Abstract
Purpose
Triple negative (TN, tumors that do not express estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2)) and HER2-overexpressing (H2E, ER−/HER2+) tumors are two particularly aggressive subtypes of breast cancer. There is a lack of knowledge regarding the etiologies of these cancers and in particular how anthropometric factors are related to risk.
Methods
We conducted a population-based case-case study consisting of 2,659 women aged 20–69 years diagnosed with invasive breast cancer from 2004–2012. Four case groups defined based on joint ER/PR/HER2 status were included: TN, H2E, luminal A (ER+/HER2−) and luminal B (ER+/HER2+). Polytomous logistic regression was used to estimate odds ratios (ORs) and associated 95% confidence intervals (CIs) where luminal A patients served as the reference group.
Results
Obese premenopausal women (body mass index (BMI) ≥30 kg/m2) had an 82% (95% CI: 1.32–2.51) increased risk of TN breast cancer compared to women whose BMI<25 kg/m2, and those in the highest weight quartile (quartiles were categorized based on the distribution among luminal A patients) had a 79% (95% CI: 1.23–2.64) increased risk of TN disease compared to those in the lowest quartile. Among post-menopausal women obesity was associated with reduced risks of both TN (OR= 0.74, 95% CI: 0.54–1.00) and H2E (OR= 0.47, 95% CI: 0.32–0.69) cancers.
Conclusions
Our results suggest obesity has divergent impacts on risk of aggressive subtypes of breast cancer in premenopausal versus post-menopausal women, which may contribute to the higher incidence rates of TN cancers observed among younger African American and Hispanic women.
Keywords: breast cancer, triple negative, obesity, BMI
Introduction
Using gene expression profiling, five distinct molecular subtypes of breast cancer have been identified: luminal A, luminal B, HER2-overexpressing, basal-like and unclassified [1, 2]. Immunohistochemistry (IHC) -based surrogate definitions of these subtypes using protein expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) are routinely used to classify tumors as luminal (ER+), HER2-overexpressing (H2E) (ER−/HER2+) and triple negative (TN) (ER−, PR− and HER2− which serves as a surrogate for basal-like cancers). TN and H2E are two aggressive subtypes associated with poorer 5-year survival (73–89% and 52–81%, respectively) compared to luminal tumors (87–96%) [3–5].
While clinical differences across the molecular subtypes have been well described [3, 6], etiologic differences have been less studied. Several prior studies including a large pooled analysis of much of the data available worldwide have established that obesity increases risk of postmenopausal breast cancer[7–9], but decreases the risk of premenopausal breast cancer[9, 10]. Comparatively few studies[11–18] have evaluated the relationships between anthropometric factors and risks of different molecular subtypes of breast cancer. Interpretation of these studies is challenged by their relatively small sample sizes as they have included only 50–375 TN cases and 33–117 H2E cases. Nevertheless, among the larger studies[15–17] there is an increased risk of TN cancers associated with a higher BMI among premenopausal women but no differences in risk among postmenopausal women. To further our understanding regarding the impact of anthropometric factors on the risks for TN and H2E breast cancer, we conducted a population-based case-case study of breast cancer among women aged 20–69 years in western Washington and Albuquerque, New Mexico.
Materials and Methods
Patient identification
The study protocol was independently approved by the Fred Hutchinson Cancer Research Center’s and University of New Mexico’s Institutional Review Boards (IRBs). Cases consisted of women 20 to 69 years old first diagnosed with invasive breast cancer while living in the Seattle-Puget Sound, Washington (King, Pierce, and Snohomish counties) or Albuquerque, New Mexico greater metropolitan areas (Bernalillo, Sandoval, Santa Fe, Socorro, Torrance, and Valencia counties) between June 1, 2004 to June 30, 2012. Women aged 70 years or older were not included in the study because the incidence of TN and H2E tumors declines sharply after age 70 [19]. Women with incomplete tumor marker information were not eligible for the study. After ascertainment through the population-based Surveillance, Epidemiology and End Results (SEER) cancer registries serving the Seattle-Puget Sound and the state of New Mexico and review of pathology reports for potentially eligible cases, four case groups defined by joint ER/PR/HER2 status were enrolled including TN (ER−/PR−/HER2−), H2E (ER−/HER2+), luminal A (ER+/HER2−) and luminal B (ER+/HER2+). All incident TN and H2E breast cases were targeted for enrollment, but only a random sample of women with incident ER+ breast cancer, 75% of the size of the TN case group and frequency matched to the distribution of age at diagnosis, year of diagnosis, and study site of the combined TN/H2E case group were eligible for the study. This strategy was used to gain statistical efficiency while containing the cost of the study given that ER+ breast cancer is much more common than other subtypes. Subjects were included regardless of vital status and medical records were reviewed for those who were deceased at the time of enrollment through a waiver of consent.
Different approaches were used to recruit participants in New Mexico and Seattle-Puget Sound (Figure 1). In New Mexico, medical records of all 681 eligible cases were reviewed under an IRB-approved waiver of consent (participation rate: 100%) and no individual contact was made with participants. At the Seattle site, two sources were used to recruit cases: (1) 461 eligible cases identified from prior studies with overlapping eligibility criteria (the design and methods have been described previously[20, 21]); and (2) de novo enrolled cases. Self-reported data obtained through structured interviewer-administered questionnaires were available from all 461 previously enrolled women and medical records were reviewed for 450 of them. In addition, 2,363 new cases meeting our eligibility criteria were identified. They were first sent a letter describing the study’s purpose and procedure, followed several days later by a telephone call from one of our trained interviewers to answer questions and obtain consent for interview and/or medical records review. A total of 1,568 of these 2,363 women (65.4%) were enrolled including 1,050 for whom both interview and medical records data were available, 355 for whom medical record data only were available, and 163 for whom only interview data were available. Among the total of 2,710 breast cases enrolled at both sites, 51 cases missing height and/or weight data from both sources were excluded from this analysis. Thus our final analytic sample size consisted of 2,659 cases including 1,275 TN, 772 luminal A, 129 luminal B and 483 H2E breast cancer cases.
