Abstract
Following more than two decades of effort, reflectance confocal microscopy (RCM) imaging of skin was granted codes for reimbursement by the US Centers for Medicare and Medicaid Services. Dermatologists in the USA have started billing and receiving reimbursement for the imaging procedure and for the reading and interpretation of images. RCM imaging combined with dermoscopic examination is guiding the triage of lesions into those that appear benign, which are being spared from biopsy, against those that appear suspicious, which are then biopsied. Thus far, a few thousand patients have been spared from biopsy of benign lesions. The journey of RCM imaging from bench to bedside is certainly a success story, but still much more work lies ahead toward wider dissemination, acceptance, and adoption. We present a brief review of RCM imaging and highlight key challenges and opportunities. The success of RCM imaging paves the way for other emerging optical technologies, as well—and our bet for the future is on multimodal approaches.
Keywords: reflectance confocal microscopy, skin, skin cancer, melanoma, non-melanoma, reimbursement codes
Introduction
On January 1st, 2016, following more than two decades of research and development, commercialization, translational studies and clinical trials, reflectance confocal microscopy (RCM) imaging of skin was granted category I current procedural terminology (CPT) reimbursement codes (96931–96936) by the US Centers for Medicare and Medicaid Services (CMS) [1]. Dermatologists in the USA have started billing and receiving reimbursement for the imaging procedure and for the reading and interpretation of images. Leading up to this milestone, a core worldwide group of pioneering “early adopter” dermatologists has been testing and implementing RCM imaging to actively guide patient care. RCM imaging combined with dermoscopic examination is guiding the triage of lesions into those that appear benign, which are being spared from biopsy, against those that appear suspicious, which are then biopsied and treated. At this point, the initial impact is promising: a few thousand patients have been spared from biopsy of benign lesions and these lesions are now being noninvasively monitored.
The CPT codes are designed to parallel those that are currently in use for routine biopsy and pathology (Table 1), and are categorized under “Special Dermatological Procedures” [1]. The first three codes are for imaging on a single lesion on a patient. One code (96931) is for image acquisition, followed by reading the images and producing a report. (Images must be in the format of mosaics, as further explained below.) The first code is applicable when all three procedures are carried out by a single clinician. The second code (96932) is for image acquisition only, and third code (96933) is for only reading and producing a report. These two codes are applicable when each procedure is carried out by a separate clinician. Then, there are three equivalent codes (96934, 96935, 96936) for each additional lesion, for those cases in which patients present multiple lesions. At the time of this writing, a preliminary physician fee schedule for 2017, with proposed values for all six codes, is available [1]. The final fee schedule, which will, of course, vary by region, will be determined in November and December, 2016 and will officially go into effect on January 1st, 2017.
Table 1. Category I Current Procedural Terminology (CPT) Reimbursement Codes for Reflectance Confocal Microscopy (RCM) Imaging of Skin, Granted by the US Centers for Medicare and Medicaid Services (CMS), On January1st, 2016.
| CPT code | Procedure |
|---|---|
| 96931 | RCM imaging of cellular and sub-cellular detail in skin. Image acquisition, interpretation and report for the first lesion |
| 96932 | Image acquisition for the first lesion |
| 96933 | Interpretation and report for the first lesion |
| 96934 | Image acquisition, interpretation and report for each additional lesion |
| 96935 | Image acquisition for each additional lesion |
| 96936 | Interpretation and report for each additional lesion |
At the time of this writing, CMS released a preliminary physician fee schedule for 2017, with proposed reimbursement values for all six codes [1]. The final fee schedule will be determined in November and December, 2016 and will officially go into effecton January 1st, 2017.
The journey of RCM imaging from bench to bedside, along with the newly received CPT codes, is certainly a success story of technology development, academic-industry partnership, clinical collaboration on a global scale, and advancing patient care. However, our work is far from over. Challenges lie ahead—and must be addressed—toward wider dissemination, adoption, and clinical impact. We present a brief history of the RCM imagingjourney and highlight key challenges and opportunities: current practice and pitfalls for guiding diagnosis, emerging role in guiding therapy with potentially larger impact, building smaller, lower-cost and easier-to-use microscopes, creating a platform for training and education, and harnessing multimodal approaches to enhance current cellular-imaging capability with molecular and functional contrast. The success of RCM imaging paves the way for other emerging optical technologies, as well—and our bet for the future is on multimodal approaches.
The “ABCDE” Rule and Dermoscopy to Guide Diagnosis
Approximately 3.6 million new cases of skin cancer are diagnosed in the USA every year, and another million in the rest of the world [2–4]. In the 1980s, dermatologists, relying mainly on visual inspection, augmented by a magnifying glass, determined the gross clinical morphologic features to discriminate malignant skin lesions from benign ones, producing the widely known “ABCDE” rule for pigmented lesions [5]. However, early detection of many skin cancers remained difficult. To improve the sensitivity for early detection, while limiting the number of unnecessary biopsies of benign lesions, dermatologists explored approaches to further augment visual inspection. One approach that has greatly impacted skin cancer detection is dermoscopy. The dermoscope is a hand held microscope with 4–10× magnification that permits observation of subsurface morphologic features that are not visible to the unaided eye. In the 1990s, advances in dermoscopy led to clear improvements in diagnostic accuracy [6–9]. An experienced dermatologist can detect melanocytic lesions and pigmented basal cell carcinomas with sensitivity and specificity of about 90%. However, for lightly- or non-pigmented melanocytic lesions (hypomelanotic and amelanotic lesions), for non-pigmented non-melanocyticlesions, for featureless or feature-poor lesions and for small lesions, the specificity tends to be quite variable and can be much lower (32–90%).
Clinical experience, dermoscopic knowledge, lesion type, and patient characteristics all impact the ultimate diagnostic accuracy and benign-to-malignant biopsy ratio. Not surprisingly, this ratio is quite variable, ranging from as high as 2-to-1 to as low as 47-to-1 in adults, to an astonishing 600-to-1 in children and adolescents [10–17]. Needless to say, the fear of missing a skin cancer, particularly a melanoma, results in the biopsy of millions of benign lesions each year. Although a biopsy of skin is a relatively minor procedure, it is invasive, leaves a scar, associated with some degree of morbidity and is expensive. Most lesions in high-risk individuals, including those with the atypical mole syndrome which present hundreds of nevi, are benign, and most skin cancers, including melanomas, arise de novo, which makes biopsy or excision of all lesions impractical and even unethical. Thus, dermatologists must frequently decide on which potentially malignant lesions to biopsy, while avoiding the biopsy of potentially benign lesions. The natural question that then arises is whether technologies beyond dermoscopy can further improve our ability to detect skin cancer. Toward this end, noninvasive high-resolution optical approaches offer attractive possibilities. Such approaches can be performed directly on the patient, in real-time, at the bedside, and, likely, at reduced (or, at least, justifiable) cost.
Beyond Dermoscopy: Higher-Resolution Sub-Surface Approaches
A number of optical imaging and spectroscopic technologies have been developed for noninvasive detection of skin cancers [18–21]. These include RCM, optical coherence tomography (OCT), diffuse reflectance spectroscopy including multi- and hyper-spectral approaches, two-photon microscopy, Raman spectroscopy, and multi-modal spectroscopy [22–61]. Of these, RCM imaging is currently the furthest along in clinical settings, with level I and level II evidence supporting its role in enabling dermatologists to discriminate benign from malignant lesions with high sensitivity and specificity. The five studies that were assessed by the American Academy of Dermatology (who supported the development of the CPT codes and their reimbursement values) and by the American Medical Association (who presented to the CMS) demonstrated level IIa, level IIb, and level Ib evidence [22,23,27,28,32].
Reflectance Confocal Microscopy
RCM imaging noninvasively shows nuclear and cellular-level morphology in human skin in vivo [62–68]. With optical sectioning of 2–5 μm and resolution of 0.5–1.0 μm, imaging of the epidermis and the underlying papillary dermis in small fields-of-view of, typically, 0.5 × 0.5 mm2 and down to depths of 100–200 μm is routinely performed. Imaging is based on the detection of singly backscattered photons from the optical section and contrast is due to the relative variations in refractive indices and sizes of organelles and microstructures [66–69]. Strong signal and bright contrast is obtained particularly from melanin, keratin, and collagen. Imaging is in en face planes, oriented parallel to the skin surface. Imaging in depth is performed by acquisition of a stack of images. At any depth of interest, a two-dimensional matrix of images is acquired and stitched together into a mosaic to increase the field of view, to up to 8 × 8mm2. Mosaicking overcomes the inherent limitation of small field-of-view and enables display of larger areas of tissue. In recognition of this quintessential feature, CMS's mandate for use of the CPT codes is acquisition of several mosaics at different depths (followed by reading and interpretation to render a diagnosis). The powerful ability to acquire mosaics and stacks with RCM imaging in real-time facilitates noninvasive probing of a relatively large volume of tissue in vivo. By comparison, in pathology, the usual examination of a few 5 μm-thin sections from, say, a 2 mm-biopsy within, say, an equivalently sized 8 × 8 mm2 lesion, samples less than 1% of the volume. The significantly larger sampling of tissue with RCM imaging is likely to help in the detection of focal malignancies and regions with sparse tumor, which may then facilitate, to some extent, higher level of sensitivity.
The depth of RCM imaging is limited to about 200 μm, which reaches into the papillary dermis. Nonetheless, this depth routinely includes and allows for the examination of the dermal–epidermal junction, which is usually at depths of 50–150 μm. For dermatologists and pathologists, the dermal–epidermal junction is of key interest, as almost all skin cancers originate and spread from the basal cell layer at this location. Another limitation is that the imaging relies on endogenous reflectance at a single illumination wavelength, and the resulting contrast is grayscale with limited specificity for microstructure. Thus, the dermal–epidermal junction can be difficult to visualize, particularly in lightly pigmented skin or when disrupted or effaced in lesional skin. Melanin cannot be easily distinguished from keratin, and melanocytes cannot be easily differentiated from keratinocytes, or Langerhans cells [69,70]. Neither can different types of inflammatory cells such as melanophages be differentiated from lymphocytes. Nonetheless, despite these limitations, the pioneering early adopter dermatologists learned to read images and distinguish types of cells and microstructural detail based on differences in morphology. As a result, we have witnessed tremendous progress in translation of RCM imaging for detection of skin cancers.
