Abstract
The mechanical loading environment influences the development and maturation of joints. In this study, the influence of imbalanced muscular loading on joint development was studied using localized chemical denervation of hip stabilizing muscle groups in neonatal mice. It was hypothesized that imbalanced muscle loading, targeting either gluteal muscles or quadriceps muscles, would lead to bilateral hip joint asymmetry, as measured by acetabular coverage, femoral head volume and bone morphometry, and femoral-acetabular shape. The contralateral hip joints as well as age-matched, uninjected mice were used as controls. Altered bone development was analyzed using micro-computed tomography, histology, and image registration techniques at postnatal days (P) 28, 56, and 120. This study found that unilateral muscle unloading led to reduced acetabular coverage of the femoral head, lower total volume, lower bone volume ratio, and lower mineral density, at all three time points. Histologically, the femoral head was smaller in unloaded hips, with thinner triradiate cartilage at P28 and thinner cortical bone at P120 compared to contralateral hips. Morphological shape changes were evident in unloaded hips at P56. Unloaded hips had lower trabecular thickness and increased trabecular spacing of the femoral head compared to contralateral hips. The present study suggests that decreased muscle loading of the hip leads to altered bone and joint shape and growth during postnatal maturation. Statement of Clinical Significance: Adaptations from altered muscle loading during postnatal growth investigated in this study have implications on developmental hip disorders that result from asymmetric loading, such as patients with limb-length inequality or dysplasia.
Keywords: postnatal growth, hip, morphology, maturation, muscle loading
The mechanical loading environment influences the development and maturation of joints.1–4 For example, adaptations to bone due to increased or decreased muscle loading can influence bone shape and structure in newborns, adolescents, and adults.5,6 Furthermore, changes to the shape and structure of bones can lead to abnormal joint loading patterns, onset and progression of conditions such as acetabular dysplasia, and increased risk of osteoarthritis (OA).7 Thus far, the role of early-stage, postnatal muscle unloading on the shape, structure, and function of maturing articular joints is not well understood, particularly for the hip.
The hip joint continues to develop and mature during postnatal life and into childhood; e.g., in humans, the triradiate cartilage does not fully ossify until ages 15–18.8,9 Postnatal muscle loading at the hip is, therefore, critical for the formation of the proximal femur and acetabulum. Understanding the role of muscle unloading and/or imbalance on hip bone growth and mineralization as well as growth plate fusion (particularly of the triradiate cartilage and proximal femur) will guide our understanding of hip maturation and may provide insight into the progression of developmental hip disorders. In humans, the triradiate cartilage of the hip fuses at the time of skeletal maturity and consists of three distinct growth plates that connect the ilium, ischium, and pubis. These growth plates fuse to form the acetabulum, which is loaded orthogonal to the axis of growth plate fusion.8 Expansion of the triradiate cartilage during postnatal growth is necessary for proper joint development in humans and this expansion ends when the pelvis has fully ossified.10 Clinical case reports of premature closure of the triradiate cartilage suggest a causative association with predisposition to the development of acetabular dysplasia, or developmental dysplasia of the hip (DDH).9,11–14
Postnatal straight-legged swaddling, previously common in Japanese and Native American culture, can lead to DDH in hips that are otherwise healthy at birth, and this is analogous to muscular unloading. This disorder has been replicated in animal models and leads to hip instability and dislocation. Additionally, defective development of the hip joint with conditions, such as DDH, carries a chronic and indolent course of disease. It is estimated that 79–90% of cases of OA have definable, congenital hip joint abnormalities that lead to increased impingement or altered loading.15,16 The influence of altered mechanics on development of OA is likely even greater on development of OA in early adulthood.17 Center edge angle (corresponding to “Norberg angle”) on radiographs early in adult life have been shown to correlate with WOMAC score for symptomatic OA.18 Understanding causes and preventative measures for OA is critical, as clinical OA is estimated to affect 27 million US adults (10% of those over age 18) and cost 42.3 billion USD annually.19
Mice undergo a similar hip joint maturation process as humans and other mammals, whereby the hip follows an ordered, triradiate ossification process that converges toward the acetabulum and is completed following a period of postnatal growth.20 The pelvis emerges during development in mice as a single cartilage template, which then separates into three ossification centers that include the ilium, ischium, and pubis.20 The proximal femur of mice begins as a single chondroepiphysis at early postnatal growth, and this epiphysis later separates into two epiphyses, one of the greater trochanter and another of the femoral head.21 The rapid growth of the mouse skeleton is convenient for studying bone and joint maturation, as the separation of the proximal chondroepiphysis of the femur occurs in the first 4 weeks of postnatal growth,22 and fusion of the triradiate cartilage in the acetabulum occurs within the first 3 months of postnatal growth. The use of mouse models to study musculoskeletal growth and response to mechanical unloading is particularly beneficial, as mice are genetically similar to humans, have a relatively fast rate of postnatal maturation, and offer a number of available genetically modified tools compared to other mammalian research species.
