Abstract
Moderate-to-severe fatigue occurs in up to 94% of oncology patients undergoing active treatment. Current interventions for fatigue are not efficacious. A major impediment to the development of effective treatments is a lack of understanding of the fundamental mechanisms underlying fatigue. In the current study, differences in phenotypic characteristics and gene expression profiles were evaluated in a sample of breast cancer patients undergoing chemotherapy (CTX) who reported low (n = 19) and high (n = 25) levels of evening fatigue. Compared to the low group, patients in the high evening fatigue group reported lower functional status scores, higher comorbidity scores, and fewer prior cancer treatments. One gene was identified as upregulated and 11 as downregulated in the high evening fatigue group. Gene set analysis found 24 downregulated and 94 simultaneously up- and downregulated pathways between the two fatigue groups. Transcript origin analysis found that differential expression (DE) originated primarily from monocytes and dendritic cell types. Query of public data sources found 18 gene expression experiments with similar DE profiles. Our analyses revealed that inflammation, neurotransmitter regulation, and energy metabolism are likely mechanisms associated with evening fatigue severity; that CTX may contribute to fatigue seen in oncology patients; and that the patterns of gene expression may be shared with other models of fatigue (e.g., physical exercise and pathogen-induced sickness behavior). These results suggest that the mechanisms that underlie fatigue in oncology patients are multifactorial.
Keywords: gene expression, inflammation, cancer, fatigue, chemotherapy, diurnal variations in fatigue, breast cancer
In women undergoing chemotherapy (CTX) for breast cancer, prevalence rates for fatigue range from 30% to 94% (Alcantara-Silva, Freitas-Junior, Freitas, & Machado, 2013; Berger, Gerber, & Mayer, 2012). The severity of fatigue varies over the course of a day and displays marked interindividual variability (Dhruva et al., 2010; B. A. Fletcher et al., 2009; Miaskowski et al., 2008; Molassiotis & Chan, 2004). Some of this interindividual variability is explained by the fact that morning and evening fatigue are distinct but related symptoms (Dhruva et al., 2010, 2013; Miaskowski et al., 2008). Of note, morning and evening fatigue are distinguished by different phenotypic and genotypic characteristics. For example, in one study, a higher number of comorbid conditions was associated with more severe morning fatigue, whereas caring for children at home was associated with more severe evening fatigue (Dhruva et al., 2010). In other studies, variations in interleukin (IL) 8 and tumor necrosis factor α (TNFA) were associated with the severity of morning fatigue, whereas variations in IL 1 receptor 2 (IL1R2), IL4, IL6, and TNFA were associated with the severity of evening fatigue (Aouizerat et al., 2009; Dhruva et al., 2015; Miaskowski et al., 2010). A better understanding of the unique phenotypic and molecular characteristics associated with morning and evening fatigue would provide the means to identify high-risk patients and to develop and test interventions for these devastating symptoms.
Difficulties in the diagnosis and treatment of fatigue are related to our lack of understanding of the fundamental mechanisms that underlie this debilitating symptom. Work by our group (Aouizerat et al., 2009; Miaskowski et al., 2010) and others (Bower et al., 2009, 2013; Collado-Hidalgo, Bower, Ganz, Irwin, & Cole, 2008; Reinertsen et al., 2011) suggests that inflammation plays a role in the development of fatigue. However, while it is safe to say that the mechanisms for fatigue are multifactorial (Ryan et al., 2007), the causative pathways remain to be identified. One approach for identifying additional mechanism(s) for fatigue is to evaluate for changes in gene expression associated with this symptom.
To date, only six studies, in four independent samples, have evaluated for differences in gene expression associated with fatigue in oncology patients (Bower, Ganz, Irwin, Arevalo, & Cole, 2011; Hsiao, Araneta, Wang, & Saligan, 2013; Hsiao, Wang, Kaushal, & Saligan, 2013; Landmark-Hoyvik et al., 2009; Light et al., 2013; Saligan et al., 2013). One study evaluated changes in gene expression in pathways solely involved in mitochondrial function (Hsiao, Wang, et al., 2013). In another study (Light et al., 2013), researchers evaluated gene expression for a number of specific pathways (e.g., adrenergic, monoamine, and peptides). Of the four studies that collected whole-transcriptome measurements, three focused on select genes and pathways related to mitochondrial function (Hsiao, Wang, et al., 2013) and/or inflammation and immune function (Bower et al., 2011; Hsiao, Araneta, et al., 2013; Saligan et al., 2013). Only one study reported findings on the whole transcriptome (Landmark-Hoyvik et al., 2009). In all six studies, investigators used a general measure of fatigue and did not evaluate diurnal variability in fatigue. Additional studies are needed that apply hypothesis-generating approaches utilizing the entire transcriptome in order to identify novel pathways and processes associated with fatigue in oncology patients. Based on previous work on diurnal variations in fatigue (Aouizerat et al., 2009; Dhruva et al., 2013; Miaskowski et al., 2010), we believe that an evaluation of one dimension of fatigue (i.e., evening fatigue) may improve our ability to detect differences in gene expression between patients who do and do not experience fatigue.
In the present study, we evaluated for differences in gene expression in peripheral leukocytes of patients with low and high levels of evening fatigue. Though researchers continue to debate the role of central versus peripheral mechanisms in the development and maintenance of fatigue (Yavuzsen et al., 2009), it is the case that peripheral changes in the expression of pro-inflammatory cytokine genes can influence neural and endocrine activity (Dantzer, O’Connor, Freund, Johnson, & Kelley, 2008) and contribute to a reciprocal regulation between the neural and innate immune systems termed the “neuro-immune circuit” (Irwin & Cole, 2011). This neuro-immune circuit originates with the innate immune system (Cole, Hawkley, Arevalo, & Cacioppo, 2011; Powell, Mays, Bailey, Hanke, & Sheridan, 2011). Pro-inflammatory cytokines can cross the blood–brain barrier (Quan & Banks, 2007). In addition, increased synthesis of cytokines in the brain can occur in response to peripheral input. Therefore, studies of gene expression from peripheral leukocytes can provide valuable information on fatigue.
Evaluation of the parallel expression measures of a genome (e.g., the transcriptome) will increase our understanding of the functions of various genes as well as their contributions to the biology of an organism (Butte, 2002). Moreover, novel statistical approaches permit the identification of differential gene expression patterns at the level of a gene, a biological pathway, and the entire transcriptome. The aim of this study was to use high throughput methods to determine whether there were changes in gene expression in peripheral leukocytes associated with high and low levels of evening fatigue in a sample of patients undergoing CTX for breast cancer (N = 44). The analytic methods we employed in this study included the use of microarray data to identify genes and pathways associated with evening fatigue, the use of bioinformatic analyses to infer the cellular origin for the differences in gene expression detected in peripheral leukocytes, and an interrogation of publicly available transcriptome gene expression experiments that share a similar pattern with the gene expression differences identified in our sample.
Method
Online Supplement 1 contains a detailed description of the methods.
Patients and Settings
For this longitudinal study, we enrolled patients who were ≥18 years of age; had a diagnosis of breast, gastrointestinal, gynecological, or lung cancer; had received CTX within the preceding 4 weeks; were scheduled to receive at least two additional cycles of CTX; were able to read, write, and understand English; and gave written informed consent. We recruited patients from two comprehensive cancer centers, one Veteran’s Affairs hospital, one public hospital, and four community-based oncology practices and included the first 44 eligible patients with breast cancer.
Instruments
We used a demographic questionnaire to obtain information on age, gender, ethnicity, marital status, living arrangements, education, employment status, and income. To evaluate patients’ functional status, we used the Karnofsky Performance Status (KPS) scale (Karnofsky, Abelmann, Craver, & Burchenal, 1948). We used the Self-administered Comorbidity Questionnaire (SCQ) to evaluate the occurrence, treatment, and functional impact of common comorbid conditions (e.g., diabetes, arthritis; Sangha, Stucki, Liang, Fossel, & Katz, 2003).
Finally, to assess physical fatigue, we used the 13-item Lee Fatigue Scale (LFS; K. A. Lee, Hicks, & Nino-Murcia, 1991). The 13 items on the LFS ask respondents to rate various aspects of fatigue on a 100-mm visual analogue line, with endpoints labeled as 0 and 10. We calculated a total mean fatigue score, with higher scores indicating greater fatigue severity. Patients rated each item based on how they felt prior to going to bed each night over the previous week (i.e., evening fatigue). The LFS has well-established validity and reliability. A score of >5.6 indicates a clinically meaningful level of evening fatigue (B. S. Fletcher et al., 2008). From the first 50 patients with breast cancer enrolled in the parent study, we selected data from a subset of 44 patients, which provided the largest number of patients each for the low (LFS score < 5.6 and n = 19) and high (LFS score ≥ 5.6 and n = 25) evening fatigue groups.
Study Procedures
The Committee on Human Research at the University of California, San Francisco (UCSF) and committees at each of the study sites approved the study. A research staff member approached patients who had received at least one cycle of CTX in the infusion unit to discuss participation in the study. All participants signed written informed consent. Patients completed study questionnaires in their homes a total of 6 times over two cycles of CTX (e.g., prior to the next CTX administration [enrollment], approximately 1 week after CTX administration, and approximately 2 weeks after CTX administration, depending on the length of their CTX cycles). We used mean evening fatigue scores at the time of enrolment for the analyses in the present study. In addition to the questionnaires, we reviewed participants’ medical records for disease and treatment information.
Gene-Expression Measurements
Sample processing
Total ribonucleic acid (RNA) was extracted from whole blood collected into PAXgene RNA stabilization tubes and processed using a standard protocol (Qiagen, USA). The blood specimen was collected prior to administration of CTX. RNA concentration was measured by NanoDrop ultraviolet spectrophotometry (ThermoScientific, USA). RNA integrity was evaluated using the RNA 6000 Nano assay (Agilent, USA). All RNA samples were determined to be of good quality (i.e., RNA integrity number ≥ 8) and were retained for gene-expression profiling.
Microarray hybridization
For each sample, approximately 100 ng of total RNA was labeled using the Illumina Total Prep RNA Amplification Kit (Ambion, Foster City, CA, USA) and then hybridized to the HumanHT-12 v4.0 Expression BeadChip (47,214 transcripts; Illumina, San Diego, CA, USA). The BeadChips were scanned using the iScan system (Illumina, USA) at the UCSF Genomics Core Facility. Each HumanHT-12 BeadChip contains 12 sample BeadArrays. A total of 47 samples were measured (i.e., 44 patient specimens and 3 technical replicates). To assess for between-BeadChip variation, one sample was repeated on each of the four BeadChips at a different physical BeadArray position.