Translational Advances
A PubMed search reveals that, as of today, about 200 studies have been published on skin cancers and inflammatory conditions, and about another 200 on characterization of normal skin, pigmentary and hair disorders, wound healing, infectious disease, and oral and genital disease. Several studies reported RCM imaging to achieve sensitivity of 70–92% and specificity 84–88% for melanocytic lesions and sensitivity of 100–92% and specificity 85–97% for non-melanocytic skin lesions [22–27]. Although the sensitivity is comparable to that of dermoscopy, the specificity is two times superior, especially for lightly- and non-pigmented melanocytic lesions and for non-melanocytic lesions. Such lesions often do not present specific features on dermoscopy. The imaging was found to be particularly useful for detecting hypomelanotic and amelanotic melanomas, achieving sensitivity of 85%, and specificity 84% [26,71–73]. Level II evidence was reported that RCM imaging, as a second-level examination following dermoscopy, may significantly reduce the number of biopsies of benign lesions, by 50–68%, and, furthermore, dermoscopy combined with RCM imaging may be highly sensitive, up to 98% [26,27].
The reproducibility of diagnostic features and consensus for diagnostic accuracy was reported to be reasonably promising [29]. Image reading and diagnosis of 100 lesions, which included 55 melanocytic nevi, 20 melanomas, and 15 basal cell carcinomas, by nine dermatologists from six countries, was accomplished with average sensitivity of 89% (range 83–100%) and average specificity of 79% (range 69–91%). Average diagnostic accuracy was 83% (range 76–89%). Interestingly, diagnosis by majority (consensus of five or more readers) was accomplished with sensitivity of 100% and specificity of 80% and diagnostic accuracy of 87%, suggesting that there may be more diagnostic detail to be gleaned from collective expertise and experience than that from individuals. Experienced dermatologists showed higher sensitivity relative to that of novice readers (91% vs. 85%) but comparable specificity (80% vs. 78%). Overall, eight diagnostic features showed moderate to good reproducibility, with kappa 0.31–0.83. Obviously, there is room for improvement, which will likely happen over time: as with dermoscopy and as with any new technology, reaching widespread acceptance and consensus will require more such RCM community-wide studies, and will evolve with more training and more clinical experience, all of which will test and further refine the consistency and robustness of the current diagnostic features and approaches.
A meta-analysis of five studies, on a total of 909 patients, reported sensitivity of 93% (range 89–96%) and specificity of 76% (range 69–83%) for melanocytic lesions [74]. The analysis further predicted that, in dermoscopically concerning lesions, RCM imaging may increase specificity twofold. Another meta-analysis of six studies, involving a total of 1,126 lesions of basal cell carcinomas, reported sensitivity of 97% (range 90–99%), and specificity of 93% (range 88–96%) [75]. However, the authors caution against the relatively small number of studies for basal cell carcinomas and potential selection bias in some, and, to address this limitation, have launched a larger and randomized trial, currently in progress [76]. Meanwhile, the third and latest meta-analyses of sub-groups from a total of 21 studies involving 3,108 patients and 3,602 lesions revealed overall sensitivity of 94% (range 92–96%) and specificity of 83% (range 81–84%) for all types of skin cancers, and, in particular, sensitivity of 93% (range 90–95%) and specificity of 78% (range 76–81%) for melanomas, and sensitivity of 92% (range 87–95%) and specificity of 91% (range 84–96%) for basal cell carcinomas [77]. Other retrospective analyses claimed that dermoscopy combined with RCM imaging could “dramatically” reduce the need for biopsy of benign lesions [78–80].
Indeed, these predictions have been borne out by the results of four recent prospective studies, which assessed the number needed to treat (NNT) for dermoscopy and for dermoscopy combined with RCM imaging [31–34]. The NNT ratio is the rate of detection of ambiguous lesions that are biopsied per actually detected melanoma. In one university hospital setting, the NNT dropped from (a hypothetical) 3.73 with dermoscopy alone to 2.87 when guided by dermoscopy and RCM imaging [31], and in another similar setting, from 14.6 to 6.8 [32]. In the third report, data from over a 6-month period revealed an NNT of 19.41 in regional healthcare settings, where only dermoscopy was used, which dropped to 6.25 in a university hospital setting, where dermoscopy was combined with RCM imaging [33]. In the fourth and very latest study, the NNT was reportedtobe2.4 [34]. These results confirm that dermoscopy and RCM imaging together can decrease the NNT ratio by about two times relative to that with dermoscopy alone. In these settings, benign lesions are now not being biopsied, but, instead, noninvasively monitored.
Current Practice and Pitfalls for Guiding Diagnosis
The issuing of CPT codes by CMS ushers in the next generation of skin cancer diagnostics. RCM imaging is now advancing to daily and routine use in our Dermatology Service at Memorial Sloan Kettering Cancer Center (MSKCC). To date, the imaging has guided patient care in more than 200 cases. We present example cases to highlight the utility and pitfalls of this imaging procedure (Figs. 1–4). Three cases highlight the utility of dermoscopic examination and RCM imaging to guide patient care in various situations: to spare biopsy of a benign lesion, to confirm the need to perform biopsy of a suspicious lesion, and to provide a second level confirmation of diagnosis followed by guiding treatment choices (Figs. 1–3). The fourth case highlights a pitfall: the lack of specificity for differentiating between melanocytes and Langerhans cells in melanocytic lesions (Fig. 4).
Fig. 1.
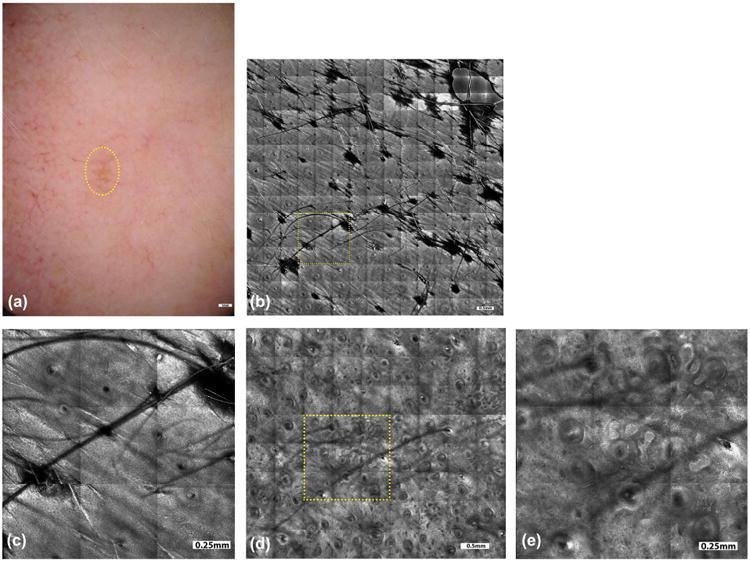
A female patient, 51 years old, noticed a pigmented lesion on her left cheek, which had not changed during the past year, but she was concerned due to previous history of melanoma. The dermoscopic picture (a, yellow circled area) appeared nebulous with no specific features. RCM imaging was performed to rule out malignant melanoma of lentigo maligna type. Mosaics (b) and magnified sub-mosaics (yellow square area, c) in the supra-basal layer showed shows regular honeycomb network of spinous cells with focal cobblestone pattern. No bright atypical pagetoid dendritic cells were identified in the superficial epidermis or around the hair follicle, which ruled out the diagnosis of lentigo maligna. Mosaics (d) and magnified sub-mosaics (yellow square area, e) at the dermal-epidermal junction showed branching tubular structures with bulbous projections or cord-like rete-ridges, lined by bright keratinocytes along with solar elastosis in the surrounding dermis. In this case, RCM imaging spared biopsy of a benign lesion and addressed the patient's concern. Follow-up imaging will be performed in 6 months to monitor the lesion. (All patient cases and figures for this paper were prepared by Drs. Manu Jain, Miguel Cordova, and Kivanc Kose in our Dermatology Service at MSKCC).
Fig. 4.
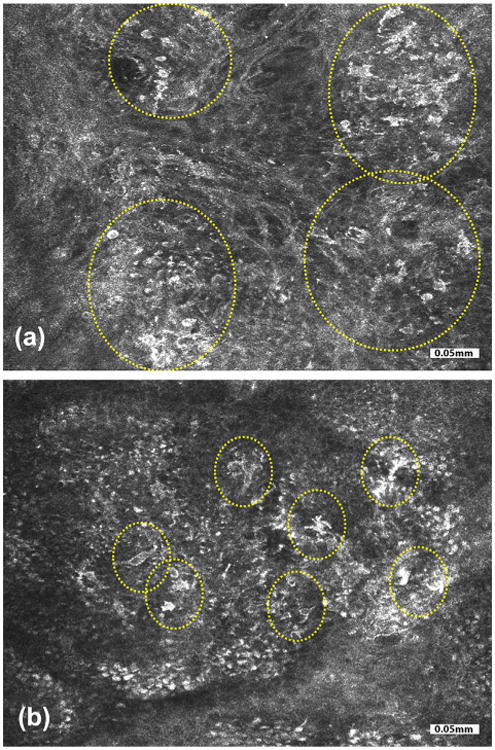
A pitfall of RCM imaging is highlighted in these two cases, each showing a compound melanocytic lesion. In one lesion (a), bright dendritic cells with roundish nuclear morphology were seen in the basal layer at the dermal-epidermal junction (yellow circled areas). Pathology and immunostaining revealed proliferation of melanocytes as either solitary cells or small and large nests situated at dermal-epidermal junction, in the overlying basal and spinous layers and underlying papillary dermis. This lesion turned out to be a melanoma. In the other lesion (b), bright pleomorphic and dendritic cells were seen in pagetoid patterns (yellow circled areas). Here, pathology and immunostaining revealed proliferation of melanocytes along dermal-epidermal junction as well as scattered Langerhans cells in the overlying epidermis. This lesion turned out to be a lentiginous compound melanocytic nevus with some architectural disorder, slight atypia, and partial regression. Details of this study, including the pathology and immunostaining correlation results, are available in reference [70]. Bright cells in pagetoid patterns in RCM images may suggest the presence of pagetoid melanocytes and is a useful feature to guide diagnosis of melanoma, but this observation can be confounded with the presence of similar appearing Langerhans cells. Bright cells in pagetoid patterns often tend to be Langerhans cells in benign nevi whereas they are usually melanocytes in melanomas.