Recent work using chick embryonic culture has shown that neonatal muscle loading influences the development of the embryonic hip joint.23 This study suggested that prenatal immobilization of the embryonic hip, induced by spinal muscle atrophy, may induce early-onset DDH.23 However, this study was unable to explore the postnatal adaptations of the hip following muscle unloading due to the global impact of muscle immobilization in the model used.23 Therefore, assessing the postnatal joint morphology following localized muscle unloading is needed. In the present study, it was hypothesized that unilateral muscular unloading (i.e., unbalanced loading) via localized denervation of hip flexors (e.g., lateral quadriceps) or hip extensors (e.g., gluteus maximus) would lead to structural and functional alterations of the hip during postnatal maturation. An in vivo model of unilateral muscle unloading of hip stabilizers was developed in neonatal mice to identify the role of muscle loading on hip development during postnatal growth. Acetabular coverage, femoral head volume, histomorphology, and bone morphometry were examined to detect differences between unloaded and contralateral hips.
METHODS
Unilateral Hip Unloading Model
All studies were performed in compliance with the Animal Studies Committee and the Department of Comparative Medicine at Washington University. CD-1 neonatal mice (postnatal day 1, P1, N = 56) were administered intramuscular injections of 0.15–0.2U botulinum toxin A (BOTOX; Allergan, Inc., Parsippany, NJ) in saline in the left hips (N = 3–10 per group/time point), with an equivolumetric dose of 0.9% saline in the contralateral hip. Botox was used to paralyze the injected muscles during postnatal growth.22 Mice were injected in the lateral aspect of their upper thigh (quad target) or the caudal aspect of the gluteus muscle groups (gluteal target) twice weekly until weaning and once per week thereafter until sacrifice at P28 and P56. Injections were made with a 28 gauge, 0.3 ml total volume insulin syringe (Becton Dickinson and Company, Franklin Lakes, NJ) and the needle was oriented as follows: for quad target, the pup was held gently by its scruff with its hindlimb in full extension. Following palpation of the thigh, the needle was inserted subcutaneously and into the muscle belly of the rectus femoris, parallel to the direction of muscle action, near the patellar tendon. For gluteal target, the pups’ hindlimbs were secured between the handler’s thumb and middle finger after placing the pup with its ventral side on the handler’s index finger. The hip bone and musculature was palpated to identify the gluteus maximus muscles and the needle was aligned parallel to the action line of the muscle and inserted subcutaneously into the muscle belly. An additional group for gluteal target unloading only was carried out through P120. Uninjected litters were used as age-matched controls for P28 and P56 time-points (5–6 per time point, N = 11 total). Mice were housed with their mothers until wean (P21) and then housed with same-sex littermates in a barrier facility with 12 h on/12 h off light cycle. Mice were monitored for distress throughout the duration of the experiment. Mice were euthanized via carbon dioxide asphyxiation. Following sacrifice, their intact hips with surrounding musculature were immediately dissected and fixed in hip extension position using 4% paraformaldehyde.
Hip Unloading with Recovery Model
A fourth group of mice (N = 7) were injected as previously described for glute target unloading. At P14, a subset of these mice ceased unilateral Botox treatment and began bilateral saline injections in an effort to encourage unloading recover (recovery group, N = 4) while the remaining mice were chronically injected (maintained unloading group, N = 3) as described in the unloading model. At P56, hips of recovery and maintained unloading groups were dissected as previously described and fixed in 4% paraformaldehyde in an anatomical position with the hips held slightly abducted and flexed.
Microcomputed Tomography and Histology
Following fixation, P28 and P56 control, gluteal target unloading, and quad target unloading hips were scanned using micro-computed tomography (μCT, standard resolution, 36 μm voxel, 45 kVp, 177 μA, and 250 ms integration time; Scanco μCT40, Brüttisellen, Switzerland). μCT scans were exported as DICOM files and analyzed in OsiriX 32-bit freeware (Pixmeo SARL, Bernex, Switzerland). Hips were digitally repositioned, oriented in craniocaudal plane, and aligned using the 3D MPR tool in OsiriX. Norberg angles (α, angle of Weiberg + 90°, Fig. 1A) for each animal’s right and left hips were measured using tools in the 3D MPR tool on at least five consecutive slices. Total volume (TV), bone volume (BV), bone volume percentage (BV/TV), and total mineral density (TMD, mg HA/cm3) of the femoral head was analyzed using Scanco software (Brüttisellen, Switzerland). High resolution scans were obtained for recovery and maintained unloading groups, as well as P120 control and gluteal target hips (high resolution setting, 20 μm voxel, 45 kVP, 177 μA) for TMD, bone mineral density (BMD), trabecular thickness (Tb.Th.), spacing (Tb.Sp.), and number (Tb.N.). Following μCT, samples were decalcified in 14% EDTA and processed for paraffin embedding and histology. Histological sections were cut at 7 μm thick sections for both left and right hips of quad target (P28 only), gluteal target (P28, P56, and P120), and controls (P28 and P56) and stained using toluidine blue.
