Abstract
Public concern regarding silver nanoparticles (AgNPs) in the environment has been increasing since they can cause adverse effects in some aquatic species. However, few data are actually available on the effects of AgNPs on the germ cells. In the present study, we used the zebrafish ovarian follicle as a model to assess the potentially adverse effects of AgNPs on oocyte maturation (germinal vesicle breakdown, GVBD) in vitro. Similar to the maturation inducing hormone (17α, 20β-dihydroxy-4-pregnen-3-one), AgNPs induced GVBD, and reduced the total cyclic adenosine monophosphate (cAMP) concentration in zebrafish ovarian follicles. The results from transmission electron microscope observation and Hoechst 33342 staining clearly indicated that AgNPs induced apoptosis in ovarian follicle cells surrounding the oocyte. Similar to AgNPs, AgNO3 also induced GVBD, decreased cAMP concentration and induced apoptosis of ovarian follicle cells. However, the results from gene expression analysis showed that transcript levels of oxidative stress related genes were more sensitive to AgNPs than AgNO3. Further more, H2O2 has an ability to induce zebrafish oocytes maturation by induction of apoptosis in ovarian follicle cells. Taken together, the results from our study indicated that oxidative stress appeared to be one of important mechanisms in AgNP induced apoptosis in ovarian follicle cells, which further triggered the GVBD.
Keywords: Silver nanoparticles, Zebrafish, Oocyte maturation, Ovarian follicle cells, Apoptosis, Oxidative stress
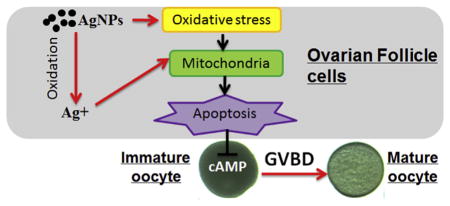
Graphical Abstract
1. Introduction
Nanomaterials are increasingly produced and utilized in a wide range of commercial products worldwide because of their novel and unique physicochemical properties that differ substantially from bulk materials of the same composition (Ahamed et al., 2010). Among various nanomaterials, silver nanoparticles (AgNPs), which have good antimicrobial efficacy against bacteria, viruses and other eukaryotic micro-organisms, are utilized in a wide variety of consumer products ranging from disinfecting medical devices and home appliances to water treatment (Poitras et al., 2015). Release of AgNPs into aquatic environments, has raised concerns on the potential adverse effects of AgNPs on human health and the environment (Benn and Westerhoff, 2008).
Aquatic organisms are susceptible to waterborne nanoparticles. Studies show that AgNPs are toxic to aquatic organisms including zebrafish (Yeo and Kang, 2008; Griffitt et al., 2009), perch (Bilberg et al., 2010), brown trout (Scown et al., 2010), Ceriodaphnia (Gao et al., 2009), Daphnia pulex (Griffitt et al., 2008) and algae (Thalassiosira weissflogii) (Miao et al., 2009). In addition to aquatic organisms, AgNPs may also pose a risk to human health. Conventional silver metals and silver salts, which have been used hitherto in silver-impregnated dressings and pharmaceuticals, are not thought to be toxic to the immune, cardiovascular, nervous or reproductive systems when they are used in reasonable amounts (Drake and Hazelwood, 2005). However, it is reported that AgNPs are highly toxic to various cultured mammalian cells (Braydich-Stolle et al., 2005; Hussain et al., 2006; Wen et al., 2007; Kawata et al., 2009), which indicates a potential toxicity of AgNPs to humans at the cellular and molecular level. Despite substantial information on the toxicity of AgNPs, studies concerning their potential effects on reproductive physiology are comparatively scarce, especially in gametogenesis which plays a vital role in ontogenesis (Gandolfi and Brevini, 2010). A study using a mouse spermatogonial stem cell line demonstrates a concentration-dependent cytotoxicity of AgNPs, whereas the corresponding soluble salts have no significant effect (Braydich-Stolle et al., 2005). One recent study shows that prepubertal male Wistar rat exposure to AgNPs causes alterations in adult sperm parameters (Mathias et al., 2015). Another study using an in vitro assay for porcine gametes shows that alloy nanoparticles with 80% silver molar fraction and pure AgNPs inhibit cumulus-oocyte maturation which implies that released Ag+ is responsible for the observed toxicity (Tiedemann et al., 2014). Although AgNPs have been shown to have adverse effects on gametogenesis, there is still a knowledge gap in the evaluation of their reproductive toxicity, especially in vertebrate oogenesis. In addition, molecular mechanisms for the toxicity of AgNPs have not yet been reported.
Zebrafish (Danio rerio), a genetic and developmental model, is widely used to evaluate the toxic potential of environmental contaminants (Segner, 2009). As in other vertebrates, the principal events responsible for the enormous growth of teleost oocytes are due essentially to the accumulation of yolk proteins within their cytoplasm (Lubzens et al., 2010). After the oocyte completes its growth, it becomes ready for maturation (the resumption of meiosis), which is accompanied by several maturation processes, e.g. breakdown of the germinal vesicle (GVBD) (Nagahama and Yamashita, 2008). Another obvious character during the maturation process is that the oocyte becomes transparent as a result of disassembly of the internal crystalline structures (Nagahama and Yamashita, 2008). The maturation-inducing hormone, 17α, 20β-dihydroxy-4-pregnen-3-one (DHP), induces oocyte maturation in vitro in most fishes (Nagahama and Yamashita, 2008). Therefore, our study aimed to assess the potential adverse effects of AgNPs during oocyte maturation in vitro. We further evaluated whether the toxicity of AgNPs is related to release of Ag+ from the AgNPs, or specific effects of AgNPs. The molecular mechanisms whereby AgNPs and Ag+ affect zebrafish oocyte maturation were also studied.
2. Materials and methods
2.1. Preparation and characterization of AgNP suspensions
Based on the method of (Choi et al., 2010b), AgNPs were synthesized from AgNO3 by appropriate mixing with NaBH4 and polyvinyl alcohol (PVA). In brief, 5 mL of 14 mM NaBH4 was added into 90 mL of 0.06% (wt) PVA solution. Afterwards, 5 mL of 14 mM AgNO3 was injected slowly at a rate of one drop per second at room temperature, and mixed immediately using a magnetic stirrer at 700 rpm in the dark. The synthesized AgNPs were then concentrated and purified with centrifugal ultrafiltration (Millipore, Amicon Ultra-15 3 K, USA), and rinsed twice with Milli-Q water (Millipore, 18.2 M cm, USA). The characterization of the synthesized AgNPs was determined as described by (Sun et al., 2013). The morphology and particle size of these AgNPs were determined using transmission electron microscopy (TEM, Hitachi H-7650, Japan) and analyzed with Image J (http://imagej.nih.gov/ij/), as shown in Supplemental Fig. S1, and its size ranged from 30 to 55 nm. The AgNP stock suspensions were quantified using inductively coupled plasma optical emission spectrometry (PerkinElmer Optima 7000 DV, USA) after nitric acid digestion, and the concentration was 120 ± 7 μg/mL. All test suspensions were prepared by dilution of the ultrasonically dispersed AgNPs (10 min at 4 °C) in Cortland medium to the desired concentration and then added to the culture medium. In order to exclude potential effects from PVA, follicle enclosed oocytes were also cultured in Cortland medium containing PVA at a concentration the same as that in AgNP stock suspensions. In order to distinguish whether possible effects are caused by the nanoparticle as such or by Ag+ ions released from the nanoparticles, oocytes were also co-incubated with silver nitrate (AgNO3). In addition, the released Ag+ from AgNPs in the Cortland medium was evaluated via inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7500cx, USA) after ultrafiltration using Amicon centrifugal ultrafilter devices (Millipore 3 kDa, USA) according to the previous study (Liu and Hurt, 2010).