Figure 1. Participants flow chart.
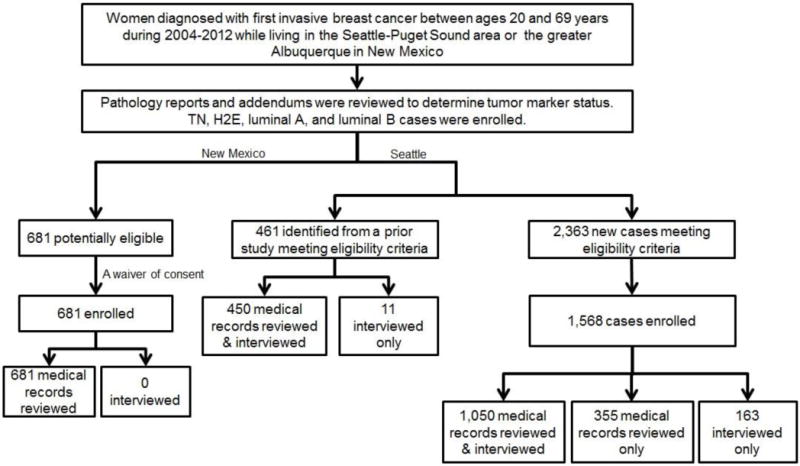
TN: triple negative. H2E: ER−/HER2+. Luminal A: ER+/HER2−. Luminal B: ER+/HER2+.
Data collection
Data on demographic, epidemiologic and clinical factors were ascertained from a detailed medical records review (both sites) and/or a structured telephone interview (Seattle only). Using the same protocol and instrument across study sites, data on medical records were abstracted from multiple sources including hospitals, oncology practices and primary care practices to obtain complete data on patient’s height and weight at the time of breast cancer diagnosis. In addition, information on a variety of breast cancer risk factors was obtained, including reproductive history, use of oral contraceptives (OCs) and menopausal hormone therapy (HT) over the five-years prior to breast cancer diagnosis, family history of breast cancer, and menopausal status. Information on stage, grade and ER/PR/HER2 status were collected from pathology, surgery and laboratory reports. A random 10% of completed abstracts were exchanged and reviewed between study sites to insure consistency in abstracting approach, methodology and coding. The primary source of data was medical records and interview data were used to supplement missing medical record data.
Statistical analysis
Body mass index (BMI, kg/m2) at diagnosis was categorized based on WHO criteria (normal: <25 kg/m2; overweight: 25.0–29.9 kg/m2; obese: ≥30 kg/m2). BMI data abstracted from medical records were available for 2,451 (92.2%) women and interview only data were available for the remaining 208 women. Among 1,452 participants whose BMI data were available from both sources, there was 79.4% agreement (kappa=0.69) within BMI categories. The level of agreement of BMI data from the two sources was similar across breast cancer subtypes (77.6%, 77.1%, 81.9% and 76.8% for luminal A, luminal B, TN and H2E cases, respectively). In general, self-reported BMI [mean: 27.2 kg/m2, standard deviation (SD): 6.5] was lower than BMI abstracted from medical records (mean: 28.3 kg/m2, SD: 6.9) among those with data from both sources. Height and weight at diagnosis were analyzed separately, categorized into quartiles based on the distribution of these variables among luminal A patients. The agreement between height and weight was 78.6% (kappa=0.71) and 75.9% (kappa=0.68) within these quartiles, respectively, with women on average self-reporting a slightly greater height [1.65 m, SD:0.07 (self-reported) vs. 1.64 m, SD: 0.07 (medical records)] and lower weight [74.03 kg, SD: 17.47 (self-reported) vs. 76.59 kg, SD: 18.79 (medical records)] than that recorded in medical records. Results did not change materially in a sensitivity analysis restricted to only data from medical records.
We used polytomous logistic regression to calculate ORs and their associated 95% confidence intervals (CIs) to estimate the risks for luminal B, TN, and H2E breast cancer relative to luminal A cancer. Luminal A patients served as the reference category for all analyses since this is the most commonly diagnosed breast cancer subtype. All analyses were performed using Stata/SE (Stata Corp, College Station, TX). All models were adjusted for age at diagnosis (5-year categories), year of diagnosis (continuous), and study site. Other variables evaluated as potential confounders (as categorized in Table 1) included race/ethnicity, first-degree family history of breast cancer, parity, health insurance, smoking status, recent HT use and recent OC use, but they did not change the risk estimates by more than 10% when individually assessed and hence were not added to our final model. Adjustment for height when evaluating weight (and vice versa) did not change ORs by more than 10% and therefore it was not included as a covariate in the final model. Analyses were further stratified by menopausal status and 50 subjects who had unknown menopausal status were excluded in this stratified analysis. Menopausal status was primarily determined based on information from medical records. Perimenopausal cases (n=223) were included in the premenopausal group. Postmenopausal women included those who had natural menopause or induced menopause by bilateral oophorectomy, and those who were using HT with unknown menstrual status (n=12). Among postmenopausal women, we evaluated HT use as a potential effect modifier focusing on the comparison between TN cases to luminal A cases. However no statistically significant interaction between HT use (collapsed into never or former users vs. current users due to small numbers after stratification) and BMI was observed (p for interaction=0.8999 on a multiplicative scale) so stratified results are not presented. Likelihood ratio testing was used to examine effect modification by other variables listed in Table 1, but none was statistically significant at p<0.05.