Fig. 3.
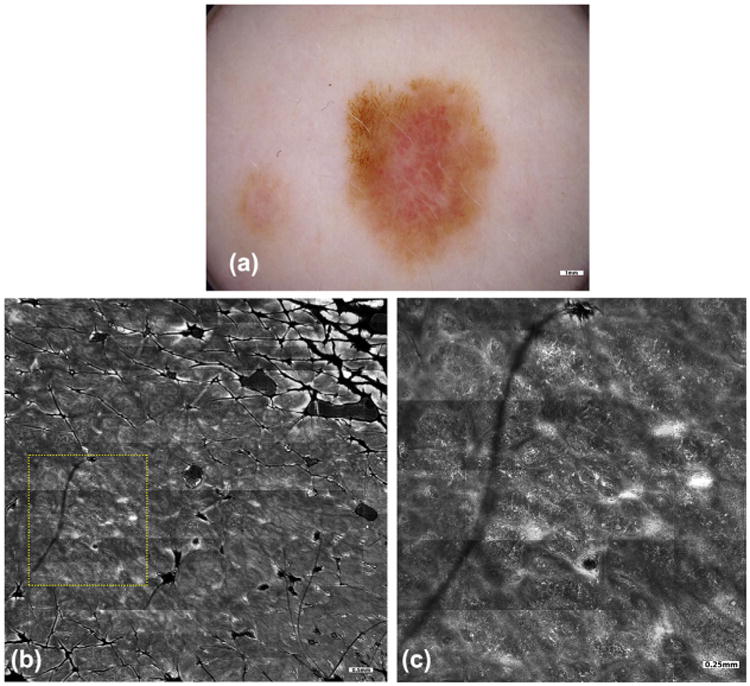
On a 38-year-old female patient with a history of melanoma and atypical mole syndrome, skin examination with total body photography revealed changes in a 1.3 cm-sized lesion on her right abdomen, relative to an earlier baseline examination. The lesion developed a focal darker area and appeared more pink incolor. Dermoscopy (a) revealed focal atypical networkinthe darker area and shiny white lines and dotted vessels in the pink area. Dermoscopic examination was supplemented with RCM imaging, given the patient's history and high risk for developing melanoma, to determine whether a nevus could be differentiated from melanoma, and perhaps avoid performing an unnecessary biopsy. Mosaics (b) and magnified sub-mosaics (yellow square area, c) revealed widespread pagetoid cells, epidermal atypia, and disarray of the spinous layers, perifollicular infiltration of atypical cells and non-edged dermal papillae. All of these features were highly suggestive of melanoma, leading to a complete excision of the lesion. The pathology confirmed the presence of melanoma in situ arising in association with a melanocytic nevus. In this case, the impact of RCM imaging was to confirm the need to perform an excisional biopsy of this lesion.
In our experience, the additional time for acquistion of RCM mosaics and stacks, followed by reading and interpretation, varies from several minutes up to an hour, depending on the size and complexity of the lesion. For diagnosis, most lesions can be imaged within 20–30 minutes, and image reading can take up to as much more time. Mapping of sub-clinical sub-surface margins of large and widespread lentigo maligna lesions can take longer. Imaging involves, typically, the acquisition of 3–5 mosaics at several levels in the epidermis and superficial dermis, followed by the additional acquisition of 2–4 stacks. For image reading and interpretation, the clinician examines each mosaic with low and high magnifications (similar to the procedure for examining pathology), inspects each of the stacks, and then writes an electronic report. Note that there is a learning curve associated with any new technology, in terms of effort, time, and utility. What remains very much work in progress is seamless integration of RCM imaging into current clinical practice and workflow, to optimize speed and efficiency. This will, no doubt, occur with wider usage and more experience.
The CPT codes provide reimbursement on a per-lesion basis. The reimbursements account for the anticipated range of time and effort for acquisition and interpretation of images, and for number of lesions. This is similar to the reimbursements for biopsy and pathology, which are on a per-lesion basis. Indeed, the currently proposed preliminary fee schedule for RCM imaging is fairly on par with that for biopsy and pathology procedures. The new fee schedule provides a starting point and will be initially defined for 3 years. Wider adoption and use by larger numbers of dermatologists, and further experience will subsequently help CMS reassess the cost in terms of time and effort, and if necessary, redefine the appropriate reimbursement for the longer term.
The use of RCM imaging appears to be clinically most indicated for lesions in the head and neck area, lesions in sun-damaged areas and lesions which appear dermoscopically regressed [34]. As with any other approach, the imaging, too, has its pitfalls [34,81,82]. Pitfalls include misdiagnosis of certain types of lesions due to either its characteristics or missing detection due to sampling errors. Nodular melanomas tend to be deeper than the maximum depth of imaging and can be missed. Nevoid melanomas with bland pathology can result in false negatives. Nevi with severe atypia can result in false positives. (However, biopsy is usually recommended to rule out melanoma in such cases, and such high-grade lesions are typically excised.) Small focal areas of melanoma in situ within a larger lesion can be missed. The presence of Langerhans cells, which cannot always be differentiated from melanocytic cells, can lead to false positives. The presence of inflammatory infiltrates can hide cellular morphology of the underlying melanoma. Certain lesions such as Spitz nevi can be confounded for melanoma and produce false positives. Certain subtypes of basal cell carcinomas such as morpheaform or infiltrative can be more difficult to detect, as thin cords of tumor cells or individual atypical tumor cells may be difficult to discern in the dermis. Many of these pitfalls are also situations in which even conventional pathology may not be conclusive or may need further expert or consensus opinion. In all such cases, if clinical and dermoscopic examination suggest a malignant or concerning lesion, and RCM imaging turns out not conclusive, then further assessment or biopsy should be performed.
Related to the clinical pitfalls and technical limitations of RCM imaging is the issue of malpractice and liability and quality control. Any liability associated with RCM imaging is expected to be no different than that potentially incurred with current use of other noninvasive imaging approaches such as dermoscopy and total body photography, and the use of other diagnostic tools for assessing skin lesions. The issue of quality control will also need to be addressed by the RCM imaging community. Similar to quality control processes in pathology and radiology, we are now developing written and standardized protocols for use on patients, with guidelines for acceptable image quality in terms of resolution, contrast and appearance of cellular and morphologic details.
Emerging Role in Guiding Therapy
Although the availability of CPT codes and the resulting foray into routine practice are certainly major steps forward, still much more work lies ahead. Beyond all the effort to date, which was focused on imaging to guide diagnosis, an emerging opportunity is imaging to guide therapy. The role and impact of RCM imaging for guiding therapy is potentially as large as—if not larger than—that for guiding diagnosis. In fact, a glimmer of this potential is already evident in the ability of RCM imaging to guide diagnosis as well as treatment of lentigo maligna lesions [83–86]. Detection of lentigo maligna is possible with sensitivity of 85% and specificity 76% [84]. On 37 complex cases of lentigo malignas, which included five lentigo maligna melanomas, the noninvasive imaging of margins guided choice of therapy. The imaging led to changes in treatment and management in 27 patients, with 11 undergoing major revisions in surgical excision and the remaining given topical or radiation therapy [85]. The imaging can also detect equivocal pigmented lesions concerning for lentigo maligna, as prospectively shown on 17 patients, with sensitivity of 100%, specificity 71% and concordance with pathology of 89% [87]. Similarly, the ability to non-invasively detect lateral margins and depth of superficial lesions as well as residual tumor margins, directly on patients, may allow the use of RCM imaging to guide and monitor Mohs surgery and surgical excision of basal cell carcinomas [88–93].
Perhaps, most exciting is the possibility that the imaging may provide “game changing” impetus to emerging newer and less invasive therapies, which do not involve conventional removal of tissue and pathology. For example, a number of studies reported the feasibility of imaging-guided laser ablation, radiation therapy, topical (Imiquimod), and photodynamic therapy of superficial, and early nodular types of basal cell carcinomas and of lentigo malignas [94–101]. The results are, for now, limited to small numbers of patients. Larger and randomized studies to test for efficacy in terms of reliability, consistency, cure rates and cosmetic outcomes remain to be carried out. Additional CPT codes, too, may be necessary to support new therapeutic applications. However, if successful in the long-term, for appropriately superficial types of lesions, one may imagine RCM imaging-guided diagnosis, treatment and monitoring to be performed all directly on patients, as a single procedure in a single visit, while reducing the need for traditional biopsy, surgery and pathology. In fact, dermatologic surgeons at MSKCC are already showing preliminary success with this approach for small superficial and nodular basal cell carcinomas [99–101].
