Abstract
Importance
Patent Ductus Arteriosus (PDA) is associated with increased mortality and worsened respiratory outcomes including bronchopulmonary dysplasia (BPD) in preterm infants. Nonsteroidal Anti-inflammatory Drugs (NSAIDs) are efficacious in closing PDA, but the effectiveness of NSAID-mediated PDA closure in improving mortality and preventing BPD is unclear.
Objective
To determine the effectiveness of NSAID treatment for PDA in reducing mortality and moderate/severe BPD at 36-weeks postmenstrual age.
Design
Within a retrospective cohort of infants discharged between January 2006–December 2013, we performed an instrumental variable analysis that incorporated provider preference-based, institutional variation in NSAID treatment frequency to determine the effect of NSAID treatment for PDA on mortality and BPD.
Setting
Neonatal Intensive Care Units (NICUs) within 25 United States’ Children’s Hospitals included in the Pediatric Health Information System.
Participants
12,018 infants born at ≤28-weeks-gestation who were admitted to the NICU on their birth date and hospitalized at least 3 days.
Exposure
Proportion of NSAID treated infants born at each infant’s institution within ±6-months of that infant’s birth.
Main Outcome and Measure
The primary composite outcome was death, moderate, or severe BPD at 36-weeks’ postmenstrual age.
Results
The instrument, the proportion of NSAID treated infants at each unique infant’s hospital within ±6-months of that infant’s birth, was significantly associated with NSAID treatment and not significantly associated with gestation, race, or gender. An individual infant’s chances of receiving NSAID treatment increased by 0.84 percentage points (95% CI: 0.8–0.9; p<0.001) for every 1 percentage point increase in the annual NSAID treatment percentage at a given hospital. Instrumental variable analysis demonstrated no significant association between NSAID treatment and the odds of mortality or BPD (OR 0.94 [95% CI: 0.70, 1.25]), mortality (0.73 [0.43, 1.13]), or BPD in survivors (1.01 [0.73, 1.45]).
Conclusions and Relevance
When we incorporated provider preference-based practice variation as an instrument to minimize the effect of unmeasured confounding, we detected no changes in the odds of mortality or moderate/severe BPD among similar ≤28-week gestation preterm infants following NSAID treatment for PDA initiated 2–28 days postnatally. Our findings are in agreement with available randomized trial evidence and support a conservative approach to PDA management.
INTRODUCTION
Patent Ductus Arteriosus (PDA) is highly prevalent in extremely preterm infants1 and associated with increased mortality and worsened respiratory outcomes including bronchopulmonary dysplasia (BPD).2 Nonsteroidal Anti-inflammatory Drugs (NSAIDs) are efficacious in closing PDA, but the effectiveness of NSAID-mediated PDA closure in improving mortality, long-term respiratory outcomes, or neurodevelopment has never been clearly demonstrated.3–6 NSAID treatment of PDA remains controversial1,7
Previous randomized clinical trials (RCTs) to examine NSAID treatment of PDA did not find a benefit of NSAID treatment on mortality, respiratory, or neurodevelopmental outcomes, but were principally powered to evaluate efficacy of ductal closure rather than longer-term outcomes including mortality and BPD.8 Several well-designed observational studies that controlled for measured confounders also did not detect an improvement in long-term outcomes following NSAID treatment for PDA.9,10
Unlike RCTs, observational investigations are prone to confounding by indication due to the inability to adjust for important, unmeasured variables. The incorporation of naturally occurring treatment variation between healthcare providers into observational data analyses has been proposed as an instrument to minimize the impact of unmeasured confounding11–13 based on the assumption that, for treatments with limited evidence, practice variation is more often due to provider and institutional preferences than differences in patient characteristics between providers and institutions. In essence, the instrumental variable (IV) analysis utilizes the premise that if a treatment is effective there should, on-average, be improvement in outcomes for similar patients as their providers’ tendency to treat increases. We previously evaluated our assumption that provider preference is a major driver of practice variation in the specific setting of preterm PDA management by electronically surveying providers at institutions participating in the Pediatric Health Information System (PHIS) database of US children’s hospitals. We documented profound differences in opinion regarding indications to treat PDA with NSAIDs.14
The objective of this investigation was to determine the effectiveness of NSAID treatment for PDA in reducing mortality and moderate/severe BPD at 36-weeks postmenstrual age. Given limited RCT evidence, the clinical importance of understanding the effectiveness of NSAID treatment to close PDA, and the current controversy surrounding the topic, we conducted an IV analysis,11–13 in which we took advantage of practice variation due to physician preference as a means to control unmeasured confounding.