Table 1.
Distribution of demographic and risk factors by breast cancer subtype
| Luminal A (n=772) n % |
Luminal B (n=129) n % |
Triple negative (n=1275) n % |
H2E (n=483) n % |
|
|---|---|---|---|---|
|
|
||||
| Year of diagnosis | ||||
| 2004–2006 | 272 (35.2) | 46 (35.7) | 426 (33.4) | 155 (32.1) |
| 2007–2008 | 206 (26.7) | 27 (20.9) | 345 (27.1) | 124 (25.7) |
| 2009–2010 | 156 (20.2) | 30 (23.3) | 288 (22.6) | 111 (23.0) |
| 2011–2012 | 138 (17.9) | 26 (20.2) | 216 (16.9) | 93 (19.3) |
| Study site | ||||
| Seattle | 586 (75.9) | 88 (68.2) | 974 (76.4) | 343 (71.0) |
| New Mexico | 186 (24.1) | 41 (31.8) | 301 (23.6) | 140 (29.0) |
| Age at diagnosis | ||||
| <40 | 96 (12.4) | 27 (20.9) | 186 (14.6) | 53 (11.0) |
| 40–49 | 207 (26.8) | 44 (34.1) | 359 (28.2) | 115 (23.8) |
| 50–59 | 269 (34.8) | 39 (30.2) | 402 (31.5) | 191 (39.5) |
| 60–69 | 200 (25.9) | 19 (14.7) | 328 (25.7) | 124 (25.7) |
| Race/Ethnicity | ||||
| Non-Hispanic white | 610 (79.4) | 93 (72.7) | 970 (76.2) | 368 (76.3) |
| Hispanic white | 76 (9.9) | 22 (17.2) | 126 (9.9) | 57 (11.8) |
| African American | 25 (3.3) | 4 (3.1) | 101 (7.9) | 22 (4.6) |
| Asian/Pacific Islander | 46 (6.0) | 5 (3.9) | 46 (3.6) | 24 (5.0) |
| Native American | 11 (1.4) | 4 (3.1) | 30 (2.4) | 11 (2.3) |
| Missing | 4 | 1 | 2 | 1 |
| Health insurance status | ||||
| Any private | 644 (84.4) | 100 (78.1) | 1035 (82.5) | 390 (82.3) |
| Any Medicaid | 54 (7.1) | 11 (8.6) | 82 (6.5) | 28 (5.9) |
| Medicare | 42 (5.5) | 11 (8.6) | 98 (7.8) | 34 (7.2) |
| No insurance | 23 (3.0) | 6 (4.7) | 39 (3.1) | 22 (4.6) |
| Missing | 9 | 1 | 21 | 9 |
| First degree family history of breast cancer | ||||
| No | 575 (76.7) | 101 (80.2) | 972 (77.5) | 379 (80.1) |
| Yes | 175 (23.3) | 25 (19.8) | 282 (22.5) | 94 (19.9) |
| Missing | 22 | 3 | 21 | 10 |
| Menopausal status | ||||
| Pre-menopausal | 368 (48.4) | 76 (60.8) | 574 (45.7) | 199 (41.9) |
| Post-menopausal | 396 (51.8) | 49 (39.2) | 683 (54.3) | 276 (58.1) |
| Unknown or missing | 8 | 4 | 18 | 8 |
| Number of full term pregnancies | ||||
| 0 | 190 (24.6) | 39 (30.2) | 287 (22.5) | 91 (18.9) |
| 1 | 128 (16.6) | 27 (20.9) | 233 (18.3) | 83 (17.3) |
| 2 | 280 (36.3) | 40 (31.0) | 428 (33.6) | 179 (37.2) |
| ≥3 | 174 (22.5) | 23 (17.8) | 326 (25.6) | 128 (26.6) |
| Missing | 0 | 0 | 1 | 2 |
| Smoking status at breast cancer diagnosis | ||||
| Never | 453 (59.0) | 68 (53.5) | 732 (57.8) | 283 (58.7) |
| Current | 106 (13.8) | 17 (13.4) | 194 (15.3) | 73 (15.1) |
| Former | 209 (27.2) | 42 (33.1) | 341 (26.9) | 126 (26.1) |
| Missing | 4 | 2 | 8 | 1 |
| Recency of menopausal hormone use at diagnosis | ||||
| Never | 618 (83.7) | 113 (90.4) | 997 (83.5) | 381 (84.3) |
| Former | 42 (5.7) | 7 (5.6) | 90 (7.5) | 38 (8.4) |
| Current estrogen only | 34 (4.6) | 5 (4.0) | 81 (6.8) | 24 (5.3) |
| Current estrogen + progestin | 44 (6.0) | 0 (0.0) | 26 (2.2) | 9 (2.0) |
| Missing | 34 | 4 | 81 | 31 |
| Recency of hormonal oral contraceptive use at diagnosis | ||||
| Never | 619 (82.8) | 105 (85.4) | 995 (81.7) | 408 (88.1) |
| Former | 38 (5.1) | 8 (6.5) | 87 (7.1) | 20 (4.3) |
| Current | 61 (8.2) | 6 (4.9) | 76 (6.2) | 24 (5.2) |
| Ever with unknown end date | 30 (4.0) | 4 (3.3) | 60 (4.9) | 11 (2.4) |
| Missing | 24 | 6 | 57 | 20 |
Results
Luminal B cases were somewhat younger, more frequently Hispanic, premenopausal, nulliparous, never users of HT, and less likely to be never smokers and to have private health insurance compared to women with other breast cancer subtypes (Table 1). Women with TN cancers were somewhat more likely to be younger, African American, and to be current users of estrogen only HT. H2E cases were somewhat more likely to be postmenopausal, to have three or more full-term pregnancies and to have used OCs. Luminal A cases were somewhat more likely to be non-Hispanic white, to have private insurance, and to be current estrogen+progestin HT users and OC users compared to women with the other breast cancer subtypes.