Microarray preprocessing and normalization
Summary-level data from the uncorrected, nonnormalized, and nontransformed summary intensities at the probe level were calculated. Data preparation and analyses were conducted using two well-established protocols (Gentleman et al., 2004; Luo, Friedman, Shedden, Hankenson, & Woolf, 2009). The quality control procedures and associated results are described in detail in Online Supplement 1 and summarized in Supplemental Figure S1 . None of the samples displayed unusual distance between arrays or array signal intensity distributions. Background correction, quantile normalization, and log2 transformation were performed using limma (Smyth, 2005). Probes with insufficient expression measurements were excluded, leaving 34,267 assays spanning 16,980 genes for analysis (Online Supplement 2, Supplemental Figure S1, panel B). The reliability of the expression measurements was supported by a high level of correlation between quadruplicate arrays across all filtered assays (mean pairwise Pearson’s ρ = .95). These values were significantly higher than those observed between all samples (mean pairwise Pearson’s ρ = .92, Welch two sample t-test p < 1.31 × 10−12). Finally, potential clustering of samples was evaluated by principal components analysis. No obvious clustering by fatigue group was observed (data not shown) and no adjustment for batch effects was warranted.
Data Analyses
Demographic and clinical data
Demographic and clinical data were analyzed using SPSS version 22 (IBM, Armonk, NY) and Stata version 13.0 (StataCorp, College Station, TX). Descriptive statistics and frequency distributions were calculated for demographic and clinical characteristics as well as for fatigue severity. The two evening-fatigue groups were defined using a score of 5.6 on the LFS, which is the cut-off score indicating clinically meaningful fatigue, with scores < 5.6 indicating low evening fatigue (n = 19) and scores ≥ 5.6 indicating high evening fatigue (n = 25). Independent sample t-tests, Mann-Whitney U tests, χ2 tests, and Fisher’s exact tests were used to evaluate for differences in demographic and clinical characteristics between the two evening-fatigue groups. Effect size was calculated using Cohen’s d statistic (Cohen, 1988).
Differential gene expression
Differential expression (DE) of genes can offer insights into the biological processes that influence interindividual variability in evening fatigue. Although numerous approaches are used to identify between-group differences in DE (Jeanmougin et al., 2010), we selected two well-known methods, namely, the t-test using GenePattern and an estimation of gene-by-gene variance with limma (Figure 1, blue outline).
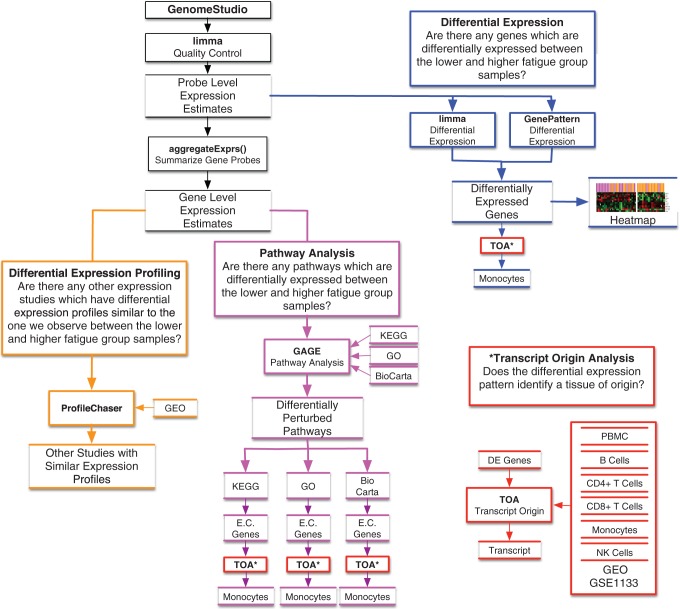
Figure 1.
Experimental approach and analysis plan. The diagram illustrates the analysis plan for the study, which includes differential expression (blue outline), differential expression profiling (orange outline), pathway analysis (purple outline), transcript origin analysis (red outline), and ProfileChaser (orange outline). Closed boxes denote analysis or software, and open boxes denote data or results. aggregateExprs() = a function contained in the PGSEA R (version 1.44.0) statistical software package; DE = differentially expressed; E.C. genes = essential contributing genes; GAGE = Generally Applicable Gene Set Enrichment; GEO = gene expression omnibus; GO = gene ontogeny; GSE = GEO series; KEGG = Kyoto Encyclopedia of Genes; NK = natural killer; PBMC = peripheral blood mononuclear cell (dendritic); TOA = transcript origin analysis.
Unsupervised clustering was used to evaluate the resolution of the two evening-fatigue groups by the DE genes identified using limma and GenePattern. Evening-fatigue group membership was then mapped onto the samples to visualize the degree to which the DE genes distinguished between patients with high versus low levels of evening fatigue (Figure 1, blue outline).
Differential pathway perturbation
Since pathway analysis is performed at the level of the gene, a summary signal estimate of expression was calculated from all valid probes spanning each gene (Reimers, 2010). Summary signal intensities were obtained for 16,980 genes. Differential pathway perturbation was performed using competitive analysis with the R package gageData ((Release 3.2) generally applicable gene set enrichment; Luo et al., 2009).By excluding genes with insufficient background expression levels and utilizing a whole-genome gene-expression microarray, we minimized the impact of known limitations and spurious results associated with competitive approaches (Figure 1, purple outline; Tripathi, Glazko, & Emmert-Streib, 2013).
Pathways and gene sets were defined using the 177 Kyoto Encyclopedia of Genes (KEGG; Aoki-Kinoshita & Kanehisa, 2007), 259 BioCarta (Nishimura, 2001), and 17,202 gene ontology (GO; Harris et al., 2004) annotated sets provided by the gageData R package. Pathways model the complex interactions between genes in a biological setting and are not expected to be solely simultaneously all up- (or down-) regulated. Rather, perturbations are more likely to consist of a mixture of up- and downregulation. As such, we tested for differential perturbations under three models: upregulation, downregulation, and both (simultaneous up/down or “2-D”). While all of the genes in each pathway were included in this analysis, only a subset of these genes had discernible expression changes above background (termed “essential contributing [EC] genes”).
Transcript origin analysis (TOA)
Peripheral blood contains a heterogeneous population of nucleated immune cells (e.g., B cells, CD4+ T cells, CD8+ T cells, dendritic cells, monocytes, and natural killer [NK] cells). Each cell type is involved in unique biological processes and expresses different subsets of genes (i.e., genes involved in different biological pathways). In order to identify the cell type(s) of origin for genes and/or pathways that were significantly DE between the high- and low-evening-fatigue groups, we used the TOA test (Figure 1, red outline), as described by Cole, Hawkley, Arevalo, and Cacioppo (2011), using Python. Our implementation was diagnostic for B cells, CD4+ T cells, dendritic cells, monocytes, and NK cells (Supplemental Table S1). We were not able to sufficiently validate for diagnosticity of CD8+ T cells and consider any results that identified this class of cells as unreliable.
Objective query of publicly available transcriptome experiments
To better categorize and understand the biological significance of the molecular signatures associated with evening fatigue, we employed a data-driven approach to leverage the collection of over 1,800 data sets available in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) and to identify curated data sets with similar DE patterns (Figure 1, orange outline). Specifically, we employed ProfileChaser (Engreitz et al., 2010; http://profilechaser.stanford.edu/) to identify DE profiles that existed in GEO similar to the ones that we identified between the low- and high-evening-fatigue groups.
Two independent reviewers curated the abstracts for each published study from the candidate profiles with similar DE patterns for conceptual relevance to evening fatigue. If either reviewer selected an abstract for consideration, it was included for further evaluation. Then, the factor comparison identified by ProfileChaser was evaluated in order to exclude uninterpretable comparisons. While a large number of significant hits were expected, false positives needed to be identified and culled (Engreitz et al., 2010). As hypothesized, many of the significantly similar profiles were not appropriate for our study due to the content-agnostic fashion (i.e., no pairwise comparisons are excluded) in which the universe of target profiles were generated (e.g., a “sample ID” factor that splits samples based on the individual ID of samples) or were not easily interpretable in the context of our current study (e.g., a factor splitting sample based on expression at different time points of a yeast colony’s development). Manuscripts from profile matches for all studies of interest to either reviewer (K. M. K and B. E. A) were retained. From this list, we collected and reviewed the full manuscripts and supplemental materials.
Results
Patient Characteristics
The total sample consisted of 44 women undergoing CTX for breast cancer. As shown in Table 1, the majority of the patients were college graduates and Caucasian with a mean age of 56.1 (±9.5) years. Patients had a mean KPS score of 80.9 (±12.9) and a mean SCQ score of 5.8 (±3.5). The mean fatigue score for the total sample was 5.6 (±2.2). The mean scores for the low- (n = 19) and the high- (n = 25) evening-fatigue groups were 3.7 (±1.7) and 7.1 (±1.2), respectively (p < .001).
Table 1.
Demographic and Clinical Characteristics of the Low- and High-Evening-Fatigue Groups.