2.2. Zebrafish stock
The experimental fish were Tüebingen strain, which were housed in the zebrafish facility (ESSEN, China), maintained under constant temperature (28 °C), photoperiod (14 L:10D, lights on at 08:00), salinity (conductivity 500–1200 μS), and pH (7.2–7.6). The fish were fed three times per day with commercial tropical fish food (Otohime B2, Reed Mariculture, CA, USA), using standard conditions for this species (Westerfield, 2000). Experimental protocols were approved by the Institutional Animal Care and Use Committee of Xiamen University.
2.3. Isolation of ovarian follicles
Gravid female zebrafish were euthanized in melting ice and humanely sacrificed by severing the spinal cord following deep anesthetization with 0.01% tricaine methanesulfonate (Sigma-Aldrich, China). The ovaries were removed and washed several times in Cortland medium (pH 7.7) containing (weight in g/L): NaCl, 7.25; KCl, 0.38; CaCl2·2H2O, 0.26; NaHCO3, 1.0; Na2HPO4, 0.46; MgCl2·6H2O, 0.23; MgSO4·7H2O, 0.23; bovine serum albumen, 1; Hepes, 5.20; penicillin, 0.03; and streptomycin, 0.05 (Pang and Ge, 2002). Individual ovarian follicles enclosing fully-grown immature oocytes (Ø = 550–650 μm) were carefully separated using fine forceps and blades without damaging ovarian follicle cell layers. The diameters of the ovarian follicles were measured with an ocular micrometer under a dissecting microscope. Appropriately sized and healthy oocytes were selected and pooled for each experiment. The selected oocytes were pre-incubated in culture medium at 28 °C for 2 h. After pre-incubation, only oocytes showing normal morphology were randomly transferred to 24-well plates (NEST, USA) for further exposure treatments.
2.4. Assay of in vitro ovarian follicle incubation and oocyte maturation
To examine the effect of AgNPs or AgNO3 on oocyte maturation, groups of 30 ovarian follicles were placed in each well and incubated with 2 mL Cortland medium containing different reagents. All incubation was conducted in triplicate for 4 h at 28 °C. To assess the maturation processes, germinal vesicles were examined under a binocular microscope after placing the follicles in clearing solution, or the GVBD was assessed by scoring the oocytes that became transparent (Pang and Ge, 2002).
2.5. Cyclic adenosine monophosphate (cAMP) measurement
In order to examine the effect of AgNPs or AgNO3 on the camp concentration of ovarian follicles, the follicles were treated with 30 μg/mL AgNPs or 10 μg/mL AgNO3 for 2 h. Control and DHP (10 ng/mL) treated groups were also examined at the same time. Groups of 50–60 ovarian follicles were placed in each well. Samples were collected and analyzed with a cAMP EIA kit, purchased from the Caymen Chemical Company (Ann Arbor, MI), following the manufacturer’s instructions.
2.6. TEM
For TEM investigation, ovarian follicles treated with 30 μg/mL AgNPs or 10 μg/mL AgNO3 for 2 h were carefully collected and immediately fixed (for 1 h at 4 °C) using 2.5% glutaraldehyde (phosphate buffered preparation). After fixation, the tissue was rinsed three times (15 min each time) with 0.1 M phosphate buffer, post-fixed with 1% OsO4 fixative for 2–3 h, dehydrated through an ethanol series, and embedded via propylene oxide in Taab epoxy resin (Taab Ltd, Aldermaston, UK). Thereafter, 60–80 nm thick sections of the fixed tissue were cut and double-stained with 3% uranyl acetate, followed by lead citrate, and then examined using TEM (JEM2100HC, JEOL Ltd, Tokyo, Japan).
2.7. Hoechst 33342 staining
The changes of nuclear chromatin morphology are indicative of apoptosis and can be detected using fluorescent microscopy with Hoechst 33342 (1 μg/mL) fluorescent staining. In addition to control and DHP (10 ng/mL) treatments, ovarian follicles were treated with 10 and 30 μg/mL AgNPs or 10 μg/mL AgNO3. The follicles were collected after 1, 2, 3 and 4 h treatment, and incubated with Hoechst 33342 in 2 mL buffer containing 10 mM Hepes/KOH (pH 7.4), 140 mM NaCl, and 2.5 mM CaCl2 for 10 min at 4 °C. Morpho-logical changes of the nuclear chromatin were observed under a fluorescence microscope (M165 FC, Leica Microsystems, Germany).
2.8. Gene expression analysis
In addition to the control and DHP (10 ng/mL) treatments, groups of 30 ovarian follicles were treated with AgNPs (10 μg/mL) or AgNO3 (1 μg/mL) for 2 h. Total RNA was extracted from the ovarian follicle samples using RNAzol reagent (MRC, Cincinnati, OH, USA). The same amount of total RNA (2.0 μg) was used for synthesis of the first strand cDNAs using a Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific, USA) and following the manufacturer’s instructions. The relative expression levels of caspase3, caspase9, gst2 (glutathione S transferase pi 2), hsp70 (heat shock protein 70), mt2 (metallothionein 2) and house-keeping-gene β-actin were determined using real-time quantitative PCR (qPCR) and gene-specific primers (shown in Supplemental, Table 1S), which have been examined for their specificity and amplification efficiency on serial dilutions of respective target gene plasmid DNA (1 × 102–1 × 107 copies/μL). The qPCR was performed in a 20 μL reaction on a 7500 FAST real-time PCR detection system (Applied Biosystems, USA) using default setting. Copies of β-actin, which showed no significant difference among different stages (data not shown), were used as an internal control. The relative mRNA levels of the target genes were determined using the comparative Ct method (Schmittgen and Livak, 2008).
2.9. Statistical analysis
All data were presented as means ± standard error of the mean. Student’s t-test was conducted to determine any significant differences between two groups. The analyses were performed using the GraphPad Prism 5 software package (GraphPad Software, San Diego, CA).
