-induced obese mice with tempol for 6 weeks significantly corrected mechanical sensitivity compared with untreated obese and diabetic mice, respectively. However, treating diabetic mice with tempol only improved mechanical sensitivity partially and this remained significantly impaired compared with control mice (Figure 3).
Figure 1.
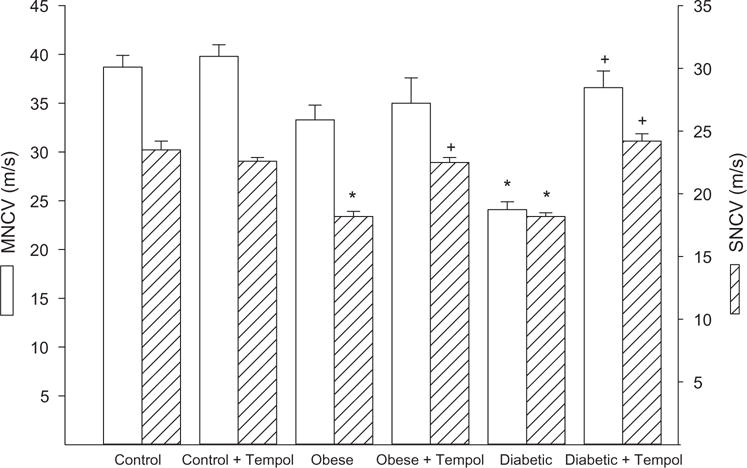
Motor and sensory nerve conduction velocity for control, diet-induced obese, and type 2 diabetic mice treated with or without tempol. Motor and sensory nerve conduction was determined as described in the Methods section. Data are the mean ± S.E.M. The number of mice in each group was the same as shown in Table 1. (*p < 0.05, compared with control mice; + p < 0.05, compared with untreated obese or diabetic mice, respectively.)
Figure 2.
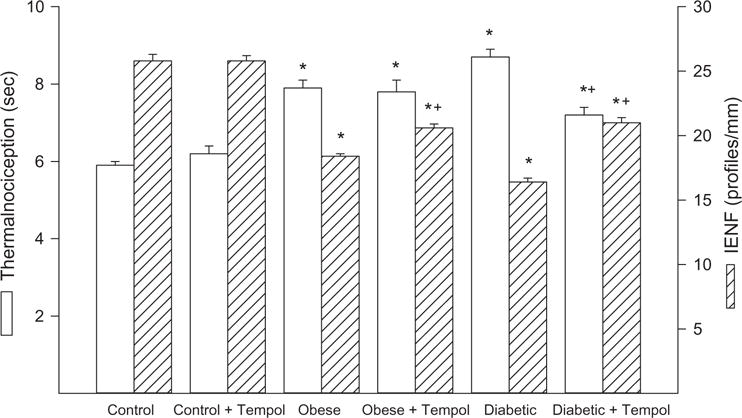
Intraepidermal nerve fiber density and thermal nociception for control, diet-induced obese and type 2 diabetic mice treated with or without tempol. Intraepidermal nerve fiber density and thermal nociception were determined as described in the Methods section. Data are the mean ± S.E.M. The number of mice in each group was the same as shown in Table 1. (*p < 0.05, compared with control mice; + p < 0.05, compared with untreated obese or diabetic mice, respectively.)
Figure 3.
