Abstract
The orexigenic neuropeptide MCH is well-positioned to play a key role in connecting brain reward and homoeostatic systems due to its synthesis in hypothalamic circuitry and receptor expression throughout the cortico-striatal reward circuit. Here we examined whether targeted-deletion of the MCH receptor (MCH-1R) in gene-targeted heterozygote and knock-out mice (KO), or systemic treatment with pharmacological agents designed to antagonize MCH-1R in C57BL/6J mice would disrupt two putative forms of reward learning that rely on different neural circuitries: Conditioned reinforcement (CRf) and Pavlovian-instrumental transfer (PIT). Mice were trained to discriminate between presentations of a reward-paired cue (CS+) and an unpaired CS−. Following normal acquisition of the Pavlovian discrimination in all mice, we assessed the capacity for the CS+ to act as a reinforcer for new nose-poke learning (CRf). Pharmacological disruption in control mice, and genetic deletion in KO mice impaired CRf test performance, suggesting MCH-1R is necessary for initiating and maintaining behaviors that are under the control of conditioned reinforcers. To examine a dissociable form of reward learning (PIT), a naïve group of mice were trained in separate Pavlovian and instrumental lever training sessions followed by PIT test. For all mice the CS+ was capable of augmenting ongoing lever responding relative to CS− periods. These results suggest a role for MCH in guiding behavior based on the conditioned reinforcing value of a cue, but not on its incentive motivational value.
Keywords: conditioned reinforcement, Pavlovian-instrumental transfer, MCH-1R, antagonist, mice
Introduction
The maintenance of homeostatic control represents a key component of biological adaptation and demands the integration of a variety of hormonal, visceral and sensorimotor control mechanisms. At the same time, learned reward signals or exposure to palatable food rewards can influence internal regulatory mechanisms (Berthoud et al., 2011; Holland & Petrovich, 2005). Due to its expression in hypothalamic systems and mesolimbic circuitry (Bittencourt et al., 1992; Guyon et al., 2009) the 19-amino acid peptide Melanin Concentrating Hormone (MCH) and its receptor (MCH-1R) likely play a key role in connecting brain reward and regulatory systems.
MCH is primarily synthesized in the lateral hypothalamus (LH) and zona incerta. Acting through its G protein-coupled receptor MCH-1R, this orexigenic peptide is upregulated during periods of food withdrawal or in hypoleptinemic ob/ob mice (Presse et al., 1996; Qu et al., 1996), increases food intake following central infusions (Gomori et al., 2003; Della-Zuana et al., 2002) and leads to marked hyperphagia following transgenic overexpression (Ludwig et al., 2001). Conversely, MCH-1R antagonism or deletion of the peptide results in hypophagia (Shimada et al., 1998; Mashiko et al., 2005), whereas MCH-1R deficient mice are lean, hyperactive and have altered metabolism (Marsh et al., 2002). Although MCH synthesis is restricted to the hypothalamus, MCH-1R is expressed throughout mesolimbic reward circuitry (Chung et al., 2009a), with its expression in ventral striatal nucleus accumbens shell (ACBS) medium spiny neurons receiving particular attention (Georgescu et al., 2005; Chung et al., 2009b; Sears et al., 2010). Within the ACBS MCH-1R is thought to modulate excitability of this region since application of MCH reduces phosphorylation of GluR1 Ser845 and other PKA targets (Sears et al., 2010) and leads to an increase in food intake (Georgescu et al., 2005; Kelley, 2004)
Because this pattern of expression includes brain systems implicated in the attribution of incentive value to cues associated with food reward (Mackintosh, 1974; Berridge et al., 2010), here we sought to further delineate the role of MCH-1R in reward learning. Specifically, we studied the modulatory role of MCH on performance in two incentive tasks, conditioned reinforcement (CRf) and Pavlovian-instrumental transfer (PIT) using a lack-of-function approach in MCH-1R gene knockout mice and pharmacological antagonism of that receptor in C57BL/6J mice. It should be noted that MCH-R1 is the solely functional MCH receptor in rodents (Tan et al., 2002) thus MCH-1R KO represent a complete deletion of MCH function in this engineered mouse model.
CRf reflects the acquisition of conditioned incentive value to a reward-paired CS – rendering it capable of supporting new learning (Mackintosh, 1974). CRf is thought to be dependent on ACBS, basolateral amygdala and the orbitofrontal cortex (Cador et al., 1989; Pears et al., 2003; Taylor & Robbins, 1986). Alternatively, PIT reflects the capacity of conditioned stimuli to modulate the performance of ongoing instrumental behavior (Rescorla & Solomon, 1967) and depends on mesolimbic circuitry including the central nucleus of the amygdala, midbrain ventral tegmental area and the ACBS (Holland & Gallagher, 2003; Lex & Hauber, 2008). Our results point to a role for MCH in guiding behavior based on the conditioned reinforcing properties of a reward-paired cue.
Materials and Methods
Subjects
The inactivation of the MCH-1R allele, the generation of knockout animals and the genotyping method have been described previously (Adamantidis et al., 2005). Heterozygous MCH-1R+/− mice were backcrossed a minimum of eight-times to C57BL/6J strain. For CRf, naïve age-matched C57BL6/J strain (n=37), MCH-1R−/− Knockout (KO) (n = 14), heterozygote+/− (HET) (n = 8) and wild-type+/+ (WT) (n = 14) mice were used. PIT training and testing was conducted using age-matched male C57BL6/J strain mice (n=21) and, KO (n = 10), HET (Het) (n = 7) and WT (n = 7) mice.
