Abstract
Various biomarkers of acute kidney injury (AKI) have been discovered and characterized in the recent past. These molecules can be detected in urine or blood and signify structural damage to the kidney. Clinically, they are proposed as adjunct diagnostics to serum creatinine and urinary output to improve the early detection, differential diagnosis and prognostic assessment of AKI. The most obvious requirements for a biomarker include its reflection of the underlying pathophysiology of the disease. Hence, a biomarker of AKI should derive from the injured kidney and reflect a molecular process intimately connected with tissue injury. Here, we provide an overview of the basic pathophysiology, the cellular sources and the clinical performance of the most important currently proposed biomarkers of AKI: neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), liver-type fatty acid-binding protein (L-FABP), interleukin-18 (IL-18), insulin-like growth factor-binding protein 7 (IGFBP7), tissue inhibitor of metalloproteinase 2 (TIMP-2) and calprotectin (S100A8/9). We also acknowledge each biomarker’s advantages and disadvantages as well as important knowledge gaps and perspectives for future studies.
Keywords: acute kidney injury, biomarkers, calprotectin, kidney injury molecule 1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), tissue inhibitor of metalloproteinase-2 (TIMP-2) and IGF-binding protein 7 (IGFBP7)
Acute kidney injury (AKI) is a common and potentially life-threatening condition (Bellomo et al. 2012, Kellum et al. 2012). It is associated with elevated short-term morbidity and mortality as well as with unfavourable long-term outcomes caused by the development of chronic kidney disease (CKD) or the occurrence of cardiovascular events (Bihorac et al. 2009, Chawla et al. 2014a,b, Wu et al. 2014). At the time of AKI diagnosis, a number of diagnostic and therapeutic measures are needed. These measures include the determination of the underlying cause of AKI and the initiation of specific and supportive therapeutic measures, such as antibiotic therapy for sepsis, immunosuppression for autoimmune disease, an adjustment of nephrotoxic drugs or directed fluid management (Finfer et al. 2004, National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network et al., 2006, Murugan et al. 2010).
As early institution of these measures is critical for their effectiveness, efforts have been made to identify subtle insults to the kidney that do not cause measurable functional decline, that is subclinical forms of AKI, and to identify indicators of a particular risk of AKI (Kellum et al. 2012, Ronco et al. 2012, Chawla et al. 2015). This is also reflected in The Kidney Disease: Improving Goal Outcomes (KDIGO) clinical practice guidelines for AKI, which not only include patients with AKI but also patients who are at risk for the development of AKI (Khwaja 2012). Despite these efforts and the consensus that specific measures must be undertaken to identify patients with subclinical AKI in order to protect them from poor long-term outcomes, there is still little implementation of this knowledge in daily clinical practice. Currently, the standard diagnostic tools for the detection of AKI are monitoring of urinary output and serum creatinine concentration (sCr), both of which are markers of kidney function but not kidney injury (Waikar et al. 2009). Accordingly, AKI is defined by an increase of serum creatinine by ≥0.3 mg dL−1 in 48 h or an increase by ≥1.5-fold from a known or assumed baseline or by a decrease of urinary output to less than 0.5 mL kg−1 h−1 for 6 h (Khwaja 2012). In clinical reality, however, serial measurements of serum creatinine are often unavailable complicating the differentiation between AKI and CKD. In addition, creatinine and urinary output when measured at presentation do not always predict adverse outcomes, such as hospital mortality or a requirement for renal replacement therapy (RRT) (Mehta et al. 2002, Bell et al. 2009). Furthermore, this definition does not account for the aetiology of AKI. Most importantly, it does not differentiate between quickly reversible, volume-sensitive reductions in glomerular filtration rate (=pre-renal AKI) and primary structural injury to the kidney (=intrinsic AKI). Furthermore, the peak height of sCr, which defines AKI severity according to current criteria, may not be the best approach to understanding AKI because according to Coca et al., the duration of azotaemia correlates with mortality better than the peak height of sCr (Coca et al. 2010). The patients with the most elevated creatinine could be those with the best muscle mass, that is the best health status at baseline. Long periods of azotaemia may correlate with time-consuming repair of the renal tubule, while short periods of azotaemia may correlate with rapidly reversible hemodynamic variation. In addition, the lack of specificity is the concern over sensitivity because a healthy renal reserve would blunt the rise in creatinine. In fact, removal or damage of a portion of a kidney may not elevate sCr, despite loss of renal mass. The shortcomings of serum creatinine as a biomarker for AKI are widely acknowledged (Devarajan 2007, Jo et al. 2007, Bennett et al. 2008).