| Characteristic | Total Sample (N = 44), Mean (SD) | Low Evening Fatigue (n = 19; 43.2%), Mean (SD) | High Evening Fatigue (n = 25; 56.8%), Mean (SD) | Statistics |
|---|---|---|---|---|
| Age (years) | 56.1 (9.5) | 57.3 (7.8) | 55.2 (10.7) | t = 0.72, p = .474 |
| Education (years) | 16.4 (2.4) | 15.9 (2.1) | 16.8 (2.5) | t = −1.27, p = .211 |
| Body mass index (kg/m2) | 27.1 (6.4) | 25.4 (3.9) | 28.4 (7.6) | t = −1.64, p = .111 |
| Karnofsky performance status score | 80.9 (12.9) | 86.3 (11.6) | 76.8 (12.5) | t = 2.58, p = .014 |
| Self-Administered Comorbidity Questionnaire score | 5.8 (3.5) | 4.5 (2.8) | 6.8 (3.8) | t = −2.23, p = .031 |
| Time since diagnosis (years)a | 4.6 (7.7) | 5.5 (6.1) | 3.9 (8.8) | U, p = .142 |
| Number of prior cancer treatments | 2.1 (1.8) | 2.8 (1.9) | 1.6 (1.5) | t = 2.32, p = .025 |
| Number of metastatic sites including lymph node involvementb | 1.6 (1.7) | 2.1 (1.9) | 1.3 (1.4) | t = 1.56, p = .128 |
| Number of metastatic sites excluding lymph node involvement | 1.1 (1.4) | 1.4 (1.5) | 0.8 (1.2) | t = 1.26, p = .215 |
| LFS evening fatigue score | 5.6 (2.2) | 3.7 (1.7) | 7.1 (1.2) | t = −7.66, p < .001 |
| % (n) | % (n) | % (n) | ||
| Self-reported ethnicity | χ2 = 4.96, p = .292 | |||
| White | 77.3 (35) | 68.4 (13) | 80.0 (20) | |
| Asian/Pacific Islander | 11.4 (5) | 15.8 (3) | 8.0 (2) | |
| Black non-Hispanic | 4.5 (2) | 10.5 (2) | 4.0 (1) | |
| Hispanic/mixed/other | 6.8 (3) | 5.3 (1) | 8.0 (2) | |
| Married or partnered (% yes) | 72.7 (32) | 84.2 (16) | 64.0 (16) | FE, p = .181 |
| Lives alone (% yes) | 15.9 (7) | 10.5 (2) | 20.0 (5) | FE, p = .680 |
| Currently employed (% yes) | 29.5 (13) | 31.6 (6) | 28.0 (7) | FE, p = 1.00 |
| Annual household income | χ2 = 2.83, p = .419 | |||
| <$30,000 | 2.8 (1) | 0.0 (0) | 4.3 (1) | |
| $30,000–70,000 | 16.7 (6) | 23.1 (3) | 13.0 (3) | |
| $70,000–100,000 | 8.3 (3) | 0.0 (0) | 13.0 (3) | |
| >$100,000 | 72.2 (26) | 76.9 (10) | 69.6 (16) | |
| Exercise on a regular basis (% yes) | 77.3 (34) | 78.9 (15) | 76.0 (19) | FE, p = 1.00 |
| Child care responsibilities (% yes) | 28.9 (11) | 17.6 (3) | 38.1 (8) | FE, p = .282 |
| Elder care responsibilities (% yes) | 13.9 (5) | 12.5 (2) | 15.0 (3) | FE, p = 1.00 |
| AJCC status | χ2 = 1.36, p = .715 | |||
| Stage 0 | 59.1 (26) | 63.2 (12) | 56.0 (14) | |
| Stage I | 4.5 (2) | 5.2 (1) | 4.0 (1) | |
| Stage IIA, IIB | 18.2 (8) | 10.5 (2) | 25.0 (6) | |
| Stage IIA, IIIB, IIIC, IV | 18.2 (8) | 21.1 (4) | 16.7 (4) | |
| Prior cancer treatmentc | χ2 = 8.58, p=.035 | |||
| No prior treatment | 14.3 (6) | 11.8 (2) | 16.0 (4) | |
| Only surgery, CTX, or RT | 42.9 (18) | 23.5 (4) | 56.0 (14) | |
| Surgery and CTX, or surgery and RT, or CTX and RT | 9.5 (4) | 5.9 (1) | 12.0 (3) | |
| Surgery and CTX and RT | 33.3 (14) | 58.8 (10) | 16.0 (4) | |
| Metastatic sites | χ2 = 6.99, p = .073 | |||
| No metastasis | 36.4 (16) | 31.6 (6) | 40.0 (10) | |
| Only lymph node metastasis | 20.5 (9) | 15.8 (3) | 24.0 (6) | |
| Only metastatic disease in sites other than lymph nodes | 9.1 (4) | 0.0 (0) | 16.0 (4) | |
| Metastatic disease in lymph nodes and other sites | 34.1 (15) | 52.6 (10) | 20.0 (5) |
Note. AJCC = American Joint Committee on Cancer; CTX = chemotherapy; FE = Fisher’s exact; LFS = Lee Fatigue Scale; RT = radiation therapy; U = Mann-Whitney test; χ2 = chi-square test.
aMedian time since diagnosis was 0.45, 3.75, and 0.37 years for the total sample, the low-evening-fatigue group, and the high-evening-fatigue group, respectively. bTotal number of metastatic sites evaluated was 9. cPost hoc contrasts failed to reveal the subgroup(s) underlying the differences in prior cancer treatments observed in the high- compared to the low-evening-fatigue groups.
Differences in Demographic and Clinical Characteristics Between the Two Fatigue Groups
Table 1 summarizes the differences in demographic and clinical characteristics between the low- and high-evening-fatigue groups. Compared to the low group, patients in the high-evening-fatigue group reported a lower KPS score, had a higher comorbidity score, and had a lower number of prior cancer treatments.
Differences in Gene Expression Between the Two Fatigue Groups
We performed the transcriptomic analysis using the Human HT-12 Expression BeadChip. We identified one DE gene by limma and 11 by GenePattern (Table 2). Among these 12 genes, 1 was upregulated and 11 were downregulated in the high-evening-fatigue group. A heatmap of the two-class cluster analysis of these 12 genes revealed that the DE genes noticeably, but incompletely, distinguished between the low- and high-evening-fatigue groups (Supplemental Figure S2).
Table 2.
Differentially Expressed Genes.
| Probe | Symbol | Name | Fold Changea | ENTREZ Gene ID | Directionb | p adjusted c | Primary Roled |
|---|---|---|---|---|---|---|---|
| Limma | |||||||
| ILMN_1684308 | DEFB103B | Defensin, β 103B (DEFB103B) | 0.10 | 55894 | Down | .0489 | I |
| Gene pattern | |||||||
| ILMN_1873793 | Hs.650028 | cDNA FLJ25030 fis, clone CBL02631 | 0.18 | Up | < .0001 | U | |
| ILMN_1655418 | CAPNS1 | Calpain, small subunit 1 | 0.58 | 826 | Down | < .0001 | I |
| ILMN_1705605 | MGC13005 | PREDICTED: Homo sapiens hypothetical protein MGC13005 | 0.83 | Down | < .0001 | U | |
| ILMN_1728799 | FBP1 | Fructose-1,6-bisphosphatase 1 | 0.75 | 2203 | Down | < .0001 | M |
| ILMN_1808821 | COMMD9 | COMM domain containing 9 Transcription elongation factor A (SII)-like 1, transcript variant | 0.59 | 29099 | Down | < .0001 | I |
| ILMN_2398408 | TCEAL1 | 2 | 0.18 | 9338 | Down | < .0001 | I/M |
| ILMN_1653115 | ECH1 | Enoyl Coenzyme A hydratase 1, peroxisomal | 0.46 | 1891 | Down | < .0001 | M |
| ILMN_1759652 | C1orf61 | chromosome 1 open reading frame 61 | 0.16 | 10485 | Down | < .0001 | U |
| ILMN_1666269 | CTSZ | Cathepsin Z Yip1 interacting factor homolog B (S. cerevisiae), transcript | 0.77 | 1522 | Down | < .0001 | I |
| ILMN_2363668 | YIF1B | variant 4 | 0.55 | 90522 | Down | < .0001 | I/M/S |
| ILMN_1791211 | DOK2 | Docking protein 2, 56kDa | 0.75 | 9046 | Down | < .0001 | I |
Note. aFold change of the log2-transformed, background-corrected, quantile-normalized intensity gene expression values in the high- as compared to low-evening-fatigue group. bDirection of difference in gene expression in the high- as compared to the low-evening-fatigue group. cPadjusted = Benjamini-Hochberg adjusted p value. dThe primary functional role(s) of a given gene is identified as follows: M = metabolism, protein synthesis (n = 4); I = immune activation and immune cell replenishment (n = 7); S = serotonergic activation (n = 1); and U = unknown (n = 3).
Of the DE genes identified, three (i.e., cDNA FLJ25030 fis, clone CBL02631 [Hs.650028]; Homo sapiens hypothetical protein MGC13005; and chromosome 1 open reading frame 61 [C1orf61]) do not have established functional roles. However, the remaining eight genes can be categorized into three groups based on their known functions (summarized in Supplemental Table S2): immune activation (i.e., Calpain, small subunit 1 [CAPSN1]; COMM domain containing 9 [COMMD9]; Cathepsin Z [CTSZ]; Defensin, β 103B [DEF103B]; Docking protein 2, 56kDa [DOK2]; Transcription elongation factor A [SII]-like 1, transcript variant 2 [TCEAL1]; Yip1 interacting factor homolog B [S. cerevisiae], transcript variant 4 [YIF1B]), energy metabolism and physical activity (i.e., TCEAL1, Enoyl Coenzyme A hydratase 1, peroxisomal [ECH1]; YIF1B, Fructose-1,5-bisphosphatase 1 [FBP1]), and serotonergic activation (i.e., YIF1B).
Pathways Differentially Perturbed Between the Two Fatigue Groups
We performed gene set analysis to discover differences between the low- and high-evening-fatigue groups in perturbations of genes that operate together in pathways. KEGG pathways are the primary focus of this article, given their superior depth of annotation and rich usage in pathway analysis. We identified no upregulated, 24 downregulated, and 94 2-D perturbed KEGG pathways that differentiated between the two evening fatigue groups. Table 3 lists these pathways, but includes only those downregulated pathways that were not identified as 2-D perturbed (i.e., 5 of 24). Supplemental Table S3 includes a listing of all 19 KEGG downregulated, GO, and BioCarta analyses.
Table 3.
Pathways Differentially Perturbed Between the Low- and High-Evening-Fatigue Groups.