METHODS
Data Source
We derived the study cohort from neonates included in the PHIS database (Children’s Hospital Association; Shawnee Mission, KS) with discharge dates between January 1, 2006-December 31, 2013 from participating US children’s hospitals. The Nationwide Children’s Hospital Institutional Review Board approved the study.
Study Cohort
Our cohort was restricted to ≤28-weeks gestation infants. To prevent referral bias due to older infants referred from other hospitals for PDA management or management of complications associated with mortality or BPD, we only included infants who were admitted on their birth date. Postnatal day 2 was considered the first potential day of NSAID treatment for PDA, since prophylactic indomethacin treatment is normally initiated on days 0–1. Thus, we excluded those infants hospitalized for <3 days to ensure all infants survived to be eligible for treatment on day 2 (Supplement eFigure 1). We excluded those without a recorded discharge status (home, transfer, death), which was needed to calculate mortality and BPD outcomes. Since our IV analysis was dependent upon an accurate determination of the annual proportion of NSAID administration within a given hospital, we only included neonatal intensive care units (NICUs) with an average of at least 21 infants meeting inclusion criteria/year (≥150 infants over the 7-year study) in our primary analysis.
Since physician diagnosis of PDA is highly variable,14,15 we did not require infants in our primary analysis to have an ICD-9 PDA diagnosis. Physicians who, based on personal preference, are less likely to treat PDA with NSAIDs may also be less likely to evaluate for and diagnose PDAs. A physician’s degree of early surveillance affects PDA diagnosis, which is time dependent since all infants have a patent ductus at birth but at 25–28 weeks gestation, 35% close within one week.1 Most physicians at the NICUs included within PHIS use echocardiograms to inform their PDA treatment decisions, but some base treatment on the presence of a murmur, a less-sensitive method.14 Regardless of actual PDA presence, we assume that treated infants were more likely to receive a PDA diagnosis. Therefore, restricting our analysis solely to infants formally diagnosed with PDA may introduce bias. Since our IV analysis is based upon physician preference-based variation in the annual proportion of NSAID administration per hospital, inclusion of infants without a PDA diagnosis should not bias our IV-based results. We evaluated this assumption in a sensitivity analysis.
Variables
In addition to demographic data, PHIS contains a record of medication administration, procedures, and respiratory treatments for each day of an infant’s hospitalization as determined from billing records. Thompson-Reuters Healthcare (Ann Arbor, MI), the PHIS data processing partner, maps each hospital’s daily charge codes to a common classification system, the Clinical Transaction Classification (CTC) codes to ensure comparability of charge-level data between institutions. Supplement eTable 1 shows the CTC codes we evaluated.
Gestational Age
Gestational age (GA) at delivery was defined by using a multistep process. GA was classified into groups: ≤24-weeks, 25–26 weeks, and 27–28 weeks, based on International Classification of Diseases, Ninth Revision, (ICD-9) codes (Supplement eTable 1). If the ICD-9 for GA was missing, we relied on GA as recorded in the patient’s demographic file. Since GA determination (≤28-weeks) was necessary for inclusion, we excluded infants when both forms of GA records were missing or when there was any discordance between ICD-9 and demographic information (n=6).
Definition of NSAID Treatment for PDA
An infant was considered to have been treated with NSAIDs for PDA when the first indomethacin or ibuprofen dose was given between 2–28 calendar days inclusive postnatally (birth date=day 0) or if the infant received NSAID (indomethacin and/or ibuprofen) on ≥4 total days within the first 28 postnatal days. This allowed us to separate PDA treatment doses from prophylactic indomethacin dosing to prevent intraventricular hemorrhage (IVH), which is typically started on the date of birth (started on day 0 or day 1 if a late night admission) and given daily for three consecutive days. PDA treatment with NSAIDs for infants that did not receive indomethacin prophylaxis presumably would begin on or after postnatal day 2. Any infant that did receive indomethacin prophylaxis would presumably receive more than 3 total NSAID doses if subsequently treated for PDA. Therefore, infants that received prophylactic indomethacin were still eligible for this study. We did not consider NSAID doses beyond 28 postnatal days since older infants might be treated with ibuprofen for pain control and most RCTs investigating NSAID treatment of PDA have focused on treatment within the first postnatal month of age.1,3,4,16 We evaluated treatment with either indomethacin or ibuprofen since both have similar efficacy in closing PDA.4,17
Definition of BPD Outcome
We used each infant’s daily respiratory support modality record to determine BPD outcomes at 36-weeks postmenstrual age according to the National Institutes of Health consensus definition,18 as recently clarified by Poindexter and Jobe.19 We merged high frequency and conventional ventilation codes to create a binary, composite code designating daily administration of any invasive mechanical ventilation. We likewise created a composite code to designate daily administration of any non-invasive positive pressure ventilation modality inclusive of: continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), intermittent positive pressure breathing (IPPB), or noninvasive positive pressure ventilatory support (NPPV).