3. Results
3.1. Effects of AgNPs and Ag+ on oocyte maturation
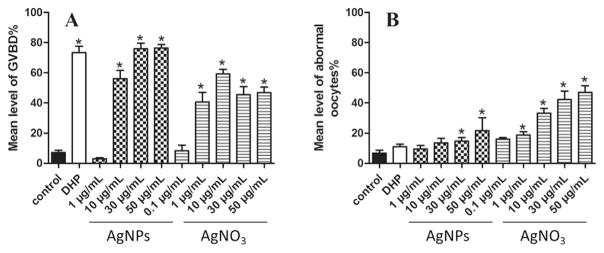
Neither AgNPs nor Ag+ inhibited DHP-induced GVBD (data not shown), but similar to DHP, both significantly induced GVBD (Fig. 1). As control, PVA had no effects on the ovarian follicles. At a concentration of 1 μg/mL, AgNPs had some effects on GVBD, but 1 μg/mL AgNO3 exhibited a significant induction effect on GVBD. However, at high dosage groups (30 and 50 μg/mL), AgNPs showed a stronger induction effect on oocyte maturation than AgNO3 (Fig. 2A). Besides, some ovarian follicles exhibited abnormal morphology in response to AgNPs or AgNO3 (as shown in Supplemental Fig. S2). Significant increases of abnormal rates in response to AgNPs or AgNO3 required at least 30 μg/mL or 1 μg/mL (Fig. 2B). Moreover, at the same dosage, AgNO3 induced higher rates of abnormal oocytes than AgNPs. These results indicated that AgNO3 was more cytotoxic than AgNPs to the oocyte. It is worth noting that, when 1 μg/mL AgNPs were spiked into the culture medium, the released Ag+ (data not shown) was below method detection limit (0.5 ng/mL). When 10 and 30 μg/mL AgNPs were spiked into the culture medium, concentration of dissolved silver had a sharp rise in a short time (8–15 ng/mL), and then gradually reached stable (Fig. S3). Because the highest GVBD rates were obtained at 30 μg/mL AgNPs or 10 μg/mL AgNO3, the subsequent experiments were performed at these concentrations unless otherwise indicated.
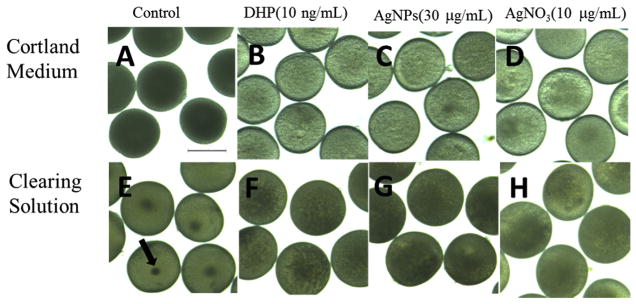
Fig. 1. Silver nanoparticles (AgNPs) or AgNO3 induce oocyte maturation in zebrafish.
Ovarian follicles enclosing fully-grown immature oocytes remain opaque in the control group (A) whereas they become transparent after the exposure of DHP (B), AgNPs (C) or AgNO3 (D). After clearing solution treatment, germinal vesicles are observed at the center of oocytes in the control group (E), whereas they disappear after the treatment of DHP (F), AgNPs (G) or AgNO3 (H). The arrow indicates the germinal vesicle. Scale bar: 500 μm.
Fig. 2. Silver nanoparticles (AgNPs) or AgNO3 induce oocyte maturation and abnormal development of zebrafish oocytes.
Ovarian follicles enclosing fully-grown immature oocytes were incubated with Cortland medium only (control), the medium containing DHP (10 ng/mL), or various concentrations of AgNPs or AgNO3. Each treatment group contains at least 30 oocytes. Percentages of oocytes undergoing germinal vesicle breakdown, or those with abnormal appearance were determined after a 4 h exposure. Experiments were repeated six times, and the data are expressed as average of six replicates (mean ± SE, n = 6). Bars marked with asterisk are significantly different from the control group (p < 0.05, Student’s t-test).
3.2. AgNPs or Ag+ reduced cAMP concentration in oocytes
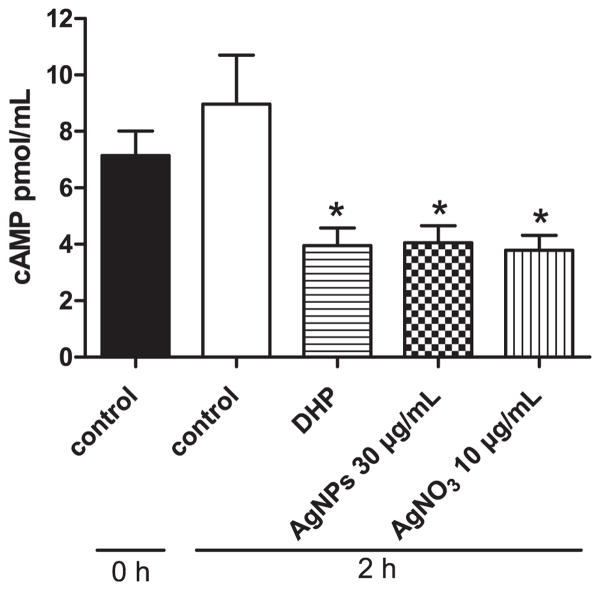
It is well established that a high concentration of cAMP in fish oocytes is essential for maintaining meiotic arrest, and treatment with maturation inducing hormone causes a decrease in adenylyl cyclase activity (Pace and Thomas, 2005). Therefore, to determine whether AgNPs or AgNO3 may act via the same mechanism to induce oocyte maturation, we further investigated the effects of AgNPs and AgNO3 on cAMP concentration in treated oocytes. As expected, oocytes treated with DHP (10 ng/mL) caused a significant decrease in cAMP production compared to the control. Similarly, oocytes treated with 30 μg/mL AgNPs, or 10 μg/mL AgNO3 also resulted in a significant decrease in cAMP concentration (Fig. 3). These results clearly indicated that AgNPs or AgNO3 induced oocyte maturation in the zebrafish by decreasing the cAMP concentration.
Fig. 3. Silver nanoparticles (AgNPs) or AgNO3 inhibit cAMP production in zebrafish ovarian follicles.
The concentrations of cAMP were determined from a pooled extraction of 50–60 ovarian follicles enclosing fully-grown immature oocytes that were incubated with Cortland medium (control), the medium containing DHP (10 ng/mL), AgNPs (30 μg/mL), or AgNO3 (10 μg/mL) for 2 h. The experiment was repeated six times and data was expressed as the average of six replicates (mean ± SE, n = 6). Bars marked with asterisk are significantly different from the control group (p < 0.05, Student’s t-test).