Next Generation of Microscopes
Although the size and cost of commercially available RCM imaging devices for skin (Vivascope, Caliber Imaging & Diagnostics) are shrinking, currently available microscopes are still too large, expensive and cumbersome to support wider dissemination, and thus remain mostly confined to tertiary academic healthcare centers. An entirely new class of smaller, lower-cost and easier-to-use microscopes is needed for a “quantum leap” in dissemination. Toward this objective, new devices based on divided-pupil line-scanning and miniaturized micro-electro-mechanical systems' approaches are being engineered [102–104]. Both approaches show promise for imaging skin, as seen in bench-top studies, but are yet to be developed for clinical use.
Training and Education
Training the next generation of dermatologists and pathologists to read and interpret mosaics, either at the bedside or remotely, is critical for wider acceptance and adoption of RCM imaging. Although slowly increasing, the number of clinicians who can interpret with high accuracy and consistency is small. Although the morphologic features are similar to those of pathology, a substantial investment of effort is still required to review a large number of cases and become proficient in interpreting the en face grayscale mosaics. In a set of 100 lesions read by nine dermatologists, those with more than 3 years' experience performed with higher sensitivity than those with less (94% vs. 85%) while the specificity was on par (80% vs. 78%) [29]. In another set of 334 lesions, a dermatologist with 9 years' experience read with sensitivity of 97% and specificity 80%, whereas a novice with less than a year's experience demonstrated sensitivity of 93% and specificity 64% [28]. Not surprisingly, with more experience, in a follow up set of 119 lesions, the same (but now less novice) reader performed better, with sensitivity of 100% and specificity 92% [30].
Moreover, the current procedure for image acquisition, typically performed by clinical staff, requires the ability to first locate the topmost surface of skin (stratum corneum) and then visually identify the clinically most relevant depths and acquire mosaics, with at least one at the dermal–epidermal junction. This purely visual and qualitative approach is subject to user expertise and opinion, and therefore necessitates a significant investment in training. An alternative is to use a semi-automated image-acquisition procedure that captures mosaics at pre-defined depths. But this approach carries the risk of missing the clinically most important layers and/or the dermal–epidermal junction. Therefore, some combination of both approaches will likely produce the most accurately and consistently located set of mosaics. Based on the collective experience of the core group of early adopter dermatologists, a consensus is evolving for a standardized approach for imaging, which involves acquisition of mosaics, to display large areas of lesions, at a few discrete user-selected depths encompassing the dermal–epidermal junction, followed by acquisition of stacks with finely-spaced images, to display lesion morphology with depth. This experience and evolving consensus led to CMS's determination of the imaging approach—mandatory acquisition of 3–5 mosaics, optional acquisition of 2–4 stacks—for use of the CPT codes. Consequently, our next phase of work is now necessarily focused on standardizing, implementing, and disseminating this approach. Training of new users by the early adopters is in progress.
Beyond such efforts, quantitative approaches to supplement and guide the traditional visual approach may help to shorten the learning curve. Research is, in fact, already in progress to create computational models and machine learning-based algorithms for both image acquisition and image analysis [105–110]. Such models and algorithms may eventually produce quantitative tools to guide standardized acquisition, reading and diagnosis, as well as, serve as a platform for training and education.
Multimodal Approaches
Other technologies that are currently in translation may compete with RCM imaging [18–21]. More likely, though, we will see synergistic collaboration, with their complementary advantages in molecular and functional contrast adding to the cellular-level detail (and thus overcoming the limitations) of RCM imaging. OCT (Michaelson Diagnostics) images faster and deeper in skin, and can detect basal cell carcinomas with sensitivity 79–84% and specificity 85–96% [35–37]. High definition-OCT (SkinTell) can detect melanoma, based on optical properties, with higher diagnostic accuracy (82–95%) and negative predictive value (89–97%) than those based on morphology [37]. Two-photon microscopy (DermaInspect) provides exquisite specificity of contrast, and can detect melanocytic lesions with sensitivity of 71–76% and specificity of 97–72% [38]. Raman spectroscopy (Verisante Technology) provides molecular specificity over large fields-of-view, and can detect both melanocytic and non-melanocytic skin lesions with sensitivity of 99–90% and specificity 20–75% [39,40]. Diffuse reflectance spectroscopy provides biochemical and functional specificity. One multispectral approach (Mela-Find) performed with sensitivity of 98% and specificity 9.9% (relative to dermatologists' specificity of 3.7%, in a clinical trial that included mostly atypical lesions and a relatively small number of benign lesions) for detection of melanocytic lesions [41–45]. Other studies indicated range of sensitivity of 78–96% and specificity 46–9%, depending on use of MelaFind alone or in combination with clinical examination and dermoscopy [41,45]. Another multispectral approach, called spectro-photometric intracutaneous analysis (SIAscopy or MoleMate), performs with sensitivity of 84–98% and specificityof84–46% for melanocytic and non-melanocytic lesions [46–49]. Other developments include multi- and hyper-spectroscopy, reporting range of sensitivities from 69% to 97% and specificities from 72% to 87% [50–56]. Multimodal spectral approaches, combining reflectance, fluorescence, and Raman, can classify melanocytic and non-melanocytic lesions with sensitivity 90–100% and specificity 71% to 100% [57,58]. A new polarization-sensitive hyper-spectral imaging approach (SkinSpect) is in clinical testing [59–61].
What will be interesting to see is how these approaches advance and are combined to ultimately produce the best solutions for rapid detection over larger and deeper fields-of-view, with cellular and molecular contrast, and both high sensitivity and high specificity. The integration of dermoscopy and RCM imaging is already an excellent example, dermoscopy combined with OCT is showing promise with sensitivity of 96% and specificity of 75% for basal cell carcinomas [111,112], and preliminary but quite fascinating work on integration of other modes is well on its way [57,58,113–121].
Potential Long-term Global Impact
Finally, the success of RCM imaging paves the way for all other emerging noninvasive optical technologies. If the initial success of RCM imaging is sustained, what might be the potential long-term global impact of optical multimodal approaches? Currently, an NNT of five may be expected with the use of dermoscopy by experienced dermatologists in most cancer care settings. With the added help of RCM imaging, the NNT will reduce by about 50%, to about 2.5. Given the estimated global incidence rate of ∼4.6 million new cases of skin cancer every year, the potential savings can be on the order of ten million biopsies per year. Of course, this will take time and may happen only in the distant future. Meanwhile, in the immediate future, if the NNT drops by even a rather modest level, say, between 1% and 10%, the savings may still amount to between 200,000 and two million biopsies per year worldwide. Surely, if emerging optical multimodal approaches succeed for noninvasive detection, we may anticipate that, in the long run, substantial benefit can accrue to very large numbers of patients.
Fig. 2.
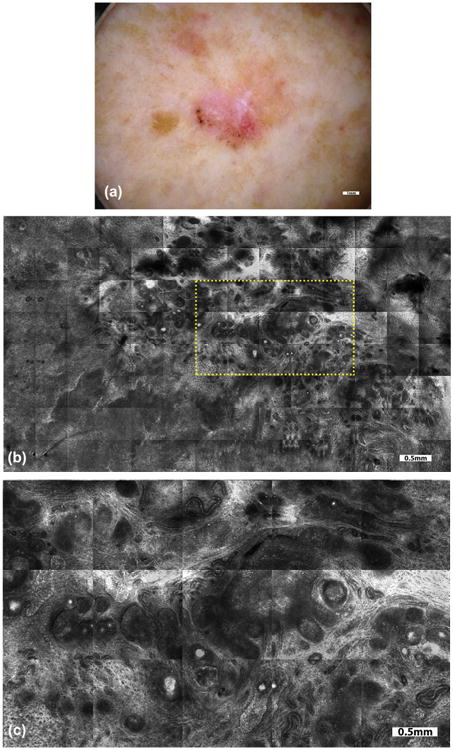
On a 76-year-old female patient, with a history of melanoma and squamous cell carcinoma, skin examination revealed an irregular 1.3 cm-sized pigmented lesion on the left posterior lower leg. Dermoscopy (a) revealed leaf-like structures, concentric globules, and shiny white blotches and strands. Dermoscopic examination was supplemented with RCM imaging to further guide and confirm diagnosis. Mosaics (b) and magnified sub-mosaics (yellow rectangular area, c) revealed streaming of nuclei with tumor nests and vessels, indicating the presence of superficial and nodular basal cell carcinoma (BCC). Based on RCM imaging, the option of proceeding to complete excision versus biopsy and subsequent topical (Imiquimod) treatment was discussed. The patient chose the latter option, and the saucerized specimen confirmed the presence of superficial and nodular BCC with clear margins. In this case, the impact of RCM imaging was to confirm the diagnosis of BCC and guide decision on definitive treatment.
Acknowledgments
Funding support for the initial bench-top instrumentation development work at Massachusetts General Hospital (Wellman Laboratories of Photomedicine) was provided by the Department of Energy and Whitaker Foundation. Some of the subsequent work, to traverse the so-called “valley of death”—initial technology development and initial commercialization at Caliber Imaging and Diagnostics (formerly, Lucid Inc.) and early translational/clinical studies at MSKCC (Dermatology Service)—was supported by grants from the NIH (NCI and NIBIB).
Contract grant sponsor: Department of Energy and Whitaker Foundation; Contract grant sponsor: NIH (NCI and NIBIB).
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and have disclosed the following: Milind Rajadhyaksha is a former employee of, and owns equity in, Caliber Imaging and Diagnostics (formerly, Lucid Inc.), the company that manufactures and sells the VivaScope reflectance confocal microscope. The VivaScope is the commercial version of an original laboratory prototype that was developed by Rajadhyaksha when he was at Wellman Laboratories of Photomedicine, Massachusetts General Hospital, Harvard Medical School. Allan Halpern serves on the scientific advisory board of Caliber Imaging and Diagnostics. None for Ashfaq Marghoob, Anthony Rossi and Kishwer Nehal.