To be diagnosed with moderate/severe BPD, an infant had to receive respiratory support via invasive mechanical ventilation, non-invasive ventilation, or supplemental oxygen for 28 total, but not necessarily consecutive days19 and remain on invasive or non-invasive ventilation (severe BPD), or supplemental oxygen (moderate BPD), at 36-weeks postmenstrual age. For those infants transferred out prior to 36-weeks postmenstrual age, we adapted a method of imputing BPD at transfer from the method the NICHD neonatal research network used to create their validated BPD prediction tool.20 We imputed a diagnosis of severe BPD if the infant was receiving mechanical or positive pressure ventilation at transfer (n=129) and a diagnosis of moderate BPD (n=316) if the infant was receiving oxygen.
Statistical Analysis
Our primary composite outcome was death or moderate/severe BPD at 36-weeks postmenstrual age. First, we estimated the effect of NSAID treatment on mortality/BPD using a generalized estimating equation (GEE) to adjust for gestational age, with heteroskedasticity-robust standard errors to account for clustering by institution.
Then, we incorporated periods of hospital-specific NSAID treatment for PDA surrounding each infant’s birth as an instrumental variable (IV) to obtain an unbiased (exogenous) treatment effect11–13 under the assumption that each preterm infant born in a period of higher preference-based NSAID treatment for PDA has a higher likelihood of treatment than infants born during periods of lower treatment. The IV estimates apply to those infants that would be treated with NSAIDs based on their institution’s inclination to treat PDA with NSAIDs.
For every unique infant in the cohort, we created an IV varying between 0–1, by incorporating the proportion of infants who were born at that infant’s institution within ±6-months of their birth (excluding that unique infant) and treated for PDA with an NSAID per our definition. We used a 2-stage least squares (2SLS) regression model, in which the first-stage predicted treatment based on the value of the IV and the second-stage subsequently incorporated the probability of treatment as determined in the first-stage. These models included heteroskedasticity-robust standard errors and adjusted for birth gestation. Since the standard output of 2SLS models are risk differences, we calculated marginal odds ratios for comparison with the non-instrumented GEE output, then calculated 95% confidence intervals via bias-corrected bootstrapping.21
A valid instrument should strongly affect or be associated with treatment by sharing a common cause and should have no direct or indirect effect on the outcome except through its association with the treatment.13 If an instrument does not strongly predict receipt of treatment it is considered weak and can increase bias.13 We tested our instrument’s strength by evaluating its effect on measured covariate balance, assessing its association with actual NSAID treatment, and conducting an F-Test to test whether its inclusion significantly contributed to the first-stage of the IV model.13
To evaluate the sensitivity of our results, we ran additional IV models including sex, race/ethnicity, and a binary variable indicating prophylactic indomethacin treatment on the date of birth. We also reran all analyses after restricting the cohort only to infants with an ICD-9 PDA diagnosis. We conducted a time-varying Cox regression analysis22–24 to evaluate the impact of immortal time bias on our non-instrumented GEE analysis, then tested the effects of varying hospital sample-size cutoffs on our instrumented estimates. Finally, we reran our analysis with an instrument that incorporated hospital-level NSAID treatment frequency in the 12-months prior to each infant’s birth rather than a ±6-month interval. All analyses were conducted using Stata 14.1 (College Station, Texas).
RESULTS
The cohort included 12,018 infants within 25 hospitals (eFigure 1). Table 1 shows the covariate balance among treated and untreated infants. Overall, 32% of infants were treated with NSAID for PDA, with 27% receiving indomethacin and 7% ibuprofen.
Table 1.