| KEGG ID | KEGG Pathway Description | p Value | q Value | Fundamental Rolea | TOAb |
|---|---|---|---|---|---|
| Downregulated | |||||
| hsa00280 | Valine, leucine, and isoleucine degradation | 1.007E-03 | 1.612E-02 | M | M |
| hsa00410 | β-Alanine metabolism | 1.946E-03 | 2.616E-02 | M | B |
| hsa00970 | Aminoacyl-tRNA biosynthesis | 2.610E-03 | 2.984E-02 | D | NK |
| hsa00640 | Propanoate metabolism | 4.953E-03 | 4.055E-02 | M | DC |
| hsa00980 | Metabolism of xenobiotics by cytochrome P450 | 5.068E-03 | 4.055E-02 | M | M |
| 2-D perturbed | |||||
| hsa04145 | Phagosome | 1.626E-56 | 2.601E-54 | M | M |
| hsa04612 | Antigen processing and presentation | 8.693E-51 | 6.955E-49 | I | DC |
| hsa04640 | Hematopoietic cell lineage | 5.206E-47 | 2.777E-45 | I | DC |
| hsa04142 | Lysosome | 1.416E-36 | 5.663E-35 | M | M |
| hsa04380 | Osteoclast differentiation | 2.512E-35 | 8.038E-34 | I | M |
| hsa03010 | Ribosome | 5.333E-32 | 1.422E-30 | D | B |
| hsa00190 | Oxidative phosphorylation | 3.901E-24 | 8.916E-23 | M | M |
| hsa04141 | Protein processing in endoplasmic reticulum | 1.087E-22 | 2.174E-21 | M | DC |
| hsa04514 | Cell adhesion molecules (CAMs) | 3.541E-22 | 6.296E-21 | I | M |
| hsa03040 | Spliceosome | 5.169E-22 | 8.270E-21 | M | DC |
| hsa04650 | Natural killer cell mediated cytotoxicity | 3.430E-20 | 4.990E-19 | I | M |
| hsa04062 | Chemokine signaling pathway | 1.390E-18 | 1.853E-17 | I | id |
| hsa04660 | T-cell receptor signaling pathway | 2.625E-18 | 3.230E-17 | I | NK |
| hsa04144 | Endocytosis | 9.210E-18 | 1.053E-16 | M | NK |
| hsa04666 | Fc γ R-mediated phagocytosis | 5.354E-17 | 5.711E-16 | I | M |
| hsa04670 | Leukocyte transendothelial migration | 2.283E-16 | 2.283E-15 | I | id |
| hsa04662 | B-cell receptor signaling pathway | 2.625E-16 | 2.471E-15 | I | M |
| hsa03050 | Proteasome | 2.087E-15 | 1.855E-14 | M | M |
| hsa04672 | Intestinal immune network for IgA production | 5.226E-14 | 4.401E-13 | I | DC |
| hsa04620 | Toll-like receptor signaling pathway | 8.205E-14 | 6.564E-13 | I | M |
| hsa04920 | Adipocytokine signaling pathway | 3.765E-13 | 2.869E-12 | I | M |
| hsa04621 | NOD-like receptor signaling pathway | 1.408E-12 | 1.024E-11 | I | DC |
| hsa04210 | Apoptosis | 2.992E-11 | 2.081E-10 | D | M |
| hsa00051 | Fructose and mannose metabolism | 3.914E-11 | 2.610E-10 | M | id |
| hsa04722 | Neurotrophin signaling pathway | 2.686E-10 | 1.719E-09 | I | M |
| hsa00240 | Pyrimidine metabolism | 1.290E-09 | 7.937E-09 | M | id |
| hsa03018 | RNA degradation | 2.241E-08 | 1.328E-07 | M | DC |
| hsa00030 | Pentose phosphate pathway | 3.228E-08 | 1.845E-07 | M | DC |
| hsa04910 | Insulin signaling pathway | 4.674E-08 | 2.579E-07 | M | NK |
| hsa00520 | Amino sugar and nucleotide sugar metabolism | 5.161E-08 | 2.752E-07 | M | id |
| hsa04330 | Notch signaling pathway | 6.687E-08 | 3.451E-07 | I | DC |
| hsa00010 | Glycolysis/gluconeogenesis | 7.849E-08 | 3.925E-07 | M | DC |
| hsa00480 | Glutathione metabolism | 1.485E-07 | 7.201E-07 | M | M |
| hsa04630 | Jak-STAT signaling pathway | 1.625E-07 | 7.649E-07 | I | DC |
| hsa00910 | Nitrogen metabolism | 1.804E-07 | 8.248E-07 | M | M |
| hsa04120 | Ubiquitin-mediated proteolysis | 4.660E-07 | 2.071E-06 | M | DC |
| hsa00052 | Galactose metabolism | 9.313E-07 | 4.027E-06 | M | DC |
| hsa00230 | Purine metabolism | 1.036E-06 | 4.364E-06 | M | DC |
| hsa04623 | Cytosolic DNA-sensing pathway | 4.788E-06 | 1.964E-05 | I | M |
| hsa04110 | Cell cycle | 5.049E-06 | 2.020E-05 | D | NK |
| hsa04146 | Peroxisome | 1.758E-05 | 6.861E-05 | M | M |
| hsa00760 | Nicotinate and nicotinamide metabolism | 1.870E-05 | 7.125E-05 | M | M |
| hsa03013 | RNA transport | 2.209E-05 | 8.221E-05 | M | NK |
| hsa04070 | Phosphatidylinositol signaling system | 2.989E-05 | 1.087E-04 | I | NK |
| hsa00020 | Citrate cycle (TCA cycle) | 3.237E-05 | 1.151E-04 | M | M |
| hsa04010 | MAPK signaling pathway | 4.111E-05 | 1.430E-04 | I | M |
| hsa03015 | mRNA surveillance pathway | 5.529E-05 | 1.882E-04 | M | id |
| hsa03410 | Base excision repair | 6.074E-05 | 2.025E-04 | D | id |
| hsa00532 | Glycosaminoglycan biosynthesis—chondroitin sulfate | 7.306E-05 | 2.386E-04 | M | id |
| hsa04966 | Collecting duct acid secretion | 7.703E-05 | 2.465E-04 | M | M |
| hsa04810 | Regulation of actin cytoskeleton | 9.502E-05 | 2.981E-04 | D | M |
| hsa04973 | Carbohydrate digestion and absorption | 9.893E-05 | 3.044E-04 | M | DC |
| hsa04130 | SNARE interactions in vesicular transport | 1.226E-04 | 3.703E-04 | M | M |
| hsa04710 | Circadian rhythm—mammal | 1.460E-04 | 4.325E-04 | C | NK |
| hsa04664 | Fc epsilon RI signaling pathway | 1.702E-04 | 4.950E-04 | I | DC |
| hsa00563 | Glycosylphosphatidylinositol (GPI)-anchor biosynthesis | 2.136E-04 | 6.102E-04 | M | DC |
| hsa00620 | Pyruvate metabolism | 2.266E-04 | 6.360E-04 | M | DC |
| hsa03450 | Non-homologous end-joining | 2.368E-04 | 6.533E-04 | D | id |
| hsa04520 | Adherens junction | 2.501E-04 | 6.781E-04 | I | NK |
| hsa00511 | Other glycan degradation | 3.379E-04 | 9.012E-04 | M | M |
| hsa00071 | Fatty acid metabolism | 3.661E-04 | 9.516E-04 | M | DC |
| hsa04964 | Proximal tubule bicarbonate reclamation | 3.687E-04 | 9.516E-04 | M | NK |
| hsa04012 | ErbB signaling pathway | 4.779E-04 | 1.214E-03 | I | id |
| hsa00604 | Glycosphingolipid biosynthesis—ganglio series | 5.013E-04 | 1.253E-03 | M | B |
| hsa00564 | Glycerophospholipid metabolism | 9.206E-04 | 2.266E-03 | M | id |
| hsa04370 | VEGF signaling pathway | 2.024E-03 | 4.907E-03 | I | id |
| hsa04960 | Aldosterone-regulated sodium reabsorption | 2.122E-03 | 5.061E-03 | M | id |
| hsa00270 | Cysteine and methionine metabolism | 2.151E-03 | 5.061E-03 | M | id |
| hsa03060 | Protein export | 2.188E-03 | 5.073E-03 | M | DC |
| hsa03320 | PPAR signaling pathway | 2.616E-03 | 5.979E-03 | M | M |
| hsa04622 | RIG-I-like receptor signaling pathway | 3.298E-03 | 7.433E-03 | I | id |
| hsa03030 | DNA replication | 3.354E-03 | 7.452E-03 | D | DC |
| hsa04971 | Gastric acid secretion | 5.221E-03 | 1.144E-02 | M | id |
| hsa04150 | mTOR signaling pathway | 5.602E-03 | 1.211E-02 | I | id |
| hsa04510 | Focal adhesion | 6.216E-03 | 1.326E-02 | I | M |
| hsa00561 | Glycerolipid metabolism | 7.320E-03 | 1.541E-02 | M | M |
| hsa03008 | Ribosome biogenesis in eukaryotes | 7.485E-03 | 1.555E-02 | M | DC |
| hsa00860 | Porphyrin and chlorophyll metabolism | 7.594E-03 | 1.558E-02 | M | NK |
| hsa04530 | Tight junction | 7.703E-03 | 1.560E-02 | I | B |
| hsa00900 | Terpenoid backbone biosynthesis | 8.277E-03 | 1.655E-02 | M | NK |
| hsa03420 | Nucleotide excision repair | 8.925E-03 | 1.763E-02 | D | NK |
| hsa00330 | Arginine and proline metabolism | 9.351E-03 | 1.825E-02 | M | DC |
| hsa04962 | Vasopressin-regulated water reabsorption | 1.022E-02 | 1.971E-02 | M | NK |
| hsa04310 | Wnt signaling pathway | 1.085E-02 | 2.048E-02 | I | id |
| hsa04720 | Long-term potentiation | 1.088E-02 | 2.048E-02 | L | id |
| hsa00510 | N-Glycan biosynthesis | 1.298E-02 | 2.414E-02 | M | id |
| hsa00770 | Pantothenate and CoA biosynthesis | 1.365E-02 | 2.511E-02 | M | M |
| hsa00380 | Tryptophan metabolism | 1.760E-02 | 3.199E-02 | M | M |
| hsa00531 | Glycosaminoglycan degradation | 1.889E-02 | 3.395E-02 | M | M |
| hsa00450 | Selenocompound metabolism | 2.839E-02 | 5.047E-02 | M | M |
| hsa04614 | Renin–angiotensin system | 2.943E-02 | 5.174E-02 | I | id |
| hsa00790 | Folate biosynthesis | 3.327E-02 | 5.787E-02 | M | id |
| hsa04260 | Cardiac muscle contraction | 4.953E-02 | 8.521E-02 | L | M |
| hsa03020 | RNA polymerase | 5.568E-02 | 9.477E-02 | D | id |
Note. 2-D = simultaneous two-dimensional perturbation (up and down); id = insufficient data; KEGG = Kyoto Encyclopedia of Genes and Genomes; TOA = transcript origin analysis; NOD = nonobese diabetic; STAT = signal transducer and activator of transcription; TCA = tricarboxylic acid; MAPK = mitogen-activated protein kinases; SNARE = Soluble N-ethylmaleimide-sensitive factor Attachment Protein Receptor; VEGF = Vascular endothelial growth factor; PPAR = peroxisome proliferator-activated receptors; RIG = retinoic acid inducible gene; mTOR = Mammalian target of rapamycin.
aThe primary functional role of a given pathway is identified as follows: M = cellular metabolism, protein synthesis (n = 56); I = immune activation and immune-cell replenishment (n = 30); D = DNA synthesis and repair, cell division (n = 10); L = long-term potentiation (n = 2); and C = circadian dysfunction (n = 1). bTranscript of origin analysis (TOA) was employed in order to infer the cell type from which a differentially expressed pathway originates. The cell types identified by TOA are abbreviated as follows: M = monocytes (n = 34); DC = dendritic cells (n = 24); id = insufficient data to perform TOA (n = 22); NK = natural killer cells (n = 14); and B = B cells (n = 4).
Differentially expressed pathways were broadly categorized into those associated with immune cell replenishment and activation (n = 30); cellular metabolism and protein synthesis (n = 56); DNA synthesis, repair and cell division (n = 10); and neurological activity (n = 3). From the TOA, we inferred that the DE pathways originated from B cells, dendritic cells, monocytes, and NK cells. Only a small number of DE pathways were identified as originating from CD4+ T cells.
When we compared the two fatigue groups, we identified significantly differentially perturbed cytokine pathways from KEGG (KEGG: hsa04920), GO (GO: 0019221, GO: 0071345, GO: 0034097, GO: 0019221, GO: 0071345, GO: 0034097), and BioCarta (gata3pathway), as well as inflammation and immune-response pathways from GO (GO: 0006954, GO:0002472, GO:0002252) and BioCarta (il1rpathway, il2pathway, il3pathway, il4pathway, il6pathway, il10pathway, il22pathway, nthipathway; Table 3 and Supplemental Table S3).