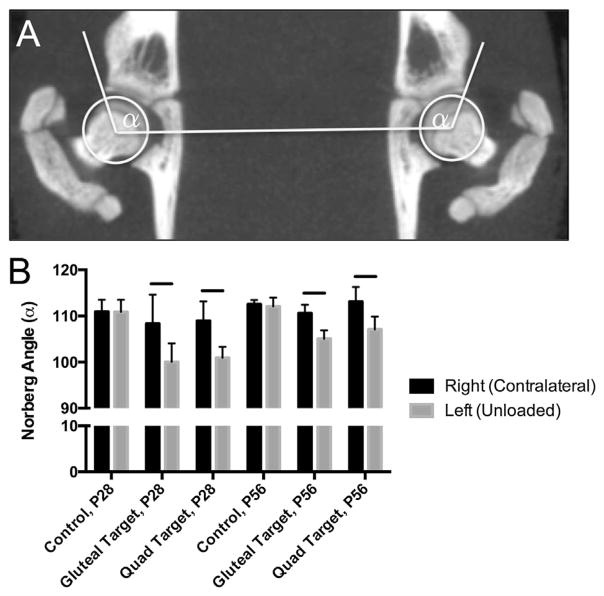
Figure 1.
(A) Representative bilateral measurement of Norberg angles (α) from reoriented, frontal plane microCT image stacks. (B) Norberg angle measurements at P28 and P56 for control, gluteal-target, and quad-target unloading for right (contralateral) and left (unloaded) hips. Solid line indicates significant differences between groups (p <0.05).
Image Registration and Shape Comparisons
Image registration was performed using the Image Registration Toolkit (IRTK, since upgraded to MIRTK https://biomedia.doc.ic.ac.uk/software/mirtk/, last accessed March 2016, London, UK).24 Data from both left and right hips at P56 and P120 (P56 control, P56 gluteal target, P120 control, and P120 gluteal target) were included for image registration. For each specimen, both left and right proximal femur and acetabular region of the pelvis were virtually segmented using Mimics software (Materialise, Leuven, Belgium) in preparation for image registration. All right-sided femora and pelvises were mirrored to enable comparison with the contralateral counterparts. Within each group, multiple rigid registrations and transformations were performed to align all femora or pelvises in the group in exactly the same orientation and position. Next, an atlas image was created to provide an average of the aligned input images, thereby providing an average representation of the shape for the left or right side of that treatment group. Within each group (e.g., P120 gluteal target), four atlases were created; left femur, right femur, left pelvis, and right pelvis. The left and right atlases of the same rudiments within each group were then aligned with respect to each other using a further iteration of rigid registration and transformation. Each atlas was thresholded (using the same parameters within each comparison set) in order to remove noise due to sub-optimal alignment of a minority of datasets, or due to shapes that are significantly different from the standard shape for that particular group. Finally, ImageJ (http://rsbweb.nih.gov/ij/, last accessed March 2016)25 was used to calculate the pixel differences between each set of atlases, with the output of this step demonstrating region specific size and shape differences between the left and right sides for all groups.
Statistical Analysis
All statistical comparisons were performed using Prism 6 (version 6.0d, Graphpad, LaJolla, CA). Two-way ANOVA with Holm–Sidak multiple comparisons tests (1 – β = 0.80; α = 0.05) were used to compare Norberg angles, TV, BV, BV/TV, and TMD for control, quad target, and gluteal target animals at P28 and P56 time points with repeated measures (left and right limbs paired). Linear regression was performed to test goodness of fit and to compare slopes and intercepts that describe the relationship between Norberg angle (α) and bone morphometric outcomes (TV, BV/TV, and TMD) for gluteal-target unloaded and contralateral groups as well as for combined gluteal-target unloaded/contralateral data (P28 and P56 combined; α = 0.05). For trabecular outcomes at P120 (unloaded/contralateral), paired t tests were used to compare total volume, BV/TV, and TMD of the trabecular bone of the femoral head, as well as Tb.Th., Tb.N., and Tb.Sp. of the contralateral and unloaded hips (α = 0.05). For trabecular outcomes at P56 for the recovery and maintained unloading groups, two-way ANOVA with repeated measures was used to compare left versus right proximal femur bone morphometry (TV, BV, BV/TV, TMD, TbSp, TbN, and TbTh) between recovery and maintained unloading groups (α = 0.05).
RESULTS
All animals responded well to the injections and were used in analyses. Postnatal imbalanced loading via isolated paralysis of either the the hip flexors (i.e., quadriceps) or hip extensors/stabilizers (i.e., gluteus maximus) led to a decreased Norberg angle at P28 and P56 compared to the contralateral side (Fig. 1B). No statistical differences in Norberg angle were found between the contralateral limb and uninjected, age-matched controls at P28 or P56. Norberg angle did not differ between left and right femoro-acetabular joints for control hips (Fig. 1B).