Balance of Covariates Across Treatment Groups
| Cohort N=12018 | Treatment Frequency | |
|---|---|---|
| Treated with NSAIDs | Untreated | |
|
n=3888 (32%) |
n=8130 (68%) |
|
| n (%) | n (%) | |
| Gestational Age | ||
| 27–28 weeks (n=5405; 45%) |
1325 (34%) | 4080 (50%) |
| 25–26 weeks (n=4217; 35%) | 1548 (40%) | 2669 (33%) |
| 24-weeks (n=1607; 13%) |
698 (18%) | 909 (11%) |
| <24-weeks (n=789; 7%) |
317 (8%) | 472 (6%) |
| Gender | ||
| Male (n=6370; 53%) |
2068 (53%) | 4302 (53%) |
| Female (n=5648; 47%) |
1820 (47%) | 3828 (47%) |
| Ethnicity | ||
| White (n=4995; 42%) |
1572 (40%) | 3423 (42%) |
| African American (n=3176; 26%) |
947 (24%) | 2229 (27%) |
| Hispanic (n=1823; 15%) |
671 (17%) | 1152 (14%) |
| Other (n=1555; 13%) |
558 (14%) | 997 (12%) |
| Missing (n=469; 4%) |
140 (4%) | 329 (4%) |
Variation in NSAID treatment for PDA
NSAID treatment varied by both hospital (median use: 34.0%; 25th%–75th%: 21.5%–40.5%; range: 16.1%-54.7%) and year (Figure 1). The overall, annual percentage of infants treated with NSAID dropped from 2005 (40.6%) to 2013 (24.1%). Temporary reductions in indomethacin and ibuprofen use corresponded to reported indomethacin shortages in late 200925 and 2011–201325,26 and an ibuprofen recall from July 2010-November 201227 (Figure 1).28
Figure 1. Variation in NSAID Use Over Time.
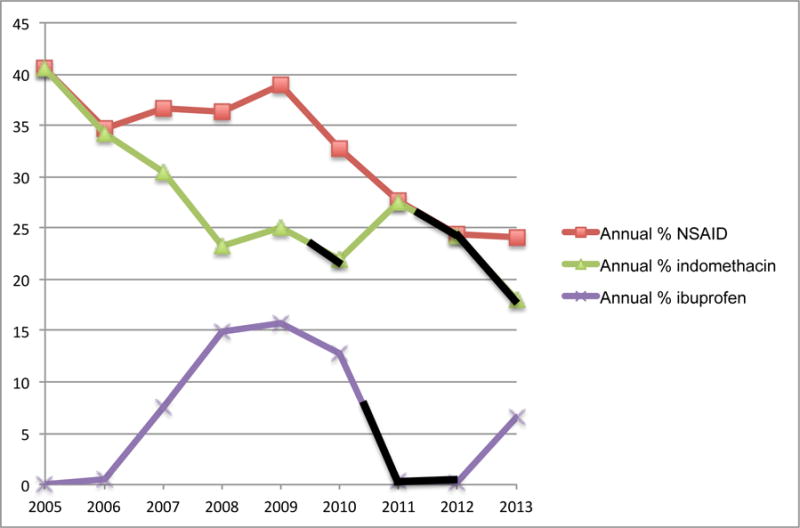
Annual percent of infants treated with NSAID for PDA. Black lines indicate periods of reported drug shortages. Ibuprofen use first increases in 2006 following United States’ Food and Drug Administration approval for PDA treatment.28
Multivariable Regression Estimates
Unadjusted bivariate analyses are shown in supplement eTable 2. Supplement eTable 3 shows the unadjusted relationship of GA, sex, and race/ethnicity with NSAID treatment status. We excluded race/ethnicity and sex from our final models because they were not confounders, as they were unassociated with receipt of NSAID treatment. However these variables were included in sensitivity analyses (below).
The non-instrumented GEE models, controlling only for gestation and hospital-effect, showed a significant association between NSAID treatment and increased mortality/BPD (composite outcome) and between NSAID treatment and increased moderate/severe BPD among survivors. NSAID treatment was associated with decreased mortality (Table 2) (Model Output: Supplement eTable 4).
Table 2.
Mortality and Bronchopulmonary Dysplasia According to NSAID Treatment of PDA
| GEE | Instrumental Variable | ||
|---|---|---|---|
| Odds Ratio (95% CI) |
Odds Ratio (95% CI)c |
Risk Difference (95% CI) |
|
| Mortality or Moderate/Severe BPDa | 1.31 (1.15, 1.49) p<0.001 |
0.94 (0.70, 1.25) | −0.01 (−0.09, 0.06) p= 0.69 |
| Mortality | 0.57 (0.49, 0.67) p<0.001 |
0.73 (0.43, 1.13) | −0.03 (−0.08, 0.01) p=0.18 |
| Moderate/Severe BPDa among survivorsb | 1.52 (1.35, 1.72) p<0.001 |
1.01 (0.73, 1.45) | 0.003 (−0.07, 0.08) p=0.94 |
All models are adjusted for gestational age. Odds Ratios are marginal (population-averaged).
