Summary
1. Global reactive nitrogen (N) is projected to further increase in the coming years. Previous studies have demonstrated that N enrichment weakens the temporal stability of the ecosystem and the primary productivity through decreased biodiversity and species asynchrony. Mowing is a globally common practise in grasslands; and infrequent mowing can maintain or increase plant diversity under N enrichment conditions. However, it is unclear how infrequent mowing affects ecosystem stability in the face of N enrichment.
2. By independently manipulating the frequency (twice vs. monthly additions per year) and rate (i.e. 0, 1, 2, 3, 5, 10, 15, 20, and 50 g N m−2 year−1) of NH4NO3 inputs and mowing (unmown vs. mown) over 3 years (2011–2013) in a temperate grassland of northern China, we aimed to examine the interactive effects of N enrichment and mowing on ecosystem stability.
3. The results show that mowing maintained a positive relationship between species richness and ecosystem stability despite N addition, but that it exacerbated the negative effects of N addition on ecosystem stability. Mowing increased mean primary productivity and plant species richness, but it also increased the synchrony of population fluctuations and the variability of primary productivity under N enrichment, thereby contributing to a decline in the ecosystem stability.
4. Thus, our study reveals that infrequent mowing can buffer the negative effects of N enrichment on biodiversity to some extent and further increase the primary productivity, but it exacerbates the loss of ecosystem stability with N enrichment, thereby threatening local and/or semiarid regional food security.
Keywords: biodiversity, biomass harvest, clipping, community stability, hay, nitrogen addition, nitrogen addition frequency, productivity, steppe
Introduction
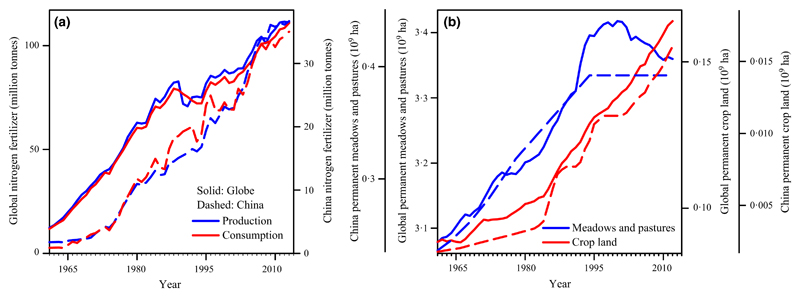
Nitrogen (N) fertilization is a common practise in the world’s semi-natural and managed grasslands and provides important benefits to modern agriculture (Erisman et al. 2008; Gibson 2009). For example, fertilization of natural grasslands and pastures in Argentina, Brazil, China, the Netherlands, Russia, the United Kingdom, the United States of America, Uruguay, and other countries has significantly increased plant and animal production (Tilman 1987; Suttie, Reynolds & Batello 2005; Zhang et al. 2015). However, intentional (fertilizer application) or unintentional (atmospheric deposition) reactive N enrichment is also one of the major causes of species extinction and changes in species composition in grassland ecosystems (Mountford, Lakhani & Kirkham 1993; Stevens et al. 2004; Clark & Tilman 2008; Socher et al. 2013; Zhang et al. 2014), and it threatens ecosystem stability (defined as the inverse of the coefficient of temporal variability of net primary productivity: Tilman & Downing 1994; Yang et al. 2012; Hautier et al. 2015; Zhang et al. 2016a). The production and consumption of N fertilizers has increased in most developing countries, such as Brazil and China, and at the global scale since the 1960s (Fig. 1a) (International Fertilizer Industry Association 2015). Because of the rising global human population and the lack of technologies to improve nitrogen utilization efficiency, the application of reactive N is expected to further increase in the coming decades at a rate of 1·4% per year globally (The Food and Agriculture Organization of the United Nations 2015). Consequently, global atmospheric N deposition will continue to increase accordingly (Galloway et al. 2008).
Fig. 1.
Temporal trends in N fertilizer, permanent meadows and pastures, and permanent crop lands. (a) Temporal trends in the annual amount and consumption of N fertilizer for most nations around the world (globe) and for China during 1961–2013, indicating that global N deposition has increased in the past decades. (b) Temporal trends in the land area of permanent meadows and pastures or crops globally and for mainland China during 1961–2012. Solid and dashed lines correspond to the data at global scale and China national scale, respectively. Data in (a) and (b) were obtained from the International Fertilizer Industry Association (2015) and the FAOSTAT (2014), respectively.
Biomass harvest (mowing) is one of the oldest and most widespread practises in grassland management because it produces hay, which can be stored for on-farm/agricultural use. Hay is also a sellable commodity as it is easy to transport and store (Suttie, Reynolds & Batello 2005; Mladkova et al. 2015). Over the past half century, cropland area increased significantly in the mainland of China and across the globe (Fig. 1b). The area of land in permanent meadows and pastures that is used for hay production increased from the 1960s to the 1990s but has remained stable during the past two decades (i.e. from 1995 to 2012) in the mainland of China (Fig. 1b) (FAOSTAT 2014). As mowing can increase light availability for small, subdominant plant species and thus affect their germination rates, it is now considered a crucial management strategy for restoring plant diversity under increased N deposition/fertilization scenarios (Collins et al. 1998; Storkey et al. 2015; Jones et al. 2017). Infrequent mowing after plant reproduction can reduce the negative effects of N enrichment on species richness (Socher et al. 2013; Jones et al. 2017), and thereby partly restore species richness (Collins et al. 1998; Härdtle et al. 2006; Stevens 2016). By contrast, however, intensive mowing can have negative effects on species richness (Blüthgen et al. 2012; Socher et al. 2012) because frequent mowing before flowering and/or seed release decreases seed production, seed bank diversity, and germination quality and quantity (Socher et al. 2012, 2013).
Biodiversity, in particular plant species richness, plays an important role in ecosystem stability by increasing the asynchrony of species fluctuations (Hector et al. 2010; Loreau & de Mazancourt 2013; Hautier et al. 2015). The portfolio effect (Tilman, Lehman & Bristow 1998) is the phenomenological outcome of this asynchrony (Loreau 2010), and highlights the positive effect of biodiversity on ecosystem stability. As previous studies reported opposite impacts of N enrichment and mowing on species richness (Collins et al. 1998; Yang et al. 2012; Storkey et al. 2015; Jones et al. 2017), how the combination of mowing and N enrichment may affect plant species richness and ecosystem stability remains unclear.
In this study, we use a unique dataset of plant species richness (m−2) and above-ground net primary productivity (ANPP; g m−2 year−1) in a temperate grassland in China to examine the interactive effects of mowing and N enrichment on ecosystem stability. We hypothesized that (i) N enrichment would have negative effects on biodiversity, based on the results of previous studies (Stevens et al. 2004; Clark & Tilman 2008; Hautier, Niklaus & Hector 2009; Zhang et al. 2014), (ii) N enrichment would decrease ecosystem stability via a reduction in species richness and species asynchrony, and (iii) infrequent mowing, which increases species richness (Collins et al. 1998; Jones et al. 2017), would increase ecosystem stability, based on the positive relationship between species richness and ecosystem stability in the temperate grasslands studied (Yang et al. 2012; Zhang et al. 2016a).