For all experiments, mice were tested at approximately 3 months of age and were housed three or four to a cage under a 12 h light/dark cycle (lights on at 7:00 A.M to 7:00 P.M). Prior to food deprivation mice weighed between 25 and 35 grams. Consistent with previous studies (Chen et al., 2002; Marsh et al., 2002) there were significant differences in baseline weight due to MCH-1R deletion; the weight of WT mice (32.25 ± 0.71 g) was significantly greater then both HET (28.2 ± 0.7 g) and KO mice (28.62 ± 0.68 g)- (p’s<0.01). Food deprivation began 5 d prior to the start of each experiment by restricting access to a single daily meal pellet. Behavioral training and testing were completed in the light cycle between 9:00 A.M and 5:00 P.M. Animal procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and under the auspices of the Johns Hopkins Animal Care and Use Committee.
Apparatus
Behavioral procedures were conducted in eight individual chambers (53 cm × 35 cm × 35 cm LWH) with aluminum front and back walls, clear polycarbonate sides, and a floor made of 17.8 mm stainless steel rods spaced 0.5 cm apart (Med Associates, St. Albans, VT). The ceiling was also polycarbonate and contained an infrared locomotor activity detector (Colbourn Instruments, Allentown, PA; Model H24-61MC) positioned above a hole in the roof panel. The activity monitor recorded the change in position of the subjects’ infrared body heat signature. A food cup was recessed in the center of one end wall into which 50µl of liquid reward could be delivered. A vacuum was attached to the bottom of the food cup which could be released via an attached solenoid. An infrared photocell placed inside the food cup monitored the time spent and number of head entries made into the food cup. An audio generator, which could emit either a 3-kHz tone or white noise (each 80 dB) was mounted on the outside of the chamber on the wall opposite the food cup. Chamber illumination was provided by a 28 V, 100 mA house light mounted on the inside wall of the sound attenuating chamber. During CRf testing phase, the chambers were fitted with two nose-poke devices, each 12 mm in diameter, and located at identical heights on the left and right sides of the food cup. Each nose-poke device contained a yellow stimulus LED located at the rear of the recessed hole and a photo beam sensor to monitor nose-poke entries. For PIT, within each chamber retractable ultra-sensitive mouse levers (Med Associates, St. Albans, VT) were available to the right and left sides of the food cup. An IBM-compatible computer equipped with Med-PC software (Med Associates, St. Albans, VT) controlled and recorded all stimuli and responses.
Drugs
For CRf and PIT, C57/BL6J mice were treated with the MCH-1R receptor antagonist PMC-3881-PI (Peptides International; Bednarek et al., 2002) at a dose appropriate to their group allocation (10 mg/kg or 30 mg/kg). PMC-3881-PI was dissolved in sterile sodium chloride solution (0.9%) and administered at a volume of 10 ml/kg intraperitoneally (IP). For the control condition, matched volumes of 0.9% saline were administered IP.
Behavioral Training Procedures
(1) Conditioned reinforcement
The experiment was run separately for C57BL/6J mice and MCH-1R mutant mice. Mice were first trained to consume rewards delivered to the food cup (50µl of 10 % w/v sucrose solution) where 60 deliveries of sucrose occurred on a random time (RT) 30 s schedule each day for two days. Next, the mice received single daily Pavlovian training sessions for a total of 12 days. Each session was approximately 45 min long and consisted of fourteen separate 10 s presentations each of a tone and of a white noise, with a variable intertrial interval (ITI) of 30 s. For half the mice in each group, sucrose was delivered to the food cup for the final 5 s of the tone (the reinforced CS, or CS+) but not for the noise (the non-reinforced CS, or CS−). For the remaining mice, the noise served as CS+ and the tone served as CS−. Any sucrose remaining at the end of CS+ presentation was vacuumed out at that time. CS+ and CS− trials were intermixed in pseudorandom sequences determined by the computer. The response measure reflected the number of food cup entries prior to each CS (Pre-CS) and the initial 5s window following cue presentation.
After completion of Pavlovian training, mice received a single 40 min conditioned reinforcement test session. At this stage the C57BL/6J mice were divided into groups (saline n = 18; 10 mg/kg n = 7; 30 mg/kg n = 12) counterbalanced according to their Pavlovian conditioning histories and performance. Twenty-minutes prior to the CRf test, half the mice from each group were injected with either saline (control group) or the relevant dose of MCH-1R antagonist PMC-3881-PI. During this test, illuminated nose-poke ports were made available on both sides of the food cup. For half the mice in each group, each nose-poke to the left port resulted in brief (3 s) presentation of the tone cue, and each right-nose poke response produced a 3 s noise presentation. Nose pokes made during a cue presentation were recorded but had no programmed consequences. For the remaining mice, the response-stimulus contingencies were reversed. The Pavlovian conditioning histories of tone and noise (as CS+ or CS−) were counterbalanced with respect to left or right nose poke responses. If the CS+ acquired the ability to serve as a conditioned reinforcer, then more nose-pokes that produced the previously reinforced CS+ would be performed than nose-pokes that produced the previously nonreinforced CS−.
(2). Pavlovian-instrumental transfer
The experiment was run separately for C57BL/6J mice and MCH-1R mutant mice. Initially mice received food cup training, identical to that administered for the conditioned reinforcement experiments. Following food cup training, mice received one Pavlovian training session each day for three days. Each session consisted of 10 pseudorandomly presented 2 min cues: 5 reward-paired (CS+; either tone or clicker) and 5 non-reward-paired (CS−). For half the mice the tone served as CS+ and the clicker as CS−, while the remaining mice received the opposite contingencies. Sucrose was delivered on an RT 30 s schedule during the CS+, but not during the CS− trials. Any remaining sucrose at the end of a CS+ trial was removed via the vacuum. Trials were separated by a 2 min variable ITI. The response measure reflected the percentage of time in the food cup during the 10 s period prior to CS presentations (Pre-CS) and the initial 10 s CS presentation period.