3.3. Effects of AgNPs or Ag+ on ultrastructure of ovarian follicle cells
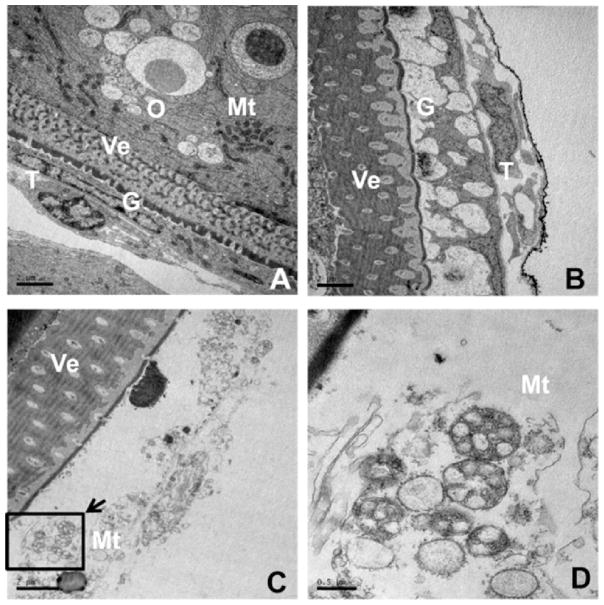
In vertebrates, cAMP generated in ovarian follicle cells can enter the oocyte via heterologous gap junctions connecting follicle cells and the oocyte. Therefore, we further determined whether AgNPs or AgNO3 had cytotoxic effects on the follicle cells. We first examined the ultrastructure of the ovarian follicle. As shown in Fig. 4A, the zebrafish oocytes as in other vertebrates are surrounded by ovarian follicle cells layers that include two major cell layers, an outer thecal cell layer and an inner granulosa cell layer. However, after exposure to AgNPs (30 μg/mL) and AgNO3 (10 μg/mL), both thecal and granulosa cells revealed irregular cell morphology, and exhibited disorganization in the cytoplasm, nuclear condensation and fragmentation (Fig. 4B and C). In addition, the follicle cells of the AgNO3 treatment group showed acute vacuolation, and the mitochondria swelled but had an intact inner mitochondrial membrane (Fig. 4D). These results suggested that ovarian follicle cells underwent apoptosis.
Fig. 4.
Effects of silver nanoparticles (AgNPs) or AgNO3 on ultrastructure of ovarian follicle cells. Ovarian follicles enclosing fully-grown immature oocytes were incubated with Cortland medium containing designed reagents for 2 h. (A), control group showing normal appearance of ovarian follicle cells (Scale bar, 2 μm); (B) AgNPs (30 μg/mL) and (C) AgNO3 (10 μg/mL) treated group showing ovarian follicle cells have irregular cell morphology, acute vacuolation, nuclear condensation and fragmentation (Scale bar, 2 μm); (D) a high magnification image of the area indicated in C showing a novel mitochondrial swelling with intact inner mitochondrial membrane (Scale bar, 0.5 μm). G: granulosa, Mt: mito-chondria, O: oocyte, T: thecal cell, Ve: vitelline envelope.
3.4. Effects of AgNPs and Ag+ on induction of apoptosis in ovarian follicle cells
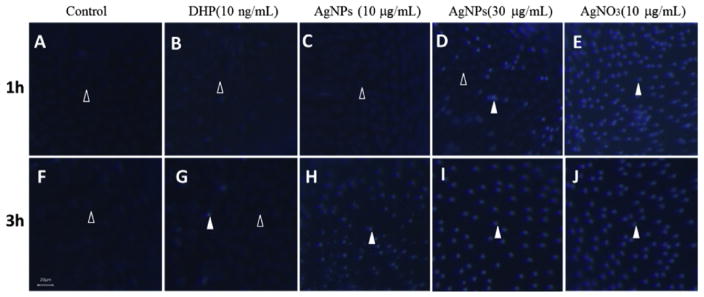
In order to further Confirm that both AgNPs and AgNO3 induced apoptosis in ovarian follicle cells, we used Hoechst 33342 to reveal that ovarian follicle cells surrounding the oocyte had undergone apoptosis. Within 3 h exposure in both control and DHP (10 ng/mL) groups, most of the follicle cells appeared to be intact and of oval shape and their nuclei were stained with a weak blue fluorescence, and only a few of the follicles showed apoptosis features, such as a spherical bead shape and bright blue fluorescence (Fig. 5A and B). In contrast, a vast majority of the follicle cells underwent apoptosis after 3 h exposure to AgNPs at 10 μg/mL (Fig. 5C), 1 h exposure to AgNPs at 30 μg/mL (Fig. 5D), and 1 h exposure to AgNO3 at 10 μg/mL (Fig. 5E).
Fig. 5. Effects of silver nanoparticles (AgNPs) or AgNO3 on apoptosis in ovarian follicle cells.
Ovarian follicles enclosing fully-grown immature oocytes were incubated with Cortland medium (control, panel A,F); or medium containing DHP (10 ng/mL, panel B,G); AgNPs (10 μg/mL, panel C,H); AgNPs (30 μg/mL, panel D,I); and AgNO3 (10 μg/mL, panel E,J) that were collected 1 or 3 h following treatment. After Hoechst 33342 staining, the representative fluorescence images were taken to showing the follicle cells layer surrounding the oocytes. Open arrowhead indicated the intact follicle cells with oval shape and their nuclei were stained with a weak blue fluorescence; Filled arrowhead indicated the follicle cells exhibit apoptosis features such as spherical bead shape and bright blue fluorescence when treated with AgNPs or AgNO3. Scale bar, 20 μm.
3.5. Effects of AgNPs and Ag+ exposure on gene expression in ovarian follicles
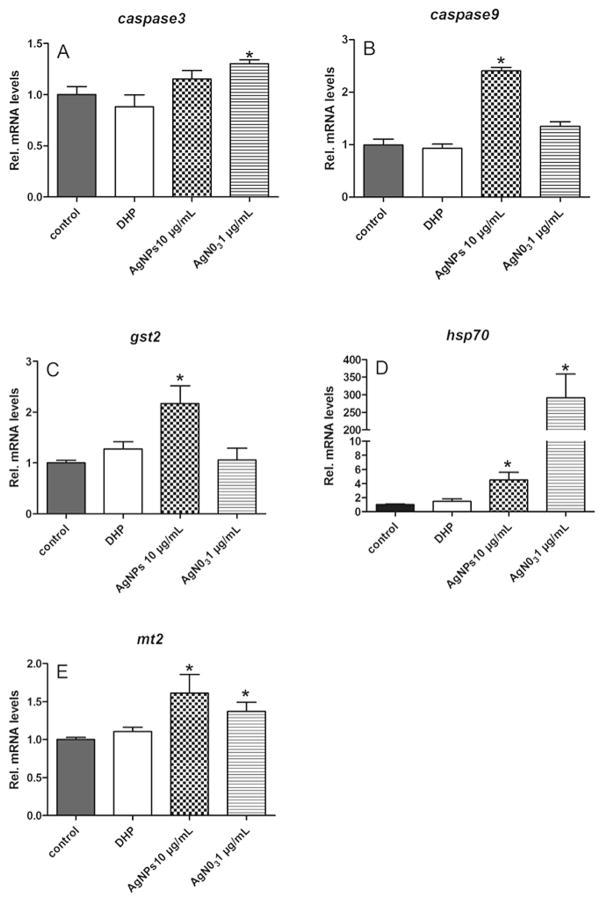
To elucidate the molecular mechanisms of AgNPs (10 μg/mL) and AgNO3 (1 μg/mL) induced apoptosis of follicle cells, the expression of several candidate genes relevant to oxidative stress (gst2, hsp70 and mt2) and apoptosis (caspase3, caspase9) were determined using qPCR. The results showed that DHP had no effects on the expression of all tested genes. The AgNPs and AgNO3 significantly induced the upregulation of transcript levels of caspase9 and caspase3, respectively (Fig. 6A and B). A significant increase in gst2 transcript level was observed only in the AgNPs treatment group (Fig. 6C). The most sensitive gene to silver was hsp70, the expression of which increased ~5 and ~300 fold in response to AgNPs and AgNO3 (Fig. 6D). Similarly, the expression levels of mt2 transcripts increased significantly in response to AgNPs or AgNO3 treatment (Fig. 6E).