Materials and methods
Study Site
The field experiment was carried out at a grassland near the Inner Mongolia Grassland Ecosystem Research Station (116°14′E, 43°13′N), Inner Mongolia, China. A 50-ha field had been fenced since 1999 to exclude large animals from grazing. The topography of the experimental area was flat, with an elevation range of 1255–1260 m. The mean annual temperature was 0·9 °C, with a mean monthly temperature that ranged from −21·3 °C in January to 19·7 °C in July for the period of 1983–2013. Mean annual precipitation was 348·5 mm, with 81·8% falling in from May to September (here after termed the growing season). Temperature and precipitation, in the growing seasons of 2011 to 2013, ranged from 15·1 to 16·0 °C and from 244·6 to 406·7 mm, respectively. The soil was classified as Haplic Calcisol based on the FAO soil classification system. The plant community was dominated by rhizomatous perennial C3 grass, i.e. Leymus chinensis (Trin.) Tzvel, and perennial C3 bunchgrass, i.e. Stipa grandis P. Smirn., which together accounted for more than 60% of the total above-ground biomass (Zhang et al. 2015). There were about 50 vascular plant species, averaging eight species per m2 in the control plots across 2008–2013 (Zhang et al. 2014, 2016b). This ecosystem had received no fertilizer or mowing before this experiment. The ambient total (wet and dry) N deposition in this region was less than 1·5 g N m−2 year−1 in the 2000s (Zhu et al. 2015).
Experimental Design
The experiment was established during September 2008, and followed a randomized complete block design that consisted of nine rates (i.e. 0, 1, 2, 3, 5, 10, 15, 20, and 50 g N m−2 year−1) crossed with two frequencies (two times per year vs. monthly) (Zhang et al. 2014) of N addition and mowing (unmown vs. mown) (Zhang et al. 2013). The use of higher N addition rates served as a proxy for N fertilization activities and/or long-term extreme N enrichment in a temperate grassland ecosystem. The higher frequency (i.e. monthly: 12 times per year) of N addition was used to simulate atmospheric N deposition (Smith, Knapp & Collins 2009), which occurs continuously throughout the year (Aneja et al. 2001). During the growing season from May to October, fertilizer was weighed and mixed with purified water (9·0 L total for all treatments receiving water: either 9·0 L once in June or 1·5 L monthly from May to October), and sprinkled evenly using a sprayer to each plot to simulate wet N deposition. It was estimated that less than 1 mm of water was added to each plot annually, except the control plots, which had no water added. In winter (from November to April), NH4NO3 was mixed with sand (because of low amount of added NH4NO3 at low N rates in 12 N additions per year; 0·5 kg total for each treatment receiving sand: either 0·5 kg once in November or 0·08 kg monthly from November to April) and broadcast uniformly by hand. Sand was sieved through less than 1 mm in size, hydrochloric acid dipped, washed in purified water, and then heated at 120 °C for 24 h in an oven. To avoid otherwise potentially confounding effects, the plots received the same amount of water and sand, regardless of whether they received the high or low frequency of N addition treatment. An untreated control (control, with no added N, water, sand, or mowing) was used to detect the influence of water, sand addition, and mowing. In addition, a mowing only control (mowing control, with no added N, water, and sand) was included to detect the influence of added water and sand with mowing, which was also compared with the untreated control. Each plot was 8 × 8 m in area. Hence, there were 38 experimental treatments in total, with 10 replicate blocks for each treatment (i.e. 380 treatment plots in total). Plots were mown annually, in late August (after reproduction had finished for most species), simulating typical hay-cutting management in this region. Single mowing was performed with a mower at a 10-cm height. The harvested above-ground biomass was removed immediately after mowing.
Plant Data Collection
The ANPP of the community was estimated from peak above-ground plant biomass, which is an acceptable approximation for ANPP in this region as above-ground plant tissues die during the winter season. Because of a lack of labourers in 2009 and in 2010, plant above-ground biomass was sampled only from 2011 to 2013. This was conducted between 10 and 15 August using a 0·5 m × 2 m rectangle, which was randomly placed in each plot without a spatial overlap of quadrats amongst years and at least 50 cm inside the border of each plot to avoid edge effects. All living vascular plants were sorted according to species, oven-dried at 65 °C for 48 h to a constant weight, and then weighed. Species richness (number of plant species per m2) was recorded in the same quadrat in which above-ground biomass was measured. Hence, there were about 12 540 plant above-ground biomass measurements within 1140 plots in the study.
Shannon-Diversity (H′)
H′ was measured as
where N is species richness, and bi is the ANPP of species i in the community.
Evenness
Evenness was calculated as (1/D)/N, where N is species richness, and D is Simpson’s dominance index (Smith & Wilson 1996). D was calculated as
where bi is the ANPP of species i in the community.
Ecosystem Stability
The temporal ecosystem stability in each plot was defined as μ/σ (Lehman & Tilman 2000), where μ and σ are the inter-annual mean and standard deviation of the ecosystem ANPP, respectively.
Species Asynchrony
Species asynchrony was quantified as
where σ2 is the temporal variance in ecosystem ANPP, and σbi is the temporal standard deviation in the ANPP of species i in a community with N species over years (Loreau & de Mazancourt 2008). This measure of species asynchrony ranges between 0 (perfect synchrony) and 1 (perfect asynchrony) (Loreau & de Mazancourt 2008).
The Mean-Variance Scaling Relationship (Taylor’s Power Law)
This relationship has the form σi2 = cmz, where σi2 is the variance in the ANPP of species i in a community, c is a constant, m is the average (mean) of species ANPP, and z is the scaling coefficient (Tilman, Lehman & Bristow 1998).
Statistical Analyses
Ecosystem stability, ecosystem mean (μ), and ecosystem variance (σ2) were natural log transformed to ensure normality and homogeneity before the analyses. A two-way analysis of covariance (ANCOVA) was used to explore the effects of the frequency and the rate of N addition, mowing, and their interactions on species richness, the temporal stability of ecosystem ANPP, ecosystem mean (μ), ecosystem variance (σ2), species asynchrony, evenness, and Shannon-diversity (H′), using the rate of N addition as a continuous variable (Table 1).
Table 1.
Effects of treatments and their interactions on variables
| Species richness |
Ecosystem stability |
Ecosystem μ |
Ecosystem σ2 |
Asynchrony |
Evenness |
H′ |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | P | F | P | F | P | F | P | F | P | F | P | F | P | |
| N | 1355 | 216·4 | <0·001 | 75·5 | <0·001 | 1138·0 | <0·001 | 243·7 | <0·001 | 18·5 | <0·001 | 17·2 | <0·001 | 120·5 | <0·001 |
| F | 1355 | 3·5 | 0·064 | 3·7 | 0·056 | 0·3 | 0·594 | 2·9 | 0·088 | 2·3 | 0·133 | 0·6 | 0·426 | 2·1 | 0·145 |
| M | 1355 | 107·0 | <0·001 | 4·1 | 0·044 | 3·4 | 0·066 | 5·4 | 0·021 | 7·4 | 0·007 | 14·7 | <0·001 | 33·7 | <0·001 |
| F × M | 1355 | 1·7 | 0·196 | 0·2 | 0·686 | 1·6 | 0·209 | 0·4 | 0·510 | 0·1 | 0·805 | 0·8 | 0·386 | 0·3 | 0·606 |
Results of the two-way ANCOVA for the effects of the rate (N) and the frequency (F) of N addition, mowing (M), and their interactions on species richness, the temporal stability of ecosystem above-ground net primary productivity, ecosystem mean (μ), ecosystem variance (σ2), species asynchrony, evenness, and Shannon-diversity (H′), using the rate of N addition as a continuous variable. d.f., degrees of freedom. F- and P-values are given.