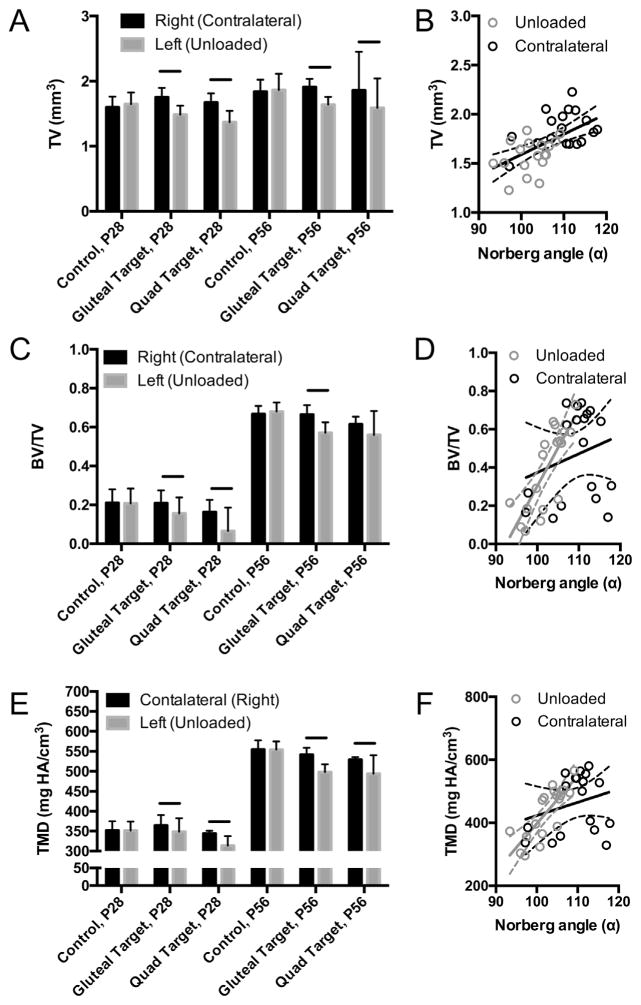
Hips that developed with imbalanced loading had significantly smaller TV (Fig. 2A) and reduced TMD (Fig. 2E) compared to their contralateral sides, for both quad and gluteal target groups, at both P28 and P56. No statistical differences in TV were observed between gluteal target unloading and contralateral data using linear regression (Fig. 2B); however, after gluteal target unloaded and contralateral data were pooled for linear regression, there was a significant relationship between TV and Norberg angle (R2 = 0.3471, p <0.001; Fig. 2B). BV/TV was significantly lower in femoral heads from unloaded hips compared to contralateral hips at P28; however, BV/TV was only significantly lower in unloaded hips compared to contralateral hips at P56 for the gluteal target group (Fig. 2C). A significant linear relationship was observed between BV/TV and Norberg angle for unloaded, but not contralateral, hips (R2 = 0.6351, p <0.0001; Fig. 2D). Pooled BV/TV and Norberg angle comparisons using linear regression was statistically significant (R2 = 0.2139, p = 0.0045). TMD of the femoral head was significantly lower for gluteal target and quad target, compared to contralateral hips, at P28 and P56 (Fig. 2E). A significant linear relationship was observed between TMD and Norberg angle for unloaded hips (R2 = 0.6428, p <0.001; Fig. 2F), but not contralateral hips (Fig. 2F). Pooled TMD and Norberg angle comparisons using linear regression was statistically significant (R2 = 0.2295, p = 0.0031). No statistical differences were found between the contralateral limb and uninjected, age-matched controls for TV, BV/TV, or TMD at P28 or P56. However, BV/TV and TMD significantly increased from P28 to P56 for all groups (Fig. 2C and E). Femoral head TV, BV/TV, and TMD did not vary between left and right control hips at either P28 or P56 (Fig. 2).
Figure 2.
Bone morphometric outcomes of P28 and P56 control, gluteal-target, and quad-target groups of the right (contralateral) and left (unloaded) hips. (A) Total volume (mm3) of the proximal femur for right and left hips with (B) linear relationship between Norberg angle (α) and total volume (mm3) of unloaded (gray dots) and contralateral (black dots) gluteal-targeted proximal femurs. (C) Bone volume ratio (BV/TV), and (D) tissue mineral density (TMD) was measured for the femoral head/neck using microCT. Solid line in A, C, and D indicates significant differences between groups (p <0.05). Solid and dotted black line in B represents the linear correlation for combined unloaded and contralateral hips; respective solid and dotted gray (unloaded) and black (contralateral) lines in D and E represent the linear correlation and 95% confidence intervals for BV/TV and TMD of gluteal unloaded and contralateral hips, respectively.
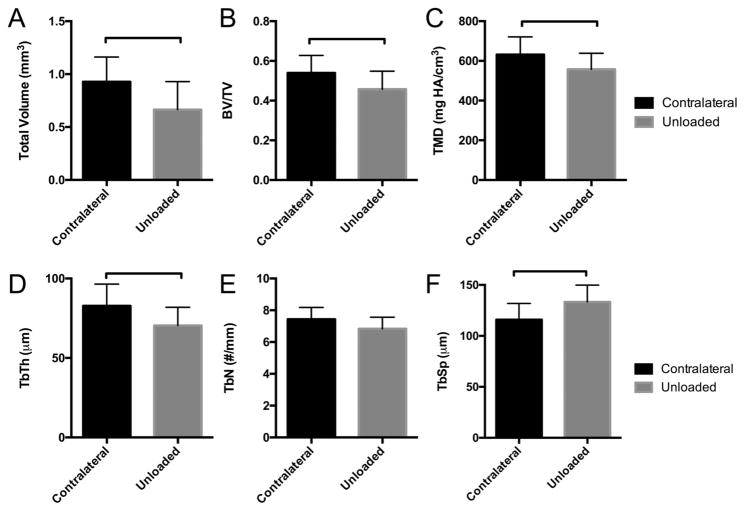
The trabecular structure of the femoral head was examined in contralateral and unloaded hips at P56 and P120 (Fig. 3). Trabecular bone adaptations included decreased trabecular volume in the femoral head (Fig. 3A), consistent with observations at earlier time points when including the cortical bone in the analysis (Fig. 2A). Similarly, trabecular BV/TV and TMD were significantly lower in unloaded femoral heads compared to contralateral femoral heads at P120 (Fig. 3B and C). Tb.Th. was significantly reduced (Fig. 3D) and Tb.Sp. was significantly increased (Fig. 3F) for unloaded hips compared to contralateral hips. The number of trabeculae (Tb.N.) did not differ significantly between groups at this time point (Fig. 3E).