Fig. 6. Exposure to silver nanoparticles (AgNPs) and AgNO3 affect gene expression in ovarian follicles.
Total RNA was extracted from a group of 30 ovarian follicles enclosing fully-grown immature oocytes that was incubated with Cortland medium as a control, or the medium containing DHP (10 ng/mL), AgNPs (10 μg/mL), or AgNO3 (1 μg/mL) for 2 h. Transcripts of caspase3, caspase9, gst2, hsp70, and mt2 were determined using qPCR and normalized to an internal reference gene (β-actin). Data are expressed as the mean ± SE (n = 6) relative to respective transcript levels measured in the control group. Bars marked with an asterisk are significantly different from the control group (p < 0.05, Student’s t-test).
3.6. H2O2 induced oocytes maturation by induction of apoptosis inovarian follicle cells
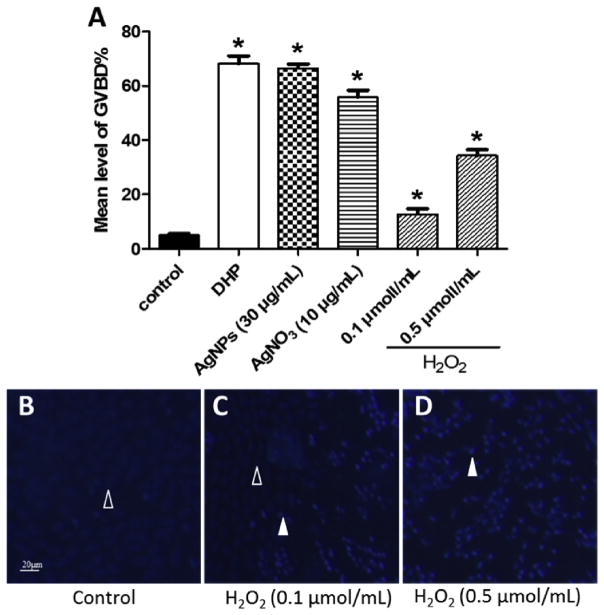
To confirm that the oxidative stress appeared to be an important mechanism in AgNPs inducing apoptosis of ovarian follicle cells, we further examine the effects of H2O2 on zebrafish oocytes maturation. The results showed that H2O2 exhibited a significant induction effect on the oocytes maturation, although the GVBD rates of H2O2 groups were significantly lower than that of DHP, AgNPs and AgNO3 groups (Fig. 7A). Besides, Hoechst 33342 staining revealed that ovarian follicle cells surrounding the oocyte had undergone apoptosis (Fig. 7B, C, D).
Fig. 7. Effects of H2O2 on zebrafish oocyte maturation and apoptosis in ovarian follicle cells.
(A) Ovarian follicles enclosing fully-grown immature oocytes were incubated with Cortland medium only (control), the medium containing DHP (10 ng/mL), AgNPs (30 μg/mL) or AgNO3 (10 μg/mL) or H2O2 (0.1 and 0.5 μmol/mL) for 4 h. Each treatment group contains at least 30 oocytes. Experiments were repeated six times, and the data are expressed as average of six replicates (mean ± SE, n = 6). Bars marked with asterisk are significantly different from the control group (p < 0.05, Student’s t-test). (B–D) Ovarian follicles enclosing fully-grown immature oocytes were incubated with Cortland medium (control); or H2O2 (0.1 and 0.5 μ,ol/mL) for 3 h. After Hoechst 33342 staining, the representative fluorescence images were taken to showing the follicle cells layer surrounding the oocytes. Open arrowhead indicated the intact follicle cells with oval shape and their nuclei were stained with a weak blue fluorescence; Filled arrowhead indicated the follicle cells exhibit apoptosis features such as spherical bead shape and bright blue fluorescence when treated with H2O2. Scale bar, 20 μm.
4. Discussion
The use of engineered nanoparticles has risen exponentially over the last decade. However, our understanding concerning the possible adverse effects of nanoparticles is still incomplete. The potential disrupting effects of environmental contaminants on germ cells have received significant attention, especially regarding the possible disrupting effects which may not only affect the person or animal directly exposed to the environmental contaminants but can also be passed on to the following generations (Diamanti-Kandarakis et al., 2009). However, toxicological testing of nano-particles on germ cells has so far been frequently neglected.
In fish, fully grown oocytes are arrested at the prophase of the first meiotic division. When such oocytes are cultured in medium supplemented with DHP, oocyte maturation (also named as GVBD) is induced in vitro in a wide variety of teleosts (Nagahama, 1994). Using this standardized in vitro system, in a small pilot study, we examined whether AgNPs would affect the process of GVBD induced by DHP. After 4 h co-incubation with AgNPs (50 μg/mL) and DHP (10 ng/mL), the results showed that AgNPs has no inhibitive effects on zebrafish oocyte maturation (data not shown). However, we observed a significant increase of oocyte maturation rate in the AgNPs treatment group.
So far, few studies have been published concerning the impact of nanoparticles on ovarian follicle. Hou et al. (2009). examined the effect of titanium dioxide nanoparticles on ovarian follicle development and oocyte maturation of rats. Hsieh et al. (2009). studied the influence of CdSe-core quantum dots on oocyte maturation, fertilization, and subsequent pre- and post-implantation development in the mouse. One recent study investigated the reprotoxicity of gold, silver and gold–silver alloy nanoparticles on porcine cumulus-oocyte-complexes (Tiedemann et al., 2014). These studies report inhibitive effects of the respective nanoparticles on oocyte maturation, which are different from the results observed in our study. In order to explain this result, we examined the effects of AgNPs on the cAMP concentrations in the ovarian follicle, because a decrease of cAMP concentrations in oocytes of vertebrates is required for the resumption of meiosis (Dekel and Beers, 1978; Pace and Thomas, 2005; Nagahama and Yamashita, 2008). Our results showed that, similar to DHP, AgNPs also induced a reduction of total cAMP concentrations in zebrafish ovarian follicles, which likely led to meiosis resumption and oocyte maturation. Furthermore, results from TEM and Hoechst 33342 staining suggested that AgNPs induced apoptosis of ovarian follicle cells. Previous studies in zebrafish and carp (Cyprinus carpio) have shown that removal of ovarian follicle layer led to a striking increase in spontaneous GVBD (Pang and Thomas, 2010; Majumder et al., 2015). In teleost, the inhibitory influence of ovarian follicle cells on GVBD is mediated, at least partly, through increases in cAMP concentrations via estrogen produced by the ovarian follicle cells (Pang et al., 2008; Pang and Thomas, 2010; Majumder et al., 2015). It is possible that apoptosis of the ovarian follicle cells induced by the AgNPs resulted in termination of the transfer of cAMP from the follicle cells to the oocyte and production of estrogen, both of which result in a decrease of cAMP concentration in the oocyte that triggered the resumption of meiosis.