As N enrichment impacted on both plant species richness and ecosystem stability simultaneously (Table 1), it is likely to hide the specific effects of diversity on stability (Huston 1997; Loreau 1998). For testing the effects of N addition and mowing (treatments) on diversity-dependent variables, ANCOVAs were performed (Yang et al. 2012; Zhang et al. 2016a). Firstly, a three-way ANCOVA was used to test the effects of species richness, the rate and frequency of N addition, mowing, and their interactions on ecosystem stability, ecosystem mean (μ), ecosystem variance (σ2), species asynchrony, evenness, and H′, using the rate of N addition as a continuous variable (Table 2). Secondly, because there were no interactive effects of treatment × species richness on ecosystem stability, species asynchrony, evenness, and H′ (Table 2), two-way ANCOVA was employed, using the frequency of N addition and mowing as fixed variables, species richness as a covariate, and the rate of N addition as a continuous variable (Table 3). Because evenness was affected by neither the rate of N addition nor mowing (Table 1; all P > 0·4), evenness was excluded from further regression analyses. In addition, because the frequency of N addition had no effect on the above-mentioned variables (Tables 2 and 3; all P > 0·05), values were combined in further analyses (i.e. 20 replicates for each rate of N addition under either unmown or mown treatment). Two-way ANCOVA was employed to test whether mowing affected the slopes as a function of the rate of N addition (species richness or H′). In these mentioned two-way ANCOVAs, Type I was used and F-test was the interaction effect of mowing (H′) and variables (N rates, species richness, species asynchrony, ecosystem stability, etc.).
Table 2.
Effects of species richness, treatment, and their interactions
| Ecosystem stability |
Ecosystem μ |
Ecosystem σ2 |
Asynchrony |
Evenness |
H′ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | P | F | P | F | P | F | P | F | P | F | P | |
| N | 1276 | 42·5 | <0·001 | 609·3 | <0·001 | 138·6 | <0·001 | 7·8 | 0·006 | 0·7 | 0·405 | 4·8 | 0·029 |
| F | 1276 | 2·6 | 0·110 | 1·0 | 0·317 | 1·8 | 0·182 | 5·0 | 0·026 | 0·2 | 0·690 | 0·9 | 0·334 |
| M | 1276 | 5·4 | 0·021 | 3·2 | 0·076 | 7·0 | 0·009 | 8·9 | 0·003 | 1·5 | 0·226 | 0·4 | 0·515 |
| R | 26 276 | 2·3 | 0·001 | 1·8 | 0·011 | 2·6 | <0·001 | 0·8 | 0·739 | 2·3 | 0·001 | 7·1 | <0·001 |
| F × M | 1276 | 0·1 | 0·781 | 1·2 | 0·265 | 0·0 | 0·980 | 0·0 | 0·881 | 0·8 | 0·373 | 0·7 | 0·397 |
| F × R | 22 276 | 0·9 | 0·660 | 0·8 | 0·671 | 0·8 | 0·706 | 1·3 | 0·166 | 1·1 | 0·327 | 1·3 | 0·145 |
| M × R | 18 276 | 1·5 | 0·074 | 2·0 | 0·009 | 1·8 | 0·021 | 1·0 | 0·439 | 1·0 | 0·436 | 1·3 | 0·191 |
| F × M × R | 13 276 | 1·4 | 0·152 | 0·6 | 0·848 | 1·5 | 0·114 | 1·0 | 0·462 | 1·3 | 0·200 | 1·6 | 0·073 |
Results of the three-way ANCOVA (with the treatment × species richness interaction term) for the effects of the rate (N) and the frequency (F) of N addition, mowing (M), species richness (R), and their interactions on the temporal stability of ecosystem above-ground net primary productivity, ecosystem mean (μ), ecosystem variance (σ2), species asynchrony, evenness, and Shannon-diversity (H'), using the rate of N addition as a continuous variable. d.f., degrees of freedom. F- and P-values are given.
Table 3.
Effects of treatments and their interactions on diversity-dependent variables
| Ecosystem stability |
Asynchrony |
Evenness |
H′ |
||||||
|---|---|---|---|---|---|---|---|---|---|
| d.f. | F | P | F | P | F | P | F | P | |
| R | 1354 | 0·7 | 0·394 | 0·8 | 0·370 | 30·7 | <0·001 | 169·2 | <0·001 |
| N | 1354 | 40·0 | <0·001 | 8·1 | 0·005 | 0·0 | 0·991 | 6·3 | 0·013 |
| F | 1354 | 3·3 | 0·069 | 2·0 | 0·159 | 0·1 | 0·779 | 0·2 | 0·627 |
| M | 1354 | 4·8 | 0·030 | 7·9 | 0·005 | 0·7 | 0·409 | 0·0 | 0·933 |
| F × M | 1354 | 0·2 | 0·645 | 0·1 | 0·759 | 0·3 | 0·603 | 0·1 | 0·789 |
Results of two-way ANCOVA (without treatment × species richness interaction term) for the effects of species richness (R), the rate (N) and the frequency (F) of N addition, mowing (M), and their interactions on ecosystem stability, species asynchrony, evenness, and H', using species richness as a covariate and the rate of N addition as a continuous variable. d.f., degrees of freedom. F- and P-values are given.
Structural equation modelling (SEM) was employed to estimate the effects of N addition and annual mowing (biomass removing) via the alterations in species richness and species asynchrony, on ecosystem stability. Data were fitted to the model using the maximum likelihood estimation method. Adequacy of the model was determined using a chi-squared test, root square mean errors of approximation (RMSEA), and Akaike information criteria (AIC). Adequate model fits are indicated by a non-significant chi-squared test (P > 0·05), low RMSEA (<0·08), and AIC (Grace 2006).