Generalized Estimating Equation (GEE) model incorporating: Gaussian family, Logit link, and Exchangeable correlation structure with heteroskedasticity- robust standard errors (Huber-White sandwich estimator) to generate population-averaged (marginal) odds ratios
Two-Stage Least Squares (2SLS) Instrumental Variable Model with heteroskedasticity-robust standard errors (Huber-White sandwich estimator).
Bias-corrected Bootstrap 95% Confidence Intervals constructed using 2000 replications with replacement
Denominator for BPD among survivors= 10065 survivors
Note that BPD imputed among transfers [Imputation for infants transferred prior to 36-weeks corrected gestation (n=945 infants; 8.3%)]
Strength of the Instrument
Testing demonstrated that our instrument was strongly related to actual receipt of NSAID treatment. The first stage of our IV model showed that for every 1 percentage point increase in our instrument, the percentage of infant’s treated with NSAIDs at a given hospital within ±6-months of each infant’s birth, an individual infant’s chances of receiving NSAID treatment increased by 0.84 percentage points (95% CI: 0.8–0.9; p<0.001). The F-test13 (1,12013)= 932 indicated our instrument contributed significantly to the IV model (F-test >10 desirable). There were no significant associations between the instrument and gestation, sex, or ethnicity (Supplement eTable 5) suggesting that NICUs administering NSAIDs more frequently did not see sicker patients. Table 3 shows the relationship between the percentile of infants within given GA categories and outcome measures, and increasing quartiles of the instrument. A standardized difference of > ±0.1 indicates significant correlation.29 As expected, the percentile of infants receiving NSAIDs is significantly and directly correlated with increasing instrument values. However, GA distribution and outcomes are uncorrelated.
Table 3.
Balance of Gestational Age and Outcomes Across Treatment Groups and Levels of the Instrument
| Instrument Quartilesa: Percent NSAID Treatment for PDA at Each Unique Infant’s Hospital Within ±6-Months of Birth | Standardized Difference (Between Two Lowest and Two Highest Quartiles) | ||||
|---|---|---|---|---|---|
| Covariates |
0–22.6% (n=2996) |
>22.6–33.3% (n=2943) |
>33.3–41.3% (n=3072) |
>41.3–92.3% (n=3007) |
|
| Received NSAID Treatment | 518 (17.3%) | 821 (27.9%) | 1147 (37.3%) | 1402 (46.6%) | 0.424 |
| Gestational Age | |||||
| 27–28 weeks | 1307 (43.6%) | 1331 (45.2%) | 1392 (45.3%) | 1375 (45.7%) | 0.022 |
| 25–26 weeks | 1061 (35.4%) | 1058 (36.0%) | 1070 (34.8%) | 1028 (34.2%) | −0.024 |
| 24-weeks | 421 (14.1%) | 379 (12.9%) | 390 (12.7%) | 417 (13.9%) | −0.006 |
| <24-weeks | 207 (6.9%) | 175 (6.0%) | 220 (7.2%) | 187 (6.2%) | 0.011 |
| Outcomes | |||||
| Mortality or Moderate/Severe BPD | 1471 (49.1%) | 1265 (43.0%) | 1367 (44.5%) | 1408 (46.8%) | −0.008 |
| Mortality | 374 (12.5%) | 351 (11.9%) | 388 (12.6%) | 355 (11.8%) | 0.000 |
| Moderate/Severe BPDb | 1097 (41.8%) | 914 (35.3%) | 979 (36.5%) | 1053 (39.7%) | −0.010 |
Percentiles were derived using column n as the denominator.
median 33.3%; 25th–75th: 22.6%, 41.3%; range 0–92.3%
Denominator for percent with Moderate/Severe BPD is based on the Quartile n of survivors until discharge. Quartile 1 (0–22.6%)=2622; Quartile 2 (>22.6–33.3%)=2592; Quartile 3 (>33.3–41.3%)=2684; Quartile 4 (>41.3–92.3%)=2652
Instrumental Variable Results
2SLS IV models incorporating the annual proportion of NSAID treatment for PDA at each infant’s hospital demonstrated no significant difference in odds between NSAID treatment and mortality or BPD, between NSAID treatment and mortality, or between NSAID treatment and BPD in survivors (Table 2) (Model Output: Supplement eTable 4).