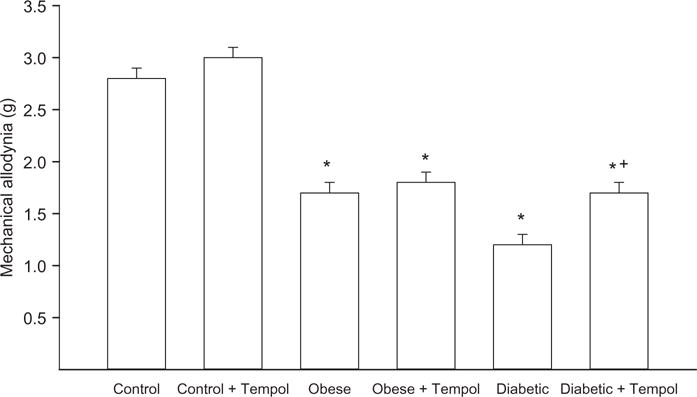
Mechanical sensitivity for control, diet-induced obese, and type 2 diabetic mice treated with or without tempol. Mechanical sensitivity was determined as described in the Methods section. Data are the mean ± S.E.M. The number of mice in each group was the same as shown in Table 1. (*p < 0.05, compared with control mice; +p < 0.05, compared with untreated obese and diabetic mice, respectively.)
Discussion
In a review article by Wilcox and Pearlman, they described tempol to be the most extensively studied nitroxide and to be broadly effective in detoxifying reactive oxygen species in cell and animal studies [26]. Tempol has been shown to preserve mitochondria against oxidative damage and improve tissue oxygenation, improve insulin sensitivity, vascular reactivity, renal function and erectile dysfunction in rodent models of diabetes, improve cardiac function and reduce hypertension in high-fat fed models of obesity, and protect heart and brain from ischemia/reperfusion injury [6,15,16,18–20,27–30]. The toxicity of tempol in rodents seems limited but, apart from external application, there are no reports of administration of tempol to humans [27,31].
In the present study, we report that treating a mouse model of type 2 diabetes with tempol improved or slowed the progression of many neuropathic endpoints of diabetic peripheral neuropathy [21]. This study may be the first evidence of tempol improving peripheral neuropathy in an animal model of diabetes. However, tempol treatment of diet-induced obese mice was only partially effective on these same endpoints. Treating diet-induced obese mice with tempol improved sensory nerve conduction velocity and partially improved intraepidermal nerve fiber density but did not improve thermal nociception or mechanical allodynia. Tempol treatment of both diet-induced obese mice and diabetic mice corrected markers of oxidative stress in the liver and sciatic nerve. This suggests that mechanisms other than oxidative stress are contributing to the neuropathology associated with obesity.
It has been previously reported that tempol can improve cognitive dysfunction in diabetic rats [32]. Other studies have shown that tempol improves cognitive deficits in other disease states. Tempol was found to protect against cerebral oxidative stress and improve cerebral circulation and cognitive dysfunction in uremic mice and transgenic mice that overexpress amyloid beta peptide [33,34]. Tempol also improved mitochondrial and neurobehavioral deficits in an experimental animal model of Huntington’s disease [35].
Tempol lowered blood glucose levels in diabetic mice. The blood glucose level after 6 weeks of untreated hyperglycemia in mice randomized to the tempol treatment group was 408 ± 12 and after 6 weeks of treatment with tempol blood glucose levels declined to 343 ± 28 (n = 12, p < 0.08). Blood glucose in diabetic mice randomized to the no treatment group after 6 weeks of no treatment was 417 ± 11 and after 6 more weeks of no treatment increased to 437 ± 28. Tempol has been shown to improve insulin sensitivity in obese Zucker rats and rats fed a high-fat high-sucrose diet, although there is no evidence in the literature demonstrating that tempol improves insulin sensitivity in a rodent model of type 2 diabetes [13,36,37]. The impact of the minor lowering of blood glucose in diabetic mice treated with tempol on diabetic peripheral neuropathy would likely be minimal. Tempol treatment had little effect on serum hyper cholesterol in diet-induced obese mice and diabetic mice but did lower liver nitrotyrosine and sciatic nerve hydroxynonenal levels, an indication of tempol reducing oxidative stress. In other studies, tempol has also been shown to reduce nitrotyrosine levels in the kidney and retina of diabetic rats [17,38,39]. Neutralization of reactive oxygen species is the action of tempol in many biological systems.