Prior to multiple hypothesis correction, we identified several cytokine-related genes that were DE between the fatigue groups. Among 57 (94 probes) of the 70 measured genes that are involved in the adipocytokine signaling pathway (KEGG: hsa04920), 5 had probes identified by limma to be DE prior to statistical correction: retinoid X receptor, γ (RXRG); v-akt murine thymoma viral oncogene homolog 3 (AKT3); acyl-CoA synthetase long-chain family member 4 (ACSL4); inhibitor of κ light polypeptide gene enhancer in B cells, kinase γ (IKBKG); and signal transducer and activator of transcription 3 (acute-phase response factor; STAT3; all p < .03). Among 114 (160 probes) of the 265 measured genes that are involved in the cytokine–cytokine receptor interaction pathway (KEGG: ksa04060), two had probes identified by limma to be DE prior to statistical correction: platelet factor 4 (PF4) and chemokine (C-X-C motif) receptor 5 (CXCR5; all p < .02). Gene-expression studies of fatigue in a larger sample might result in the detection of cytokine gene expression differences that survive statistical correction for multiple testing.
Differentially perturbed pathways related to DNA synthesis, repair, and cell division included DNA replication (hsa03030), cell cycle (hsa04110), nucleotide excision repair (hsa03420), and base excision repair (hsa03410; Table 3 and Supplemental Table S3).
Cellular metabolism and protein synthesis pathways that were differentially perturbed include glycolysis/gluconeogenesis (hsa00010), oxidative phosphorylation (hsa000190), fructose and mannose metabolism (hsa00051), amino sugar and nucleotide sugar metabolism (hsa00520) and the citrate cycle (TCA cycle; hsa00020) from KEGG, and mitochondrial matrix (GO:0005759), membrane (GO:0031966), inner membrane (GO:0005743) and envelope (GO:0005740), generation of precursor metabolites and energy (GO:0006091), oxidative phosphorylation (GO:0006119), and respiratory chain (GO:0070469) from GO (Table 3 and Supplemental Table S3).
Finally, differentially perturbed pathways related to neurotransmission included long-term potentiation (hsa04720), soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) interactions in vesicular transport (hsa04130), mitogen-activated protein kinase (MAPK) signaling (hsa04010) and epidermal growth factor receptor (avian erythroblastic leukemia viral [v-erb-b] oncogene homolog; ErbB) signaling (hsa04012) pathways in KEGG and the γ-aminobutyric acid (GABA) receptor lifecycle (gabapathway) and MAPKinase signaling (mapkpathway) pathways in BioCarta (Table 3 and Supplemental Table S3).
TOA
Peripheral blood contains a heterogeneous population of nucleated cells from which gene expression data may be derived. TOA is used to identify the cell lineage with the highest degree of statistical significance for a group of DE genes (Cole et al., 2011). The cell types that can be distinguished include B cells, CD4+ T cells, dendritic cells, monocytes, and NK cells. In the present study, we successfully mapped diagnosticity scores for 12 out of the combined set of the 13 genes that were DE between fatigue groups (see Table 2), 70 of 77 EC genes from all 22 downregulated KEGG pathways, and 907 of 980 EC genes from 94 2-D-perturbed KEGG pathways (Supplemental Table S3). In total, using TOA we were able to unambiguously infer the origin of 77 of the 99 (78%) differentially perturbed KEGG pathways listed in Table 3. While the individual perturbed pathways originated primarily from monocytes, some originated from dendritic cells, NK cells, B cells, and CD4+ T cells (Table 4). The transcript origin for each differentially perturbed pathway is identified in Supplemental Table S3 and summarized in Supplemental Table S4.
Table 4.
Summary of Transcript Origin Analysis Results for Differentially Perturbed Gene Sets.
| Grouping | KEGG, Down | KEGG, 2-D | GO, Down | GO, -D | BioCarta, Down | BioCarta, 2-D |
|---|---|---|---|---|---|---|
| SPS | 22 | 94 | 249 | 2,685 | 1 | 67 |
| SPS with E.C. genes | 22 | 94 | 249 | 2,685 | 1 | 67 |
| SPS with TOA signal | 22 | 72 | 230 | 2,201 | 1 | 45 |
| Monocytes | 15 | 13 | 84 | 1,081 | 0 | 16 |
| Dendritic cells | 14 | 23 | 80 | 617 | 0 | 13 |
| NK cells | 5 | 13 | 32 | 226 | 1 | 10 |
| B cells | 2 | 3 | 31 | 213 | 0 | 4 |
| CD4+ T cells | 0 | 0 | 3 | 33 | 0 | 0 |
Note. E.C. genes = essential contributing genes: genes with substantial or above-background expression changes in the set; GO = gene ontogeny; KEGG = Kyoto Encyclopedia of Genes and Genomes; NK = natural killer; SPS = significantly perturbed sets; TOA = transcript origin analysis; and 2-D = simultaneous two-dimensional perturbation (up and down).
Identification of Similar Whole-transcriptome Gene Expression Experiments
ProfileChaser was used to identify publicly available gene expression studies and associated publications that shared a whole-transcriptome pattern of differential gene expression similar to that which we found in the present study. The significant GEO data sets (GDSs) identified across all five rounds of the split analyses (n = 108) were retained (Supplemental Tables S5 and S6). Two of the authors (B. E. A and K. M. K) independently reviewed abstracts for the original source publications obtained from the 108 GDSs (n = 102) for their relevance to fatigue. Based on this review of the abstracts, they excluded all but 44 complete publications to evaluate. After full evaluation of the experimental profiles of these 44 manuscripts, we retained 20. We reviewed these 20 publications, representing 18 unique GDSs, and categorized them as providing “strong” (n = 14), “moderate” (n = 1), or “weak” (n = 5) insights into the mechanism(s) that contribute to fatigue (Table S6).
Discussion
Differences in Demographic and Clinical Characteristics
This study is the first to evaluate for differences in gene expression and perturbed pathways in breast cancer patients who reported low versus high levels of evening fatigue during CTX. The between-group differences in fatigue severity scores represent not only statistically significant, but also clinically meaningful differences (Cohen’s d = 1.5; Osoba, 1999). Consistent with previous reports, patients in the high-fatigue group had a poorer functional status (Dhruva et al., 2013; Hofso, Miaskowski, Bjordal, Cooper, & Rustoen, 2012) and a more severe comorbidity profile (Berger et al., 2012).
The more surprising and intriguing finding was the association we identified between a lower number of previous cancer treatments and membership in the high-evening-fatigue group. While this finding needs to be validated in an independent sample, we can postulate a number of plausible explanations. Patients who are in later stages of their disease trajectory may experience a “response shift” in their perception of fatigue. First used in oncology to describe changes over time in quality of life (QOL) (Sprangers & Schwartz, 1999 ), a “response shift” is an age-related psychological shift that represents a change in a person’s internal framework for the assessment of experiences (Costanzo, Ryff, & Singer, 2009). In the present context, patients in the low-fatigue group who had received prior cancer treatments may have changed their internal conceptualization of fatigue based on their previous experiences with the symptom. An alternative hypothesis is that, with prolonged cancer treatment, patients may develop tolerance to the physiologic responses that contribute to the development of fatigue. Finally, there may be a selection bias, where more women who had previous cancer treatments (e.g., due to tolerance or increased survival) volunteered to participate in this study. Longitudinal studies that assess both phenotypic and epigenetic trajectories associated with fatigue are needed to confirm or refute these hypotheses.
Differences in Gene Expression
In the present study, we performed data-driven analyses at the levels of the gene, the pathway, and the entire transcriptome to evaluate for differences in gene expression between patients who reported high versus low levels of evening fatigue (Figure 1). Using TOA, we inferred that differentially expressed genes and perturbed pathways originated from several cell types (Supplemental Table S4), with the majority of the signals originating from monocytes, dendritic cells, B cells, and NK cells (Table 4). This profile is consistent with those reported in gene expression experiments that have evaluated the effects of exhaustive physical exercise (Supplemental Table S6). In addition, the expression profiles for the high-evening-fatigue group were consistent with those reported in a number of gene expression studies that evaluated sickness behavior (Calvano et al., 2005; Dennehy, 2007; Foteinou, Calvano, Lowry, & Androulakis, 2010; Rodriguez et al., 2007; Wurfel et al., 2005), circadian rhythm disruption (Bailey et al., 2009; Yang, Wang, Valladares, Hannenhalli, & Bucan, 2007), and mechanisms of neuro-inflammation (Irwin & Cole, 2011). Of note, the DE genes (Table 2) and pathways (Table 3) we identified using transcriptomic analyses in the current study have similar biological qualities in that these genes play roles in sickness behavior, inflammation, mitochondrial dysfunction, circadian rhythm disruption, and serotonin regulation. Taken together, our results and those of others described below provide a more complete picture of the mechanisms that underlie evening fatigue in oncology patients.
We identified 12 genes that were DE between the low- and high-evening-fatigue groups (Table 2). While the expression patterns of these 12 genes distinguished between patients in the two fatigue groups (Supplemental Figure S2), this distinction was not perfect, suggesting that additional genes remain to be identified. TOA of this set of 12 DE genes revealed that the expression patterns originated predominantly from monocytes. This finding is consistent with previous reports that noted that altered cytokine production in monocytes is associated with fatigue in oncology patients with breast cancer (Collado-Hidalgo, Bower, Ganz, Cole, & Irwin, 2006; Saligan & Kim, 2012).
Inflammation and Immune Response
In general, cytoxic CTX kills rapidly proliferating cancer cells (Mitchison, 2012). While the targets are cancer cells, CTX also depletes other rapidly dividing cells, including peripheral leukocytes. The need for peripheral blood counts to recover is the primary reason that CTX regimens are administered in cycles. In addition, immune system effectors are impacted during and following CTX (Saligan & Kim, 2012). For example, increased CD4 T-cell counts, which may result in a prolonged pro-inflammatory state, are associated with increased fatigue in breast cancer survivors (Bower, Ganz, Aziz, Fahey, & Cole, 2003). Downregulation of DEF103B in the high-evening-fatigue group in the present study may have favored the production of pro-inflammatory cytokines, which are associated with increased fatigue severity (Aouizerat et al., 2009; Bower et al., 2011; Jager, Sleijfer, & van der Rijt, 2008; Miaskowski et al., 2010). Decreased expression of DOK2 in the high-evening-fatigue group may have resulted in prolonged immune cell activation, which could lead to higher levels of evening fatigue. Decreased ECH1 gene expression in patients with higher evening fatigue may be associated with decreased energy production and delayed immune system recovery following CTX. While the function of YIF1B in peripheral leukocytes is unknown, reduction of YIF1B in the high-evening-fatigue group may have resulted in decreases in 5-Hydroxytryptamine (Serotonin) Receptor 1A, G Protein-Coupled (HTR1A) expression, intracellular cAMP, and immune activation (i.e., the decrease in YIF1B creates a pro-inflammatory state that results in fatigue). While it is not known whether lower YIF1B gene expression in the periphery is associated with lower gene expression in the central nervous system, increasing evidence suggests that peripheral gene expression reflects system-wide biology (Liew, Ma, Tang, Zheng, & Dempsey, 2006).