Sensitivity Analyses
Outcome estimates remained similar, detecting no effect of NSAID treatment on mortality/BPD outcomes, when we adjusted for race/ethnicity, sex, and prophylactic indomethacin treatment, and also when we restricted the cohort to only those infants with an ICD-9 PDA diagnosis. An instrument that incorporated hospital-level NSAID treatment frequency in the 12-months preceding an infant’s birth provided similar results as an instrument based on a ±6-month interval from birth (Supplemental eTable 6). Our IV results were also robust to inclusion of hospitals with smaller sample-sizes than permitted in our primary analysis (Supplemental eTable 7). Alternate IV modeling specifications (Bivariate probit IV and generalized structural equation models)13,30,31 produced results similar to the 2SLS models (Supplement eTable 8). Time-varying Cox regression models demonstrated that a high early mortality rate (immortal time bias) strongly influenced our non-instrumented GEE estimates (Supplement eTable 9).
DISCUSSION
We found no significant effect on mortality or BPD at 36-weeks after controlling for unmeasured confounders using IV analysis. This result aligns with meta-analyses constructed from the secondary outcomes of randomized trials and well-designed observational investigations.3–5,9,10,32,33
Our instrument was both a strong predictor of NSAID treatment and unassociated with our measured risk factors (gestation, sex, ethnicity) for mortality and BPD. No statistical test can completely verify the assumption that an instrument has no effect on the outcome apart from its association with the treatment.11–13 Therefore, prior to this study, we conducted an investigation to evaluate our assumption that caregiver preferences are the main contributors to variation in NSAID treatment for PDA. We surveyed NICU leaders at all hospitals included within our analysis and found wide variation in hospital-specific reasons for closing/not-closing PDA with NSAIDs. In addition to wide between-hospital variation, respondents noted variation between physicians in preferences for NSAID treatment of PDA within 70% (n=32) of hospitals.14
Immortal Time Bias and Confounding by Indication due to Illness Severity
Our non-instrumented and presumably biased GEE estimates differ considerably from our IV results. Although highly implausible based on existing literature our GEE estimates (40% mortality odds reduction, 50% BPD odds increase) are remarkably similar to multivariable estimates from another large database cohort with limited markers for early severity of illness.34 We attribute the discrepancy between IV and non-instrumented analyses to confounding by indication due to severity of illness35 and immortal time bias, a period of follow-up when mortality cannot occur in treated subjects who must by default survive until treatment.23 PHIS lacks acuity indexes such as neonatal acute physiology (SNAP) scores, which might enable statistical adjustment for early severity of illness. Severely ill infants are less likely to be treated with NSAIDs due to renal and hematological concerns.36 Immortal time bias, present in our cohort (Supplement eTable 9, eFigure 2) and common in pharmacoepidemiological investigations,23 leads to an erroneous, implausibly high reduction in mortality following treatment.24 A unique feature of IV analysis is that it robust to both confounding by indication and immortal time bias.
Strengths and Limitations
Our investigation benefits from a large representative dataset with a generous sample size (N=12,018) and the use of advanced methods to minimize unmeasured confounding. One limitation is that we must assume our provider preference-based instrument is unrelated to mortality/BPD risk except through its impact on whether an infant receives NSAID treatment, and that institutions with increased NSAID treatment of PDA are not also increased or decreased users of another treatment that independently influences mortality and/or BPD risk.21 IVs should produce unbiased estimates, but that validity comes with reduced precision and larger confidence intervals relative to non-instrumented regression analyses. However, our IV point estimates and 95% CI widths are similar in magnitude to those of RCT meta-analyses and other large observational studies that also showed no effect of NSAID treatment for PDA on mortality or moderate/severe BPD.3,4,9
Instead of focusing on NSAID treatment for PDA at only a single point in time, our observational design allowed us to pragmatically assess real-world treatment patterns between 2–28 days postnatal age. As in a RCT, our estimates apply to the population-average of all infants studied. Although NSAID treatment of all infants with PDA does not appear beneficial, our findings do not imply that some infants with certain risk factors may not benefit from PDA closure. Likewise, subgroups may exist for whom avoiding NSAIDs is beneficial.
Conclusions
When we incorporated provider preference-based practice variation as an instrument to minimize the effect of unmeasured confounding, we detected no changes in the odds of mortality or moderate/severe BPD among ≤28-week gestation newborns following NSAID treatment for PDA initiated between 2–28 days postnatal age. Our result is in agreement with available RCT evidence and previous well-designed observational investigations that similarly detected no effect.
Supplementary Material
Acknowledgments
Jonathan Slaughter had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding/Support: This investigation was supported by grant 5K08HL121182 from the National Heart Lung and Blood Institute at the National Institutes of Health (principal investigator: Slaughter). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- CHA
Children’s Hospital Association
- CI
confidence interval
- CPAP
continuous positive airway pressure
- GEE
Generalized Estimating Equation
- ICD-9
International Classification of Diseases, Ninth Revision
- IV
instrumental variable
- IVH
intraventricular hemorrhage
- NEC
necrotizing enterocolitis
- NICU
neonatal intensive care unit
- NSAID
nonsteroidal anti-inflammatory drug
- PDA
patent ductus arteriosus
- PHIS
Pediatric Health Information System
Footnotes
The authors (Jonathan Slaughter, Patricia Reagan, Thomas Newman, Mark Klebanoff) all report no conflicts of interest (financial interests, activities, relationships, affiliations) in regards to this manuscript.