Figure 3.
Trabecular bone morphometry of P120 femoral heads for gluteal-target contralateral and unloaded hips. (A) Total volume of the trabecular bone in the femoral head (mm3), (B) BV/TV, (C) tissue mineral density (TMD), and (D–E) trabecular parameters, i.e., thickness (Tb.Th.), number (Tb.N.), and spacing (Tb.Sp.), respectively, were compared between contralateral and unloaded groups. Solid bar indicates significant difference between groups (p <0.05).
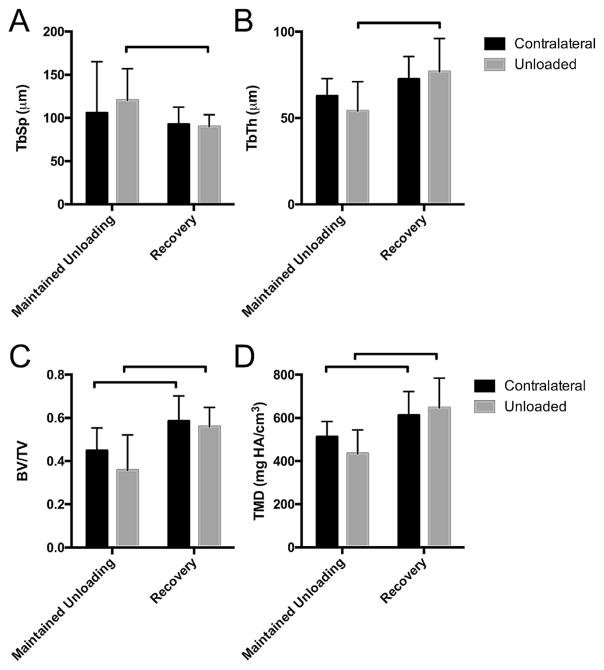
The trabecular bone morphometry were significantly different in mice that were allowed to recovery during postnatal growth after an unloading period of 2 weeks compared to hips from age-matched littermates receiving maintained unloading (Fig. 4). Specifically, TMD and BV/TV were significantly higher in both contralateral and short-term unloaded hips following a period of recovery compared to the contralateral and unloaded hips of age-matched littermates that received maintained unloading during postnatal growth (Fig. 4C and D). A period of recovery also resulted in decreased Tb.Sp. and increased Tb.Th. compared to maintained unloading for unloaded limbs (Fig. 4A and B).
Figure 4.
Recovery of bone morphometry after 2 weeks of postnatal gluteal-target unloading, compared to maintained unloading, at P56. (A) Tb.Sp. was significantly lower and (B) Tb.Th. was significantly higher for hips that were allowed to recover from postnatal unloading compared to maintained unloading. (C and D) Contralateral and unloaded hips demonstrated higher BV/TV and TMD following a period of recovery compared to maintained unloading. Bar indicates significant difference between group (p <0.05).
Shape differences between right (contralateral) and left (unloaded) hips were visualized between thresholded atlases of rigidly registered datasets (Fig. 5). Color mapping and pixel density indicated shape differences between the groups; magenta pixels indicate that more growth occurred in that region for the right (contralateral) bone whereas turquoise pixels indicate that more growth occurred in that region for the left (unloaded) bone. Control hips at P56 are shown in Figure 5A, C, and E, and the gluteal-target hips are shown in Figure 5B, D, and F. For control hips, there are mild or no differences in growth patterning between left and right hips, as indicated by the amount of white pixels (e.g., matching size). However, for the gluteal-target hips, the contralateral acetabulum was markedly larger compared to the unloaded acetabulum (Fig. 5B). The greater and lesser trochanter, as well as the medial, articulating surface of the femoral head, were larger in the contralateral hip compared to the unloaded hip, indicated by more magenta patterning on these surfaces (Fig. 5D). Surprisingly, the proximal surface of the femoral head as well as the proximal femoral neck displayed more pronounced outgrowths in the unloaded group compared to the contralateral group, indicated by more turquoise patterning on this surface (Fig. 5F).
Figure 5.
Shape comparisons using image registered atlases from microCT images of the (A and B) acetabulum, (C and D) posterior view of the proximal femur, and (E and F) anterior view of the proximal femur at P56. Right (magenta) and left (turquoise) control hip overlay registrations are shown in A, C, and E; contralateral (magenta) and unloaded (turquoise) hip overlay registrations are shown in B, D, and F. Magenta pixels show localization where the contralateral/right atlas is larger, whereas turquoise pixels represent localization of a larger unloaded/left atlas.