Sensory nerve conduction velocity and both motor and sensory nerve conduction velocities were fully protected in diet-induced obese mice and diabetic mice treated with tempol, respectively. In previous studies, we have shown that nerve conduction velocity is significantly decreased at 4 weeks after the onset of hyperglycemia in type 2 diabetic mice (unpublished observation). Thus, in these studies, treatment with tempol was able to reverse the slowing of nerve conduction velocity in a mouse modeling type 2 diabetes. In contrast, our data imply that tempol treatment of type 2 diabetic mice slowed the progression of loss of intraepidermal nerve fibers and thermal and mechanical deficits. In these mice, loss of intraepidermal nerve fibers and thermal hypoalgesia occurs after 6–8 weeks of hyperglycemia [21]. Treatment of diabetic mice after 6 weeks of untreated diabetes significantly improved these neuropathic endpoints compared with untreated diabetic mice but they remained significantly impaired compared with control mice after 6 weeks of treatment. It is possible that a longer duration of treatment or initiating treatment earlier would have had a greater impact on these endpoints.
In conclusion, our studies provide evidence that tempol, a superoxide dismutase mimetic, can either reverse or slow progression of neuropathic endpoints associated with obesity and to a greater extent in a mouse modeling type 2 diabetes.
Acknowledgments
Funding
This material is based upon work supported in part by the U.S. Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Rehabilitation Research and Development Service (RX000889-05) and by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK107339-02 from NIH.
Footnotes
Disclosure statement
There are no conflicts of interest to be declared by any of the authors. The content of this manuscript is new and solely the responsibility of the authors and does not necessarily represent the official views of the granting agencies.
References
- 1.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Rosen P, Nawroth PP, King G, Moller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- 3.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280:C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 4.van Dam PS. Oxidative stress and diabetic neuropathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2002;18:176–184. doi: 10.1002/dmrr.287. [DOI] [PubMed] [Google Scholar]
- 5.Schnackenberg CG. Physiological and pathophysiological roles of oxygen radicals in the renal microvasculature. Am J Physiol Regul Integr Comp Physiol. 2002;282:R335–R342. doi: 10.1152/ajpregu.00605.2001. [DOI] [PubMed] [Google Scholar]
- 6.Kawakami T, Urakami S, Hirata H, Tanaka Y, Nakajima K, Enokida H, et al. Superoxide dismutase analog (Tempol: 4-hydroxy-2,2,6,6-tetramethylpiperdine 1-oxyl) treatment restores erectile function in diabetes-induced impotence. Int J Impot Res. 2009;21:348–355. doi: 10.1038/ijir.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yorek MA. The role of oxidative stress in diabetic vascular and neural disease. Free Radic Res. 2003;37:471–480. doi: 10.1080/1071576031000083161. [DOI] [PubMed] [Google Scholar]
- 8.Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135–159. [PubMed] [Google Scholar]
- 9.Doggrell SA. Therapeutic potential of selective superoxide dismutase mimetics. Drugs Fut. 2002;27:385–390. [Google Scholar]
- 10.Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, et al. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 11.Salvemini D, Riley DP. M40403. Drugs Fut. 2000;25:1027–1033. [Google Scholar]