References
- 1.Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. The preliminary physician fee schedule for 2017 is available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html. [Google Scholar]
- 2.Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer AB, Coldiron BM. Incidence estimate of nonmelanoma skin cancer in the United States 2006. Arch Dermatol. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 3.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166:1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 4.Nikolaou V, Stratigos AJ. Emerging trends in the epidemiology of melanoma. Br J Dermatol. 2014;170:11–19. doi: 10.1111/bjd.12492. [DOI] [PubMed] [Google Scholar]
- 5.Rigel DS, Russak J, Friedman R. The evolution of melanoma diagnosis: 25 years beyond the ABCDs. CA Cancer J Clin. 2010;60:301–316. doi: 10.3322/caac.20074. [DOI] [PubMed] [Google Scholar]
- 6.Argenziano G, Soyer HP, Chimenti S, Talamini R, Corona R, Sera F, Binder M, Cerroni L, De Rosa G, Ferrara G, Hofmann-Wellenhof R, Landthaler M, Menzies SW, Pehamberger H, Piccolo D, Rabinovitz HS, Schiffner R, Staibano S, Stolz W, Bartenjev I, Blum A, Braun R, Cabo H, Carli P, De Giorgi V, Fleming MG, Grichnik JM, Grin CM, Halpern AC, Johr R, Katz B, Kenet RO, Kittler H, Kreusch J, Malvehy J, Mazzocchetti G, Oliviero M, Ozdemir F, Peris K, Perotti R, Perusquia A, Pizzichetta MA, Puig S, Rao B, Rubegni P, Saida T, Scalvenzi M, Seidenari S, Stanganelli I, Tanaka M, Westerhoff K, Wolf IH, Braun-Falco O, Kerl H, Nishikawa T, Wolff K, Kopf AW. Dermoscopy of pigmented skin lesions: Results of a consensus meeting via the Internet. J Am Acad Dermatol. 2003;48:679–693. doi: 10.1067/mjd.2003.281. [DOI] [PubMed] [Google Scholar]
- 7.Pagnanelli G, Soyer HP, Argenziano G, Talamini R, Barbati R, Bianchi L, Campione E, Carboni I, Carrozzo AM, Chimenti MS, de Simoni I, Falcomatá V, Filipe Neto I, Francesconi F, Ginebri A, Hagman J, Marulli GC, Palamara F, Vidolin AP, Piemonte P, Soda R, Chimenti S. Diagnosis of pigmented skin lesions by dermoscopy: Web-based training improves diagnostic performance of non-experts. Br J Dermatol. 2003;148:698–702. doi: 10.1046/j.1365-2133.2003.05168.x. [DOI] [PubMed] [Google Scholar]
- 8.Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: A meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669–676. doi: 10.1111/j.1365-2133.2008.08713.x. [DOI] [PubMed] [Google Scholar]
- 9.Menzies SW, Kreusch J, Byth K, Pizzichetta MA, Marghoob A, Braun R, Malvehy J, Puig S, Argenziano G, Zalaudek I, Rabinovitz HS, Oliviero M, Cabo H, Ahlgrimm-Siess V, Avramidis M, Guitera P, Soyer HP, Ghigliotti G, Tanaka M, Perusquia AM, Pagnanelli G, Bono R, Thomas L, Pellacani G, Langford D, Piccolo D, Terstappen K, Stanganelli I, Llambrich A, Johr R. Dermoscopic evaluation of amelanotic and hypomelanotic melanoma. Arch Dermatol. 2008;144:1120–1127. doi: 10.1001/archderm.144.9.1120. [DOI] [PubMed] [Google Scholar]
- 10.Soares TF, Laman SD, Yiannias JA, Connolly SM, Lim KK, Wu Q, Swanson DL. Factors leading to the biopsy of 1547 pigmented lesions at Mayo clinic, Scottsdale, Arizona, in 2005. Int J Dermatol. 2009;48:1053–1056. doi: 10.1111/j.1365-4632.2009.04137.x. [DOI] [PubMed] [Google Scholar]
- 11.Kovalyshyn I, Dusza SW, Siamas K, Halpern AC, Argenziano G, Marghoob AA. The impact of physician screening on melanoma detection. Arch Dermatol. 2011;147:1269–1275. doi: 10.1001/archdermatol.2011.181. [DOI] [PubMed] [Google Scholar]
- 12.Waldmann A, Nolte S, Geller AC, Katalinic A, Weinstock MA, Volkmer B, Greinert R, Breitbart EW. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012;148:903–910. doi: 10.1001/archdermatol.2012.893. [DOI] [PubMed] [Google Scholar]
- 13.Argenziano G, Cerroni L, Zalaudek I, Staibano S, Hofmann-Wellenhof R, Arpaia N, Bakos RM, Balme B, Bandic J, Bandelloni R, Brunasso AM, Cabo H, Calcara DA, Carlos-Ortega B, Carvalho AC, Casas G, Dong H, Ferrara G, Filotico R, Gomez G, Halpern A, Ilardi G, Ishiko A, Kandiloglu G, Kawasaki H, Kobayashi K, Koga H, Kovalyshyn I, Langford D, Liu X, Marghoob AA, Mascolo M, Massone C, Mazzoni L, Menzies S, Minagawa A, Nugnes L, Ozdemir F, Pellacani G, Seidenari S, Siamas K, Stanganelli I, Stoecker WV, Tanaka M, Thomas L, Tschandl P, Kittler H. Accuracy in melanoma detection: A 10-year multicenter survey. J Am Acad Dermatol. 2012;67:54–59. doi: 10.1016/j.jaad.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Menzies SW, Stevenson ML, Altamura D, Byth K. Variables predicting change in benign melanocytic nevi undergoing short-term dermoscopic imaging. Arch Dermatol. 2011;147:655–659. doi: 10.1001/archdermatol.2011.133. [DOI] [PubMed] [Google Scholar]
- 15.Cohen B. To biopsy or not to biopsy changing moles in children and adolescents: Are we removing too many pigmented nevi in this age group? Arch Dermatol. 2011;147:659–660. doi: 10.1001/archdermatol.2011.154. [DOI] [PubMed] [Google Scholar]
- 16.Moscarella E, Zalaudek I, Cerroni L, Sperduti I, Catricala C, Smolle J, Hofmann-Wellenhof R, Sgambato A, Pellacani G, Argenziano G. Excised melanocytic lesions in children and adolescents—A 10-year survey. Br J Dermatol. 2012;167:368–373. doi: 10.1111/j.1365-2133.2012.10952.x. [DOI] [PubMed] [Google Scholar]
- 17.Oliveria SA, Selvam N, Mehregan D, Marchetti MA, Divan HA, Dasgeb B, Halpern AC. Biopsies of nevi in children and adolescents in the United States, 2009 through 2013. JAMA Dermatol. 2015;151:447–448. doi: 10.1001/jamadermatol.2014.4576. [DOI] [PubMed] [Google Scholar]
- 18.March J, Hand M, Grossman D. Practical application of new technologies for melanoma diagnosis: Part I. Noninvasive approaches. J Am Acad Dermatol. 2015;72:929–41. 941–942. doi: 10.1016/j.jaad.2015.02.1138. [DOI] [PubMed] [Google Scholar]
- 19.Menge TD, Pellacani G. Advances in noninvasive imaging of melanoma. Semin Cutan Med Surg. 2016;35:18–24. doi: 10.12788/j.sder.2016.003. [DOI] [PubMed] [Google Scholar]
- 20.Hibler BP, Qi Q, Rossi AM. Current state of imaging in dermatology. Semin Cutan Med Surg. 2016;35:2–8. doi: 10.12788/j.sder.2016.001. [DOI] [PubMed] [Google Scholar]
- 21.Giavedoni P, Puig S, Carrera C. Noninvasive imaging for nonmelanoma skin cancer. Semin Cutan Med Surg. 2016;3:31–41. doi: 10.12788/j.sder.2016.014. [DOI] [PubMed] [Google Scholar]
- 22.Nori S, Rius-Dıaz F, Cuevas J, Goldgeier M, Jaen P, Torres A, Gonzalez S. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: A multicenter study. J Am Acad Dermatol. 2004;51:923–930. doi: 10.1016/j.jaad.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Astner S, Gonzalez E, Cheung A, Rius-Diaz F, González S. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986–992. doi: 10.1016/j.jaad.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Pellacani G, Guitera P, Longo C, Avramidis M, Seidenari S, Menzies S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759–2765. doi: 10.1038/sj.jid.5700993. [DOI] [PubMed] [Google Scholar]
- 25.Gerger A, Hofmann-Wellenhof R, Langsenlehner U, Richtig E, Koller S, Weger W, Ahlgrimm-Siess V, Horn M, Samonigg H, Smolle J. In vivo confocal laser scanning microscopy of melanocytic skin tumours: Diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158:329–333. doi: 10.1111/j.1365-2133.2007.08389.x. [DOI] [PubMed] [Google Scholar]
- 26.Guitera P, Pellacani G, Longo C, Seidenari S, Avramidis M, Menzies SW. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129:131–138. doi: 10.1038/jid.2008.193. [DOI] [PubMed] [Google Scholar]
- 27.Guitera P, Menzies SW, Longo C, Cesinaro AM, Scolyer RA, Pellacani G. In vivo confocal microscopy for diagnosis of melanoma and basal cell carcinoma using a two-step method: Analysis of 710 consecutive clinically equivocal cases. J Invest Dermatol. 2012;132:2386–2394. doi: 10.1038/jid.2012.172. [DOI] [PubMed] [Google Scholar]
- 28.Rao BK, Mateus R, Wassef C, Pellacani G. In vivo confocal microscopy in clinical practice: Comparison of bedside diagnostic accuracy of a trained physician and distant diagnosis of an expert reader. J Am Acad Dermatol. 2013;69:295–300. doi: 10.1016/j.jaad.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 29.Farnetani F, Scope A, Braun RP, Gonzalez S, Guitera P, Malvehy J, Manfredini M, Marghoob AA, Moscarella E, Oliviero M, Puig S, Rabinovitz HS, Stanganelli I, Longo C, Malagoli C, Vinceti M, Pellacani G. Skin cancer diagnosis with reflectance confocal microscopy: Reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075–1080. doi: 10.1001/jamadermatol.2015.0810. [DOI] [PubMed] [Google Scholar]