The exact mechanism by which AgNPs inflict damage to organisms is still a matter of debate. Although the toxicity seems to be driven by oxidation and inflammation, it is unclear whether silver in its nanoparticulate form is responsible for the toxic effects, as some studies claim, or whether they are caused solely by silver ions dissolving in the course of oxidation of the metal. A recent study in pigs implies that release of Ag+ is responsible for the observed inhibition effects of AgNPs on cumulus-oocyte maturation (Tiedemann et al., 2014). Therefore, we also examined whether Ag+ would induce zebrafish oocyte maturation. The results showed that, similar to AgNPs, AgNO3 also induced oocyte maturation, decreased cAMP concentrations, and induced apoptosis in ovarian follicle cells. We observed that follicle cells treated with Ag+ showed a novel mitochondrial swelling while the inner membrane was still partially visible. This novel ultrastructure has been reported in a study that examined the effect of Ag+ isolated rat liver mitochondria, and suggests that Ag+ promotes a nonclassical permeability transition and releases apoptogenic cytochrome c which triggers the apoptotic cascade (Almofti et al., 2003). In the meantime, our results indicated that silver ion release rate was about 1‰ in the culture medium containing AgNps, and AgNO3 was more toxic than AgNPs to both follicle cells and oocytes. Taken together, although both AgNPs and AgNO3 induced apoptosis in follicle cells, the molecular mechanisms induced by these two compounds might have been different. Therefore, gene expression analysis was performed in order to distinguish the impacts of AgNPs from Ag+ ions (Griffitt et al., 2009; Poynton et al., 2012).
Caspases (cysteine-aspartic acid proteases) are activated during apoptosis in many cells and are known to play a vital role in both the initiation and execution of apoptosis. The upregulation of caspase3 and caspase9 in response to AgNO3 and AgNPs, respectively, further proved that both AgNPs and AgNO3 induced apoptosis in ovarian follicle cells. HSPs have been classified as stress response proteins owing to their induction by several types of cellular stress such as infection and inflammation (Bouwmeester et al., 2011). In addition, the HSPs may be involved in preventing cells from apoptosis (Li et al., 2009). In the present study, both AgNPs and AgNO3 exposure induced up-regulation of hsp70, which was in agreement with the previous studies showing an up-regulation of HSP genes after AgNPs and Ag+ ion exposure (small hsps, hsp40, hsp70, hsp90 and hsp110 family) (Foldbjerg et al., 2012). Therefore, hsp70 is a sensitive biomarker of both AgNPs and AgNO3 inducing cellular damage e.g. apoptosis. It is reported in several studies that genes from the metallothionein superfamily that are involved in metal binding and the response to metal exposure are up-regulated (Kawata et al., 2009; Lim et al., 2012; Ding et al., 2014; Eom et al., 2014). A study in zebrafish demonstrates that mt mRNA expression in liver increases after treatment with AgNPs (Choi et al., 2010a). As reported, results from our study showed that both AgNPs and AgNO3 induced an upregulation of mt2 transcript levels. Because the cysteines of metallothioneins bind oxidant radicals such as superoxide and hydroxyl radicals (Kumari et al., 1998; Formigare et al., 2007), it is possible that, in the ovarian follicle, mt2 is induced mainly as a response to oxidative stress. Therefore, we further analyzed the expression of glutathione S-transferases (gst), which catalyzes the conjugation of glutathione (GSH) with various electrophilic substances, and plays a role preventing oxidative damage by conjugating breakdown products of lipid peroxides to GSH (Ketterer et al., 1983). Results from our study showed that AgNPs, but not AgNO3, induced the up-regulation of gst2 transcript levels. The results from gene expression analyses suggested that AgNPs-induced apoptosis of ovarian follicle cells may mainly be due to the increase of oxidative stress. Previous studies in mammalian liver cells indicated that AgNPs and silver nanoclusters cause cytotoxicity by oxidative stress-induced apoptosis and damage to cellular components (Piao et al., 2011). Based on these information, we applied H2O2 to increase the oxidative stress of ovarian follicles. The results indicated that H2O2 had an ability to induce zebrafish oocytes maturation by induction of apoptosis in ovarian follicle cells. Thus, oxidative stress appeared to be one of mechanism in AgNPs inducing apoptosis of follicle cells.
It has been demonstrated that the bioaccumulation of AgNPs by marine medaka (Oryzias melastigma) from the waterborne phase was very low (Wang and Wang, 2014b). However, dietary exposure of AgNPs may also act as a potential uptake route of AgNPs in fish. Recent studies in zebrafish (Rahmani, et al., 2016) and marine medaka (Wang and Wang, 2014a) have provided evidence that AgNPs can transfer from brine shrimp (Artemia salina) nauplii to the fish by dietary exposure. Although the amount of assimilated Ag (from AgNPs contaminated food) is small, it still generated toxic effects (e.g., inhibition of Na+/K+-ATPase and SOD activity, reduction of total body length, and water content) on marine medaka. Although we can’t provide direct evidence to approve the quality of zebrafish oocyte is damaged by AgNPs, study in mammals have clear demonstrated that ROS-induced mitochondrial damage has widespread impacts on multiple aspects of oocyte quality (Boudoures and Moley, 2015). Therefore, results from present study may suggest a potential environmental risky of trophic transfer of AgNPs into the fish, and AgNPs-generated free radicals further affect oocyte quality.
In summary, our results confirmed that in vitro maturation of fish oocytes represented a very sensitive system for the exploration of nanotoxicology in which even subtle effects could be visualized. To our knowledge, this is the first report of AgNPs and Ag+ inducing the maturation of fish oocytes. The results of gene expression patterns suggested that oxidative stress appeared to be one of important mechanisms in AgNP-induced apoptosis in ovarian follicle cells. Further study is necessary to examine the quality of mature oocytes affected by AgNPs and Ag+ using other animal models.
Supplementary Material
Highlights.
Both AgNPs and AgNO3 unexpectedly induced zebrafish oocyte maturation in vitro.
Both AgNPs and AgNO3 reduced total cAMP concentration in zebrafish ovarian follicles.
Both AgNPs and AgNO3 induced apoptosis in the follicle cells surrounding the oocyte.
H2O2 induced oocytes maturation by induction of apoptosis in the follicle cells.
AgNP-induced apoptosis of the follicle cells may mainly due to the oxidative stress.
Acknowledgments
This work was supported by the Natural Science Foundation of Fujian Province (No. 2013J05029), the National Natural Science Foundation of China (No. 31201977) and Xiamen Southern Oceanographic Center (No. 14GZY019NF19). Professor John Hodgkiss is thanked for editing the English.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.chemosphere.2016.12.016.