AMOS 22.0 (Amos Development Co., Greene, ME, USA) was used for the SEM analysis. All statistical analyses were carried out using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Effects of N Enrichment
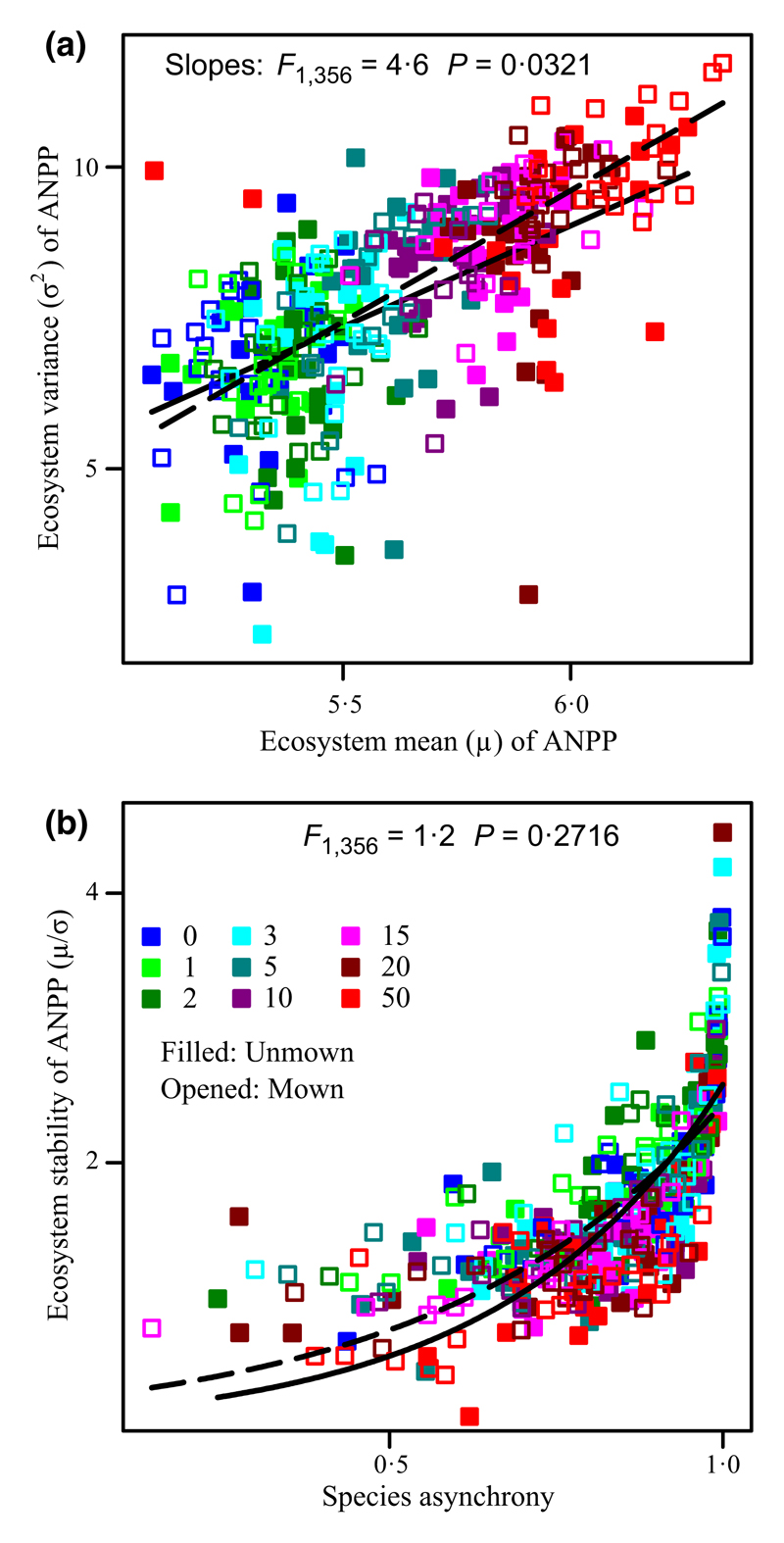
Irrespective of mowing, ecosystem stability (Fig. 2a; F1,358 = 74·5, P < 0·0001; R2 = 0·17), species richness (Fig. 2b; F1,358 = 165·8, P < 0·0001; R2 = 0·32), Shannon-diversity (H′; Fig. 3a; F1,358 = 110·3, P < 0·0001; R2 = 0·24), and species asynchrony (Fig. 2c; F1,358 = 18·2, P < 0·0001; R2 = 0·05) were all significantly reduced by increasing the rate of N addition, whereas ecosystem mean ANPP (Fig. 3b; F1,358 = 1130·8, P < 0·0001; R2 = 0·76) and ecosystem variance (Fig. 3c; F1,358 = 239·8, P < 0·0001; R2 = 0·40) increased significantly. Ecosystem mean ANPP and ecosystem variance were positively correlated (Fig. 4a; F1,358 = 299·9, P < 0·0001; R2 = 0·46). The rate of increase of the ecosystem mean ANPP with increasing the rate of N addition was slower than that of the ecosystem variance (i.e. smaller slope; F1,716 = 138·8, P < 0·0001).
Fig. 2.
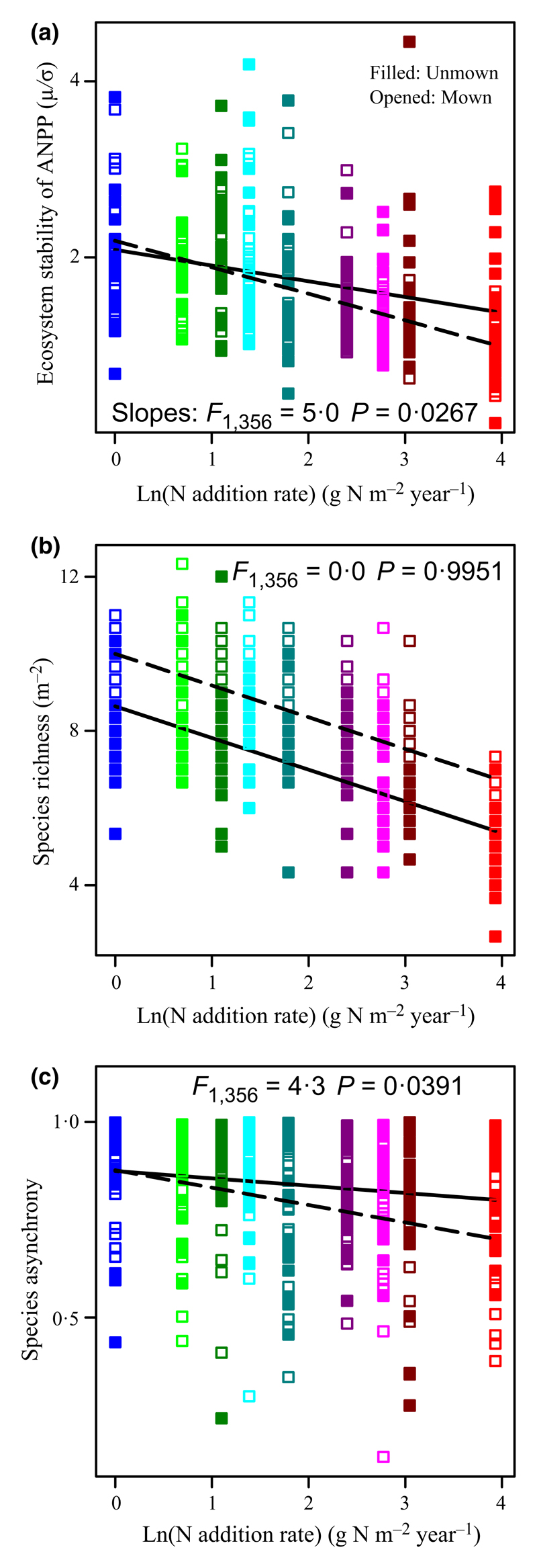
Effects of N and mowing on ecosystem stability, species richness, and species asynchrony. Changes in (a) the temporal stability of ecosystem above-ground net primary productivity (ANPP), (b) species richness (number of species per m2), and (c) species asynchrony according to the rate of N addition (different colours; g N m–2 year–1) and mowing (filled = unmown, opened = annual mown). Solid and dashed lines correspond to unmown and mowing treatments, respectively. F- and P-values are given to demonstrate the effects of mowing on the slopes.
Fig. 3.