- 30.Giambrone D, Alamgir M, Masud A, Bronsnick T, Rao B. The diagnostic accuracy of in vivo confocal microscopy in clinical practice. J Am Acad Dermatol. 2015;73:317–319. doi: 10.1016/j.jaad.2015.03.052. [DOI] [PubMed] [Google Scholar]
- 31.Alarcon I, Carrera C, Palou J, Alos L, Malvehy J, Puig S. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170:802–808. doi: 10.1111/bjd.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pellacani G, Pepe P, Casari A, Longo C. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: A longitudinal prospective study. Br J Dermatol. 2014;171:1044–1051. doi: 10.1111/bjd.13148. [DOI] [PubMed] [Google Scholar]
- 33.Pellacani G, Witkowski A, Cesinaro AM, Losi A, Colombo GL, Campagna A, Longo C, Piana S, De Carvalho N, Giusti F, Farnetani F. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J Eur Acad Dermatol Venereol. 2016;30:413–419. doi: 10.1111/jdv.13408. [DOI] [PubMed] [Google Scholar]
- 34.Borsari S, Pampena R, Lallas A, Kyrgidis A, Moscarella E, Benati E, Raucci M, Pellacani G, Zalaudek I, Argenziano G, Longo C. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;31 doi: 10.1001/jamadermatol.2016.1188. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Cheng HM, Guitera P. Systematic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371–1380. doi: 10.1111/bjd.14042. [DOI] [PubMed] [Google Scholar]
- 36.Cheng HM, Lo S, Scolyer R, Meekings A, Carlos G, Guitera P. Accuracy of optical coherence tomography for the diagnosis of superficial basal cell carcinoma—A prospective, consecutive, cohort study of 168 cases. Br J Dermatol. 2016 doi: 10.1111/bjd.14714. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Boone MA, Suppa M, Dhaenens F, Miyamoto M, Marneffe A, Jemec GB, Del Marmol V, Nebosis R. In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch Dermatol Res. 2016;308:7–20. doi: 10.1007/s00403-015-1608-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dimitrow E, Ziemer M, Koehler MJ, Norgauer J, König K, Elsner P, Kaatz M. Sensitivity and specificity of multiphoton laser tomography for in vivo and ex vivo diagnosis of malignant melanoma. J Invest Dermatol. 2009;129:1752–1758. doi: 10.1038/jid.2008.439. [DOI] [PubMed] [Google Scholar]
- 39.Lui H, Zhao J, McLean D, Zeng H. Real-time Raman spectroscopy for in vivo skin cancer diagnosis. Cancer Res. 2012;72:2491–2500. doi: 10.1158/0008-5472.CAN-11-4061. [DOI] [PubMed] [Google Scholar]
- 40.Zhao J, Zeng H, Kalia S, Lui H. Wavenumber selection based analysis in Raman spectroscopy improves skin cancer diagnostic specificity. Analyst. 2016;141:1034–1043. doi: 10.1039/c5an02073e. [DOI] [PubMed] [Google Scholar]
- 41.Friedman RJ, Gutkowicz-Krusin D, Farber MJ, Warycha M, Schneider-Kels L, Papastathis N, Mihm MC, Googe P, King R, Prieto VG, Kopf AW, Polsky D, Rabinovitz H, Oliviero M, CArmand Cognetta A, Rigel DS, Marghoob A, Rivers J, Johr R, Grant-Kels JM, Tsao H. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476–482. doi: 10.1001/archderm.144.4.476. [DOI] [PubMed] [Google Scholar]
- 42.Monheit G, Cognetta AB, Ferris L, Rabinovitz H, Gross K, Martini M, Grichnik JM, Mihm M, Prieto VG, Googe P, King R, Toledano A, Kabelev N, Wojton M, Gutkowicz-Krusin D. The performance of MelaFind: A prospective multicenter study. Arch Dermatol. 2011;147:188–194. doi: 10.1001/archdermatol.2010.302. [DOI] [PubMed] [Google Scholar]
- 43.Wells R, Gutkowicz-Krusin D, Veledar E, Toledano A, Chen SC. Comparison of diagnostic and management sensitivity to melanoma between dermatologists and MelaFind: A pilot study. Arch Dermatol. 2012;148:1083–1084. doi: 10.1001/archdermatol.2012.946. [DOI] [PubMed] [Google Scholar]
- 44.Cukras AR. On the comparison of diagnosis and management of melanoma between dermatologists and MelaFind. JAMA Dermatol. 2013;149:622–623. doi: 10.1001/jamadermatol.2013.3405. [DOI] [PubMed] [Google Scholar]
- 45.Hauschild A, Chen SC, Weichenthal M, Blum A, King HC, Goldsmith J, Scharfstein D, Gutkowicz-Krusin D. To excise or not: Impact of MelaFind on German dermatologists' decisions to biopsy atypical lesions. J Dtsch Dermatol Ges. 2014;12:606–614. doi: 10.1111/ddg.12362. Epub. [DOI] [PubMed] [Google Scholar]
- 46.Emery JD, Hunter J, Hall PN, Watson AJ, Moncrieff M, Walter FM. Accuracy of SIAscopy for pigmented skin lesions encountered in primary care: development and validation of a new diagnostic algorithm. BMC Dermatol. 2010;10:9. doi: 10.1186/1471-5945-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walter FM, Morris HC, Humphrys E, Hall PN, Kinmonth AL, Prevost AT, Wilson EC, Burrows N, Norris P, Johnson M, Emery J. Protocol for the Molemate UK trial: A randomised controlled trial of the Molemate system in the management of pigmented skin lesions in primary care [ISRCTN 79932379] BMC Fam Pract. 2010;11:36. doi: 10.1186/1471-2296-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hacioglu S, Saricaoglu H, Baskan EB, Uner SI, Aydogan K, Tunali S. The value of spectrophotometric intracutaneous analysis in the noninvasive diagnosis of nonmelanoma skin cancers. Clin Exp Dermatol. 2013;38:464–469. doi: 10.1111/j.1365-2230.2012.04460.x. [DOI] [PubMed] [Google Scholar]
- 49.Sgouros D, Lallas A, Julian Y, Rigopoulos D, Zalaudek I, Longo C, Moscarella E, Simonetti V, Argenziano G. Assessment of SIAscopy in the triage of suspicious skin tumours. Skin Res Technol. 2014;20:440–444. doi: 10.1111/srt.12138. [DOI] [PubMed] [Google Scholar]
- 50.Murphy BW, Webster RJ, Turlach BA, Quirk CJ, Clay CD, Heenan PJ, Sampson DD. Toward the discrimination of early melanoma from common and dysplastic nevus using fiber optic diffuse reflectance spectroscopy. J Biomed Opt. 2005;10:064020. doi: 10.1117/1.2135799. [DOI] [PubMed] [Google Scholar]
- 51.Swanson DL, Laman SD, Biryulina M, Nielsen KP, Ryzhikov G, Stamnes JJ, Hamre B, Zhao L, Castellana FS, Stamnes K. Optical transfer diagnosis of pigmented lesions: A pilot study. Skin Res Technol. 2009;15:330–337. doi: 10.1111/j.1600-0846.2009.00367.x. [DOI] [PubMed] [Google Scholar]
- 52.Swanson DL, Laman SD, Biryulina M, Ryzhikov G, Stamnes JJ, Hamre B, Zhao L, Sommersten E, Castellana FS, Stamnes K. Optical transfer diagnosis of pigmented lesions. Dermatol Surg. 2010;36:1979–1986. doi: 10.1111/j.1524-4725.2010.01808.x. [DOI] [PubMed] [Google Scholar]
- 53.Diebele I, Kuzmina I, Lihachev A, Kapostinsh J, Derjabo A, Valeine L, Spigulis J. Clinical evaluation of melanomas and common nevi by spectral imaging. Biomed Opt Express. 2012;3:467–472. doi: 10.1364/BOE.3.000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagaoka T, Nakamura A, Okutani H, Kiyohara Y, Sota T. A possible melanoma discrimination index based on hyper-spectral data: a pilot study. Skin Res Technol. 2012;18:301–310. doi: 10.1111/j.1600-0846.2011.00571.x. [DOI] [PubMed] [Google Scholar]
- 55.Nagaoka T, Nakamura A, Okutani H, Kiyohara Y, Koga H, Saida T, Sota T. Hyperspectroscopic screening of melanoma on acral volar skin. Skin Res Technol. 2013;19:290–296. doi: 10.1111/j.1600-0846.2012.00642.x. [DOI] [PubMed] [Google Scholar]
- 56.Nagaoka T, Kiyohara Y, Koga H, Nakamura A, Saida T, Sota T. Modification of a melanoma discrimination index derived from hyperspectral data: a clinical trial conducted in 2 centers between march 2011 and december 2013. Skin Res Technol. 21:278–283. doi: 10.1111/srt.12188. [DOI] [PubMed] [Google Scholar]
- 57.Lim L, Nichols B, Migden MR, Rajaram N, Reichenberg JS, Markey MK, Ross MI, Tunnell JW. Clinical study of noninvasive in vivo melanoma and nonmelanoma skin cancers using multimodal spectral diagnosis. J Biomed Opt. 2014;19:117003. doi: 10.1117/1.JBO.19.11.117003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajaram N, Reichenberg JS, Migden MR, Nguyen TH, Tunnell JW. Pilot clinical study for quantitative spectral diagnosis of non-melanoma skin cancer. Lasers Surg Med. 2010;42:716–727. doi: 10.1002/lsm.21009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vasefi F, MacKinnon N, Saager RB, Durkin AJ, Chave R, Lindsley EH, Farkas DL. Polarization-sensitive hyperspectral imaging in vivo: A multimode dermoscope for skin analysis. Sci Rep. 2014;4:4924. doi: 10.1038/srep04924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MacKinnon N, Vasefi F, Booth N, Farkas DL. Melanoma detection using smartphone and multimode hyperspectral imaging Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues IX. In: Farkas Daniel L, Nicolau Dan V, Leif Robert C., editors. Proc of SPIE 2016. Vol. 9711. SPIE BiOS; 2016. Apr 6, paper 971117. [Google Scholar]