Compared to luminal A cases, no differences in risk were observed with respect to increasing quartiles of BMI, weight, or height across the breast cancer subtypes based on p-values for trend (Table 2). However, when stratified by menopausal status (Table 2) both weight and BMI were positively associated with risk for TN breast cancer among premenopausal women (p- trend: 0.012 and 0.004, respectively) with a 5% increase in risk observed per 5 kg increase in weight and a 16% increase in risk per 5 kg/m2 increase in BMI. In contrast, we found a 9% and a 16% decrease in risk per 5 kg/m2 increase in BMI for TN and H2E cancers, respectively, among postmenopausal women. A 6% decrease in risk per 5 kg increase in weight was also observed for H2E disease. Height was not associated with differential risk of breast cancer subtypes among either premenopausal or postmenopausal women.
Table 2.
The relationship between body size at diagnosis and breast cancer subtypes, overall and by menopausal status†
| Luminal A | Luminal B | Triple negative | H2E | ||||
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | n (%) | OR (95% CI) | |
|
| |||||||
|
Overall
| |||||||
| BMI (kg/m2) | |||||||
| <25 | 283 (36.7) | 54 (41.9) | 1.00 (ref) | 435 (34.1) | 1.00 (ref) | 190 (39.3) | 1.00 (ref) |
| 25–29.9 | 221 (28.6) | 40 (31.0) | 0.96 (0.61–1.51) | 379 (29.7) | 1.12 (0.90–1.41) | 147 (30.4) | 0.97 (0.73–1.28) |
| ≥30 | 268 (34.7) | 35 (27.1) | 0.73 (0.46–1.15) | 461 (36.2) | 1.14 (0.92–1.42) | 146 (30.2) | 0.79 (0.60–1.04) |
| p for trend | 0.285 | 0.571 | 0.134 | ||||
| continuous (per 5kg/m2) | 0.93 (0.80–1.07) | 1.02 (0.96–1.08) | 0.94 (0.86–1.02) | ||||
| Weight (kg) | |||||||
| Q1(<62.6) | 191 (24.7) | 36 (27.9) | 1.00 (ref) | 288 (22.6) | 1.00 (ref) | 143 (29.6) | 1.00 (ref) |
| Q2(62.6–72.6) | 195 (25.3) | 34 (26.4) | 0.94 (0.56–1.56) | 309 (24.2) | 1.06 (0.82–1.37) | 112 (23.2) | 0.76 (0.55–1.04) |
| Q3(72.6–86.4) | 193 (25.0) | 33 (25.6) | 0.94 (0.56–1.58) | 345 (27.1) | 1.20 (0.93–1.55) | 125 (25.9) | 0.85 (0.62–1.16) |
| Q4(>86.4) | 193 (25.0) | 26 (20.2) | 0.78 (0.45–1.35) | 333 (26.1) | 1.17 (0.90–1.51) | 103 (21.3) | 0.71 (0.51–0.98)* |
| p for trend | 0.445 | 0.543 | 0.077 | ||||
| continuous (per 5kg) | 0.98 (0.93–1.03) | 1.01 (0.98–1.03) | 0.97 (0.94–1.00) | ||||
| Height (m) | |||||||
| Q1(<1.60) | 188(24.4) | 31 (24.0) | 1.00 (ref) | 316 (24.8) | 1.00 (ref) | 147 (30.4) | 1.00 (ref) |
| Q2(1.60–1.64) | 195 (25.3) | 24 (18.6) | 0.77 (0.43–1.36) | 322 (25.3) | 0.98 (0.75–1.26) | 117 (24.2) | 0.78 (0.57–1.07) |
| Q3(1.64–1.68) | 123 (15.9) | 26 (20.2) | 1.36 (0.76–2.42) | 194 (15.2) | 0.94 (0.70–1.25) | 73 (15.1) | 0.78 (0.54–1.12) |
| Q4(>1.68) | 266 (34.5) | 48 (37.2) | 1.08 (0.66–1.79) | 443 (34.7) | 0.98 (0.77–1.25) | 146 (30.2) | 0.73 (0.54–0.98)* |
| p for trend | 0.409 | 0.718 | 0.166 | ||||
| continuous (per 5cm) | 1.06 (0.92–1.21) | 0.99 (0.93–1.05) | 0.94 (0.87–1.02) | ||||
|
| |||||||
| Among premenopausal women | |||||||
|
| |||||||
| BMI (kg/m2) | |||||||
| <25 | 172 (46.7) | 36 (47.4) | 1.00 (ref) | 213 (37.1) | 1.00 (ref) | 80 (40.2) | 1.00 (ref) |
| 25–29.9 | 105 (28.5) | 25 (32.9) | 1.14 (0.64–2.01) | 161 (28.0) | 1.27 (0.92–1.75) | 59 (29.6) | 1.24 (0.81–1.88) |
| ≥30 | 91 (24.7) | 15 (19.7) | 0.78 (0.40–1.50) | 200 (34.8) | 1.82 (1.32–2.51)* | 60 (30.2) | 1.41 (0.92–2.16) |