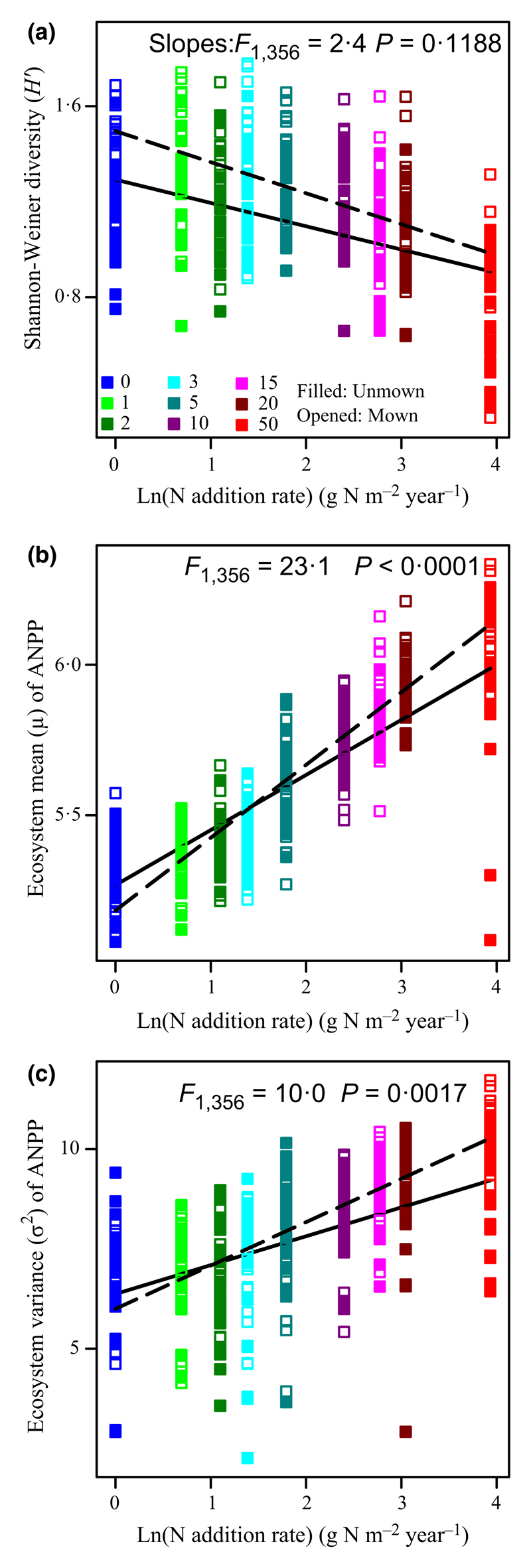
Effects of N and mowing on diversity, ecosystem mean, and ecosystem variance. Changes in (a) Shannon-diversity index (H′), (b) ecosystem mean above-ground net primary productivity (ANPP) (μ), and (c) ecosystem variance (σ2). F- and P-values are given to demonstrate the effects of mowing on the slopes. See symbols in Fig. 2.
Fig. 4.
Mowing accelerated the changes in ecosystem variance with N enrichment. (a) Ecosystem mean above-ground net primary productivity (ANPP) (μ) was positively associated with ecosystem variance (σ2). The slope in mown treatment was greater, suggesting that mowing decreased ecosystem stability via increases in the variance-to-mean ratio under N enrichment conditions. (b) Species asynchrony promoted ecosystem stability, indicating that lower species asynchrony was likely to have contributed to the reduced ecosystem stability under mowing after N enrichment. F- and P-values are given to demonstrate the effects of mowing on the slopes. See symbols in Fig. 2.
Diversity and Diversity–Stability Relationship
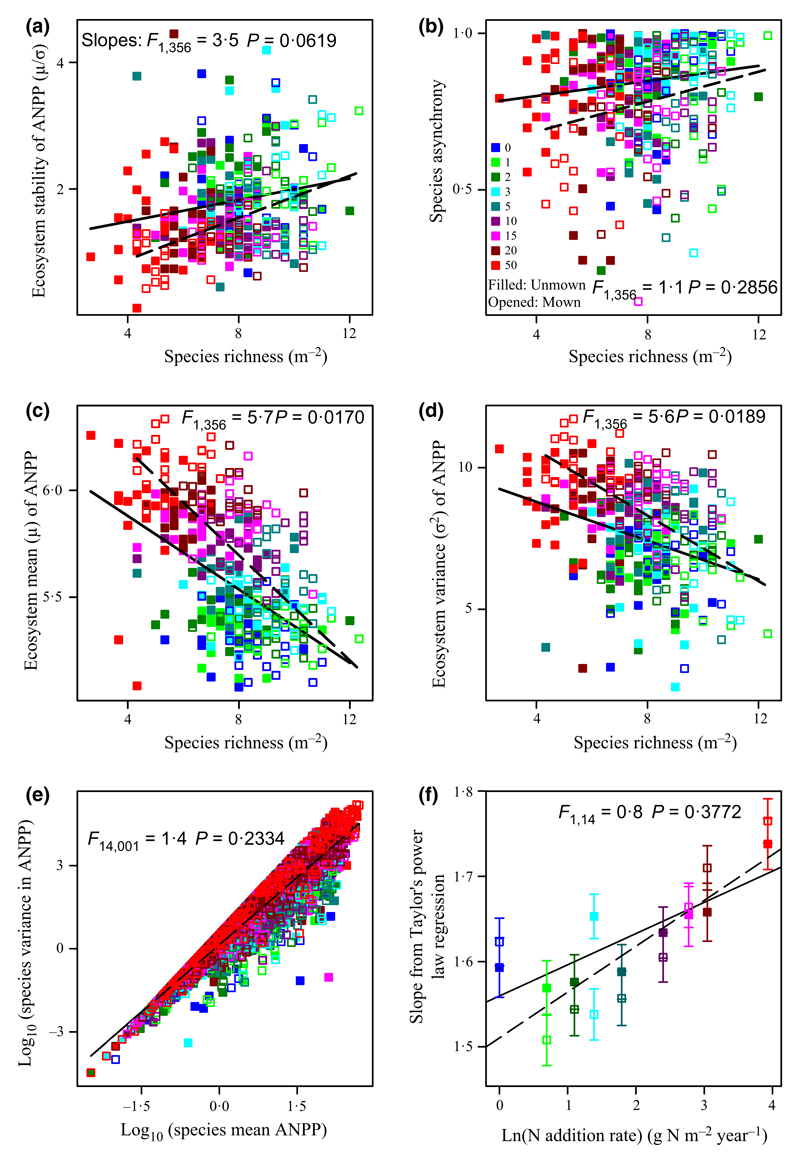
Mowing significantly increased both species richness and H′ (Table 1). Species richness was positively associated with the ecosystem stability (Fig. 5a; F1,358 = 20·0, P < 0·0001) and species asynchrony (Fig. 5b; F1,358 = 4·1, P = 0·0431), whilst species asynchrony was positively related to ecosystem stability (Fig. 4b; F1,358 = 440·8, P < 0·0001; R2 = 0·55). Species richness was negatively correlated with ecosystem mean ANPP (Fig. 5c; F1,358 = 126·7, P < 0·0001; R2 = 0·26) and ecosystem variance (Fig. 5d; F1,358 = 53·6, P < 0·0001; R2 = 0·13). The results were similar when using H′ instead of species richness. We also found that ecosystem stability, species richness, and species asynchrony were positively correlated (Figs 4b and 5a,b), whereas N addition reduced ecosystem stability, species richness, and species asynchrony (Fig. 2a–c).
Fig. 5.
Biodiversity effects on ecosystem stability. (a) Species richness was positively associated with ecosystem stability. (b) Species richness was positively associated with species asynchrony. Species richness was negatively associated with (c) ecosystem mean above-ground net primary productivity (ANPP) (μ) and (d) ecosystem variance (σ2). (e), Mean-variance scaling relationships. (f), Slope (z) from Taylor’s power law regression increased with increases in the rate of N addition. F- and P-values are given to demonstrate the effects of mowing on the slopes. Note: mowing did not alter the slopes of the relationship between Shannon–Weiner diversity (H′) and ecosystem stability too (F1,356 = 3·6 P = 0·0575; Type I, the interaction effect of mowing × H′). See symbols in Fig. 2.
In addition, log-transformed values of the variance of species ANPP were positively correlated with log-transformed values of its mean (Fig. 5e). The scaling coefficient z of the mean-variance scaling relationship was 1·640 and 1·622 for unmown and mown plots in combination with N enrichment, respectively (Fig. 5e). There was a similar scaling coefficient z between mown and unmown plots (Fig. 5e; F1,4001 = 1·4 P = 0·2334). Moreover, coefficient z increased with the rate of N addition (Fig. 5f; both P < 0·05, R2 > 0·60) and had similar slopes between mown and unmown plots (Fig. 5f; F1,14 = 0·8, P = 0·3772). As the portfolio effect theory requires that the scaling coefficient of this mean-variance scaling relationship should be between one and two, our study highlights the portfolio effect of biodiversity on promoting ecosystem stability.