In addition to the identification of these DE genes with plausible inflammatory and immune mechanisms for evening fatigue, we identified a number of DE pathways associated with immune cell recovery following CTX. Consistent with established associations between inflammatory cytokines and fatigue in oncology patients (Aouizerat et al., 2009; Bower et al., 2009; Miaskowski et al., 2010), we identified cytokine pathways as well as inflammation-related pathways that were significantly differentially perturbed between the two fatigue groups. In addition, our finding that the BioCarta pathway (nthipathway) was significantly differentially perturbed between the two groups (Supplemental Table S3) is consistent with the overrepresentation of the NF-κβ response elements Bower, Ganz, Irwin, Arevalo, and Cole (2011) reported in breast cancer survivors. Common elements (i.e., genes) of the identified KEGG, GO, and Biocarta pathways in this study that have been identified as harboring polymorphisms associated with fatigue in oncology patients include IL1B (Bower et al., 2013; Collado-Hidalgo et al., 2008; Reinertsen et al., 2011), IL4 (Doong et al., 2015), IL6 (Bower et al., 2013; Miaskowski et al., 2010; Reinertsen et al., 2011), and TNFA (Aouizerat et al., 2009; Bower et al., 2013; Dhruva et al., 2015). Future genetic association studies should be conducted that include other members of the above-identified pathways.
Although we observed DE genes and differentially perturbed pathways related to inflammation, we did not detect DE cytokine genes. This finding is consistent with previous research that also failed to find an association between cytokine gene expression and levels of fatigue (Landmark-Hoyvik et al., 2009; Reinertsen et al., 2011). The lack of detectable differences in gene expression despite the associations repeatedly reported between cytokine genes and fatigue may be due to the timing of cytokine gene expression in relation to the experience of fatigue (i.e., the change in expression may occur prior to the perception of fatigue). Alternatively, the DE genes and perturbed pathways we detected in the current study may represent up- and/or downstream events in relation to cytokine gene expression. Finally, our conservative adjustment for multiple hypothesis testing may have resulted in the exclusion of gene expression signals that would have been identified with a larger sample.
Circadian Rhythm
The co-occurrence of (Davidson, MacLean, Brundage, & Schulze, 2002) and common genetic risk factors for (Aouizerat et al., 2009; Miaskowski et al., 2010) fatigue and sleep disturbance suggest that their mechanisms may overlap. Circadian influences on immune function may be particularly relevant for explaining the relationship between sleep and fatigue in oncology patients undergoing CTX. Haimovich et al. (2010) observed altered expression of circadian-clock genes in peripheral leukocytes in healthy individuals who experienced sickness behavior when exposed to endotoxin. The majority of immune cells (including NK cells) demonstrate circadian rhythmicity in healthy individuals (Mazzoccoli et al., 2011). This rhythmicity may be perturbed during CTX. Our finding that the KEGG circadian-rhythm pathway (hs04710) was differentially perturbed in the high-fatigue group supports this hypothesis. NK cells were the cell type of origin for this perturbation (Table 3). This observation is bolstered by the observation in a previous study of malfunctioning NK cells in patients with chronic fatigue syndrome (Meeus, Mistiaen, Lambrecht, & Nijs, 2009), and the relationship between circadian influences on immune cells and fatigue warrants further study.
Neurotransmission
An unexpected finding from the pathway analyses we conducted in the present study was the identification of relationships between fatigue and pathways that participate in regulation of neurotransmission, including long-term potentiation of neurons (hsa04720 and hsa04260), GABA receptor life cyle (gabapathway), SNARE interactions in vesicular transport (hsa04130), and ErbB signaling pathway (hsa04012; Table 3 and Supplemental Table S3). It should be noted that long-term potentiation of neurons may be a misnomer for the first of these pathways because this pathway shares genes with others that are not related to neurons. In addition, the long-term potentiation of neurons has not been characterized specifically in peripheral blood mononuclear cell. Centrally, long-term potentiation plays an important role in a number of physiologic processes (e.g., learning, memory, and pain). While GABA has an effect in peripheral tissue (Erdo & Wolff, 1990), it is the main inhibitory neurotransmitter in the cortex (Petroff, 2002). Serotonin, another neurotransmitter, modulates GABA. SNARE proteins are involved in cell signaling in neurons (Gotte & von Mollard, 1998). The ErbB family of receptor tyrosine kinases performs a complex array of functions including regulation of neurotransmitter receptors (Bublil & Yarden, 2007). However, many patients with breast cancer undergo treatment to block ErbB2 (Yarden & Sliwkowski, 2001), so this particular pattern may reflect differences in treatment rather than levels of fatigue. While these results, combined with the potential roles of the DE genes CAPSN1 and YIF1B in neurotransmission, are intriguing, it is unclear whether these peripherally perturbed genes and pathways reflect changes in gene expression in the central nervous system (Cole, 2013; Liew et al., 2006) that are associated with fatigue. If replicated in an independent sample, these pathways would warrant further study.
Energy Metabolism
Regulation and control of the expression of energy metabolism genes occur through a variety of processes that cancer or its treatment may alter (Andrews, Morrow, Hickok, Roscoe, & Stone, 2004). Radiation treatment or CTX may result in a decrease in adenosine triphosphate (ATP) regeneration. This disruption of ATP metabolism may lead to a reduction in mechanical ability (Ryan et al., 2007). The differences in expression patterns in pathways related to mitochondrial or energy metabolism found in our study parallel a previous report of associations between fatigue and mitochondrial-function genes in patients receiving radiation therapy for prostate cancer (Hsiao, Wang, et al., 2013). The decreased expression of FBP1 in our high-fatigue group may be associated with decreased energy production and an attempt to increase intracellular glucose in order to restore energy reserves. Importantly, mitochondrial dysfunction has pleiotropic effects (Chan, 2006) and is closely tied with other processes (e.g., inflammation; Liu et al., 2012).
Similarities in Whole-transcriptome DE With Other Studies
An evaluation of the studies we identified with ProfileChaser suggests that the mechanisms underlying fatigue in oncology patients are multifactorial. The mechanism of cytokine-induced sickness behavior (B. N. Lee et al., 2004) has a long-standing association with common symptoms experienced by oncology patients, including fatigue. Interrogation of publicly available transcriptome data sets using ProfileChaser revealed a preponderance of similarities between our gene expression study and studies that featured an inflammatory component. Of the 20 studies we identified using ProfileChaser and retained after in-depth review, 5 employed various experimental models (e.g., healthy adults and acute pediatric viral infection) that incorporated an endotoxin or viral challenge (Calvano et al., 2005; Foteinou et al., 2010; Rodriguez et al., 2007; Wang et al., 2007; Wurfel et al., 2005). In addition to pathogen-mediated induction of cytokines, four studies employed a more direct induction of cytokines (e.g., interferon and IL-2) in a wide variety of experimental models (e.g., multiple sclerosis and rheumatoid arthritis; Indraccolo et al., 2007; Zhang, Martino, & Faulon, 2007). Interestingly, in one murine-model study that focused on radiation therapy (RT), investigators found a transcriptome profile similar to the one we found in the present study, which supports the extant literature that cancer treatments induce a pro-inflammatory response in peripheral leukocytes that is associated with increased fatigue (Goldrath, Luckey, Park, Benoist, & Mathis, 2004).
One study described differences in the transcriptomes of healthy volunteers who underwent exhaustive as compared to no or moderate physical exercise (Buttner, Mosig, Lechtermann, Funke, & Mooren, 2007). The differences in gene expression between our low- and high-fatigue groups are similar to the differences Buttner et al. found between the nonexhaustive and exhaustive exercise groups. These findings suggest that exhaustive exercise may result in differences in gene expression that overlap with those that occur in cancer patients with high evening fatigue.
In one novel study we identified using ProfileChaser, Yang, Wang, Valladares, Hannenhalli, and Bucan (2007) found diurnal variations in gene expression in the prefrontal cortex of rodents. This finding is of interest because fatigue also displays a diurnal variability (B. A. Fletcher et al., 2009; Miaskowski et al., 2008). In general, evening fatigue is more severe than morning fatigue and is associated with different risk factors (Dhruva et al., 2010). The prefrontal cortex plays an important role in the regulation of sleep and fatigue. Yang et al.’s (2007) findings corroborate our finding of DE genes known to be involved in diurnal processes (i.e., COMMD9 and CTSZ). A complementary study identified diurnal variations in gene expression in the pineal gland of rodents (Bailey et al., 2009), which plays an important role in chronobiology and in diurnal variability of genes involved in immune/inflammatory responses.
To date, only two gene expression studies of fatigue in breast cancer survivors have been published (Bower et al., 2011; Landmark-Hoyvik et al., 2009). While our findings and those of the previous studies identified differences between patients reporting low and high levels of fatigue in gene expression related to immune function and mitochondrial dysregulation, the specific genes and pathways differed. The lack of congruence among these studies may be due in part to different designs and study populations. The studies by Bower et al. and Landmark-Høyvik et al. evaluated breast cancer survivors with persistent fatigue and did not account for diurnal variations in fatigue severity. Bower et al. evaluated and discussed only genes responsive to NF-κβ or downregulation of glucocorticoid-responsiveness. The analytic approaches we employed in our study and those that Landmark-Høyvik et al. used were different. ProfileChaser did not identify either of these studies because one (Bower et al.) did not exist in the GEO and the other (Landmark-Hoyvik et al.) had been submitted but not curated as a GEO data set (which are used exclusively by ProfileChaser).
Limitations
Although our study findings do not provide direct evidence of the causal mechanisms for evening fatigue in breast cancer patients, they do offer strong candidates for future functional as well as intervention studies. Several limitations need to be acknowledged. While the sample size for this study was adequate and slightly larger than that of the typical gene expression study (Bower et al., 2011), use of a larger independent sample might allow investigators to identify additional DE genes and pathways that differentiate between patients with high and low levels of evening fatigue. While all of the gene expression studies of fatigue in oncology patients, including our own, identified differences in genes involved in inflammation, the specific genes and pathways identified differed between studies, which may be due to differences in the patient populations studied (e.g., patient receiving CTX vs. survivors). The present study sample is heterogeneous for type of treatment received, disease stage, and number of previous CTX cycles. Finally, because the preponderance of DE genes and pathways that we and previous researchers identified are related to inflammation, one could hypothesize that this finding may be a by-product of the tissues studied (i.e., peripheral leukocytes). However, the transciptome analyses (i.e., ProfileChaser) identified gene expression studies that were performed in nonimmune cells, in numerous tissues, and under different experimental conditions. Until they are replicated, the current findings must be viewed as preliminary.