- 12.Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Lund DD, Salvemini D, Yorek MA. Effect of M40403 treatment of diabetic rats on endoneurial blood flow, motor nerve conduction velocity and vascular function of epineurial arterioles of the sciatic nerve. Br J Pharmacol. 2001;134:21–29. doi: 10.1038/sj.bjp.0704216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banday AA, Marwaha A, Tallam LS, Lokhandwala MF. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor-G-protein coupling and function in obese Zucker rats. Diabetes. 2005;54:2219–2226. doi: 10.2337/diabetes.54.7.2219. [DOI] [PubMed] [Google Scholar]
- 14.Freidja ML, Vessieres E, Toutain B, Guihot AL, Custaud MA, Loufrani L, et al. AGEs breaking and antioxidant treatment improves endothelium-dependent dilation without effect on flow-mediated remodeling of resistance arteries in old Zucker diabetic rats. Cardiovasc Diabetol. 2014;13:55–71. doi: 10.1186/1475-2840-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oniki H, Goto K, Fujii K, Kansui Y, Murakami N, Ohtsubo T, et al. Effects of the superoxide dismutase mimetic tempol on impaired endothelium-dependent and endothelium-independent relaxations in type II diabetic rats. Clin Exp Hypertens. 2013;35:112–119. doi: 10.3109/10641963.2012.702829. [DOI] [PubMed] [Google Scholar]
- 16.Luan J, Li W, Han J, Zhang W, Gong H, Ma R. Renal protection of in vivo administration of tempol in streptozotocin-induced diabetic rats. J Pharmacol Sci. 2012;119:167–176. doi: 10.1254/jphs.12002fp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosales MA, Silva KC, Lopes de Faria JB, Lopes de Faria JM. Exogenous SOD mimetic tempol ameliorates the early retinal changes reestablishing the redox status in diabetic hypertensive rats. Invest Ophthalmol Vis Sci. 2010;51:4327–4336. doi: 10.1167/iovs.09-4690. [DOI] [PubMed] [Google Scholar]
- 18.Ebenezer PJ, Mariappan N, Elks CM, Haque M, Francis J. Diet-induced renal changes in Zucker rats are ameliorated by the superoxide dismutase mimetic TEMPOL. Obesity (Silver Spring) 2009;17:1994–2002. doi: 10.1038/oby.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnackenberg CG, Wilcox CS. The SOD mimetic tempol restores vasodilation in afferent arterioles of experimental diabetes. Kidney Int. 2001;59:1859–1864. doi: 10.1046/j.1523-1755.2001.0590051859.x. [DOI] [PubMed] [Google Scholar]
- 20.Haj-Yehia AI, Nassar T, Assaf P, Nassar H, Anggard EE. Effects of the superoxide dismutase-mimic compound TEMPOL on oxidant stress-mediated endothelial dysfunction. Antioxid Redox Signal. 1999;1:221–232. doi: 10.1089/ars.1999.1.2-221. [DOI] [PubMed] [Google Scholar]
- 21.Yorek MS, Obrosov A, Shevalye H, Holmes A, Harper MM, Kardon RH, Yorek MA. Effect of diet-induced obesity or type 1 or type 2 diabetes on corneal nerves and peripheral neuropathy in C57Bl/6J mice. J Peripher Nerv Syst. 2015;20:24–31. doi: 10.1111/jns.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obrosova IG, Drel VR, Oltman CL, Mashtalir N, Tibrewala J, Groves JT, Yorek MA. Role of nitrosative stress in early neuropathy and vascular dysfunction in streptozotocin-diabetic rats. Am J Physiol Endocrinol Metab. 2007;293:E1645–E1655. doi: 10.1152/ajpendo.00479.2007. [DOI] [PubMed] [Google Scholar]
- 23.Stavniichuk R, Shevalye H, Lupachyk S, Obrosov A, Groves JT, Obrosova IG, Yorek MA. Peroxynitrite and protein nitration in the pathogenesis of diabetic peripheral neuropathy. Diabetes Metab Res Rev. 2014;30:669–678. doi: 10.1002/dmrr.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber D, Kneschke N, Grimm S, Bergheim I, Breusing N, Grune T. Rapid and sensitive determination of protein–nitrotyrosine by ELISA: application to human plasma. Free Radic Res. 2012;46:276–285. doi: 10.3109/10715762.2011.652627. [DOI] [PubMed] [Google Scholar]