- 61.Vasefi F, MacKinnon N, Saager R, Kelly KM, Maly T, Chave R, Booth N, Durkin AJ, Farkas DL. Multimode optical dermoscopy (SkinSpect) analysis for skin with melanocytic nevus Imaging, Manipulation, and Analysis of Biomolecules, Cells, and Tissues IX. In: Farkas Daniel L, Nicolau Dan V, Leif Robert C., editors. Proc of SPIE 2016. Vol. 9711. SPIE BiOS; 2016. Apr 6, paper 971110. [Google Scholar]
- 62.New KC, Petroll WM, Boyde A, Martin L, Corcuff P, Leveque JL, Lemp MA, Cavanagh HD, Jester JV. In vivo imaging of human teeth and skin using real-time confocal microscopy. Scanning. 1991;13:369–372. [Google Scholar]
- 63.Corcuff P, Lévêque JL. In vivo vision of the human skin with the tandem scanning microscope. Dermatology. 1993;186:50–54. doi: 10.1159/000247302. [DOI] [PubMed] [Google Scholar]
- 64.Corcuff P, Bertrand C, Leveque JL. Morphometry of human epidermis in vivo by real-time confocal microscopy. Arch Dermatol Res. 1993;285:475–481. doi: 10.1007/BF00376820. [DOI] [PubMed] [Google Scholar]
- 65.Bertrand C, Corcuff P. In vivo spatio-temporal visualization of the human skin by real-time confocal microscopy. Scanning. 1994;16:150–154. doi: 10.1002/sca.4950160301. [DOI] [PubMed] [Google Scholar]
- 66.Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR. In vivo confocal scanning laser microscopy of human skin: Melanin provides strong contrast. J Invest Dermatol. 1995;104:946–952. doi: 10.1111/1523-1747.ep12606215. [DOI] [PubMed] [Google Scholar]
- 67.Rajadhyaksha M, Anderson RR, Webb RH. Video-rate confocal scanning laser microscope for imaging human tissues in vivo. Appl Opt. 1999;38:2105–2115. doi: 10.1364/ao.38.002105. [DOI] [PubMed] [Google Scholar]
- 68.Rajadhyaksha M, González S, Zavislan JM, Anderson RR, Webb RH. In vivo confocal scanning laser microscopy of human skin II: Advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113:293–303. doi: 10.1046/j.1523-1747.1999.00690.x. [DOI] [PubMed] [Google Scholar]
- 69.Rajadhyaksha M, Gonzalez S, Zavislan JM. Detectability of contrast agents for confocal reflectance imaging of skin and microcirculation. J Biomed Opt. 2004;9:323–331. doi: 10.1117/1.1646175. [DOI] [PubMed] [Google Scholar]
- 70.Hashemi P, Pulitzer MP, Scope A, Kovalyshyn I, Halpern AC, Marghoob AA. Langerhans cells and melanocytes share similar morphologic features under in vivo reflectance confocal microscopy: A challenge for melanoma diagnosis. J Am Acad Dermatol. 2012;66:452–462. doi: 10.1016/j.jaad.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Busam KJ, Hester K, Charles C, Sachs DL, Antonescu CR, Gonzalez S, Halpern AC. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923–929. [PubMed] [Google Scholar]
- 72.Losi A, Longo C, Cesinaro AM, Benati E, Witkowski A, Guitera P, Pellacani G. Hyporeflective pagetoid cells: A new clue for amelanotic melanoma diagnosis by reflectance confocal microscopy. Br J Dermatol. 2014;171:48–54. doi: 10.1111/bjd.12781. [DOI] [PubMed] [Google Scholar]
- 73.Guitera P, Menzies SW, Argenziano G, Longo C, Losi A, Drummond M, Scolyer RA, Pellacani G. Dermoscopy and in vivo confocal microscopy are complimentary techniques for the diagnosis of difficult amelanotic and light colored skin lesions. Br J Dermatol. 2016;13 doi: 10.1111/bjd.14749. [DOI] [PubMed] [Google Scholar]
- 74.Stevenson AD, Mickan S, Mallett S, Ayya M. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19–27. doi: 10.5826/dpc.0304a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kadouch DJ, Schram ME, Leeflang MM, Limpens J, Spuls PI, de Rie MA. In vivo confocal microscopy of basal cell carcinoma: A systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol. 2015;29:1890–1897. doi: 10.1111/jdv.13224. [DOI] [PubMed] [Google Scholar]
- 76.Kadouch DJ, Wolkerstorfer A, Elshot Y, Zupan-Kajcovski B, Crijns MB, Starink MV, Bekkenk MW, van der Wal AC, Spuls PI, de Rie MA. Treatment of basal cell carcinoma using a one-stop-shop with reflectance confocal microscopy: Study design and protocol of a randomized controlled multicenter trial. JMIR Res Protoc. 2015;4:e109. doi: 10.2196/resprot.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiong YD, Ma S, Li X, Zhong X, Duan C, Chen Q. A metaanalysis of reflectance confocal microscopy for the diagnosis of malignant skin tumours. J Eur Acad Dermatol Venereol. 2016;30:1295–1302. doi: 10.1111/jdv.13712. [DOI] [PubMed] [Google Scholar]
- 78.Stanganelli I, Longo C, Mazzoni L, Magi S, Medri M, Lanzanova G, Farnetani F, Pellacani G. Integration of reflectance confocal microscopy in sequential dermoscopy follow-up improves melanoma detection accuracy. Br J Dermatol. 2015;172:365–371. doi: 10.1111/bjd.13373. [DOI] [PubMed] [Google Scholar]
- 79.Ferrari B, Pupelli G, Farnetani F, De Carvalho NT, Longo C, Reggiani C, Argenziano G, Pellacani G. Dermoscopic difficult lesions: An objective evaluation of reflectance confocal microscopy impact for accurate diagnosis. J Eur Acad Dermatol Venereol. 2015;29:1135–1140. doi: 10.1111/jdv.12769. [DOI] [PubMed] [Google Scholar]
- 80.Lovatto L, Carrera C, Salerni G, Alos L, Malvehy J, Puig S. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol. 2015;29:1918–1925. doi: 10.1111/jdv.13067. [DOI] [PubMed] [Google Scholar]
- 81.Busam KJ, Marghoob AA, Halpern A. Melanoma diagnosis by confocal microscopy: Promise and pitfalls. J Invest Dermatol. 2005;125:vii–ix. doi: 10.1111/j.0022-202X.2005.23865.x. [DOI] [PubMed] [Google Scholar]
- 82.Scope A, Longo C. Recognizing the benefits and pitfalls of reflectance confocal microscopy in melanoma diagnosis. Dermatol Pract Concept. 2014;4:67–71. doi: 10.5826/dpc.0403a13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen CS, Elias M, Busam K, Rajadhyaksha M, Marghoob AA. Multimodal in vivo optical imaging, including confocal microscopy, facilitates presurgical margin mapping for clinically complex lentigo maligna melanoma. Br J Dermatol. 2005;153:1031–1036. doi: 10.1111/j.1365-2133.2005.06831.x. [DOI] [PubMed] [Google Scholar]
- 84.Guitera P, Pellacani G, Crotty KA, Scolyer RA, Li LX, Bassoli S, Vinceti M, Rabinovitz H, Longo C, Menzies SW. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080–2091. doi: 10.1038/jid.2010.84. [DOI] [PubMed] [Google Scholar]
- 85.Guitera P, Moloney FJ, Menzies SW, Stretch JR, Quinn MJ, Hong A, Fogarty G, Scolyer RA. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692–698. doi: 10.1001/jamadermatol.2013.2301. [DOI] [PubMed] [Google Scholar]
- 86.Guitera P, Haydu LE, Menzies SW, Scolyer RA, Hong A, Fogarty GB, Gallardo F, Segura S. Surveillance for treatment failure of lentigo maligna with dermoscopy and in vivo confocal microscopy: New descriptors. Br J Dermatol. 2014;170:1305–1312. doi: 10.1111/bjd.12839. [DOI] [PubMed] [Google Scholar]
- 87.Menge TD, Hibler BP, Cordova MA, Nehal KS, Rossi AM. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): A prospective study. J Am Acad Dermatol. 2016;74:1114–1120. doi: 10.1016/j.jaad.2015.12.045. [DOI] [PubMed] [Google Scholar]
- 88.Marra DE, Torres A, Schanbacher CF, Gonzalez S. Detection of residual basal cell carcinoma by in vivo confocal microscopy. Dermatol Surg. 2005;31:538–541. doi: 10.1111/j.1524-4725.2005.31157. [DOI] [PubMed] [Google Scholar]
- 89.Scope A, Mahmood U, Gareau DS, Kenkre M, Lieb JA, Nehal KS, Rajadhyaksha M. In vivo reflectance confocal microscopy of shave biopsy wounds: Feasibility of intraoperative mapping of cancer margins. Br J Dermatol. 2010;163:1218–1228. doi: 10.1111/j.1365-2133.2010.10063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Webber SA, Wurm EM, Douglas NC, Lambie D, Longo C, Pellacani G, Soyer HP. Effectiveness and limitations of reflectance confocal microscopy in detecting persistence of basal cell carcinomas: A preliminary study. Australas J Dermatol. 2011;52:179–185. doi: 10.1111/j.1440-0960.2011.00769.x. [DOI] [PubMed] [Google Scholar]
- 91.Pan ZY, Lin JR, Cheng TT, Wu JQ, Wu WY. In vivo reflectance confocal microscopy of basal cell carcinoma: Feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38:1945–1950. doi: 10.1111/j.1524-4725.2012.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Flores ES, Cordova M, Kose K, Phillips W, Rossi A, Nehal K, Rajadhyaksha M. Intraoperative imaging during Mohs surgery with reflectance confocal microscopy: Initial clinical experience. J Biomed Opt. 2015;20:61103. doi: 10.1117/1.JBO.20.6.061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Venturini M, Gualdi G, Zanca A, Lorenzi L, Pellacani G, Calzavara-Pinton PG. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br J Dermatol. 2016;174:380–385. doi: 10.1111/bjd.14244. [DOI] [PubMed] [Google Scholar]