Next, mice were trained to perform a lever-press for the sucrose reward for three 30-min sessions, each conducted on a separate day. At this time both levers were available; presses on the lever designated active resulted in sucrose delivery, while presses on the other (inactive) lever did not. Active and inactive lever locations (i.e., left and right-side) were fully counterbalanced across mice. To facilitate lever responding on the first day, a small amount of sweetened condensed milk solution was placed on the active lever. Each response on the active lever resulted in sucrose delivery (i.e. a continuous reinforcement schedule). Subsequently, mice received alternating Pavlovian and instrumental training sessions every 3 days. Pavlovian sessions continued as previously described, while the response-reinforcement schedule associated with the active lever in instrumental training gradually increased to a variable interval (VI) 30 s (instrumental sessions 4–6), VI 60 s (sessions 7–9) and VI 90 s (sessions 10–12). Training was complete when mice received 12 separate sessions each of Pavlovian and instrumental training.
Twenty-four hrs following the final training session, C57BL/6J mice were divided into groups (saline n = 8; 10 mg/kg n = 8; 30 mg/kg n = 4) counterbalanced according to their Pavlovian conditioning histories. Twenty-minutes prior to the PIT test, half the mice from each group were injected with either the MCH-1R antagonist, at the dose relevant to their group assignment, while the remaining mice were injected with saline. For the test, all mice had access to both levers, and following a 6-min extinction period, each cue (CS+, CS−) was presented 5 times in an alternating order, separated by 2-min fixed intervals, with the criterion that the same cue would not be repeated more than twice consecutively. We expected Pavlovian-instrumental transfer to be expressed as an augmentation of the rate of ongoing lever-pressing by CS+, but not CS−.
Results
Conditioned reinforcement: MCH-1R mutant mice
The deletion of MCH-1R had no effect on the acquisition of a simple Pavlovian discrimination, as compared to wild-type mice (Figure 1). Three-way group X cue X block ANOVA revealed a main effect of cue (F(1,35) = 30.42, p<0.001), block (F(3,105) = 32.87, p<0.001), and a cue X block interaction (F(3,105) = 32.87, p<0.001). No effects of group or its interaction among any of the other variables were revealed (F’s<1; p’s>0.42). Thus, prior to conditioned reinforcement testing all mice showed similar Pavlovian conditioning.
Figure 1. Pavlovian conditioning for conditioned reinforcement following genetic deletion of MCH-1R.
WT, HET and KO mice showed a comparable increase in food cup responding during CS+ cue relative to the CS−. Data are presented in food cup responses/min during the initial 5s of CS presentation - Pre-CS (5 s prior to CS). Error bars indicate standard error of the mean (SEM).
Test Stage
While deletion of MCH-1R had no effect on Pavlovian training, it had a profound effect on conditioned reinforcement (Figure 2a). Both WT and HET showed a clear preference for the CS+ eliciting nose-poke relative to the CS− poke during the test session. By contrast, discriminative responding was entirely absent in KO mice (Figure 2a). Three-way ANOVA with variables of group, cue and bin revealed no main effect of group (F<1), a main effect of cue (F(1,35) = 19.52, p<0.0001, and, importantly, a significant group X cue interaction (F(2,35) = 3.28, p<0.05). Further comparisons revealed a clear conditioned reinforcement effect in both WT (F(1,35) = 11.79, p<0.01) and HET (F(1,35) = 12.16, p<0.01) groups, but not KO mice (F<1). The nature of the impairment appeared to be enhanced responding to the CS− eliciting poke in KO relative to WT mice (F(1,35) = 7.21, p=0.01). The same conclusion, that MCH-1R deletion disrupted conditioned reinforcement, can be drawn from an analysis of discrimination ratio scores (responding for CS+/[responding for CS+ plus responding for CS−]), in which scores significantly greater than 0.5 indicate greater responding to the CS+ eliciting poke. Adopting this analysis revealed significantly greater responding for the CS+ eliciting poke than CS− (one-sample t test, HO = 0.5) in WT (0.70 ± 0.03; t(14) = 5.02, p<0.001) and HET (0.69 ± 0.03; t(8) = 5.66, p<0.001), but not KO mice (0.55 ± 0.05; t(13) = 1.00, p=0.33).
Figure 2. Conditioned reinforcement following genetic deletion of MCH-1R.
(a) WT and HET mice showed a preference in responding to the CS+ eliciting poke relative to the CS− poke. By contrast, KO mice showed comparable nose-poke responding for both CS+ and CS− eliciting pokes. (b–d) Within session nose-poke responding for CS+ and CS− eliciting poke (in 5-min bins), during the test phase for (b) WT, (c) HET and (d) KO. *Indicates significant CRf in WT (F(1,35) = 11.79, p<0.01) and HET mice (F(1,35) = 12.16, p<0.01). # indicates greater CS− responding in KO compared to WT mice (F(1,35) = 7.21, p=0.01). Error bars indicate SEM.