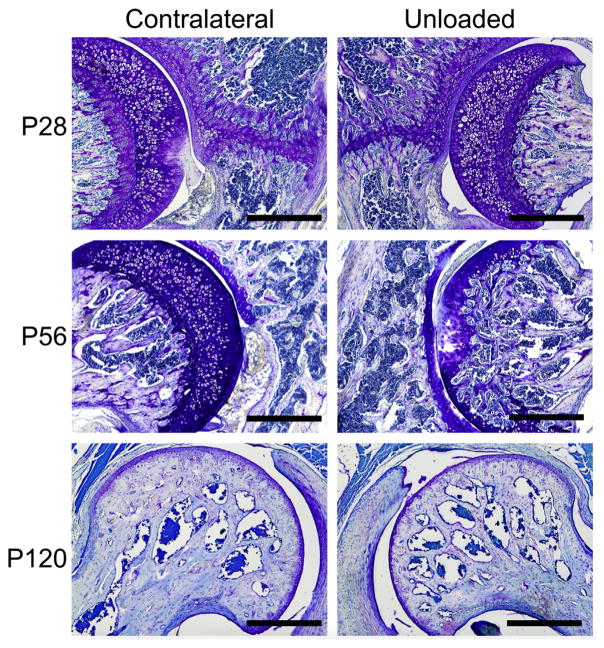
At all three time points, the histology of the hip joint reflected the shape adaptions in femoral head following imbalanced loading (Fig. 6, top row). At P28, the femoral head appeared smaller than the contralateral control following quad target unloading (Fig. 6). The triradiate cartilage of the pelvis, along with its adjacent trabeculae, appeared thinner on the unloaded size compared to the contralateral side (Fig. 6, top row). At P56, the triradiate cartilage was fused for both contralateral and unloaded sides, and the femoral head of the unloaded side appeared consistently smaller (Fig. 6, middle row). Additionally, at P56, the cartilaginous secondary ossification zone of the femoral head was not yet fully mineralized in all contralateral hips; however, the femoral head had mostly mineralized its secondary ossification center in the unloaded hips (Fig. 6, middle row). At P120, the femoral head of the contralateral hip was fully mineralized and mature, with thick subchondral/cortical bone and thickened trabeculae (Fig. 6, bottom row). The femoral head of the unloaded hip, however, was less densely mineralized, had thinner trabeculae, and thinner subchondral/cortical bone (Fig. 6, bottom row). Similar to the P28 and P56 unloaded hips, the P120 unloaded hips were smaller than their contralateral hips (Fig. 6, bottom row).
Figure 6.
Histological sections stained with toluidine blue for P28, P56, and P120 contralateral and unloaded hips from the gluteal-target group. Note the reduced femoral head size for the unloaded group at all three time points. Additionally, thickness of the triradiate cartilage was smaller for P28 unloaded hips compared to contra-lateral hips, and fusion of the femoral physis was accelerated for P56 unloaded hips compared to contralateral hips. Scale bar = 500 μm.
DISCUSSION
Unilateral postnatal unloading of key hip-stabilizing muscle groups led to decreased acetabular coverage (indicated by decreased Norberg angles), decreased bone accumulation of the femoral head (indicated by decreased TMD), and altered size and shape of the unloaded hip compared to contralateral hips. These changes demonstrate the importance of bilateral dynamic and balanced loading during postnatal maturation of the hip joint for proper joint development.
Unilateral adaptations of bone and joints during postnatal growth can have a dramatic impact on the long-term health of bones and joints into adulthood. Recently, femoral head volume has been negatively correlated with cephalad displacement of the femoral head.26 Unilateral vascular insufficiency has also been correlated with increased risk of femoral neck and acetabular deformities at maturation.27 Additionally, medial bowing and premature fusion of the femoral physis have been associated with poor outcomes following maturation of the hip in cases of congenital dislocation.27 The diameter of the acetabulum is controlled by the expansion of triradiate cartilage,8 and the depth of the acetabulum is controlled by pressures from the femoral head.28 Premature, unilateral closure and smaller hips may lead to altered gait and degenerative outcomes.7,10,11 Conversely, asymmetric growth plate expansion of murine long bones of the hindlimb has been shown to be modulated by the delivery of heat, which led to limb length discrepancy and the potential for altered loading patterns between left and right hips.29 Adaptations to loading during postnatal growth may influence proliferation and differentiation of growth plate cells,29 particularly of the triradiate cartilage and proximal femur, and future work should explore this further.
Shape differences between unloaded and contralateral hips may provide insight into the contact areas between the acetabulum and femoral head during stages of linear growth and, concomitantly, following unloading. Because the acetabulum serves as the articulating point of the pelvis and femur, the acetabulum adapts during growth to both constrain the femoral head within the socket but also allow full range of motion without impingement. Increasing coverage of the femoral head by the acetabulum likely leads to increased stability of the joint. It was hypothesized in the study that acetabular coverage would decrease following muscle unloading. We found that there were regions of significant loss of acetabular coverage as a result of unloading, which were in line with changes in the shape of the proximal femur. This study demonstrated that changes in muscular loading of hip stabilizers may lead to altered contact mechanics of the proximal femur and acetabulum.
Results from long-term paralysis (P120) validated the results from earlier time points in this study, and also allowed for more detailed analysis of trabecular morphology. The analysis of trabeculae was not performed at earlier time points for a few reasons. At P28, trabeculae were not yet established in the femoral head and therefore trabecular morphology was not determined. Likewise, high variability existed at P56, with some hips showing full, bilateral mineralization of the femoral head and others showing incomplete fusion of the secondary ossification center (as shown in Fig. 3). MicroCT images, capable of discerning gross differences in bone mineralization and shape, were obtained at a lower resolution for P28 and P56 compared to P120. At P120, mice had fully matured, mineralized bones, allowing for full characterization of trabecular morphology.