Conclusion
This study is novel because we evaluated differences between patients with high and low levels of evening fatigue at the levels of genes, pathways, and the entire transcriptome. Our analyses revealed that pathways involved in inflammation, neurotransmitter regulation, and energy metabolism are likely associated with evening-fatigue severity; that CTX may contribute to the severity of evening fatigue; and that the patterns of gene expression may be shared with other models of fatigue (e.g., physical exercise and pathogen-induced sickness behavior). Importantly, our findings suggest that the molecular mechanisms associated with evening fatigue are multifactorial and that these mechanisms interact among themselves (e.g., neurotransmitter regulation and inflammation, inflammation, and mitochondrial dysfunction). Future research should evaluate this potential interplay among pathways to determine the mechanisms that underlie evening fatigue.
Footnotes
Author Contributions: Kord M. Kober, Christine Miaskowski, and Bradley Aouizerat contributed to design and interpretation; drafted and critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Laura Dunn, Judy Mastick, Bruce Cooper, Dale Langford, Michelle Melisko, Alan Venook, Lee-May Chen, Fay Wright, Marilyn Hammer, Brian Schmidt, and Jon Levine contributed to conception, design, and interpretation; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by a grant from the National Cancer Institute (NCI, CA134900). Dr. Miaskowski is funded by a grant from the NCI (K05 CA168960) and is an American Cancer Society Clinical Research Professor.
Supplemental Material: The online appendices are available at http://brn.sagepub.com/supplemental
References
- Alcantara-Silva T. R., Freitas-Junior R., Freitas N. M., Machado G. D. (2013). Fatigue related to radiotherapy for breast and/or gynaecological cancer: A systematic review. Journal of Clinical Nursing, 22, 2679–2686. doi:10.1111/jocn.12236 [DOI] [PubMed] [Google Scholar]
- Andrews P. L. R., Morrow G. R., Hickok J. T., Roscoe J., Stone P. (2004). Mechanisms and models of fatigue associated with cancer and its treatment: Evidence from preclinical and clinical studies In Armes J., Krishnasamy M., Higginson I. (Eds.), Fatigue in cancer (pp. 51–87). Oxford, England: Oxford University Press. [Google Scholar]
- Aoki-Kinoshita K. F., Kanehisa M. (2007). Gene annotation and pathway mapping in KEGG. Methods in Molecular Biology, 396, 71–91. [DOI] [PubMed] [Google Scholar]
- Aouizerat B. E., Dodd M., Lee K., West C., Paul S. M., Cooper B. A.…Miaskowski C. (2009). Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biological Research for Nursing, 11, 27–41. doi:10.1177/1099800409333871 [DOI] [PubMed] [Google Scholar]
- Bailey M. J., Coon S. L., Carter D. A., Humphries A., Kim J. S., Shi Q.…Klein D. C. (2009). Night/day changes in pineal expression of > 600 genes: Central role of adrenergic/cAMP signaling. Journal of Biological Chemistry, 284, 7606–7622. doi:10.1074/jbc.M808394200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A. M., Gerber L. H., Mayer D. K. (2012). Cancer-related fatigue: Implications for breast cancer survivors. Cancer, 118, 2261–2269. doi:10.1002/cncr.27475 [DOI] [PubMed] [Google Scholar]
- Bower J. E., Ganz P. A., Aziz N., Fahey J. L., Cole S. W. (2003). T-cell homeostasis in breast cancer survivors with persistent fatigue. Journal of the National Cancer Institute, 95, 1165–1168. [DOI] [PubMed] [Google Scholar]
- Bower J. E., Ganz P. A., Irwin M. R., Arevalo J. M., Cole S. W. (2011). Fatigue and gene expression in human leukocytes: Increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behavior and Immunity, 25, 147–150. doi:10.1016/j.bbi.2010.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J. E., Ganz P. A., Irwin M. R., Castellon S., Arevalo J., Cole S. W. (2013). Cytokine genetic variations and fatigue among patients with breast cancer. Journal of Clinical Oncology, 31, 1656–1661. doi:10.1200/JCO.2012.46.2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J. E., Ganz P. A., Tao M. L., Hu W., Belin T. R., Sepah S.…Aziz N. (2009). Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clinical Cancer Research, 15, 5534–5540. doi:1078-0432.CCR-08-2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bublil E. M., Yarden Y. (2007). The EGF receptor family: Spearheading a merger of signaling and therapeutics. Current Opinion in Cell Biology, 19, 124–134. doi:10.1016/j.ceb.2007.02.008 [DOI] [PubMed] [Google Scholar]
- Butte A. (2002). The use and analysis of microarray data. Nature Reviews in Drug Discovery, 1, 951–960. doi:10.1038/nrd961 [DOI] [PubMed] [Google Scholar]
- Buttner P., Mosig S., Lechtermann A., Funke H., Mooren F. C. (2007). Exercise affects the gene expression profiles of human white blood cells. Journal of Applied Physiology, 102, 26–36. [DOI] [PubMed] [Google Scholar]
- Calvano S. E., Xiao W., Richards D. R., Felciano R. M., Baker H. V., Cho R. J., … Host Response to Injury Large Scale Collab. Res. Program. (2005). A network-based analysis of systemic inflammation in humans. Nature, 437, 1032–1037. doi:10.1038/nature03985 [DOI] [PubMed] [Google Scholar]
- Chan D. C. (2006). Mitochondria: Dynamic organelles in disease, aging, and development. Cell, 125, 1241–1252. doi:10.1016/j.cell.2006.06.010 [DOI] [PubMed] [Google Scholar]
- Cohen J. (1988). Statistical power analysis for the behavioral sciences (2nd ed). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Cole S. W. (2013). Nervous system regulation of the cancer genome. Brain Behavior and Immunity, 30, S10–S18. doi:10.1016/j.bbi.2012.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. W., Hawkley L. C., Arevalo J. M., Cacioppo J. T. (2011). Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proceedings of the National Academy of Science U S A, 108, 3080–3085. doi:10.1073/pnas.1014218108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado-Hidalgo A., Bower J. E., Ganz P. A., Cole S. W., Irwin M. R. (2006). Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clinical Cancer Research, 12, 2759–2766. [DOI] [PubMed] [Google Scholar]
- Collado-Hidalgo A., Bower J. E., Ganz P. A., Irwin M. R., Cole S. W. (2008). Cytokine gene polymorphisms and fatigue in breast cancer survivors: Early findings. Brain Behavior and Immunity, 22, 1197–1200. doi:10.1016/j.bbi.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo E. S., Ryff C. D., Singer B. H. (2009). Psychosocial adjustment among cancer survivors: Findings from a national survey of health and well-being. Health Psychology, 28, 147–156. doi:10.1037/a0013221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J. C., Freund G. G., Johnson R. W., Kelley K. W. (2008). From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Review Neuroscience, 9, 46–56. doi:10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J. R., MacLean A. W., Brundage M. D., Schulze K. (2002). Sleep disturbance in cancer patients. Social Science and Medicine, 54, 1309–1321. [DOI] [PubMed] [Google Scholar]
- Dennehy P. H. (2007). Rotavirus vaccines—success after failure. Medicine and Health Rhode Island, 90, 321–324. [PubMed] [Google Scholar]
- Dhruva A., Aouizerat B. E., Cooper B., Paul S. M., Dodd M., West C.…Miaskowski C. (2013). Differences in morning and evening fatigue in oncology patients and their family caregivers. European Journal of Oncology Nursing, 17, 841–848. doi:10.1016/j.ejon.2013.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhruva A., Aouizerat B. E., Cooper B., Paul S. M., Dodd M., West C.…Miaskowski C. (2015). Cytokine gene associations with self-report ratings of morning and evening fatigue in oncology patients and their family caregivers. Biological Research for Nursing, 17, 175–184. doi:10.1177/1099800414534313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhruva A., Dodd M., Paul S. M., Cooper B. A., Lee K., West C.…Miaskowski C. (2010). Trajectories of fatigue in patients with breast cancer before, during, and after radiation therapy. Cancer Nursing, 33, 201–212. doi:10.1097/NCC.0b013e3181c75f2a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doong S. H., Dhruva A., Dunn L. B., West C., Paul S. M., Cooper B. A.…Miaskowski C. (2015). Associations between cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression in patients prior to breast cancer surgery. Biological Research for Nursing, 17, 237–247. doi:10.1177/1099800414550394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engreitz J. M., Morgan A. A., Dudley J. T., Chen R., Thathoo R., Altman R. B., Butte A. J. (2010). Content-based microarray search using differential expression profiles. BMC Bioinformatics, 11, 603 doi:10.1186/1471-2105-11-603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdo S. L., Wolff J. R. (1990). gamma-Aminobutyric acid outside the mammalian brain. Journal of Neurochemistry, 54, 363–372. [DOI] [PubMed] [Google Scholar]
- Fletcher B. A., Schumacher K. L., Dodd M., Paul S. M., Cooper B. A., Lee K.…Miaskowski C. (2009). Trajectories of fatigue in family caregivers of patients undergoing radiation therapy for prostate cancer. Research in Nursing and Health, 32, 125–139. doi:10.1002/nur.20312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher B. S., Paul S. M., Dodd M. J., Schumacher K., West C., Cooper B.…Miaskowski C. A. (2008). Prevalence, severity, and impact of symptoms on female family caregivers of patients at the initiation of radiation therapy for prostate cancer. Journal of Clinical Oncology, 26, 599–605. doi:10.1016/j.pmr.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Foteinou P. T., Calvano S. E., Lowry S. F., Androulakis I. P. (2010). Multiscale model for the assessment of autonomic dysfunction in human endotoxemia. Physiological Genomics, 42, 5–19. doi:10.1152/physiolgenomics.00184.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman R. C., Carey V. J., Bates D. M., Bolstad B., Dettling M., Dudoit S.…Zhang J. (2004). Bioconductor: Open software development for computational biology and bioinformatics. Genome Biology, 5, R80 doi:10.1186/gb-2004-5-10-r80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath A. W., Luckey C. J., Park R., Benoist C., Mathis D. (2004). The molecular program induced in T cells undergoing homeostatic proliferation. Proceedings of the National Academy of Science U S A, 101, 16885–16890. doi:10.1073/pnas.0407417101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotte M., von Mollard G. F. (1998). A new beat for the SNARE drum. Trends in Cell Biology, 8, 215–218. [DOI] [PubMed] [Google Scholar]