To further explore the nature of these group differences, we examined the pattern of responding during the conditioned reinforcement test. As the test session progressed both WT (Figure 2b) and HET mice (Figure 2c) maintained discriminative performance. By contrast, the disruption to conditioned reinforcement was evident throughout the test session in KO mice (Figure 2d). To analyze these differences in test performance, we separately examined responding in the first half (0–20 mins) and second half (20–40 mins) of the test session. Analysis of responding in the first half of the session revealed a cue X group interaction (F(2,35) = 3.84, p<0.05) due to an absence of conditioned reinforcement in KO mice (F(1,35) = 0.02, p=0.86) as they responded significantly more on the CS− eliciting poke than WT mice (F(1,35) = 6.58, p=0.01). In the second half of the test session, there was a trend towards an interaction between cue X group (F(2,35) = 2.50, p=0.08), again due to a failure to demonstrate conditioned reinforcement in KO mice (F(1,35) = 1.38, p=0.24). Interestingly, while KO mice showed greater responding for the CS− poke compared to WT (F(1,35) =8.61, p<0.001), HET mice showed greater CS+ responding compared to WT mice (F(1,35) = 3.84, p=0.05). However, this last observation must be viewed with caution because the relevant interaction was only marginally (p = 0.08) significant. These results indicate a pronounced conditioned reinforcement impairment in KO mice and a tendency for HET mice to show greater persistence of CS+ responding during the second half of the test. To rule out interpretations based on compensatory or developmental influences on the KO mice, we examined whether MCH-1R inactivation with the selective MCH-R1 receptor antagonist PMC-3881-PI would disrupt performance in C57BL/6J mice.
Conditioned Reinforcement: MCH-1R antagonist
Prior to drug treatment and the test stage, there were no differences in the acquisition of the Pavlovian discrimination between the groups of mice (Figure 3). Three-way group X cue X block ANOVA revealed a main effect of cue (F(1,35) = 15.13, p<0.001) and block (F(3,105) = 20.66, p<0.001) but no effect of group nor its interaction among any of the variables (Largest F-value; group X cue, F(2,35) = 1.76, p=0.18).
Figure 3. Pavlovian conditioning for conditioned reinforcement prior to treatment with MCH-1R antagonist.
Mice to-be-treated with saline, 10 mg/kg PMC-3881-PI or 30 mg/kg PMC-3881-PI showed comparable increase in food cup responding during CS+ cue relative to the CS−. Data are presented in food cup responses/min during the initial 5s of CS presentation - Pre-CS (5 s prior to CS). Error bars indicate SEM.
The primary data from this experiment are shown in Figure 4a. A disruption in MCH-1R signaling following treatment with PMC-3881-PI impaired conditioned reinforcement in the 30 mg/kg group. By contrast, there was a tendency for the lower dose (10 mg/kg) to show a facilitation of responding for the CS+ eliciting poke. Two-way group X cue ANOVA revealed no main effect of group (F(2,34) = 1.23, p=0.30), a main effect of cue (F(1,34) = 13.12, p<0.01) and an interaction between the variables (F(2,34) = 6.10, p<0.01). Simple main effects analyses revealed mice in the 30 mg/kg condition failed to show a conditioned reinforcement effect (F(1,34) = 0.31, p=0.58), whilst mice in both the saline (F(1,34) = 6.43, p=0.01) and 10 mg/kg conditions (F(1,34) = 15.46, p<0.001) showed a clear effect. In addition, mice in the 10mg/kg condition showed greater responding to the CS+ eliciting poke than both saline (F(1,34) = 4.10, p=0.05) and 30 mg/kg groups (F(1,34) = 8.21, p<0.01). Additional analyses using discrimination ratio scores (responding for CS+/[responding for CS+ plus responding for CS−]) revealed that responding was significantly greater for CS+ eliciting nose-poke (i.e., greater than 0.5) (discrimination ratio ± SEM; one-sample t test, HO = 0.5) in saline (0.63 ± 0.05; t(17) = 2.46, p<0.05) and 10 mg/kg (0.69 ± 0.06; t(6) = 2.91, p<0.05) but not in 30 mg/kg treated mice (0.53 ± 0.06; t(11) = 1.61, p=0.13).
Figure 4. Conditioned reinforcement following systemic treatment with MCH-1R antagonist.
(a) Overall responding during the conditioned reinforcement test following ip injections with saline, 10 mg/kg PMC-3881-PI or 30 mg/kg PMC-3881-PI. (b–d) Within session nose-poke responding during the test phase for (b) saline, (c) 10 mg/kg and (d) 30 mg/kg PMC-3881-PI treated mice. * indicates significant CRf effect in saline (F(1,34) = 6.43, p=0.01) and 10 mg/kg conditions (F(1,34) = 15.46, p<0.001). # indicates greater CS+ responding in 10 mg/kg compared to saline conditions (F(1,34) = 4.10, p=0.05). Error bars indicate SEM.
As with the previous experiment, we also examined responding within the test session for the first half (0–20 mins) and second half (20–40 mins) periods separately. Saline-treated mice (Figure 4b) appeared to show greater discriminative responding as the test session progressed. By contrast, mice treated with 10 mg/kg showed conditioned reinforcement throughout the test session (Figure 4c) while 30 mg/kg treated mice appeared to display greater non-discriminative responding during the first half of the test session (Figure 4d). These claims are supported statistically. An analysis of responding from the first half of the test session revealed a significant group X cue interaction (F(2,34) = 4.36, p<0.05) with 10 mg/kg group (F(1,34) = 4.93, p<0.05) but not saline (F(1,34) = 2.35, p=0.13) or 30 mg/kg (F(1,34) = 2.65, p=0.11) showing evidence of conditioned reinforcement. During this stage of the test 30 mg/kg mice showed greater CS− responding compared to other mice (smallest F-value; saline vs 30mg/kg, (F(1,34) = 3.95, p<0.05). Responses for the second half of the test session revealed a trend towards a group X cue interaction (F(2,34) = 2.93, p=0.06), due to both saline (F(1,34) = 5.82, p<0.05) and 10 mg/kg groups (F(15.38, p<0.001) but not 30 mg/kg treated mice (F(1,34) = 1.65, p=0.20) showing conditioned reinforcement. During the second half of the test stage, 10 mg/kg mice showed greater CS+ nose-poke responding relative to all other mice (smallest F-value; saline vs 10 mg/kg, F(1,34) = 3.91, p=0.05). Collectively, the results from the CRf experiments suggest a disruption in conditioned reinforcement (due to increased CS− responding) following deletion of MCH-1R gene or treatment with a high dose of the MCH-R1 antagonist. Interestingly, partial deletion of the receptor (i.e., HET animals), or a lower dose of the antagonist showed some tentative evidence for a facilitation of CS+ responding during the test stage.
