Abstract
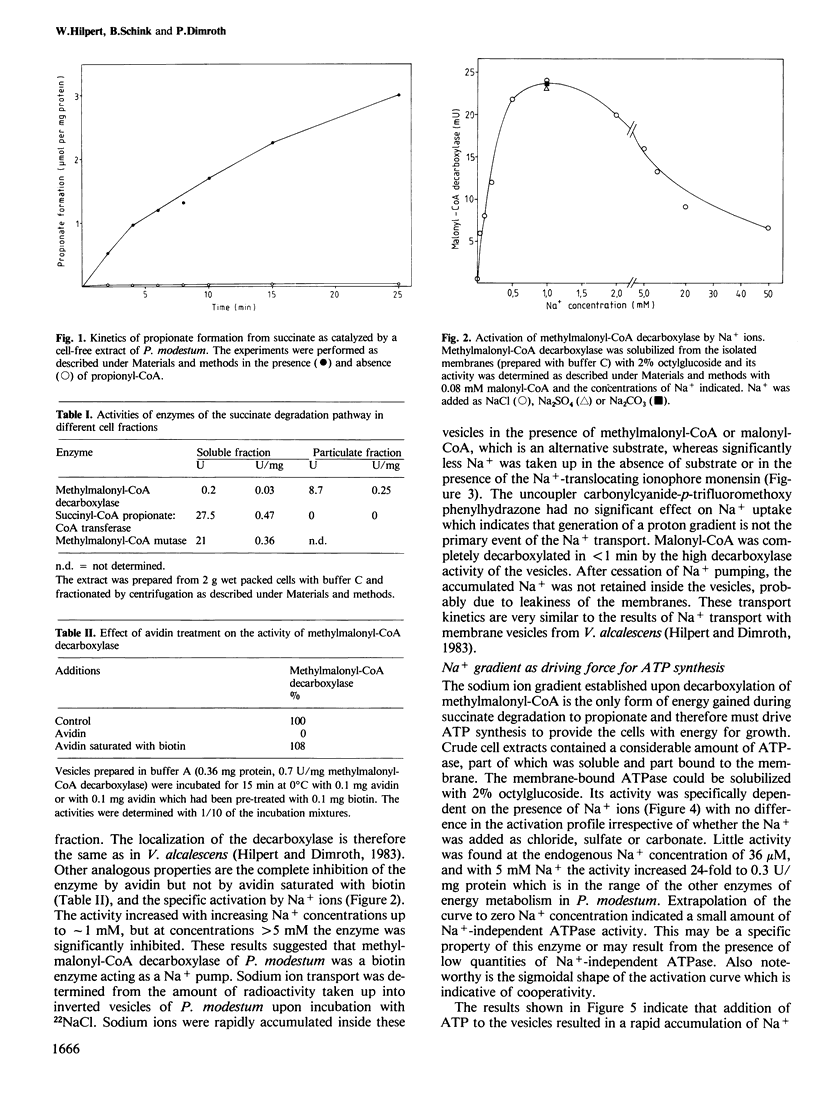
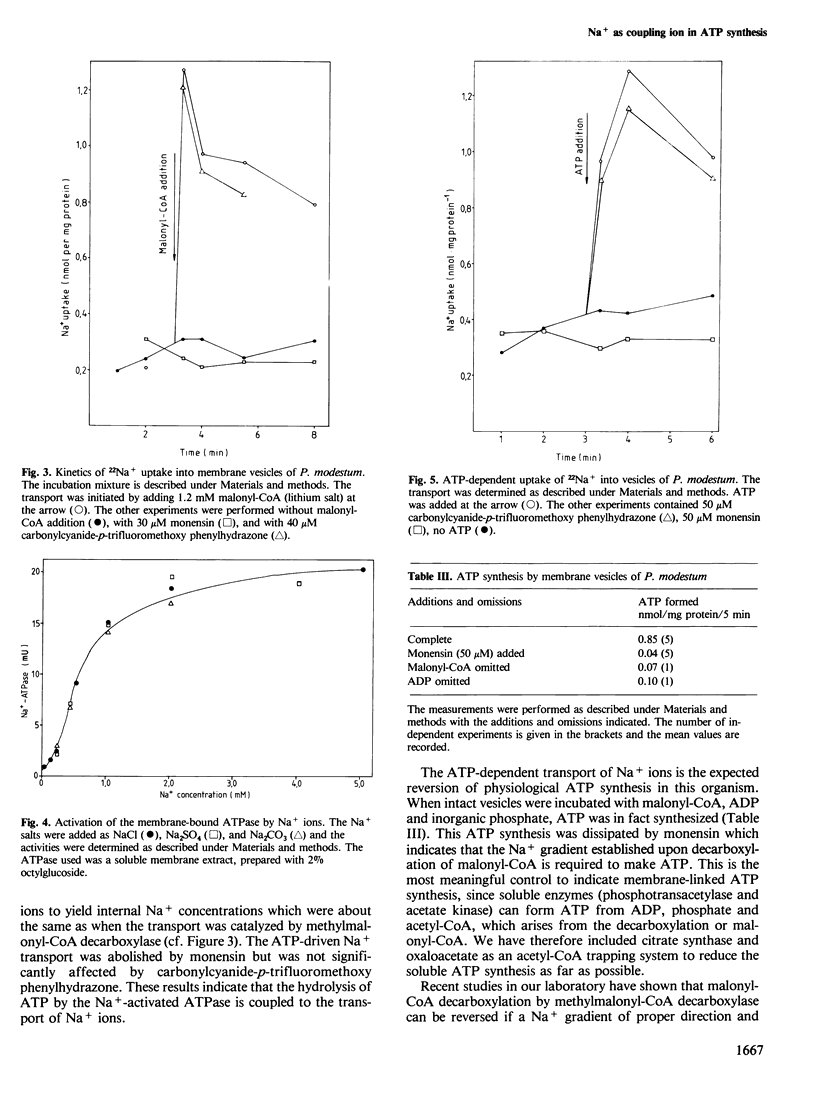
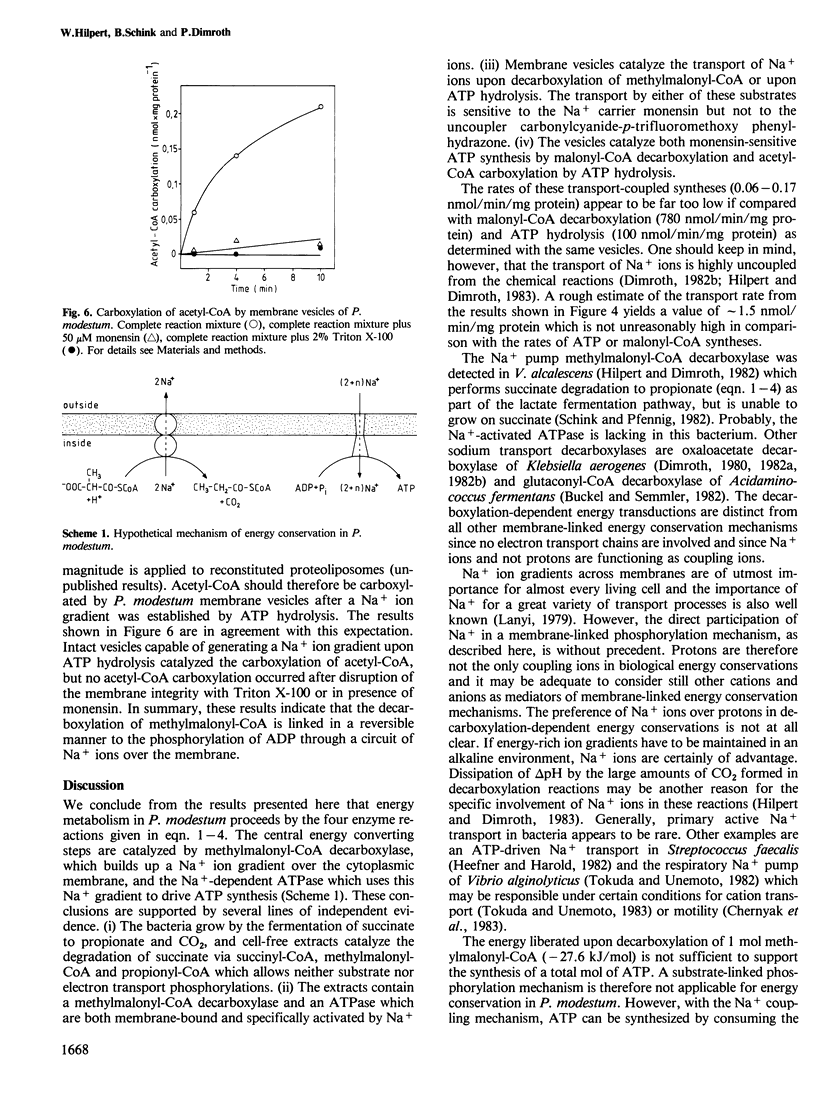
We report here a new mode of ATP synthesis in living cells. The anaerobic bacterium Propionigenium modestum gains its total energy for growth from the conversion of succinate to propionate according to: succinate + H2O → propionate + HCO3- (△Go' = -20.6 kJ/mol). The small free energy change of this reaction does not allow a substrate-linked phosphorylation mechanism, and no electron transport phosphorylation takes place. Succinate was degraded by cell-free extracts to propionate and CO2 via succinyl-CoA, methyl-malonyl-CoA and propionyl-CoA. This pathway involves a membrane-bound methylmalonyl-CoA decarboxylase which couples the exergonic decarboxylation with a Na+ ion transport across the membrane. The organism also contained a membrane-bound ATPase which was specifically activated by Na+ ions and catalyzed and transport of Na+ ions into inverted bacterial vesicles upon ATP hydrolysis. The transport was abolished by monensin but not by the uncoupler carbonylcyanide-p-trifluoromethoxy phenylhydrazone. Isolated membrane vesicles catalyzed the synthesis of ATP from ADP and inorganic phosphate when malonyl-CoA was decarboxylated and malonyl-CoA synthesis from acetyl-CoA when ATP was hydrolyzed. These syntheses were sensitive to monensin which indicates that Na+ functions as the coupling ion. We conclude from these results that ATP synthesis in P. modestum is driven by a Na+ ion gradient which is generated upon decarboxylation of methylmalonyl-CoA.