Effects of Mowing on Ecosystem Stability
Under N enrichment conditions, mowing significantly promoted the slope of the ecosystem mean ANPP with the increasing rate of N addition (Fig. 3b; F1,356 = 23·1, P < 0·0001), revealing that mowing could stimulate the average of ecosystem primary productivity across years. Unexpectedly, mowing exacerbated the negative effects of N enrichment on ecosystem stability (Table 3) and species asynchrony (Table 3). By comparisons of slopes, the data showed that mowing significantly lowered the slopes of ecosystem stability (smaller slope; Fig. 2a; F1,356 = 5·0, P = 0·0267; Type I, the interaction effects of mowing × the rate of N addition) and species asynchrony (Fig. 2c; F1,356 = 4·3, P = 0·0391; Type I, the interaction effects of mowing × the rate of N addition) vs. increasing the rate of N addition, but significantly increased the slope of ecosystem mean ANPP (Fig. 5b; F1,356 = 23·1, P < 0·0001) and ecosystem variance (Fig. 5c; F1,356 = 10·0, P = 0·0017).
Specifically, because mowing increased the slope between the ecosystem mean ANPP and ecosystem variance (Fig. 4a; F1,356 = 4·6, P = 0·0321; Type I, the interaction effects of mowing × ecosystem mean ANPP), under N enrichment conditions, mowing accelerated the decreases in ecosystem stability by enhancing the variance-to-mean ratio. Moreover, mowing, under N enrichment conditions, caused a greater significant reduction in species asynchrony (Fig. 2c) that would result in ecosystem stability decrease (Fig. 4b), but did not alter the positive effects of species asynchrony on ecosystem stability (similar slopes; Fig. 4b; F1,356 = 1·2, P = 0·2716; Type I, the interaction effects of mowing × species asynchrony). These analyses suggest that mowing exacerbated the negative effect of N enrichment on ecosystem stability, not through changes in the plant diversity but rather because of the combination of increased ecosystem variance and species synchrony.
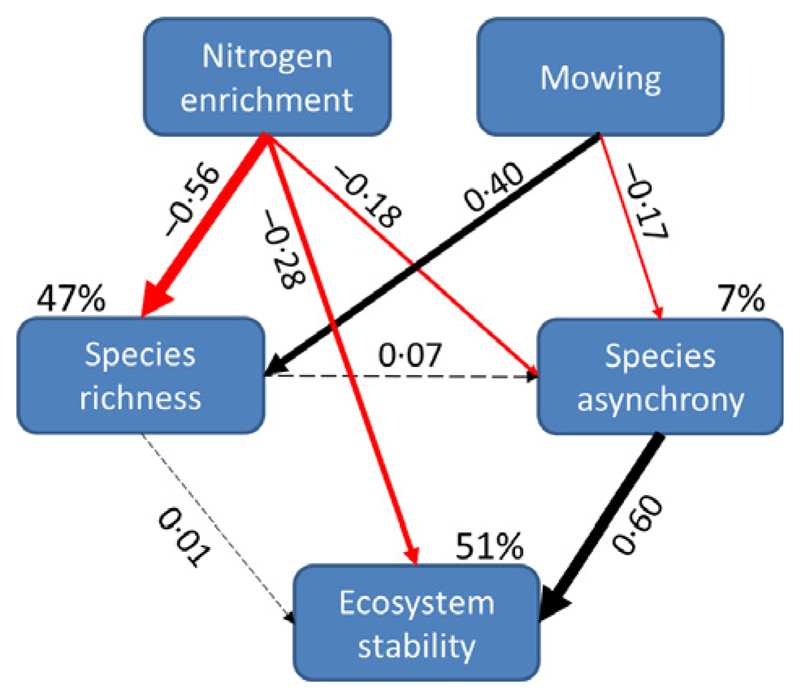
The final model fitted the data well: χ2 = 0·293, d.f. = 2, P = 0·864; RMSEA = 0·000; AIC = 26·293 (Fig. 6). From the results of the SEM (Fig. 6), it showed that N addition had significantly directly reduced species richness (standardized effect size: −0·56; P < 0·001), species asynchrony (standardized effect size: −0·18; P = 0·005), and ecosystem stability (standardized effect size: −0·28; P < 0·001). Mowing significantly directly promoted species richness (standardized effect size: 0·40; P < 0·001) whereas was significantly directly and negatively associated with species asynchrony (standardized effect size: −0·17; P = 0·004). In total, N addition had negative impacts on species richness (standardized effect size: −0·56), species asynchrony (standardized effect size: −0·22), and ecosystem stability (standardized effect size: −0·42); mowing had positive influences on species richness (Fig. 6; standardized effect size: 0·40) but had negative effects on species asynchrony (standardized effect size: −0·14), and ecosystem stability (standardized effect size: −0·08). Overall, results from the SEM revealed that mowing could have beneficial effects on species richness but would exacerbate the negative effect of N enrichment on ecosystem stability via decreased species asynchrony.
Fig. 6.
Effects of N addition and annual mowing on species richness, species asynchrony, and ecosystem stability. The final structural equation modelling (SEM) fitted the data well: χ2 = 0·293, d.f. = 2, P = 0·920; root square mean errors of approximation <0·001; AIC = 26·293. Numbers adjacent to arrows are standardized path coefficients, and width of the arrows indicates the strength of the relationship. Dashed and solid arrows indicate P > 0·1 and P < 0·01, respectively. Black and red arrows indicate positive and negative relationship, respectively. Percentages close to endogenous variables indicate the variance explained by the model (R2). The SEM revealed that annual mowing exacerbated the negative effect of N enrichment on ecosystem stability through a decrease in species asynchrony.
Discussion
In this study, we found that N enrichment had positive effects on ecosystem ANPP and negative effects on species richness, species asynchrony, and ecosystem stability, consistent with our predictions. However, contrary to our hypothesis, mowing decreased ecosystem stability under N addition because it increased species synchrony.
In line with previous studies (Hautier et al. 2015; Zhang et al. 2016a), both the mean and variability of primary productivity increased with the rate of N addition irrespective of mowing, whereas species diversity (species richness and Shannon–Weiner diversity), ecosystem stability, and species asynchrony were all significantly reduced under elevated N. The reasons for the apparent decrease in ecosystem stability following N addition had already been discussed in our previous study (see Zhang et al. 2016a). In this study, we found that the increasing rate of N addition significantly reduced species richness, Shannon–Weiner diversity, and species asynchrony, thereby associated with decreasing ecosystem stability. Moreover, based on the portfolio effect that when 2 > z > 1, diversity is expected to enhance ecosystem stability (Tilman, Lehman & Bristow 1998). In our study, the z was between one and two and increased with the increasing rate of N addition, consistent with theory (Tilman, Lehman & Bristow 1998) and previous reports (Grman et al. 2010; Zhang et al. 2016a).
Under N enrichment conditions, mowing significantly increased species richness, Shannon-Weiner diversity and the ecosystem mean ANPP, but it decreased ecosystem stability and species asynchrony. Interestingly, the study revealed that mowing with N enrichment could stimulate the average of ecosystem ANPP across years in this grassland which was not similar with previous study (Yuan et al. 2004; Niu et al. 2010). On the other hand, in line with previous studies (Collins et al. 1998; Yang et al. 2012; Storkey et al. 2015; Jones et al. 2017), we can conclude that annual infrequent mowing, which was conducted after the plants’ reproductive phase, could partially offset the negative effects of N enrichment on plant diversity. We also found that mowing did not alter the coefficient z of the mean-variance scaling relationship under N enrichment conditions. These results suggest that, regardless of N addition, mowing can still maintain the buffering effect of biodiversity on ecosystem stability.