Dr. Slaughter conceptualized and designed the study, carried out the initial analysis and interpreted the data, drafted the initial manuscript draft, and approved the final manuscript as submitted.
Dr. Reagan contributed to study conceptualization and design and the initial data analysis, interpreted the data, and revised the manuscript for important intellectual content, and approved the final manuscript as submitted.
Dr. Newman contributed to the study design, analyzed and interpreted the data, assisted in the initial manuscript draft, revised the manuscript for important intellectual content, and approved the final manuscript as submitted.
Dr. Klebanoff contributed to study conceptualization and design, analyzed and interpreted the data, revised the manuscript for important intellectual content, and approved the final manuscript as submitted.
References
- 1.Clyman RI, Couto J, Murphy GM. Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. 2012;36(2):123–129. doi: 10.1053/j.semperi.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noori S, McCoy M, Friedlich P, et al. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics. 2009;123(1):e138–e144. doi: 10.1542/peds.2008-2418. [DOI] [PubMed] [Google Scholar]
- 3.Cooke L, Steer PA, Woodgate PG. Indomethacin for asymptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev. 2003;(2):CD003745. doi: 10.1002/14651858.CD003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohlsson A, Walia R, Shah SS. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst Rev. 2015;(2):CD003481. doi: 10.1002/14651858.CD003481.pub6. [DOI] [PubMed] [Google Scholar]
- 5.Benitz WE, Committee on Fetus and Newborn American Academy of Pediatrics Patent Ductus Arteriosus in Preterm Infants. Pediatrics. 2016;137(1):e20153730. doi: 10.1542/peds.2015-3730. [DOI] [PubMed] [Google Scholar]
- 6.Bose CL, Laughon MM. Patent ductus arteriosus: lack of evidence for common treatments. Arch Dis Child Fetal Neonatal Ed. 2007;92(6):F498–502. doi: 10.1136/adc.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benitz WE. Patent ductus arteriosus: to treat or not to treat? Arch Dis Child Fetal Neonatal Ed. 2012;97(2):F80–82. doi: 10.1136/archdischild-2011-300381. [DOI] [PubMed] [Google Scholar]
- 8.Clyman RI, Chorne N. Patent ductus arteriosus: evidence for and against treatment. J Pediatr. 2007;150(3):216–219. doi: 10.1016/j.jpeds.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madan JC, Kendrick D, Hagadorn JI, Frantz ID. Patent ductus arteriosus therapy: impact on neonatal and 18-month outcome. Pediatrics. 2009;123(2):674–681. doi: 10.1542/peds.2007-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirea L, Sankaran K, Seshia M, et al. Treatment of patent ductus arteriosus and neonatal mortality/morbidities: adjustment for treatment selection bias. J Pediatr. 2012;161(4):689–694 e1. doi: 10.1016/j.jpeds.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Rassen JA, Brookhart MA, Glynn RJ, Mittleman MA, Schneeweiss S. Instrumental variables II: instrumental variable application—in 25 variations, the physician prescribing preference generally was strong and reduced covariate imbalance. J Clin Epidemiol. 2009;62(12):1233–1241. doi: 10.1016/j.jclinepi.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rassen JA, Brookhart MA, Glynn RJ, Mittleman MA, Schneeweiss S. Instrumental variables I: instrumental variables exploit natural variation in nonexperimental data to estimate causal relationships. J Clin Epidemiol. 2009;62(12):1226–1232. doi: 10.1016/j.jclinepi.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brookhart MA, Rassen JA, Schneeweiss S. Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010;19(6):537–554. doi: 10.1002/pds.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slaughter JL, Reagan PB, Bapat RV, Newman TB, Klebanoff MA. Nonsteroidal anti-inflammatory administration and patent ductus arteriosus ligation, a survey of practice preferences at US children’s hospitals. Eur J Pediatr. 2016;175(6):775–783. doi: 10.1007/s00431-016-2705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans N. Diagnosis of the preterm patent ductus arteriosus: clinical signs, biomarkers, or ultrasound? Semin Perinatol. 2012;36(2):114–122. doi: 10.1053/j.semperi.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 16.Lainwala S, Hussain N. Treatment of Patent Ductus Arteriosus with Cyclo-oxygenase Inhibitors beyond 2 Weeks of Age in Very Low Birth Weight Infants. Am J Perinatol. 2016;33(6):584–589. doi: 10.1055/s-0035-1570340. [DOI] [PubMed] [Google Scholar]