Limitations to this study include the use of a contralateral limb as an animal-matched control. Using internal controls increased the statistical power and negated the environmental factors that can affect joint maturation. However, it is likely that localized paralysis influenced the animals’ overall behavior, potentially altering the development of the contralateral limb. Additionally, the differences between contralateral and unloaded hips may be exacerbated because of the potential compensatory loading of the contralateral hip. This compensatory effect may have been the reason for differences between trabecular bone morphometry in littermate mice that had maintained unilateral unloading and mice that were allowed to recovery from unloading after 2 weeks postnatal. Despite this limitation, the contralateral hips did not differ statistically from the age-matched control hips. It is possible that altered postnatal development has lasting effects that may serve as a model for poor joint loading and subsequent arthritic disease states, such as OA. Our experiments using a period of unloading during early postnatal growth, followed by a period of recovery, showed that the mineralization of the proximal femur is able to recover from short-term periods of unloading. Although we only had a small sample size, these findings are encouraging for understanding the ability of the hip to recovery from a short-term period of unloading during postnatal growth. In an elaborate study of recovery exploring the long-term effects of Botox on shoulder girdle development, Potter et al. found that short-term supraspinatus denervation induced altered shoulder joint maturation and led to protracted bony defects with limited recovery potential.30 It is likely that growth rates and development of the shoulder and hip girdle follow different time courses, and further studies are necessary to determine the long-term significance of impaired neonatal development on the adult mice after cessation of Botox treatments. Another potential limitation to this study is the risk for targeting the gluteus medius in addition to the gluteus maximus, which could influence abduction of the hip in addition to extension. Future work could involve more careful, microinjection targeting for abductor (gluteus medius) denervation, which could potentially enhance the effects of hip dysplasia.
Adaptations in growth and function of hip joint structures play a role in the predisposition to hip OA through altered mechanics and increased local stress.15,16 Understanding the consequences of postnatal muscle imbalances on hip maturation may provide insight into proper hip development and function. This study investigated the morphological adaptations of the hip in mice subjected to unilateral hip muscle unloading. We found that sustained hip muscle unloading can impair growth and maturation of the femoro-acetabular joint. These findings shed light into the potential perturbation of postnatal musculoskeletal growth patterns driven by muscle imbalance that can influence joint alignment and loading. While not directly investigated in this study, it is possible that altered alignment and loading of the hip likely has long-term ramifications for hip joint health and warrants further investigation. There is a rising burden of hip and knee OA in young adults of which etiology is poorly understood, and this increased onset of OA at a young age can lead to an increased social and economic impact on the general population. Therefore, improving our understanding of hip joint growth and maturation in the young adult skeleton may lead to improved rehabilitation strategies and therapeutics that can go beyond total joint arthroplasty or improve implant longevity.
Acknowledgments
Grant sponsor: National Institutes of Health NRSA Postdoctoral Fellowship; Grant number: NIH NIAMS F32 AR064652-01; Grant sponsor: Children’s Discovery Institute Postdoctoral Fellowship; Grant sponsor: European Research Council under the European Union’s Seventh Framework Programme; Grant number: 336306; Grant sponsor: Washington University Musculoskeletal Research Center; Grant number: NIH P30 AR057235.
MLK was funded by the National Institutes of Health NRSA Postdoctoral Fellowship (NIH NIAMS F32 AR064652) and the Children’s Discovery Institute Postdoctoral Fellowship. NCN was funded by the European Research Council under the European Union’s Seventh Framework Programme (ERC Grant agreement n° [336306]). The Image Registration Toolkit was used under Licence from Ixico Ltd. This study was made possible in part by resources from the Washington University Musculoskeletal Research Center (NIH P30 AR057235).
Footnotes
AUTHORS’ CONTRIBUTIONS
CAF and MLK contributed to study design, data collection, data analysis, data interpretation, and manuscript preparation. NCN contributed to data analysis, data interpretation, and manuscript preparation. ST contributed to study design and manuscript preparation. All authors have read and approved the final submitted version of the document.