Unexpectedly, however, mowing exacerbated the negative effects of N enrichment on the temporal stability of ecosystem ANPP. Mowing increased the variance-to-mean ratio of ANPP under N enrichment, thereby contributing to a loss of ecosystem stability. Moreover, mowing resulted in a significantly stronger reduction in species asynchrony with N addition although it did not alter the positive effects of species asynchrony on ecosystem stability. Thus, mowing exacerbated the negative effect of N enrichment on ecosystem stability by further decreasing species asynchrony. Decreased species asynchrony because of mowing may have been caused by nutrient imbalance, ground surface warming, or increased light availability. Mowing can increase nutrient loss (such as N, phosphate, potassium, etc.) from the soil, causing nutrient imbalance (Giese et al. 2013; Liu et al. 2015), and thereby stronger competition between plants for nutrients after mowing. Mowing or biomass removal may also increase soil surface temperatures (Wan, Luo & Wallace 2002) by increasing ground irradiance, resulting in an increased synchronization of responses amongst species to climatic conditions during the growing season (Shestakova et al. 2016). Theoretically, interspecific competition can destabilize aggregate ecosystem properties (Loreau & de Mazancourt 2013). Nutrients and light are important resources for plant growth in temperate grasslands, and because mowing causes a redistribution of the available nutrients and light, it might increase interspecific competition, and thus reduce species asynchrony and ecosystem stability. As the global climate is expected to become warmer in the coming decades, species responses to climatic conditions might become more synchronous, thus decreasing the stability and predictability of ecosystem ANPP. In contrast, mowing did not alter the effect of N addition on the loss of species diversity, nor did it affect the positive relationship between species richness and ecosystem stability. Overall, the negative effect of mowing on ecosystem stability under N enrichment was not caused by differences in plant diversity, but was a result of a combination of increased ecosystem variance and species synchrony.
In conclusion, N enrichment can increase ecosystem productivity on average, but it has negative effects on species richness, species asynchrony, and ecosystem stability. Mowing can promote ecosystem mean productivity under N enrichment and be beneficial for species richness, but it tends to decrease ecosystem stability via a decrease in species asynchrony. Importantly, we found that even under N enrichment, mowing can maintain a positive relationship between species richness and ecosystem stability, highlighting the fact that maintaining biodiversity is vital for supporting ecosystem functioning and providing ecosystem services.
Acknowledgements
We thank Xiaoliang Wang, Jianjun Chen, Jinlian Wang, Minglu Rong, and others for their help to field data collection, the Inner Mongolia Grassland Ecosystem Research Station for provided facilities and meteorological data, the FAO for provided area of land in permanent meadows and pastures and crops, and the IFA for provided data of N fertilizer production and consumption. We also thank the associate editor and two anonymous referees for their constructive comments that significantly improved the manuscript. This study was supported by the Youth Fund of National Natural Science Foundation of China (31400390), the National Natural Science Foundation of China (31430016, 41573063, 31370442, and 31570469), the National Key Research Project of China (2016YFC0500202), China Post-doctoral Science Foundation funded project (2015T80153), the TULIP Laboratory of Excellence (ANR-10-LABX-41), and by the BIOSTASES Advanced Grant, funded by the European Research Council under the European Union’s Horizon 2020 Research and Innovation Programme (666971).
Footnotes
Authors’ contributions
Y.Z. and X.H. designed the research. Y.Z. collected data. Y.Z. and M.L. performed the analysis. Y.Z., M.L., N.H., G.Z., and X.H. wrote the article. All authors contributed critically to the drafts and gave final approval for publication.
Data accessibility
Data deposited in the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.sq15g (Zhang et al. 2017).
The authors declare that they have no competing interests.
References
- Aneja VP, Roelle PA, Murray GC, Southerland J, Erisman JW, Fowler D, Asman WAH, Patni N. Atmospheric nitrogen compounds II: emissions, transport, transformation, deposition and assessment. Atmospheric Environment. 2001;35:1903–1911. [Google Scholar]
- Blüthgen N, Dormann CF, Prati D, et al. A quantitative index of land-use intensity in grasslands: integrating mowing, grazing and fertilization. Basic and Applied Ecology. 2012;13:207–220. [Google Scholar]
- Clark CM, Tilman D. Loss of plant species after chronic low-level nitrogen deposition to prairie grasslands. Nature. 2008;451:712–715. doi: 10.1038/nature06503. [DOI] [PubMed] [Google Scholar]
- Collins SL, Knapp AK, Briggs JM, Blair JM, Steinauer EM. Modulation of diversity by grazing and mowing in native tallgrass prairie. Science. 1998;280:745–747. doi: 10.1126/science.280.5364.745. [DOI] [PubMed] [Google Scholar]
- Erisman JW, Sutton MA, Galloway J, Klimont Z, Winiwarter W. How a century of ammonia synthesis changed the world. Nature Geoscience. 2008;1:636–639. [Google Scholar]
- FAOSTAT. Land-use statistics for 1961-2012. [accessed 13 April 2016];2014 Available at: http://faostat.fao.org.
- Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai ZC, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA. Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science. 2008;320:889–892. doi: 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- Gibson DJ. Grasses and Grassland Ecology. Oxford University Press; Oxford, UK: 2009. [Google Scholar]
- Giese M, Brueck H, Gao YZ, et al. N balance and cycling of Inner Mongolia typical steppe: a comprehensive case study of grazing effects. Ecological Monographs. 2013;83:195–219. [Google Scholar]
- Grace JB. Structural Equation Modeling and Natural Systems. Cambridge University Press; Cambridge, UK: 2006. [Google Scholar]
- Grman E, Lau JA, Schoolmaster DR, Gross KL. Mechanisms contributing to stability in ecosystem function depend on the environmental context. Ecology Letters. 2010;13:1400–1410. doi: 10.1111/j.1461-0248.2010.01533.x. [DOI] [PubMed] [Google Scholar]
- Härdtle W, Niemeyer M, Niemeyer T, Assmann T, Fottner S. Can management compensate for atmospheric nutrient deposition in heathland ecosystems? Journal of Applied Ecology. 2006;43:759–769. [Google Scholar]
- Hautier Y, Niklaus PA, Hector A. Competition for light causes plant biodiversity loss after eutrophication. Science. 2009;324:636–638. doi: 10.1126/science.1169640. [DOI] [PubMed] [Google Scholar]
- Hautier Y, Tilman D, Isbell F, Seabloom EW, Borer ET, Reich PB. Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science. 2015;348:336–340. doi: 10.1126/science.aaa1788. [DOI] [PubMed] [Google Scholar]
- Hector A, Hautier Y, Saner P, et al. General stabilizing effects of plant diversity on grassland productivity through population asynchrony and overyielding. Ecology. 2010;91:2213–2220. doi: 10.1890/09-1162.1. [DOI] [PubMed] [Google Scholar]
- Huston MA. Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia. 1997;110:449–460. doi: 10.1007/s004420050180. [DOI] [PubMed] [Google Scholar]
- International Fertilizer Industry Association (IFA) Historical production, trade and consumption statistics from 1961. [accessed 13 April 2016];2015 Available at: http://www.fertilizer.org/statistics.