| p for trend | 0.293 | 0.004 | 0.226 | ||||
| continuous (per 5kg/m2) | 0.89 (0.72–1.11) | 1.16 (1.05–1.28)* | 1.08 (0.95–1.24) | ||||
| Weight (kg) | |||||||
| Q1(<62.6) | 116 (31.5) | 23 (30.3) | 1.00 (ref) | 142 (24.7) | 1.00 (ref) | 59 (29.6) | 1.00 (ref) |
| Q2(62.6–72.6) | 105 (28.5) | 21 (27.6) | 1.05 (0.55–2.03) | 135 (23.5) | 1.09 (0.77–1.56) | 46 (23.1) | 0.88 (0.55–1.40) |
| Q3(72.6–86.4) | 79 (21.5) | 23 (30.3) | 1.50 (0.78–2.88) | 152 (26.5) | 1.65 (1.14–2.39)* | 48 (24.1) | 1.24 (0.77–2.01) |
| Q4(>86.4) | 68 (18.5) | 9 (11.8) | 0.71 (0.31–1.63) | 145 (25.3) | 1.79 (1.23–2.64)* | 46 (23.1) | 1.32 (0.80–2.16) |
| p for trend | 0.479 | 0.012 | 0.436 | ||||
| continuous (per 5kg) | 0.97 (0.90–1.05) | 1.05 (1.01–1.08)* | 1.02 (0.97–1.07) | ||||
| Height (m) | |||||||
| Q1(<1.60) | 85 (23.1) | 13 (17.1) | 1.00 (ref) | 131 (22.8) | 1.00 (ref) | 58 (29.1) | 1.00 (ref) |
| Q2(1.60–1.64) | 74 (20.1) | 14 (18.4) | 1.30 (0.57–2.98) | 149 (26.0) | 1.29 (0.87–1.91) | 43 (21.6) | 0.83 (0.50–1.38) |
| Q3(1.64–1.68) | 60 (16.3) | 19 (25.0) | 2.21 (1.01–4.86)* | 71 (12.4) | 0.76 (0.49–1.18) | 30 (15.1) | 0.73 (0.42–1.27) |
| Q4(>1.68) | 149 (40.5) | 30 (39.5) | 1.45 (0.71–2.97) | 223 (38.9) | 0.96 (0.68–1.37) | 68 (34.2) | 0.66 (0.42–1.03) |
| p for trend | 0.290 | 0.344 | 0.215 | ||||
| continuous (per 5cm) | 1.10 (0.92–1.31) | 0.96 (0.87–1.05) | 0.92 (0.82–1.05) | ||||
|
| |||||||
| Among post-menopausal women | |||||||
|
| |||||||
| BMI (kg/m2) | |||||||
| <25 | 109 (27.5) | 16 (32.7) | 1.00 (ref) | 216 (31.6) | 1.00 (ref) | 108 (39.1) | 1.00 (ref) |
| 25–29.9 | 113 (28.5) | 13 (26.5) | 0.74 (0.34–1.61) | 213 (31.2) | 0.93 (0.67–1.29) | 86 (31.2) | 0.75 (0.51–1.11) |
| ≥30 | 174 (43.9) | 20 (40.8) | 0.78 (0.39–1.57) | 254 (37.2) | 0.74 (0.54–1.00)* | 82 (29.7) | 0.47 (0.32–0.69)* |
| p for trend | 0.918 | 0.032 | 0.002 | ||||
| continuous (per 5kg/m2) | 1.01 (0.83–1.22) | 0.91 (0.84–0.99)* | 0.84 (0.75–0.94)* | ||||
| Weight (kg) | |||||||
| Q1(<62.6) | 74 (18.7) | 11 (22.4) | 1.00 (ref) | 141 (20.6) | 1.00 (ref) | 82 (29.7) | 1.00 (ref) |
| Q2(62.6–72.6) | 89 (22.5) | 13 (26.5) | 0.87 (0.37–2.09) | 172 (25.2) | 0.97 (0.66–1.42) | 64 (23.2) | 0.63 (0.40–0.99)* |
| Q3(72.6–86.4) | 110 (27.8) | 8 (16.3) | 0.46 (0.18–1.22) | 187 (27.4) | 0.87 (0.60–1.25) | 76 (27.5) | 0.62 (0.40–0.96)* |
| Q4(>86.4) | 123 (31.1) | 17 (34.7) | 0.89 (0.39–2.03) | 183 (26.8) | 0.75 (0.52–1.08) | 54 (19.6) | 0.40 (0.25–0.62)* |
| p for trend | 0.846 | 0.079 | 0.002 | ||||
| continuous (per 5kg) | 1.01 (0.94–1.08) | 0.97 (0.94–1.00) | 0.94 (0.90–0.98)* | ||||
| Height(m) | |||||||
| Q1(<1.60) | 103 (26.0) | 17 (34.7) | 1.00 (ref) | 180 (26.4) | 1.00 (ref) | 85 (30.8) | 1.00 (ref) |
| Q2(1.60–1.64) | 118 (29.8) | 8 (16.3) | 0.40 (0.16–0.97)* | 170 (24.9) | 0.78 (0.56–1.10) | 73 (26.4) | 0.77 (0.50–1.16) |
| Q3(1.64–1.68) | 63 (15.9) | 7 (14.3) | 0.71 (0.28–1.84) | 118 (17.3) | 1.05 (0.71–1.56) | 42 (15.2) | 0.86 (0.52–1.40) |
| Q4(>1.68) | 112 (28.3) | 17 (34.7) | 0.90 (0.43–1.89) | 215 (31.5) | 1.04 (0.74–1.46) | 76 (27.5) | 0.84 (0.55–1.28) |
| p for trend | 0.929 | 0.537 | 0.658 | ||||
| continuous (per 5cm) | 1.01 (0.81–1.26) | 1.03 (0.94–1.13) | 0.98 (0.87–1.09) | ||||
Statistically significant at p<0.05.