References
- 1.Drachman D, Sokoloff L. The role of movement in embryonic joint development. Dev Biol. 1966;14:401–420. [Google Scholar]
- 2.Giorgi M, Carriero A, Shefelbine S, et al. Effects of normal and abnormal loading conditions on morphogenesis of the prenatal hip joint: application to hip dysplasia. J Biomech. 2015;48:3390–3397. doi: 10.1016/j.jbiomech.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giorgi M, Carriero A, Shefelbine S, et al. Mechanobiological simulations of prenatal joint morphogenesis. J Biomech. 2014;47:989–995. doi: 10.1016/j.jbiomech.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Carter D, Orr T, Fyhrie D, et al. Influences of mechanical stress on prenatal and postnatal skeletal development. Clin Orthop Relat Res. 1987;219:237–250. [PubMed] [Google Scholar]
- 5.Sharir A, Stern T, Rot C, et al. Muscle force regulates bone shaping for optimal load-bearing capacity during embryogenesis. Development. 2011;138:3247–3259. doi: 10.1242/dev.063768. [DOI] [PubMed] [Google Scholar]
- 6.Burr D. Muscle strength, bone mass, and age-related bone loss. J Bone Min Res. 1997;12:1547–1551. doi: 10.1359/jbmr.1997.12.10.1547. [DOI] [PubMed] [Google Scholar]
- 7.Bullough P. The role of joint architecture in the etiology of arthritis. Osteoarthritis Cartilage. 2004;12:2–9. doi: 10.1016/j.joca.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Ponsetti I. Growth and development of the acetabulum in the normal child. J Bone Joint Surg. 1978;60-A:575–585. [PubMed] [Google Scholar]
- 9.Hallel T, Salvati E. Premature clousre of the triradiate cartilage: a case report and animal experiment. Clin Orthop Relat Res. 1977;124:278–281. [PubMed] [Google Scholar]
- 10.Gepstein R, Weiss R, Hallel T. Acetabular dysplasia and hip dislocation after selective premature fusion of the triradiate cartilage. J Bone Joint Surg. 1984;66-B:334–336. doi: 10.1302/0301-620X.66B3.6725340. [DOI] [PubMed] [Google Scholar]
- 11.Blair W, Hanson C. Traumatic closure of the triradiate cartilage: report of a case. J Bone Joint Surg. 1979;61:144–145. [PubMed] [Google Scholar]
- 12.Bucholz R, Ezaki M, Ogden J. Injury to the acetabular triradiate physeal cartilage. J Bone Joint Surg. 1982;64:600–609. [PubMed] [Google Scholar]
- 13.Heeg M, Visser J, Oostvogel H. Injuries of the acetabular triradiate cartilage and sacroiliac joint. Bone Joint J. 1988;70:34–37. doi: 10.1302/0301-620X.70B1.3339056. [DOI] [PubMed] [Google Scholar]
- 14.Badina A, Vialle R, Fitoussi F, et al. Case reports: treatment of traumatic triradiate cartilage epiphysiodesis: what is the role of bridge resection? Clin Orthop Relat Res. 2013;471:3701–3705. doi: 10.1007/s11999-013-3054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris W. Etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 1986;13:20–33. [PubMed] [Google Scholar]
- 16.Ganz R, Leunig M, Leunig-Ganz K, et al. The etiology of osteoarthritis of the hip. Clin Orthop Relat Res. 2008;466:264–272. doi: 10.1007/s11999-007-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lievense A, Bierma-Zeinstra S, Verhagen A, et al. Influence of hip dysplasia on the development of osteoarthritis of the hip. Ann Rheum Dis. 2004;63:621–626. doi: 10.1136/ard.2003.009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mavcic B, Iglic A, Kralj-Iglic V, et al. Cumulative hip contact stress predicts osteoarthritis in DDH. Clin Orthop Relat Res. 2008;466:884–891. doi: 10.1007/s11999-008-0145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy L, Helmick C. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112:S13–S19. doi: 10.1097/01.NAJ.0000412646.80054.21. [DOI] [PubMed] [Google Scholar]
- 20.Pomikal C, Streicher J. 4D-analysis of early pelvic girdle development in the mouse (Mus musculus) J Morphol. 2010;271:116–126. doi: 10.1002/jmor.10785. [DOI] [PubMed] [Google Scholar]
- 21.Serrat MA, Reno PL, McCollum MA, et al. Variation in mammalian proximal femoral development: comparative analysis of two distinct ossification patterns. J Anat. 2007;210:249–258. doi: 10.1111/j.1469-7580.2007.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang W, Foster J, Rogachefsky A. Pharmacology of botulinum toxin. J Am Acad Dermatol. 2000;43:249–259. doi: 10.1067/mjd.2000.105567. [DOI] [PubMed] [Google Scholar]
- 23.Nowlan NC, Chandaria V, Sharpe J. Immobilized chicks as a model system for early-onset developmental dysplasia of the hip. J Orthop Res. 2014;32:777–785. doi: 10.1002/jor.22606. [DOI] [PubMed] [Google Scholar]
- 24.Studholme C, Hill DLG, Hawkes DJ. An overlap invariant entropy measure of 3D medical image alignment. Pattern Recogn. 1999;32:71–86. [Google Scholar]
- 25.Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- 26.Fukiage K, Fukuda A, Harada Y, et al. Femoral head volume indicates the severity of developmental dysplasia of the hip by a method using three-dimensional magnetic resonance imaging. J Pediatr Orthop B. 2015;24:286–290. doi: 10.1097/BPB.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 27.Keret D, MacEwen GD. Growth disturbance of the proximal part of the femur after treatment for congenital dislocation of the hip. J Bone Joint Surg Am. 1991;73:410–423. [PubMed] [Google Scholar]
- 28.Harrison TJ. The influence of the femoral head on pelvic growth and acetabular form in the rat. J Anat. 1961;95:12–24. [PMC free article] [PubMed] [Google Scholar]
- 29.Serrat MA, Schlierf TJ, Efaw ML, et al. Unilateral heat accelerates bone elongation and lengthens extremities of growing mice. J Orthop Res. 2015;33:692–698. doi: 10.1002/jor.22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potter R, Havlioglu N, Thomopoulos S. The developing shoulder has a limited capacity to recover after a short duration of neonatal paralysis. J Biomech. 2014;47:2314–2320. doi: 10.1016/j.jbiomech.2014.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]