- Jones L, Stevens C, Rowe EC, Payne R, Caporn SJM, Evans CD, Field C, Dale S. Can on-site management mitigate nitrogen deposition impacts in non-wooded habitats? Biological Conservation. 2017 doi: 10.1016/j.biocon.2016.06.012. [DOI] [Google Scholar]
- Lehman CL, Tilman D. Biodiversity, stability, and productivity in competitive communities. American Naturalist. 2000;156:534–552. doi: 10.1086/303402. [DOI] [PubMed] [Google Scholar]
- Liu N, Kan HM, Yang GW, Zhang YJ. Changes in plant, soil, and microbes in a typical steppe from simulated grazing: explaining potential change in soil C. Ecological Monographs. 2015;85:269–286. [Google Scholar]
- Loreau M. Biodiversity and ecosystem functioning: a mechanistic model. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:5632–5636. doi: 10.1073/pnas.95.10.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreau M. From Population to Ecosystem: Theoretical Foundations for a New Ecological Synthesis. Princeton University Press; Princeton, NJ, USA and Oxford, UK: 2010. [Google Scholar]
- Loreau M, de Mazancourt C. Species synchrony and its drivers: neutral and nonneutral community dynamics in fluctuating environments. American Naturalist. 2008;172:E48–E66. doi: 10.1086/589746. [DOI] [PubMed] [Google Scholar]
- Loreau M, de Mazancourt C. Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecology Letters. 2013;16:106–115. doi: 10.1111/ele.12073. [DOI] [PubMed] [Google Scholar]
- Mladkova P, Mladek J, Hejduk S, Hejcman M, Cruz P, Jouany C, Pakeman RJ. High-nature-value grasslands have the capacity to cope with nutrient impoverishment induced by mowing and livestock grazing. Journal of Applied Ecology. 2015;52:1073–1081. [Google Scholar]
- Mountford JO, Lakhani KH, Kirkham FW. Experimental assessment of the effects of nitrogen addition under hay-cutting and aftermath grazing on the vegetation of meadows on a Somerset peat moor. Journal of Applied Ecology. 1993;30:321–332. [Google Scholar]
- Niu SL, Wu MY, Han Y, Xia JY, Zhang Z, Yang HJ, Wan SQ. Nitrogen effects on net ecosystem carbon exchange in a temperate steppe. Global Change Biology. 2010;16:144–155. [Google Scholar]
- Shestakova TA, Gutiérrez E, Kirdyanov AV, et al. Forests synchronize their growth in contrasting Eurasian regions in response to climate warming. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:662–667. doi: 10.1073/pnas.1514717113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MD, Knapp AK, Collins SL. A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology. 2009;90:3279–3289. doi: 10.1890/08-1815.1. [DOI] [PubMed] [Google Scholar]
- Smith B, Wilson JB. A consumer’s guide to evenness indices. Oikos. 1996;76:70–82. [Google Scholar]
- Socher SA, Prati D, Boch S, Müller J, Klaus VH, Hölzel N, Fischer M. Direct and productivity-mediated indirect effects of fertilization, mowing and grazing on grassland species richness. Journal of Ecology. 2012;100:1391–1399. [Google Scholar]
- Socher SA, Prati D, Boch S, et al. Interacting effects of fertilization, mowing and grazing on plant species diversity of 1500 grasslands in Germany differ between regions. Basic and Applied Ecology. 2013;14:126–136. [Google Scholar]
- Stevens CJ. How long do ecosystems take to recover from atmospheric nitrogen deposition? Biological Conservation. 2016;200:160–167. [Google Scholar]
- Stevens CJ, Dise NB, Mountford JO, Gowing DJ. Impact of nitrogen deposition on the species richness of grasslands. Science. 2004;303:1876–1879. doi: 10.1126/science.1094678. [DOI] [PubMed] [Google Scholar]
- Storkey J, Macdonald AJ, Poulton PR, Scott T, Köhler IH, Schnyder H, Goulding KWT, Crawley MJ. Grassland biodiversity bounces back from long-term nitrogen addition. Nature. 2015;528:401–404. doi: 10.1038/nature16444. [DOI] [PubMed] [Google Scholar]
- Suttie JM, Reynolds SG, Batello C. Grasslands of the World. Food and Agriculture Organization of the United Nations; Rome, Italy: 2005. [Google Scholar]
- The Food and Agriculture Organization of the United Nations (FAO) FAO; Rome, Italy: 2015. World Fertilizer Trends and Outlook to 2018. [Google Scholar]
- Tilman D. Secondary succession and the pattern of plant dominance along experimental nitrogen gradients. Ecological Monographs. 1987;57:189–214. [Google Scholar]
- Tilman D, Downing JA. Biodiversity and stability in grasslands. Nature. 1994;367:363–365. [Google Scholar]
- Tilman D, Lehman CL, Bristow CE. Diversity-stability relationships: statistical inevitability or ecological consequence? American Naturalist. 1998;151:277–282. doi: 10.1086/286118. [DOI] [PubMed] [Google Scholar]
- Wan S, Luo Y, Wallace LL. Changes in microclimate induced by experimental warming and clipping in tallgrass prairie. Global Change Biology. 2002;8:754–768. [Google Scholar]
- Yang HJ, Jiang L, Li LH, Li A, Wu MY, Wan SQ. Diversity-dependent stability under mowing and nutrient addition: evidence from a 7-year grassland experiment. Ecology Letters. 2012;15:619–626. doi: 10.1111/j.1461-0248.2012.01778.x. [DOI] [PubMed] [Google Scholar]
- Yuan ZY, Li LH, Han XG, Jiang FH, Lin GH, Zhao MX, Ren LY. Effects of simulated grazing pattern and nitrogen supply on plant growth in a semiarid region of northern China. Acta Botanica Sinica. 2004;46:1032–1039. [Google Scholar]
- Zhang YH, He NP, Zhang GM, Huang JH, Han XG. Nitrogen deposition and Leymus chinensis leaf chlorophyll content in Inner Mongolian grassland. Acta Ecologica Sinica. 2013;33:6786–6794. [Google Scholar]
- Zhang YH, Lü XT, Isbell F, et al. Rapid plant species loss at high rates and at low frequency of N addition in temperate steppe. Global Change Biology. 2014;20:3520–3529. doi: 10.1111/gcb.12611. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Feng JC, Isbell F, Lü XT, Han XG. Productivity depends more on the rate than the frequency of N addition in a temperate grassland. Scientific Reports. 2015;5:12558. doi: 10.1038/srep12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Loreau M, Lü XT, He NP, Zhang GM, Han XG. Nitrogen enrichment weakens ecosystem stability through decreased species asynchrony and population stability in a temperate grassland. Global Change Biology. 2016a;22:1445–1455. doi: 10.1111/gcb.13140. [DOI] [PubMed] [Google Scholar]
- Zhang YH, Stevens CJ, Lü XT, He NP, Huang JH, Han XG. Fewer new species colonize at low frequency N addition in a temperate grassland. Functional Ecology. 2016b;30:1247–1256. [Google Scholar]
- Zhang YH, Loreau M, He NP, Zhang GM, Han XG. Data from: Mowing exacerbates the loss of ecosystem stability under nitrogen enrichment in a temperate grassland. Dryad Digital Repository. 2017 doi: 10.5061/dryad.sq15g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JX, He NP, Wang QF, Yuan GF, Wen D, Yu GR, Jia YL. The composition, spatial patterns, and influencing factors of atmospheric wet nitrogen deposition in Chinese terrestrial ecosystems. Science of the Total Environment. 2015;511:777–785. doi: 10.1016/j.scitotenv.2014.12.038. [DOI] [PubMed] [Google Scholar]