References
- Ahamed M, AlSalhi MS, Siddiqui MKJ. Silver nanoparticle applications and human health. Clin Chim Acta. 2010;411:1841–1848. doi: 10.1016/j.cca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Almofti MR, Ichikawa T, Yamashita K, Terada H, Shinohara Y. Silver ion induces a cyclosporine A-insensitive permeability transition in rat liver mitochondria and release of apoptogenic cytochrome C. J Biochem. 2003;134:43–49. doi: 10.1093/jb/mvg111. [DOI] [PubMed] [Google Scholar]
- Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol. 2008;42:4133–4139. doi: 10.1021/es7032718. [DOI] [PubMed] [Google Scholar]
- Bilberg K, Malte H, Wang T, Baatrup E. Silver nanoparticles and silver nitrate cause respiratory stress in Eurasian perch (Perca fluviatilis) Aquat Toxicol. 2010;96:159–165. doi: 10.1016/j.aquatox.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Boudoures AL, Moley KH. Insights into mechanisms causing the maternal age-induced decrease in oocyte quality. In: Carrell DT, Schlegel PN, Racowsky C, Gianaroli L, editors. Biennial Review of Infertility, me. Vol. 4. Springer International Publishing; Cham: 2015. pp. 43–55. [Google Scholar]
- Bouwmeester H, Poortman J, Peters RJ, Wijma E, Kramer E, Makama S, Puspitaninganindita K, Marvin HJP, Peijnenburg A, Hendriksen PJM. Characterization of translocation of silver nanoparticles and effects on whole-genome gene expression using an in vitro intestinal epithelium coculture model. ACS Nano. 2011;5:4091–4103. doi: 10.1021/nn2007145. [DOI] [PubMed] [Google Scholar]
- Braydich-Stolle L, Hussain S, Schlager JJ, Hofmann MC. In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol Sci. 2005;88:412–419. doi: 10.1093/toxsci/kfi256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JE, Kim S, Ahn JH, Youn P, Kang JS, Park K, Yi J, Ryu DY. Induction of oxidative stress and apoptosis by silver nanoparticles in the liver of adult zebrafish. Aquat Toxicol. 2010a;100:151–159. doi: 10.1016/j.aquatox.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Choi OY, Yu CP, Fernandez GE, Hu ZQ. Interactions of nanosilver with Escherichia coli cells in planktonic and biofilm cultures. Water Res. 2010b;44:6095–6103. doi: 10.1016/j.watres.2010.06.069. [DOI] [PubMed] [Google Scholar]
- Dekel N, Beers WH. Rat oocyte maturation in vitro: relief of cyclic AMP inhibition by gonadotropins. P Natl Acad Sci U S A. 1978;75:4369–4373. doi: 10.1073/pnas.75.9.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Wang ML, Chu HY, Chu MJ, Na T, Wen Y, Wu DM, Han B, Bai ZP, Chen WH, Yuan J, Wu TC, Hu ZB, Zhang ZD, Shen HB. Global gene expression profiling of human bronchial epithelial cells exposed to airborne fine particulate matter collected from Wuhan, China. Toxicol Lett. 2014;228:25–33. doi: 10.1016/j.toxlet.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Drake PL, Hazelwood KJ. Exposure-related health effects of silver and silver compounds: a review. Ann Occup Hyg. 2005;49:575–585. doi: 10.1093/annhyg/mei019. [DOI] [PubMed] [Google Scholar]
- Eom HJ, Chatterjee N, Lee J, Choi J. Integrated mRNA and micro RNA profiling reveals epigenetic mechanism of differential sensitivity of Jurkat T cells to AgNPs and Ag ions. Toxicol Lett. 2014;229:311–318. doi: 10.1016/j.toxlet.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Foldbjerg R, Irving ES, Hayashi Y, Sutherland DS, Thorsen K, Autrup H, Beer C. Global gene expression profiling of human lung epithelial cells after exposure to nanosilver. Toxicol Sci. 2012;130:145–157. doi: 10.1093/toxsci/kfs225. [DOI] [PubMed] [Google Scholar]
- Formigare A, Irato P, Santon A. Zinc, antioxidant systems and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Physiol C-Toxicol Pharmacol. 2007;146:443–459. doi: 10.1016/j.cbpc.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Gandolfi F, Brevini TAL. In vitro maturation of farm animal oocytes: a useful tool for investigating the mechanisms leading to full-term development. Reprod Fertil Dev. 2010;22:495–507. doi: 10.1071/RD09151. [DOI] [PubMed] [Google Scholar]
- Gao J, Youn S, Hovsepyan A, Llaneza VL, Wang Y, Bitton G, Bonzongo JCJ. Dispersion and toxicity of selected manufactured nanomaterials in natural river water samples: effects of water chemical composition. Environ Sci Technol. 2009;43:3322–3328. doi: 10.1021/es803315v. [DOI] [PubMed] [Google Scholar]
- Griffitt RJ, Hyndman K, Denslow ND, Barber DS. Comparison of molecular and histological changes in zebrafish gills exposed to metallic nano-particles. Toxicol Sci. 2009;107:404–415. doi: 10.1093/toxsci/kfn256. [DOI] [PubMed] [Google Scholar]
- Griffitt RJ, Luo J, Gao J, Bonzongo JC, Barber DS. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chem. 2008;27:1972–1978. doi: 10.1897/08-002.1. [DOI] [PubMed] [Google Scholar]
- Hou J, Wan XY, Wang FXGF, Liu Z, Zhang TB. Effects of titanium dioxide nanoparticles on development and maturation of rat preantral follicle in vitro. Acad J Second Mil Med Univ. 2009;29:869–873. [Google Scholar]
- Hsieh MS, Shiao NH, Chan WH. Cytotoxic effects of CdSe quantum dots on maturation of mouse oocytes, fertilization, and fetal development. Int J Mol Sci. 2009;10:2122–2135. doi: 10.3390/ijms10052122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SM, Javorina AK, Schrand AM, Duhart HM, Ali SF, Schlager JJ. The interaction of manganese nanoparticles with PC-12 cells induces dopamine depletion. Toxicol Sci. 2006;92:456–463. doi: 10.1093/toxsci/kfl020. [DOI] [PubMed] [Google Scholar]
- Kawata K, Osawa M, Okabe S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to hepG2 human hepatoma cells. Environ Sci Technol. 2009;43:6046–6051. doi: 10.1021/es900754q. [DOI] [PubMed] [Google Scholar]
- Ketterer B, Coles B, Meyer DJ. Th role of glutathione in detoxication. Environ Health Perspect. 1983;49:59–69. doi: 10.1289/ehp.834959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari MVR, Hiramatsu M, Ebadi M. Free radical scavenging actions of metallothionein isoforms I and II. Free Radic Res. 1998;29:93–101. doi: 10.1080/10715769800300111. [DOI] [PubMed] [Google Scholar]
- Li Y, Sun B, Wu H, Nie P. Effects of pure microcystin-LR on the transcription of immune related genes and heat shock proteins in larval stage of zebrafish (Danio rerio) Aquaculture. 2009;289:154–160. [Google Scholar]