- 17.Gulack BC, Laughon MM, Clark RH, Sankar MN, Hornik CP, Smith PB. Comparative effectiveness and safety of indomethacin versus ibuprofen for the treatment of patent ductus arteriosus. Early Hum Dev. 2015;91(12):725–729. doi: 10.1016/j.earlhumdev.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 19.Poindexter BB, Jobe AH. The Diagnostic Conundrum of Bronchopulmonary Dysplasia. J Pediatr. 2015;167(3):517–518. doi: 10.1016/j.jpeds.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Laughon MM, Langer JC, Bose CL, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183(12):1715–1722. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman TB, Vittinghoff E, McCulloch CE. Efficacy of phototherapy for newborns with hyperbilirubinemia: a cautionary example of an instrumental variable analysis. Med Decis Making. 2012;32(1):83–92. doi: 10.1177/0272989X11416512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosmer DW, Jr, Lemeshow S, May S. Applied Survival Analysis: Regression Modeling of Time to Event Data. 2nd. Hoboken, NJ: John Wiley and Sons, Inc; 2008. [Google Scholar]
- 23.Suissa S. Immortal time bias in pharmacoepidemiology. Am J Epidemiol. 2008;167(4):492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 24.Suissa S. Immortal time bias in observational studies of drug effects. Pharmacoepidemiol Drug Saf. 2007;16(3):241–249. doi: 10.1002/pds.1357. [DOI] [PubMed] [Google Scholar]
- 25.Thompson CA, American Society of Health-System Pharmacists Health News: Bedford Recalls Indomethacin Injection Lot 1948138 website. http://www.ashp.org/menu/News/PharmacyNews/NewsArticle.aspx?id=3560-sthash.MrpAWnGH.dpufPharmacy. Accessed August 21, 2016.
- 26.Chandramouli J, American Society of Health-System Pharmacists Current Shortages Bulletin: Indomethacin Injection [22 Oct 2014] website. http://www.ashp.org/menu/DrugShortages/CurrentShortages/Bulletin.aspx?id=596. Accessed August 21, 2016.
- 27.Chandramouli J, American Society of Health-System Pharmacists Resolved Shortages Bulletin: Ibuprofen lysine Injection [20 Nov 2012] website. http://www.ashp.org/menu/DrugShortages/ResolvedShortages/Bulletin.aspx?Source=Resolved&Type=Rss&Id=669 Accessed August 21, 2016.
- 28.United States Food and Drug Administration. Drug Approval Package: NeoProfen (Ibuprofen Lysine) Injection website. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/21-903s000TOC.cfm. Accessed August 21, 2016.
- 29.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228–1234. [Google Scholar]
- 30.Hernan MA, Robins JM. Instruments for causal inference: an epidemiologist’s dream? Epidemiology. 2006;17(4):360–372. doi: 10.1097/01.ede.0000222409.00878.37. [DOI] [PubMed] [Google Scholar]
- 31.Greenland S, Brumback B. An overview of relations among causal modelling methods. Int J Epidemiol. 2002;31(5):1030–1037. doi: 10.1093/ije/31.5.1030. [DOI] [PubMed] [Google Scholar]
- 32.Jones L, Craven P, Attia J, Thakkinstian A, Wright I. Network meta-analysis of indomethacin versus ibuprofen versus placebo for PDA in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2011;96(1):F45–52. doi: 10.1136/adc.2009.168682. [DOI] [PubMed] [Google Scholar]
- 33.Benitz WE. Treatment of persistent patent ductus arteriosus in preterm infants: time to accept the null hypothesis? J Perinatol. 2010;30(4):241–252. doi: 10.1038/jp.2010.3. [DOI] [PubMed] [Google Scholar]
- 34.Laughon M, Bose C, Clark R. Treatment strategies to prevent or close a patent ductus arteriosus in preterm infants and outcomes. J Perinatol. 2007;27(3):164–170. doi: 10.1038/sj.jp.7211662. [DOI] [PubMed] [Google Scholar]
- 35.Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB. Designing Clinical Research. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 36.Hermes-DeSantis ER, Clyman RI. Patent ductus arteriosus: pathophysiology and management. J Perinatol. 2006;26:S14–18. doi: 10.1038/sj.jp.7211465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