- Haimovich B., Calvano J., Haimovich A. D., Calvano S. E., Coyle S. M., Lowry S. F. (2010). In vivo endotoxin synchronizes and suppresses clock gene expression in human peripheral blood leukocytes. Critical Care Medicine, 38, 751–758. doi:10.1097/CCM.0b013e3181cd131c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. A., Clark J., Ireland A., Lomax J., Ashburner M., Foulger R. … Gene Ontology Consortium. (2004). The Gene Ontology (GO) database and informatics resource. Nucleic Acids Research, 32, D258–D261. doi:10.1093/nar/gkh036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofso K., Miaskowski C., Bjordal K., Cooper B. A., Rustoen T. (2012). Previous chemotherapy influences the symptom experience and quality of life of women with breast cancer prior to radiation therapy. Cancer Nursing, 35, 167–177. doi:10.1097/NCC.0b013e31821f5eb5 [DOI] [PubMed] [Google Scholar]
- Hsiao C. P., Araneta M., Wang X. M., Saligan L. N. (2013). The association of IFI27 expression and fatigue intensification during localized radiation therapy: Implication of a para-inflammatory bystander response. International Journal of Molecular Science, 14, 16943–16957. doi:10.3390/ijms140816943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C. P., Wang D., Kaushal A., Saligan L. (2013). Mitochondria-related gene expression changes are associated with fatigue in patients with nonmetastatic prostate cancer receiving external beam radiation therapy. Cancer Nursing, 36, 189–197. doi:10.1097/NCC.0b013e318263f514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indraccolo S., Pfeffer U., Minuzzo S., Esposito G., Roni V., Mandruzzato S.…Amadori A. (2007). Identification of genes selectively regulated by IFNs in endothelial cells. Journal of Immunology, 178, 1122–1135. doi:10.4049/jimmunol.178.2.1122 [DOI] [PubMed] [Google Scholar]
- Irwin M. R., Cole S. W. (2011). Reciprocal regulation of the neural and innate immune systems. Nature Review Immunology, 11, 625–632. doi:10.1038/nri3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager A., Sleijfer S., van der Rijt C. C. (2008). The pathogenesis of cancer related fatigue: Could increased activity of pro-inflammatory cytokines be the common denominator? European Journal of Cancer, 44, 175–181. doi:10.1016/j.ejca.2007.11.023 [DOI] [PubMed] [Google Scholar]
- Jeanmougin M., de Reynies A., Marisa L., Paccard C., Nuel G., Guedj M. (2010). Should we abandon the t-test in the analysis of gene expression microarray data: A comparison of variance modeling strategies. PLoS One, 5, e12336 doi:10.1371/journal.pone.0012336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnofsky D., Abelmann W. H., Craver L. V., Burchenal J. H. (1948). The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer, 1, 634–656. [Google Scholar]
- Landmark-Hoyvik H., Reinertsen K. V., Loge J. H., Fossa S. D., Borresen-Dale A. L., Dumeaux V. (2009). Alterations of gene expression in blood cells associated with chronic fatigue in breast cancer survivors. Pharmacogenomics Journal, 9, 333–340. [DOI] [PubMed] [Google Scholar]
- Lee B. N., Dantzer R., Langley K. E., Bennett G. J., Dougherty P. M., Dunn A. J.…Cleeland C. S. (2004). A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation, 11, 279–292. [DOI] [PubMed] [Google Scholar]
- Lee K. A., Hicks G., Nino-Murcia G. (1991). Validity and reliability of a scale to assess fatigue. Psychiatry Research, 36, 291–298. [DOI] [PubMed] [Google Scholar]
- Liew C. C., Ma J., Tang H. C., Zheng R., Dempsey A. A. (2006). The peripheral blood transcriptome dynamically reflects system wide biology: A potential diagnostic tool. Journal of Laboratory and Clinical Medicine, 147, 126–132. [DOI] [PubMed] [Google Scholar]
- Light K. C., Agarwal N., Iacob E., White A. T., Kinney A. Y., VanHaitsma T. A.…Light A. R. (2013). Differing leukocyte gene expression profiles associated with fatigue in patients with prostate cancer versus chronic fatigue syndrome. Psychoneuroendocrinology, 38, 2983–2995. doi:10.1016/j.psyneuen.2013.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. F., Brown C. M., El Gazzar M., McPhail L., Millet P., Rao A.…McCall C. E. (2012). Fueling the flame: Bioenergy couples metabolism and inflammation. Journal of Leukocyte Biology, 92, 499–507. doi:10.1189/jlb.0212078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Friedman M. S., Shedden K., Hankenson K. D., Woolf P. J. (2009). GAGE: Generally applicable gene set enrichment for pathway analysis. BMC Bioinformatics, 10, 161 doi:10.1186/1471-2105-10-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoccoli G., Sothern R. B., De Cata A., Giuliani F., Fontana A., Copetti M.…Tarquini R. (2011). A timetable of 24-hour patterns for human lymphocyte subpopulations. Journal of Biological Regulators and Homeostatic Agents, 25, 387–395. [PubMed] [Google Scholar]
- Meeus M., Mistiaen W., Lambrecht L., Nijs J. (2009). Immunological similarities between cancer and chronic fatigue syndrome: The common link to fatigue? Anticancer Research, 29, 4717–4726. [PubMed] [Google Scholar]
- Miaskowski C., Dodd M., Lee K., West C., Paul S. M., Cooper B. A.…Aouizerat B. E. (2010). Preliminary evidence of an association between a functional interleukin-6 polymorphism and fatigue and sleep disturbance in oncology patients and their family caregivers. Journal of Pain and Symptom Management, 40, 531–544. doi:10.1016/j.jpainsymman.2009.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C., Paul S. M., Cooper B. A., Lee K., Dodd M., West C.…Wara W. (2008). Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. Journal of Pain and Symptom Management, 35, 632–643. doi:10.1016/j.jpainsymman.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T. J. (2012). The proliferation rate paradox in antimitotic chemotherapy. Molecular Biology of the Cell, 23, 1–6. doi:10.1091/mbc.E10-04-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molassiotis A., Chan C. W. (2004). Fatigue patterns in Chinese patients receiving radiotherapy. European Journal of Oncology Nursing, 8, 334–340. [DOI] [PubMed] [Google Scholar]
- Nishimura D. (2001). BioCarta. Biotech Software & Internet Report, 2, 117–120. [Google Scholar]
- Osoba D. (1999). Interpreting the meaningfulness of changes in health-related quality of life scores: Lessons from studies in adults. International Journal of Cancer, 12, 132–137. [DOI] [PubMed] [Google Scholar]
- Petroff O. A. (2002). GABA and glutamate in the human brain. Neuroscientist, 8, 562–573. [DOI] [PubMed] [Google Scholar]
- Powell N. D., Mays J. W., Bailey M. T., Hanke M. L., Sheridan J. F. (2011). Immunogenic dendritic cells primed by social defeat enhance adaptive immunity to influenza A virus. Brain Behavior and Immunity, 25, 46–52. doi:10.1016/j.bbi.2010.07.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N., Banks W. A. (2007). Brain-immune communication pathways. Brain Behavior and Immunity, 21, 727–735. doi:10.1016/j.bbi.2007.05.005 [DOI] [PubMed] [Google Scholar]
- Reimers M. (2010). Making informed choices about microarray data analysis. PLoS Computational Biology, 6, e1000786 doi:10.1371/journal.pcbi.1000786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinertsen K. V., Grenaker Alnaes G. I., Landmark-Hoyvik H., Loge J. H., Wist E., Kristensen V. N.…Edvardsen H. (2011). Fatigued breast cancer survivors and gene polymorphisms in the inflammatory pathway. Brain Behavior and Immunity, 25, 1376–1383. doi:10.1016/j.bbi.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Rodriguez N., Mages J., Dietrich H., Wantia N., Wagner H., Lang R., Miethke T. (2007). MyD88-dependent changes in the pulmonary transcriptome after infection with Chlamydia pneumoniae. Physiological Genomics, 30, 134–145. doi:10.1152/ajplung.00011.2007 [DOI] [PubMed] [Google Scholar]
- Ryan J. L., Carroll J. K., Ryan E. P., Mustian K. M., Fiscella K., Morrow G. R. (2007). Mechanisms of cancer-related fatigue. Oncologist, 12, 22–34. doi:10.1634/theoncologist.12-S1-22 [DOI] [PubMed] [Google Scholar]
- Saligan L. N., Hsiao C. P., Wang D., Wang X. M., St John L., Kaushal A.…Dionne R. A. (2013). Upregulation of alpha-synuclein during localized radiation therapy signals the association of cancer-related fatigue with the activation of inflammatory and neuroprotective pathways. Brain Behavior and Immunity, 27, 63–70. doi:10.1016/j.bbi.2012.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saligan L. N., Kim H. S. (2012). A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain Behavior and Immunity, 26, 830–848. doi:10.1016/j.bbi.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangha O., Stucki G., Liang M. H., Fossel A. H., Katz J. N. (2003). The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis and Rheumatism, 49, 156–163. [DOI] [PubMed] [Google Scholar]
- Smyth G. (2005). Limma: Linear models for microarray data In Gentleman R. C., Carey V. J., Dudoit S., Irizarry R., Huber W. (Eds.), Bioinformatics and computational biology (pp. 397–420). New York, NY: Springer. [Google Scholar]
- Sprangers M. A., Schwartz C. E. (1999). The challenge of response shift for quality-of-life-based clinical oncology research. Annals of Oncology, 10, 747–749. [DOI] [PubMed] [Google Scholar]
- Tripathi S., Glazko G. V., Emmert-Streib F. (2013). Ensuring the statistical soundness of competitive gene set approaches: Gene filtering and genome-scale coverage are essential. Nucleic Acids Research, 41, e82 doi:10.1093/nar/gkt054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Dennehy P. H., Keyserling H. L., Tang K., Gentsch J. R., Glass R. I., Jiang B. (2007). Rotavirus infection alters peripheral T-cell homeostasis in children with acute diarrhea. Journal of Virology, 81, 3904–3912. doi:10.1128/JVI.01887-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurfel M. M., Park W. Y., Radella F., Ruzinski J., Sandstrom A., Strout J.…Martin T. R. (2005). Identification of high and low responders to lipopolysaccharide in normal subjects: An unbiased approach to identify modulators of innate immunity. Journal of Immunology, 175, 2570–2578. doi:10.4049/jimmunol.175.4.2570 [DOI] [PubMed] [Google Scholar]
- Yang S., Wang K., Valladares O., Hannenhalli S., Bucan M. (2007). Genome-wide expression profiling and bioinformatics analysis of diurnally regulated genes in the mouse prefrontal cortex. Genome Biology, 8, R247 doi:10.1186/gb-2007-8-11-r247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Sliwkowski M. X. (2001). Untangling the ErbB signalling network. Nature Reviews in Molecular and Cell Biology, 2, 127–137. doi:10.1038/35052073 [DOI] [PubMed] [Google Scholar]
- Yavuzsen T., Davis M. P., Ranganathan V. K., Walsh D., Siemionow V., Kirkova J.…Yue G. H. (2009). Cancer-related fatigue: Central or peripheral? Journal of Pain and Symptom Management, 38, 587–596. doi:10.1016/j.jpainsymman.2008.12.003 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Martino A., Faulon J. L. (2007). Identification of expression patterns of IL-2-responsive genes in the murine T cell line CTLL-2. Journal of Interferon and Cytokine Research, 27, 991–995. doi:10.1089/jir.2006.0169 [DOI] [PubMed] [Google Scholar]