- Lim DH, Jang J, Kim S, Kang T, Lee K, Choi IH. The effects of sub-lethal concentrations of silver nanoparticles on inflammatory and stress genes in human macrophages using cDNA microarray analysis. Biomaterials. 2012;33:4690–4699. doi: 10.1016/j.biomaterials.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Liu JY, Hurt RH. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ Sci Technol. 2010;44:2169–2175. doi: 10.1021/es9035557. [DOI] [PubMed] [Google Scholar]
- Lubzens E, Young G, Bobe J, Cerda J. Oogenesis in teleosts: how fish eggs are formed. Gen Comp Endocr. 2010;165:367–389. doi: 10.1016/j.ygcen.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Majumder S, Das S, Moulik SR, Mallick B, Pal P, Mukherjee D. G-protein coupled estrogen receptor (GPER) inhibits final oocyte maturation in common carp, Cyprinus carpio. Gen Comp Endocr. 2015;211:28–38. doi: 10.1016/j.ygcen.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Mathias FT, Romano RM, Kizys MML, Kasamatsu T, Giannocco G, Chiamolera MI, Dias-da-Silva MR, Romano MA. Daily exposure to silver nanoparticles during prepubertal development decreases adult sperm and reproductive parameters. Nanotoxicology. 2015;9:64–70. doi: 10.3109/17435390.2014.889237. [DOI] [PubMed] [Google Scholar]
- Miao AJ, Schwehr KA, Xu C, Zhang SJ, Luo Z, Quigg A, Santschi PH. The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ Pollut. 2009;157:3034–3041. doi: 10.1016/j.envpol.2009.05.047. [DOI] [PubMed] [Google Scholar]
- Nagahama Y. Endocrine regulation of gametogenesis in fish. Int J Dev Biol. 1994;38:217–229. [PubMed] [Google Scholar]
- Nagahama Y, Yamashita M. Regulation of oocyte maturation in fish. Dev Growth Differ. 2008;50:S195–S219. doi: 10.1111/j.1440-169X.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- Pace MC, Thomas P. Steroid-induced oocyte maturation in Atlantic croaker (Micropogonias undulatus) is dependent on activation of the phosphatidylinositol 3-kinase/Akt signal transduction pathway. Biol Reprod. 2005;73:988–996. doi: 10.1095/biolreprod.105.041400. [DOI] [PubMed] [Google Scholar]
- Pang Y, Dong J, Thomas P. Estrogen signaling characteristics of Atlantic croaker G protein-coupled receptor 30 (GPR30) and evidence it is involved in maintenance of oocyte meiotic arrest. Endocrinology. 2008;149:3410–3426. doi: 10.1210/en.2007-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang YF, Ge W. Gonadotropin and activin enhance maturational competence of oocytes in the zebrafish (Danio rerio) Biol Reprod. 2002;66:259–265. doi: 10.1095/biolreprod66.2.259. [DOI] [PubMed] [Google Scholar]
- Pang YF, Thomas P. Role of G protein-coupled estrogen receptor 1, GPER, in inhibition of oocyte maturation by endogenous estrogens in zebrafish. Dev Biol. 2010;342:194–206. doi: 10.1016/j.ydbio.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao MJ, Kang KA, Lee IK, Kim HS, Kim S, Choi JY, Choi J, Hyun JW. Silver nanoparticles induce oxidative cell damage in human liver cells through inhibition of reduced glutathione and induction of mitochondria-involved apoptosis. Toxicol Lett. 2011;201:92–100. doi: 10.1016/j.toxlet.2010.12.010. [DOI] [PubMed] [Google Scholar]
- Poitras EP, Levine MA, Harrington JM, Essader AS, Fennell TR, Snyder RW, Black SL, Sumner SS, Levine KE. Development of an analytical method for assessment of silver nanoparticle content in biological matrices by inductively coupled plasma mass spectrometry. Biol Trace Elem Res. 2015;163:184–192. doi: 10.1007/s12011-014-0141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynton HC, Lazorchak JM, Impellitteri CA, Blalock BJ, Rogers K, Allen HJ, Loguinov A, Heckman JL, Govindasmawy S. Toxicogenomic responses of nanotoxicity in Daphnia magna exposed to silver nitrate and coated silver nanoparticles. Environ Sci Technol. 2012;46:6288–6296. doi: 10.1021/es3001618. [DOI] [PubMed] [Google Scholar]
- Rahmani R, Mansouri B, Johari SA, Azadi N, Davari B, Asghari SLD. Trophic transfer potential of silver nanoparticles from Artemia salina to Danio rerio. AACL Bioflux. 2016;91:100–104. [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C-T method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Scown TM, Santos EM, Johnston BD, Gaiser B, Baalousha M, Mitov S, Lead JR, Stone V, Fernandes TF, Jepson M, van Aerle R, Tyler CR. Effects of aqueous exposure to silver nanoparticles of different sizes in rainbow trout. Toxicol Sci. 2010;115:521–534. doi: 10.1093/toxsci/kfq076. [DOI] [PubMed] [Google Scholar]
- Segner H. Zebrafish (Danio rerio) as a model organism for investigating endocrine disruption. Comp Biochem Physiol C-Toxicol Pharmacol. 2009;149:187–195. doi: 10.1016/j.cbpc.2008.10.099. [DOI] [PubMed] [Google Scholar]
- Sun Q, Li Y, Tang T, Yuan Z, Yu CP. Removal of silver nanoparticles by coagulation processes. J Hazard Mater. 2013;261:414–420. doi: 10.1016/j.jhazmat.2013.07.066. [DOI] [PubMed] [Google Scholar]
- Tiedemann D, Taylor U, Rehbock C, Jakobi J, Klein S, Kues WA, Barcikowski S, Rath D. Reprotoxicity of gold, silver, and gold-silver alloy nanoparticles on mammalian gametes. Analyst. 2014;139:931–942. doi: 10.1039/c3an01463k. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang WX. Low bioavailability of silver nanoparticles presents trophic toxicity to marine medaka (Oryzias melastigma) Environ Sci Technol. 2014a;48:8152–8161. doi: 10.1021/es500655z. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang WX. Salinity influences on the uptake of silver nano-particles and silver nitrate by marine medaka (Oryzias melastigma) Environ Toxicol Chem. 2014b;33:632–640. doi: 10.1002/etc.2471. [DOI] [PubMed] [Google Scholar]
- Wen HC, Lin YN, Jian SR, Tseng SC, Weng MX, Liu YP, Lee PT, Chen PY, Hsu RQ, Wu WF, Chou CP. Observation of growth of human fibro-blasts on silver nanoparticles. In: Meyer E, Hegner M, Gerber C, Guntherodt HJ, editors. Proceedings of the International Conference on Nano-science and Technology. Iop Publishing Ltd; Bristol: 2007. pp. 445–449. [Google Scholar]
- Westerfield M. The Zebrafish Book: a Guide for the Laboratory Use of Zebrafish (Danio rerio) University of Oregon Press; Oregon: 2000. [Google Scholar]
- Yeo MK, Kang M. Effects of nanometer sized silver materials on biological toxicity during zebrafish embryogenesis. Bull Korean Chem Soc. 2008;29:1179–1184. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.