All models adjusted for reference year, age at reference and study site.
Discussion
In this population-based case-case study with an appreciably larger sample size than any of the prior published studies, our data suggest that there is heterogeneity in the relationship between BMI and breast cancer molecular subtype risk by menopausal status. While it is well-established that obesity is a risk factor for postmenopausal breast cancer with a large pooled analysis of prospective studies suggesting 7% increased risk per 4 kg/m2 increase in BMI and 6% increase per 10 kg increase in weight,[9] our study suggests that obesity may primarily contribute to an increased risk of postmenopausal luminal disease, but not to risk of either TN or H2E breast cancer. This is consistent with earlier cohort [11] and case-control [12–14] studies, and a meta-analysis [22] that have generally observed no association between BMI and risk of ER- breast cancer among postmenopausal women. The few studies that have employed specific case-case comparisons [12, 15–17] reported no differential risk of postmenopausal TN or H2E cancers relative to luminal A subtype in relation to BMI. However, these studies had quite limited sample sizes with number of cases ranging from 143 to 375 and 61 to 117 for TN and H2E cancers, respectively.
The relationship between obesity and postmenopausal breast cancer is thought to primarily be hormonally driven[23]. The peripheral conversion of androgens to estrogen in adipose tissue is the primary source of estrogen in postmenopausal women, and obesity has been shown to directly translate to higher blood levels of circulating estrogen in older women. [23, 24] Thus, the observation that obesity may primarily impact ER+ breast cancer risk is consistent with this mechanism. The correlation between BMI and physical activity levels, a factor we did not have data to control for in this study, may also partly explain the observed associations as some prior studies [25, 26] but not all [27–30] have reported that the inverse association between physical activity and risk of postmenopausal cancers was limited to or stronger for ER+ cancers than ER- cancers. Although some earlier studies have found that the relationship between BMI and breast cancer risk among postmenopausal women is modified by HT use, with elevations in risk restricted to non-users of HT[8, 31, 32], we did not observe this in our data. A potential reason is that the numbers of HT users has dropped precipitously in recent years such that current HT users comprised <10% of our study population and consequently we had limited statistical power to evaluate this interaction.
It is also well-established that obesity is associated with a reduced risk of premenopausal breast cancer [9, 10]. However, we find that this reduction in risk may be restricted to ER+ disease as we observed that compared to women with luminal A breast cancer, obesity was associated with an increased risk of TN breast cancer. This is consistent with prior studies that reported increased risks of TN cancer with higher BMI compared to either cancer-free women [15] or women with luminal A cancers[16]. Although three other studies assessing this association did not find statistically significant relationships, all of their risk estimates were suggestive of an increased risk of TN breast cancer associated with increased BMI in young women[12, 17, 18].
In premenopausal women, ovaries are the primary source of circulating estrogen. Obesity can result in irregular and less frequent menstrual cycles among premenopausal women and thus a lower cumulative exposure to estrogen [33, 34], which may contribute to their reduction in risk of ER+ disease. Some non-hormonal pathways may also mediate the association between obesity and TN cancers among premenopausal women. Obesity is associated with inflammation, elevated levels of insulin and insulin-like growth factors and other carcinogens [35, 36] that have been linked to a higher premenopausal breast cancer risk [37–39]. However, it remains uncertain which specific factors may drive risk of particular breast cancer subtype. The potentially detrimental impact of obesity on risk of premenopausal TN cancers may partly explain the repeatedly observed higher incidence of TN cancers among young African American and Hispanic women compared to non-Hispanic whites [40, 41]. According to data from the 2009–2010 National Health and Nutrition Examination Survey, the prevalence of overweight or obesity (BMI ≥ 25 kg/m2) for women between 20–39 years of age was 74.2% for African Americans, 65.4% for Hispanics, and 50.7% for non-Hispanic whites [42].