Keywords: ATP synthesis, Propionigenium modestum, Na+ ions, methylmalonyl CoA, decarboxylation
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckel W., Semmler R. A biotin-dependent sodium pump: glutaconyl-CoA decarboxylase from Acidaminococcus fermentans. FEBS Lett. 1982 Nov 1;148(1):35–38. doi: 10.1016/0014-5793(82)81237-4. [DOI] [PubMed] [Google Scholar]
- Dawson K. A., Allison M. J., Hartman P. A. Isolation and some characteristics of anaerobic oxalate-degrading bacteria from the rumen. Appl Environ Microbiol. 1980 Oct;40(4):833–839. doi: 10.1128/aem.40.4.833-839.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimroth P. A new sodium-transport system energized by the decarboxylation of oxaloacetate. FEBS Lett. 1980 Dec 29;122(2):234–236. doi: 10.1016/0014-5793(80)80446-7. [DOI] [PubMed] [Google Scholar]
- Dimroth P. Decarboxylation and transport. Biosci Rep. 1982 Nov;2(11):849–860. doi: 10.1007/BF01114890. [DOI] [PubMed] [Google Scholar]
- Dimroth P. The generation of an electrochemical gradient of sodium ions upon decarboxylation of oxaloacetate by the membrane-bound and Na+-activated oxaloacetate decarboxylase from Klebsiella aerogenes. Eur J Biochem. 1982 Jan;121(2):443–449. doi: 10.1111/j.1432-1033.1982.tb05807.x. [DOI] [PubMed] [Google Scholar]
- Galivan J. H., Allen S. H. Methylmalonyl coenzyme A decarboxylase. Its role in succinate decarboxylation by Micrococcus lactilyticus. J Biol Chem. 1968 Mar 25;243(6):1253–1261. [PubMed] [Google Scholar]
- Heefner D. L., Harold F. M. ATP-driven sodium pump in Streptococcus faecalis. Proc Natl Acad Sci U S A. 1982 May;79(9):2798–2802. doi: 10.1073/pnas.79.9.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilpert W., Dimroth P. Conversion of the chemical energy of methylmalonyl-CoA decarboxylation into a Na+ gradient. Nature. 1982 Apr 8;296(5857):584–585. doi: 10.1038/296584a0. [DOI] [PubMed] [Google Scholar]
- Hilpert W., Dimroth P. Purification and characterization of a new sodium-transport decarboxylase. Methylmalonyl-CoA decarboxylase from Veillonella alcalescens. Eur J Biochem. 1983 May 16;132(3):579–587. doi: 10.1111/j.1432-1033.1983.tb07403.x. [DOI] [PubMed] [Google Scholar]
- Kagawa Y., Sone N. DCCD-sensitive ATPase (TF0 . F1) from a thermophilic bacterium: purification, dissociation into functional subunits, and reconstitution into vesicles capable of energy transformation. Methods Enzymol. 1979;55:364–372. doi: 10.1016/0076-6879(79)55046-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mah R. A., Smith M. R., Baresi L. Studies on an acetate-fermenting strain of Methanosarcina. Appl Environ Microbiol. 1978 Jun;35(6):1174–1184. doi: 10.1128/aem.35.6.1174-1184.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Unemoto T. Characterization of the respiration-dependent Na+ pump in the marine bacterium Vibrio alginolyticus. J Biol Chem. 1982 Sep 10;257(17):10007–10014. [PubMed] [Google Scholar]
- Tokuda H., Unemoto T. Growth of a marine Vibrio alginolyticus and moderately halophilic V. costicola becomes uncoupler resistant when the respiration-dependent Na+ pump functions. J Bacteriol. 1983 Nov;156(2):636–643. doi: 10.1128/jb.156.2.636-643.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Huser B. A., Brock T. D., Wuhrmann K. Characterization of an acetate-decarboxylating, non-hydrogen-oxidizing methane bacterium. Arch Microbiol. 1980 Jan;124(1):1–11. doi: 10.1007/BF00407022. [DOI] [PubMed] [Google Scholar]
- de Vries W., Rietveld-Struijk R. M., Stouthamer A. H. ATP formation associated with fumarate and nitrate reduction in growing cultures of Veillonella alcalescens. Antonie Van Leeuwenhoek. 1977;43(2):153–167. doi: 10.1007/BF00395670. [DOI] [PubMed] [Google Scholar]