Pavlovian-instrumental transfer: MCH-1R mutant mice
Training stage
Targeted deletion of MCH-1R gene had no effect on the acquisition of lever responding (Figure 5a). Two-way ANOVA with variables of group (WT, HET, KO), and session block (1–4) revealed a main effect of session (F(3,66) = 42.45, p<0.01), but no effect of group nor interaction (F’s<1; p’s>0.25). Similarly, in the Pavlovian training stage all mice acquired the simple discrimination, showing greater food cup responding during presentations of the rewarded CS+, compared to the non-rewarded CS− cue. There was a tendency however, for HET mice to show lower CS+ entries compared to the other groups (Figure 5b). A three-way ANOVA with group, cue (CS+, CS−) and session block (1–4) as variables revealed no main effect of group (F(2,22) = 1.11, p=0.35), main effects of cue (F(1,22) = 21.71, p<0.01) and session (F(3,66) = 2.93, p<0.05), and a three-way group X cue X session interaction (F(6,66) = 2.34, p<0.05). An examination of group differences in CS+ responding during the final block of training revealed a trend towards less responding in HET compared to KO (F(1,22)=3.64, p=0.07) but not WT mice (F(1,22) = 1.59, p=0.22).
Figure 5. Pavlovian conditioning for Pavlovian-instrumental transfer following genetic deletion of MCH-1R.
(a) Lever responses per minute during instrumental training for WT, HET and KO. (b) Percentage time in food cup for WT, HET and KO mice during CS+ cue relative to the CS−. Data are presented during the initial 10 s of CS presentation - Pre-CS (10 s prior to CS). Error bars indicate SEM.
Test Stage
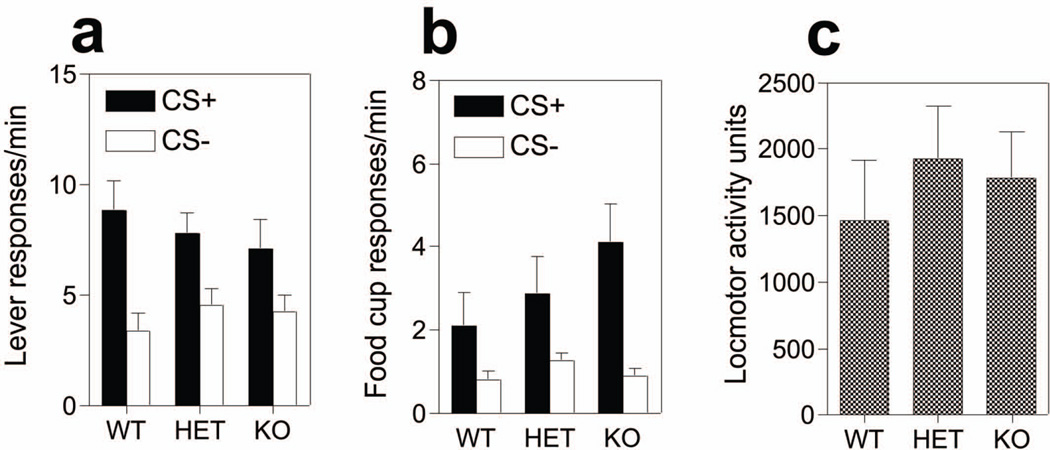
The data of primary interest from this experiment are presented in Figure 6a. All groups of mice showed evidence of a PIT effect– responding at a greater rate on the lever during presentations of the CS+ relative to the CS−. Two-way group X cue ANOVA revealed a main effect of cue (F(1,21) = 33.14, p<0.001), no effect of group (F(2,21) = 0.15, p=0.85) or group X cue interaction (F(2,21) = 1.45, p=0.25). In addition, all mice showed discriminative food cup responding to the CS+ cue compared to the CS− (Figure 6b), although there was a non-significant tendency for KO mice to show greater CS+ approach relative to other mice. ANOVA revealed a main effect of cue (F(1,21) = 14.71, p<0.001), no effect of group (F(2,21) = 1.14, p=0.34) nor interaction among the variables (F(2,21) = 1.32, p=0.28). Additional contrasts revealed no significant differences between KO mice and all other groups (F(1,21) = 2.59, p=0.12). Furthermore, locomotor activity was comparable across the groups (Figure 6c); one-way ANOVA (F(2,21) = 0.32, p=0.72).
Figure 6. Pavlovian-instrumental transfer following genetic deletion of MCH-1R.
(a) Lever responses/min during presentations of the CS+ (closed bars) and CS− (open bars) in WT, HET and KO mice. (b) Food cup responses/min during CS+ (closed bars) and CS− (open bars) in WT, HET and KO mice. (c) Locomotor activity in the intertrial no cue presentation period. Error bars indicate SEM.