It is important to acknowledge the limitations of our study. This study compared luminal B, TN and H2E cases to luminal A breast cancer cases in relation to height, weight and BMI and thus the associations obtained here should not be interpreted as risks compared to a cancer-free control population. Given the wealth of data and evidence establishing anthropometric factors as risk factors for breast cancer, the approach used here enables efficient evaluation of etiologic heterogeneity across subtypes where the case-case odds ratio is equal to the ratio of the relative risk of developing one subtype of the disease to relative risk of developing the reference subtype [43]. Recall bias is a potential concern for most case-control studies, but in this study anthropometric data for 92.2% of our sample was obtained from medical records. Further, results were unchanged when analyses were restricted to data ascertained only from medical records. Women with unknown tumor markers were excluded and misclassification of case subtypes is also possible as we abstracted ER, PR and HER2 information from cancer registries which gather data from various clinics and laboratories with potential variability in practices regarding reading and interpreting these biomarkers, however any misclassification present is likely non-differential and is unlikely to be related to anthropometric characteristics. We did not have data on some important lifestyle factors such as diet and physical activity which may be correlated with both BMI and risk of certain subtypes of breast cancer. With respect to generalizability, our study included few African American but did recruit a substantially larger number of Hispanic women than have been included in prior etiologic studies of breast cancer subtypes. Also, we reviewed pathology reports and addendums and retrieved tumor marker information for at least 94% of potentially eligible participants to minimize bias due to exclusion of cases missing tumor marker status. Since active consent was obtained from Seattle but not New Mexico participants, we compared the demographic characteristics of Seattle participants vs. non-participants. Participants were somewhat more likely to be older (proportion of women diagnosed between ages 60 and 69 years: 27.7%, 25.9%, 30.5% vs. 25.6%, 21.7% and 21.8% for ER+, H2E and TN cases among those who were enrolled and those who refused, respectively) and non-Hispanic white (88.5%, 85.4%, 82.8% vs. 74.8%, 62.5%, 73.4% for ER+, H2E and TN cases among those who were enrolled and those who refused, respectively) compared to non-participants.
Our results add to recent evidence that the role of BMI in pre- and postmenopausal breast cancer differs across the major breast cancer subtypes and adds to our understanding of the etiologies of clinically aggressive TN and H2E tumors. Further clarifying the impact of potentially modifiable risk factors such as BMI according to breast cancer subtype can help inform both the origins of known breast cancer disparities and the development of novel prevention strategies.
Acknowledgments
Funding/Support: This study was supported by National Cancer Institute 261201000029C (C.I. Li), P50 CA148143 (C.I. Li, L.S. Cook, D.A. Hill) and 261201000033C (C.L. Wiggins).
Role of the Funder: The National Cancer Institute had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
Author contributions: Lu Chen had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Study concept and design: L. Chen, L.S. Cook, C.I. Li
Acquisition, analysis or interpretation of data: L. Chen, L.S. Cook, C.I. Li, M.C. Tang, P. Porter, D.A. Hill, C.L. Wiggins, L.S. Cook, C.I. Li
Drafting of the manuscript: L. Chen
Critical revision of the manuscript for important intellectual content: L. Chen, C.I. Li, M.C. Tang, P. Porter, D.A. Hill, C.L. Wiggins, L.S. Cook
Administrative, technical, or material support: M.C. Tang, P. Porter, D.A. Hill, C.L. Wiggins, L.S. Cook, C.I. Li
Study supervision: L.S. Cook, C.I. Li
References
- 1.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 4.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7:4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawood S, Hu R, Homes MD, et al. Defining breast cancer prognosis based on molecular phenotypes: results from a large cohort study. Breast Cancer Res Treat. 2011;126:185–92. doi: 10.1007/s10549-010-1113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parise Ca, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J. 2009;15:593–602. doi: 10.1111/j.1524-4741.2009.00822.x. [DOI] [PubMed] [Google Scholar]
- 7.Trentham-Dietz A, Newcomb PA, Storer BE, et al. Body Size and Risk of Breast Cancer. Am J Epidemiol. 1997;145:1011–1019. doi: 10.1093/oxfordjournals.aje.a009057. [DOI] [PubMed] [Google Scholar]
- 8.Morimoto L, White E, Chen Z, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States) CANCER CAUSES Control. 2002;13:741–751. doi: 10.1023/A:1020239211145. [DOI] [PubMed] [Google Scholar]
- 9.van den Brandt PA, Spiegelman D, Yaun SS, et al. Pooled Analysis of Prospective Cohort Studies on Height, Weight, and Breast Cancer Risk. Am J Epidemiol. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 10.Ursin G, Longnecker MP, Haile RW, Greenland S. A Meta-Analysis of Body Mass Index and Risk of Premenopausal Breast Cancer on JSTOR. Epidemiology. 1995;6:137–41. doi: 10.1097/00001648-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Horn J, Alsaker MDK, Opdahl S, et al. Anthropometric factors and risk of molecular breast cancer subtypes among postmenopausal Norwegian women. Int J Cancer. 2014;135:2678–86. doi: 10.1002/ijc.28912. [DOI] [PubMed] [Google Scholar]
- 12.Yang XR, Sherman ME, Rimm DL, et al. Differences in risk factors for breast cancer molecular subtypes in a population-based study. Cancer Epidemiol Biomarkers Prev. 2007;16:439–443. doi: 10.1158/1055-9965.EPI-06-0806. [DOI] [PubMed] [Google Scholar]