- 94.Richtig E, Hofmann-Wellenhof R, Kopera D, El-Shabrawi-Caelen L, Ahlgrimm-Siess V. In vivo analysis of solar lentigines by reflectance confocal microscopy before and after Q-switched ruby laser treatment. Acta Derm Venereol. 2011;91:164–168. doi: 10.2340/00015555-1024. [DOI] [PubMed] [Google Scholar]
- 95.Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10. doi: 10.5826/dpc.0202a10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Longo C, Pellacani G, Tourlaki A, Galimberti M, Bencini PL. Melasma and low-energy Q-switched laser: Treatment assessment by means of in vivo confocal microscopy. Lasers Med Sci. 2014;29:1159–1163. doi: 10.1007/s10103-013-1498-8. [DOI] [PubMed] [Google Scholar]
- 97.Venturini M, Sala R, González S, Calzavara-Pinton PG. Reflectance confocal microscopy allows in vivo real-time noninvasive assessment of the outcome of methyl amino-laevulinate photodynamic therapy of basal cell carcinoma. Br J Dermatol. 2013;168:99–105. doi: 10.1111/bjd.12052. [DOI] [PubMed] [Google Scholar]
- 98.Alarcon I, Carrera C, Alos L, Palou J, Malvehy J, Puig S. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to Imiquimod. J Am Acad Dermatol. 2014;71:49–55. doi: 10.1016/j.jaad.2014.02.043. [DOI] [PubMed] [Google Scholar]
- 99.Chen CS, Sierra H, Cordova M, Rajadhyaksha M. Confocal microscopy-guided laser ablation for superficial and early nodular basal cell carcinoma: A promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994–998. doi: 10.1001/jamadermatol.2013.10225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hibler BP, Connolly KL, Cordova M, Nehal KS, Rossi AM, Barker CA. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: Response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:e543–e547. doi: 10.1016/j.prro.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hibler BP, Sierra H, Cordova M, Phillips W, Rajadhyaksha M, Nehal KS, Rossi AM. Carbon dioxide laser ablation of basal cell carcinoma with visual guidance by reflectance confocal microscopy: A proof of principle pilot study. Br J Dermatol. 2016;174:1359–1364. doi: 10.1111/bjd.14414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dwyer PJ, DiMarzio CA, Zavislan JM, Fox WJ, Rajadhyaksha M. Confocal reflectance theta line scanning microscope for imaging human skin in vivo. Opt Lett. 2006;31:942–944. doi: 10.1364/ol.31.000942. [DOI] [PubMed] [Google Scholar]
- 103.Yin C, Glaser AK, Leigh SY, Chen Y, Wei L, Pillai PC, Rosenberg MC, Abeytunge S, Peterson G, Glazowski C, Sanai N, Mandella MJ, Rajadhyaksha M, Liu JT. Miniature in vivo MEMS-based line-scanned dual-axis confocal microscope for point-of-care pathology. Biomed Opt Express. 2016;7:251–263. doi: 10.1364/BOE.7.000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arrasmith CL, Dickensheets DL, Mahadevan-Jansen A. MEMS-based handheld confocal microscope for in-vivo skin imaging. Opt Express. 2010;18:3805–3819. doi: 10.1364/OE.18.003805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wiltgen M, Gerger A, Wagner C, Smolle J. Automatic identification of diagnostic significant regions in confocal laser scanning microscopy of melanocytic skin tumors. Methods Inf Med. 2008;47:14–25. doi: 10.3414/me0463. [DOI] [PubMed] [Google Scholar]
- 106.Koller S, Wiltgen M, Ahlgrimm-Siess V, Weger W, Hofmann-Wellenhof R, Richtig E, Smolle J, Gerger A. In vivo reflectance confocal microscopy: Automated diagnostic image analysis of melanocytic skin tumours. J Eur Acad Dermatol Venereol. 2011;25:554–558. doi: 10.1111/j.1468-3083.2010.03834.x. [DOI] [PubMed] [Google Scholar]
- 107.Gareau D, Hennessy R, Wan E, Pellacani G, Jacques SL. Automated detection of malignant features in confocal microscopy on superficial spreading melanoma versus nevi. J Biomed Opt. 2010;15:061713. doi: 10.1117/1.3524301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kurugol S, Dy JG, Brooks DH, Rajadhyaksha M. Pilot study of semiautomated localization of the dermal/epidermal junction in reflectance confocal microscopy images of skin. J Biomed Opt. 2011;16:036005. doi: 10.1117/1.3549740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kurugol S, Kose K, Park B, Dy JG, Brooks DH, Rajadhyaksha M. Automated delineation of dermal-epidermal junction in reflectance confocal microscopy image stacks of human skin. J Invest Dermatol. 2015;135:710–717. doi: 10.1038/jid.2014.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kose K, Alessi-Fox C, Gill M, Dy JG, Brooks DH, Rajadhyaksha M. A machine learning method for identifying morphological patterns in reflectance confocal microscopy mosaics of melanocytic skin lesions in-vivo Photonic Therapeutics and Diagnostics XII. In: Choi Bernard, et al., editors. Proc of SPIE 2016. Vol. 9689. SPIE BiOS; 2016. Feb 29, Paper 968908. [Google Scholar]
- 111.Ulrich M, von Braunmuehl T, Kurzen H, Dirschka T, Kellner C, Sattler E, Berking C, Welzel J, Reinhold U. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: An observational study. Br J Dermatol. 2015;173:428–435. doi: 10.1111/bjd.13853. [DOI] [PubMed] [Google Scholar]
- 112.Hussain AA, Themstrup L, Nuörnberg BM, Jemec G. Adjunct use of optical coherence tomography increases the detection of recurrent basal cell carcinoma over clinical and dermoscopic examination alone. Photodiagnosis Photodyn Ther. 2016;14:178–184. doi: 10.1016/j.pdpdt.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 113.De Giorgi V, Massi D, Sestini S, Cicchi R, Pavone FS, Lotti T. Combined non-linear laser imaging (two-photon excitation fluorescence microscopy, fluorescence lifetime imaging microscopy, multispectral multiphoton microscopy) in cutaneous tumours: first experiences. J Eur Acad Dermatol Venereol. 2009;23:314–316. doi: 10.1111/j.1468-3083.2008.03045.x. [DOI] [PubMed] [Google Scholar]
- 114.Patil CA, Arrasmith CL, Mackanos MA, Dickensheets DL, Mahadevan-Jansen A. A handheld laser scanning confocal reflectance imaging-confocal Raman microspectroscopy system. Biomed Opt Express. 2012;3:488–502. doi: 10.1364/BOE.3.000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang H, Lee AM, Frehlick Z, Lui H, McLean DI, Tang S, Zeng H. Perfectly registered multiphoton and reflectance confocal video rate imaging of in vivo human skin. J Biophotonics. 2013;6:305–309. doi: 10.1002/jbio.201200067. [DOI] [PubMed] [Google Scholar]
- 116.Seidenari S, Arginelli F, Dunsby C, French P, König K, Magnoni C, Manfredini M, Talbot C, Ponti G. Multiphoton laser tomography and fluorescence lifetime imaging of basal cell carcinoma: morphologic features for non-invasive diagnostics. Exp Dermatol. 2012;21:831–836. doi: 10.1111/j.1600-0625.2012.01554.x. [DOI] [PubMed] [Google Scholar]
- 117.Graf BW, Boppart SA. Multimodal in vivo skin imaging with integrated optical coherence and multiphoton microscopy. IEEE J Sel Top Quantum Electron. 2012;18:1280–1286. doi: 10.1109/JSTQE.2011.2166377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao Y, Graf BW, Chaney EJ, Mahmassani Z, Antoniadou E, Devolder R, Kong H, Boppart MD, Boppart SA. Integrated multimodal optical microscopy for structural and functional imaging of engineered and natural skin. J Biophotonics. 2012;5:437–448. doi: 10.1002/jbio.201200003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Seidenari S, Arginelli F, Dunsby C, French PM, König K, Magnoni C, Talbot C, Ponti G. Multiphoton laser tomography and fluorescence lifetime imaging of melanoma: morphologic features and quantitative data for sensitive and specific non-invasive diagnostics. PLoS ONE. 2013;8:e70682. doi: 10.1371/journal.pone.0070682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iftimia N, Peterson G, Chang EW, Maguluri G, Fox W, Rajadhyaksha M. Combined reflectance confocal microscopy-optical coherence tomography for delineation of basal cell carcinoma margins: an ex vivo study. J Biomed Opt. 2016;21:16006. doi: 10.1117/1.JBO.21.1.016006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dickensheets DL, Kreitinger S, Peterson G, Heger M, Rajadhyaksha M. Dermoscopy-guided reflectance confocal microscopy of skin using high-NA objective lens with integrated wide-field color camera Photonic Therapeutics and Diagnostics XII. In: Choi Bernard, et al., editors. Proc of SPIE 2016. Vol. 9689. SPIE BiOS; 2016. Feb 29, Paper 96890U. [DOI] [PMC free article] [PubMed] [Google Scholar]