Pavlovian-instrumental transfer: MCH-1R antagonist
Training stage
During instrumental training mice in the 10 mg/kg group showed reduced lever responding relative to the two other groups (Figure 7a). Two-way group X block ANOVA revealed a main effect of group (F(2,18) = 4.91, p=0.01), block (F(3,54) = 42.16, p<0.001) and an interaction between the two variables (F(6,54) = 2.41, p<0.05). Simple main effects analyses revealed differences between 10 mg/kg and saline groups during blocks 2 (F(1,18) = 8.54, p<0.01) and 3 (F(1,18) = 6.77, p<0.05), and between 10 mg/kg and 30 mg/kg groups during blocks 2–4 (smallest F-value; block 4, F(1,18) = 4.47, p=0.05). There were, however, no differences between the groups during Pavlovian training (Figure 7b). Three-way group X block X cue ANOVA revealed a main effect of cue (F(1,18) = 31.86, p<0.001) and block (F(3,54) = 9.33, p<0.001). There were no effects of group nor its interaction among any of the variables (F’s<1; p’s>0.67).
Figure 7. Pavlovian conditioning for Pavlovian-instrumental transfer prior to treatment with MCH-1R antagonist.
(a) Lever responses per minute during instrumental training for mice to-be-treated with saline, 10 mg/kg PMC-3881-PI or 30 mg/kg PMC-3881-PI. (b) Percentage time in food cup for mice to-be-treated with saline, 10 mg/kg PMC-3881-PI or 30 mg/kg PMC-3881-PI during CS+ cue relative to the CS−. Error bars indicate SEM.
Test Stage
All mice showed clear evidence of a Pavlovian-instrumental transfer effect as revealed by greater responding on the lever during presentations of the CS+ relative to CS− (Figure 8a). Two way group X cue ANOVA revealed a main effect of cue (F(1,18) = 20.83, p<0.001); no other significant effects or interaction were revealed (F’s<1; p’s>0.63). These results suggest that the reduced lever training in 10 mg/kg group had no interaction on the magnitude and direction of PIT test responding. Similarly, the MCH-1R antagonist had no effect of food cup entries (Figure 8b), all groups mice showed high levels of food cup responding to the CS+ cue. ANOVA revealed a main effect of cue (F(1,18) = 20.27, p<0.001), but no other effects nor interaction were revealed (F’s<1; p’s>0.77). Finally, treatment with PMC-3881-PI had no effect on locomotor activity during the test stage (Figure 8c); no effect of group (F(1,18) = 0.98, p=0.39). The results in these PIT experiments collectively show that genetic deletion of the receptor, or pharmacological inactivation does not affect the ability of a Pavlovian CS+ to elicit simple food cup behavior or enhance ongoing instrumental performance (PIT).
Figure 8. Pavlovian-instrumental transfer following systemic treatment with MCH-1R antagonist.
Lever responses/min during presentations of the CS+ (closed bars) and CS− (open bars) in saline, 10 mg/kg PMC-3881-PI or 30 mg/kg PMC-3881-PI. (b) Food cup responses/min during CS+ (closed bars) and CS− (open bars) in saline, 10 mg/kg PMC-3881-PI or 30 mg/kg PMC-3881-PI. (c) Locomotor activity in the intertrial no cue presentation period for all mice. Error bars indicate SEM.
Discussion
This study demonstrates a critical role for MCH-1R in conditioned incentive learning. Following genetic deletion or systemic antagonism of the MCH-1R, mice failed to show a preference for responding on a novel nose-poke response for presentations of a conditioned reinforcer. On the other hand, the Pavlovian CS+ cue was capable of augmenting instrumental level responding relative to CS− periods in all groups of mice. These results suggest a dissociable role for MCH-1R in two putative forms of reward learning– CRf and PIT.
In CRf, an antecedent Pavlovian CS+ becomes capable of acquiring conditioned incentive value due to subsequent presentation of a motivationally salient reinforcer (Mackintosh, 1974). As a result, the conditioned reinforcer is capable of acting as a reward in its own right in the absence of primary reinforcer delivery (i.e., sucrose). Previous findings suggest a role for MCH in responding under progressive ratio conditions (Duncan et al., 2006; Mul et al., 2011), which assesses the amount of effort animals will expend to receive food reward (Hodos, 1961). Our results extend these findings to suggest that intact MCH-1R also appears necessary for the modulation of responding based on the conditioned reinforcing properties of a reward-paired stimulus. It is important to note that the CRf impairments following genetic deletion of MCH-1R or systemic treatment with the MCH-1R antagonist likely did not reflect (nonspecific) motoric actions, as there were no differences in response measures that might be expected to be sensitive to motor deficits, including food cup entries and locomotor activity.
MCH-1R’s have been identified in a variety of central systems important for modulating conditioned reinforcement including the orbitofrontal cortex, basolateral amygdala and ventral striatal nucleus accumbens (Cador et al., 1989; Pears et al., 2003; Taylor & Robbins, 1986). This latter region has received considerable attention as MCH-1R receptors are coexpressed with dopamine D1 and D2 receptors in ACBS medium spiny neurons (Georgescu et al., 2005; Sears et al., 2010). Moreover, consistent with a cellular excitability hypothesis of ACBS in mediating appetitive behaviors (Kelley, 2004), MCH application reduces phosphorylation of GluR1 at Ser845 and other PKA targets including p-CREB, MAPK and Darpp-32 (Sears et al., 2010). Perhaps consistent with this hypothesis, the pattern of impaired responding following deletion or inactivation of MCH-1R signaling was attributable to enhanced levels of CS− responding (Burns et al., 1993; Johnson et al., 2011). The occurrence of similar rates of nose-poke responding for CS− might reflect broad generalization of positive incentive conditioned reinforcement value to CS−, or alternatively an inability to inhibit the performance of the response that produced CS−. Perhaps a loss of ACBS MCH-1R inhibitory control might underlie this pattern of impaired CRf responding. Interestingly, under conditions of partial deletion of the MCH-1R receptor (in heterozygous mice) or the use of a lower 10 mg/kg dose of the MCH-1R antagonist, there was some evidence for mice to show enhanced responding for the CS+ eliciting poke during the second half of the CRf test. While the nature of this pattern of responding remains to be further evaluated, these results suggest differential modulation of CRf based on the severity of genetic and pharmacological manipulations.