- 13.Bandera EV, Chandran U, Hong C-C, et al. Obesity, body fat distribution, and risk of breast cancer subtypes in African American women participating in the AMBER Consortium. Breast Cancer Res Treat. 2015;150:655–666. doi: 10.1007/s10549-015-3353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phipps AI, Malone KE, Porter PL, et al. Body Size and Risk of Luminal, HER2-Overexpressing, and Triple-Negative Breast Cancer in Postmenopausal Women. Cancer Epidemiol Biomarkers Prev. 2008;17:2078–2086. doi: 10.1158/1055-9965.EPI-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudet MM, Press MF, Haile RW, et al. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res Treat. 2011;130:587–597. doi: 10.1007/s10549-011-1616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwan ML, Kushi LH, Weltzien E, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11:R31. doi: 10.1186/bcr2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millikan RC, Newman B, Tse CK, et al. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolle JM, Daling JR, White E, et al. Risk factors for triple-negative breast cancer in women under the age of 45 years. Cancer Epidemiol Biomarkers Prev. 2009;18:1157–66. doi: 10.1158/1055-9965.EPI-08-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bauer KR, Brown M, Cress RD, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–8. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 20.Li CI, Beaber EF, Tang MTC, et al. Effect of Depo-Medroxyprogesterone Acetate on Breast Cancer Risk among Women 20 to 44 Years of Age. Cancer Res. 2012;72:2028–2035. doi: 10.1158/0008-5472.CAN-11-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li CI, Malone KE, Porter PL, et al. Relationship between menopausal hormone therapy and risk of ductal, lobular, and ductal-lobular breast carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:43–50. doi: 10.1158/1055-9965.EPI-07-0558. [DOI] [PubMed] [Google Scholar]
- 22.Munsell MF, Sprague BL, Berry Da, et al. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev. 2014;36:114–136. doi: 10.1093/epirev/mxt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Key TJ, Appleby PN, Reeves GK, et al. Body Mass Index, Serum Sex Hormones, and Breast Cancer Risk in Postmenopausal Women. J Natl Cancer Inst. 2003;95:1218–26. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 24.Tchernof A. Després JP Sex steroid hormones, sex hormone-binding globulin, and obesity in men and women. Horm Metab Res. 32:526–36. doi: 10.1055/s-2007-978681. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt ME, Steindorf K, Mutschelknauss E, et al. Physical activity and postmenopausal breast cancer: effect modification by breast cancer subtypes and effective periods in life. Cancer Epidemiol Biomarkers Prev. 2008;17:3402–10. doi: 10.1158/1055-9965.EPI-08-0479. [DOI] [PubMed] [Google Scholar]
- 26.Steindorf K, Ritte R, Eomois P-P, et al. Physical activity and risk of breast cancer overall and by hormone receptor status: the European prospective investigation into cancer and nutrition. Int J cancer. 2013;132:1667–78. doi: 10.1002/ijc.27778. [DOI] [PubMed] [Google Scholar]
- 27.Phipps AI, Chlebowski RT, Prentice R, et al. Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:454–63. doi: 10.1158/1055-9965.EPI-10-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fournier A, Dos Santos G, Guillas G, et al. Recent recreational physical activity and breast cancer risk in postmenopausal women in the E3N cohort. Cancer Epidemiol Biomarkers Prev. 2014;23:1893–902. doi: 10.1158/1055-9965.EPI-14-0150. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Eliassen AH, Tamimi RM, et al. Adult body size and physical activity in relation to risk of breast cancer according to tumor androgen receptor status. Cancer Epidemiol Biomarkers Prev. 2015;24:962–8. doi: 10.1158/1055-9965.EPI-14-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enger S, Ross R, Paganini-Hill A, et al. Body size, physical activity, and breast cancer hormone receptor status: Results from two case-control studies. [PubMed] [Google Scholar]
- 31.Lahmann PH, Hoffmann K, Allen N, et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer And Nutrition (EPIC) Int J Cancer. 2004;111:762–71. doi: 10.1002/ijc.20315. [DOI] [PubMed] [Google Scholar]
- 32.Huang Z, Hankinson SE, Colditz GA, et al. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407–11. [PubMed] [Google Scholar]
- 33.Kato I, Toniolo P, Koenig KL, et al. Epidemiologic correlates with menstrual cycle length in middle aged women. Eur J Epidemiol. 1999;15:809–14. doi: 10.1023/a:1007669430686. [DOI] [PubMed] [Google Scholar]
- 34.Gerber M. Reversal of relation between body mass and endogenous estrogen concentrations with menopausal status. J Natl Cancer Inst. 1997;89:661–2. doi: 10.1093/jnci/89.9.661. [DOI] [PubMed] [Google Scholar]
- 35.Morris PG, Hudis CA, Giri D, et al. Inflammation and Increased Aromatase Expression Occur in the Breast Tissue of Obese Women with Breast Cancer. Cancer Prev Res. 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 37.Del Giudice ME, Fantus IG, Ezzat S, et al. Insulin and related factors in premenopausal breast cancer risk. Breast Cancer Res Treat. 1998;47:111–20. doi: 10.1023/a:1005831013718. [DOI] [PubMed] [Google Scholar]
- 38.Schernhammer ES, Holly JM, Pollak MN, Hankinson SE. Circulating levels of insulinlike growth factors, their binding proteins, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:699–704. doi: 10.1158/1055-9965.EPI-04-0561. [DOI] [PubMed] [Google Scholar]
- 39.Schernhammer ES, Holly JM, Hunter DJ, et al. Insulin-like growth factor-I, its binding proteins (IGFBP-1 and IGFBP-3), and growth hormone and breast cancer risk in The Nurses Health Study II. Endocr Relat Cancer. 2006;13:583–92. doi: 10.1677/erc.1.01149. [DOI] [PubMed] [Google Scholar]
- 40.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014 doi: 10.1093/jnci/dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amirikia KC, Mills P, Bush J, Newman LA. Higher population-based incidence rates of triple-negative breast cancer among young African-American women : Implications for breast cancer screening recommendations. Cancer. 2011;117:2747–53. doi: 10.1002/cncr.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 43.Begg CB, Zhang ZF. Statistical analysis of molecular epidemiology studies employing case-series. Cancer Epidemiol Biomarkers Prev. 1994;3:173–5. [PubMed] [Google Scholar]