- 25.Yorek MS, Obrosov A, Shevalye H, Lupachyk S, Harper MM, Kardon RH, Yorek MA. Effect of glycemic control on corneal nerves and peripheral neuropathy in streptozotocin-induced diabetic C57Bl/6J mice. J Peripher Nerv Syst. 2014;19:205–217. doi: 10.1111/jns.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharmacol Rev. 2008;60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther. 2010;126:119–145. doi: 10.1016/j.pharmthera.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng H, Liu X, Patel KP. Centrally mediated erectile dysfunction in rats with type 1 diabetes: role of angiotensin II and superoxide. J Sex Med. 2013;10:2165–2176. doi: 10.1111/jsm.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vessieres E, Guihot AL, Toutain B, Maquigneau M, Fassot C, Loufrani L, Henrion D. COX-2-derived prostanoids and oxidative stress additionally reduce endothelium-mediated relaxation in old type 2 diabetic rats. PLoS One. 2013;8:e68217. doi: 10.1371/journal.pone.0068217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peixoto EB, Pessoa BS, Biswas SK, Lopes de Faria JB. Antioxidant SOD memetic prevents NADPH oxidase-induced oxidative stress and renal damage in the early stage of experimental diabetes and hypertension. Am J Nephrol. 2009;29:309–318. doi: 10.1159/000163767. [DOI] [PubMed] [Google Scholar]
- 31.Simonsen U, Christensen FH, Buus NH. The effect of tempol on endothelium-dependent vasodilatation and blood pressure. Pharmacol Ther. 2009;122:109–124. doi: 10.1016/j.pharmthera.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Jabbarpour Z, Shahidi S, Saidijam M, Sarihi A, Hassanzadeh T, Esmaeili R. Effect of tempol on the passive avoidance and novel object recognition task in diabetic rats. Brain Res Bull. 2014;101:51–56. doi: 10.1016/j.brainresbull.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Fujisaki K, Tsuruya K, Yamato M, Toyonaga J, Noguchi H, Nakano T, et al. Cerebral oxidative stress induces spatial working memory dysfunction in uremic mice: neuroprotective effect of tempol. Nephrol Dial Transplant. 2014;29:529–538. doi: 10.1093/ndt/gft327. [DOI] [PubMed] [Google Scholar]
- 34.Hamel E. Cerebral circulation: function and dysfunction in Alzheimer’s disease. J Cardiovasc Pharmacol. 2015;65:317–324. doi: 10.1097/FJC.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 35.Sandhir R, Mahajan N, Mehrotra A, Aggarwal A, Sunkaria A. 4-Hydroxy tempo improves mitochondrial and neurobehavioral deficits in experimental model of Huntington’s disease. Synapse. 2015;69:128–138. doi: 10.1002/syn.21793. [DOI] [PubMed] [Google Scholar]
- 36.Bourgoin F, Bachelard H, Badeau M, Lariviere R, Nadeau A, Pitre M. Effects of tempol on endothelial and vascular dysfunctions and insulin resistance induced by a high-fat high-sucrose diet in the rat. Can J Physiol Pharmacol. 2013;91:547–561. doi: 10.1139/cjpp-2012-0273. [DOI] [PubMed] [Google Scholar]
- 37.Xiang L, Dearman J, Abram SR, Carter C, Hester RL. Insulin resistance and impaired functional vasodilation in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2008;294:H1658–H1666. doi: 10.1152/ajpheart.01206.2007. [DOI] [PubMed] [Google Scholar]
- 38.Abu-Saleh N, Awad H, Khamaisi M, Armaly Z, Karram T, Heyman SN, et al. Nephroprotective effects of TVP1022, a non-MAO inhibitor S-isomer of rasagiline, in an experimental model of diabetic renal ischemic injury. Am J Physiol Renal Physiol. 2014;306:F24–F33. doi: 10.1152/ajprenal.00379.2013. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez F, Lopez B, Perez C, Fenoy FJ, Hernandez I, Stec DE, et al. Chronic tempol treatment attenuates the renal hemodynamic effects induced by a heme oxygenase inhibitor in streptozotocin diabetic rats. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1540–R1548. doi: 10.1152/ajpregu.00847.2010. [DOI] [PubMed] [Google Scholar]