Unfortunately, we do not have bioavailability data concerning the effectiveness of the 10 mg/kg and 30 mg/kg doses in occupying central MCH-1R. Moreover, it should be noted that the apparently greater persistence of CRf in HET mice might reflect compensatory upregulation of signaling due to partial deletion of the receptor; thus resulting in more persistent CRf. By contrast, in KO animals full deletion of the receptor precludes such compensatory modulation.
Unlike the effects on conditioned reinforcement, deletion or antagonism of MCH-1R had no significant effect on the ability for a Pavlovian CS+ to augment ongoing lever responding. This process reflects conditioning of incentive motivation to the Pavlovian CS+, which is thought to exert motivational control over the instrumentally trained response (Rescorla & Solomon, 1967). Since transfer generated based on the general motivational significance of the Pavlovian excitor is also absent following lesions to areas with known MCH-1R immunoreactivity such as nucleus accumbens (e.g., Hervieu et al., 2000), targeted approaches are needed to determine specific central mechanisms of MCH-1R in reward-learning.
In recent years progress has been made in understanding interactions between reward systems and hypothalamic regulation systems (Berthoud et al., 2011; Holland & Petrovich, 2005). Several neuropeptides have been suggested to play an important role in connecting brain reward and regulatory systems including opioids, endocannabinoids, orexin and ghrelin (Berthoud et al., 2011; Crombag et al., 2010; Johnson et al., 2009). The current findings extend these assessments to support a role for MCH in not only food intake (Gomori et al., 2003; O Della-Zuana et al., 2002) but also in conditioned reinforcement. Since conditioned reinforcers are capable of modulating reward-related behaviors in the absence of food (Mackintosh, 1974), it is tempting to speculate that MCH enhances the value of conditioned reinforcers leading to facilitated acquisition of novel instrumental behaviors associated with foraging for food.
Acknowledgements
These studies were supported by NIDDK grant R21-DK84415 to A.W.J.
A.A. is supported by the Douglas Foundation, the Canadian Institute for Health Research, the Canadian Fund for Innovation, the Canadian Research Chair and the NARSAD.
References
- Adamantidis A, Thomas E, Foidart A, Tyhon A, Coumans B, Minet A, Tirelli E, Seutin V, Grisar T, Lakaye B. Disrupting the melanin-concentrating hormone receptor 1 in mice leads to cognitive deficits and alterations of NMDA receptor function. Eur. J. Neurosci. 2005;21:2837–2844. doi: 10.1111/j.1460-9568.2005.04100.x. [DOI] [PubMed] [Google Scholar]
- Bednarek MA, Hreniuk DL, Tan C, Palyha OC, MacNeil DJ, Van der Ploeg LHY, Howard AD, et al. Synthesis and biological evaluation in vitro of selective, high affinity peptide antagonists of human melanin-concentrating hormone action at human melanin-concentrating hormone receptor 1. Biochemistry. 2002;41(20):6383–6390. doi: 10.1021/bi0200514. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Research. 2010;1350:43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1266–R1277. doi: 10.1152/ajpregu.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Burns LH, Robbins TW, Everitt BJ. Differential effects of excitotoxic lesions of the basolateral amygdala, ventral subiculum and medial prefrontal cortex on responding with conditioned reinforcement and locomotor activity potentiated by intra-accumbens infusions of D-amphetamine. Behav Brain Res. 1993;55:167–183. doi: 10.1016/0166-4328(93)90113-5. [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus reward associations - interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hu C, HSU, Zhang Q, BI C, Asnicar M, Hansen M. Targeted disruption of the melanin-concentrating hormone receptor-1 results in hyperphagia and resistance to diet-induced obesity. Endocrinology. 2002;143(7):2469–2477. doi: 10.1210/endo.143.7.8903. [DOI] [PubMed] [Google Scholar]
- Chung SJ, Saito Y, Civelli O. MCH receptors/gene structure-in vivo expression. Peptides. 2009a;30:1985–1989. doi: 10.1016/j.peptides.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SJ, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, Bonci A, Civelli O. The melanin-concentrating hormone system modulates cocaine reward. Proc Natl Acad Sci USA. 2009b;106:6772–6777. doi: 10.1073/pnas.0811331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Johnson AW, Zimmer AM, Zimmer A, Holland PC. Deficits in sensory-specific devaluation task performance following genetic deletions of cannabinoid (CB1) receptor. Learn Mem. 2010;17:18–22. doi: 10.1101/lm.1610510. [DOI] [PubMed] [Google Scholar]
- Della-Zuana O, Presse F, Ortola C, Duhault J, Nahon JL, Levens N. Acute and chronic administration of melanin-concentrating hormone enhances food intake and body weight in Wistar and Sprague-Dawley rats. Int J Obes Relat Metab Disord. 2002;26:1289–1295. doi: 10.1038/sj.ijo.0802079. [DOI] [PubMed] [Google Scholar]
- Duncan E, Rider T, Jandacek R, Clegg DJ, Benoit SC, Tso P, Woods SC. The regulation of alcohol intake by melanin-concentrating hormone in rats. Pharma Biochem Behav. 2006;85:728–735. doi: 10.1016/j.pbb.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus Accumbens to modulate feeding Behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomori A, Ishihara A, Ito M, Mashiko S, Matsushita H, Yumoto M, Ito M, Tanaka T, Tokita S, Moriya M, Iwaasa H, Kanatani A. Chronic intracerebroventricular infusion of MCH causes obesity in mice. Am J Physiol Endocrinol Metab. 2003;284:E583–E588. doi: 10.1152/ajpendo.00350.2002. [DOI] [PubMed] [Google Scholar]
- Guyon A, Conductier G, Rovere C, Enfissi A, Nahon JL. Melanin-concentrating hormone producing neurons: Activities and modulations. Peptides. 2009;30:2031–2039. doi: 10.1016/j.peptides.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Hervieu G, Cluderay J, Harrison D, Meakin J, Maycox P, Nasir S, Leslie R. The distribution of the mRNA and protein products of the melanin-concentrating hormone (MCH) receptor gene, slc-1, in the central nervous system of the rat. European J Neurosci. 2000;12:1194–1216. doi: 10.1046/j.1460-9568.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur J Neurosci. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiol Behav. 2005;86:747–761. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Canter R, Gallagher M, Holland PC. Assessing the role of the growth hormone secretagogue receptor in motivational learning and food intake. Behav Neurosci. 2009;123:1058–1065. doi: 10.1037/a0016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, Crombag HS, Smith DR, Ramanan N. Effects of serum response factor (SRF) deletion on conditioned reinforcement. Behav Brain Res. 2011;220:312–318. doi: 10.1016/j.bbr.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Lex A, Hauber W. Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer. Learn & Mem. 2008;15:483–491. doi: 10.1101/lm.978708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest. 2001;107:379–386. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackintosh N. The psychology of animal learning. London: Academic; 1974. [Google Scholar]
- Marsh DJ, Weingarth DT, Novi DE, Chen HY, Trumbauer ME, Chen AS, Guan XM, Jiang MM, Feng Y, Camacho RE, Shen Z, Frazier EG, Yu H, Metzger JM, Kuca SJ, Shearman LP, Gopal-Truter S, MacNeil DJ, Strack AM, MacIntyre DE, Van der Ploeg LHT, Qian S. Melanin-concentrating hormone 1 receptor-deficient mice are lean, hyperactive, and hyperphagic and have altered metabolism. Proc Natl Acad Sci U S A. 2002;99:3240–3245. doi: 10.1073/pnas.052706899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashiko S, Ishihara A, Gomori A, Moriya R, Ito M, Iwaasa H, Matsuda M, Feng Y, Shen Z, Marsh DJ, Bednarek MA, MacNeil DJ, Kanatani A. Antiobesity effect of a melanin-concentrating hormone 1 receptor antagonist in diet-induced obese mice. Endocrinology. 2005;146:3080–3086. doi: 10.1210/en.2004-1150. [DOI] [PubMed] [Google Scholar]
- Mul JD, la Fleur SE, Toonen PW, Afrasiab-Middelman A, Binnekade R, Schetters D, Verheij MMM. Chronic loss of melanin-concentrating hormone sffects motivational aspects of feeding in the rat. PloS one. 2011;6(5):1–13. doi: 10.1371/journal.pone.0019600. e19600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears A, Parkinson J, Hopewell L. Lesions of the orbitofrontal but not medial prefrontal cortex disrupt conditioned reinforcement in primates. J Neurosci. 2003;23(35):11189–11201. doi: 10.1523/JNEUROSCI.23-35-11189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presse F, Sorokovsky I, Max JP, Nicolaidis S, Nahon JL. Melanin-concentrating hormone is a potent anorectic peptide regulated by food-deprivation and glucopenia in the rat. Neuroscience. 1996;71:735–745. doi: 10.1016/0306-4522(95)00481-5. [DOI] [PubMed] [Google Scholar]
- Qu DQ, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek J, Kanarek R, MaratosFlier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Solomon RL. 2-Process Learning Theory - Relationships between pavlovian conditioning and instrumental learning. Psych Rev. 1967;74(3):151. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Sears RM, Liu R-J, Narayanan NS, Sharf R, Yeckel MF, Laubach M, Aghajanian GK, DiLeone RJ. Regulation of Nucleus Accumbens Activity by the Hypothalamic Neuropeptide Melanin-Concentrating Hormone. J Neurosci. 2010;30(24):8263–8273. doi: 10.1523/JNEUROSCI.5858-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- Tan CP, Sano H, Iwaasa H, Pan J, Sailer AW, Hreniuk DL, Feighner SD, Palyha OC, Pong SS, Figueroa DJ, Austin CP, Jiang MM, Yu H, Ito J, Ito M, Ito M, Guan XM, MacNeil DJ, Kanatani A, Van der Ploeg LH, Howard AD. Melanin-concentrating hormone receptor subtypes 1 and 2: Species-specific gene expression. Genomics. 2002;79(6):785–792. doi: 10.1006/geno.2002.6771. [DOI] [PubMed] [Google Scholar]
- Presse F, Sorokovsky I, Max J, Nicolaidis S, Nahon JL. Melanin-concentrating hormone is a potent anorectic peptide regulated by food-deprivation and glucopenia in the rat. Neuroscience. 1996;71(3):735–745. doi: 10.1016/0306-4522(95)00481-5. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology. 1986;90:390–397. doi: 10.1007/BF00179197. [DOI] [PubMed] [Google Scholar]