One major advance to detect AKI at an earlier stage would be the implementation of new reliable biomarkers that identify AKI earlier than conventional tests or that detect subclinical AKI (Hoste et al. 2006, Cruz et al. 2013, Chawla et al. 2014a,b, Wu et al. 2014). Within the past years, several new potential biomarker molecules that are measureable in urine or plasma samples of patients with AKI have been discovered including neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule 1 (KIM-1), interleukin-18 (IL-18), liver-type fatty acid-binding protein (L-FABP), tissue inhibitor of metalloproteinase-2 (TIMP-2), insulin-like growth factor-binding protein 7 (IGFBP7) and calprotectin (Heller et al. 2011, Kashani et al. 2013, Singer et al. 2013, Shao et al. 2014, Lin et al. 2015, Medić et al. 2015, Xu et al. 2015, Zhou et al. 2016). The purpose of this article is to review biological and physiological data on new biomarkers and to summarize clinical studies that investigate these new biomarkers.
NGAL
NGAL is a 25-kDa protein of the lipocalin family. Human NGAL exists as a monomer and a 45-kDa homodimer as well as it exists as a 135-kDa heterodimer where it is conjugated to gelatinase and is specific to neutrophils. The monomer is the gene product itself, which is very rapidly secreted from stimulated or damaged epithelial cells. In some cells, where the monomer is stored, the protein can homodimerize such as in neutrophils (Cai et al. 2010). The known functions of NGAL are related to its ability to bind iron–siderophore complexes. It exerts a bacteriostatic function of the innate immune system by sequestering iron–siderophore complexes and thereby preventing iron uptake by bacteria (Goetz et al. 2002, Flo et al. 2004). This function can also be co-opted to transport iron to the cytoplasm via catecholate–iron complexes where it activates or represses iron-responsive genes (Yang et al. 2002, Bao et al. 2010).
NGAL is expressed at very low constant levels in different cell types. NGAL is highly upregulated on mRNA and protein level after ischaemic or toxic kidney injury in human neonates, children and adults and in every animal model where it has been studied (Mishra et al. 2003, 2004, Supavekin et al. 2003). Hence, the biology of NGAL is evolutionarily conserved in AKI probably because it confers essential protection against infection (Paragas et al. 2014). The elevation of NGAL is detectable as early as 3 h after the injury and it peaks at approx. 6–12 h after injury depending on the severity of injury. The elevation can persist up to 5 days after the initial injury when the injury is severe (Mishra et al. 2005, Parikh et al. 2011).
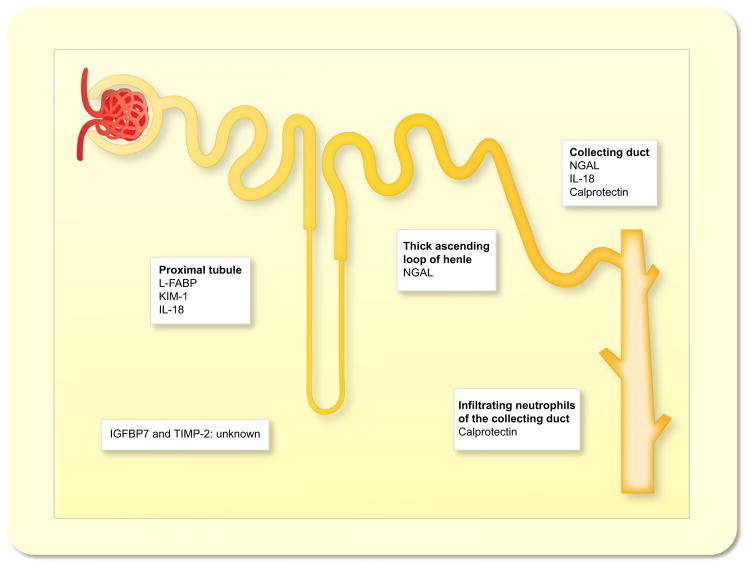
In vivo data have suggested that the thick ascending loop of Henle and the intercalated cells of the collecting duct are the primary sites of NGAL production in the kidney (Schmidt-Ott et al. 2007, Paragas et al. 2014) (Fig. 1). Kidney injury leads to an apical and basolateral secretion from kidney epithelia (Mori et al. 2005, Schmidt-Ott et al. 2007, Schmidt-Ott 2011). NGAL is filtered by the glomerulus and is reabsorbed by the proximal tubule in a megalin-dependent manner (Hvidberg et al. 2005) (Fig. 2). A decrease in tubular reabsorption after AKI may lead to a further increase in urinary NGAL concentration. The evidence that NGAL after AKI originates from the injured kidney and not from other tissues was produced by two animal models using a luciferase-based in vivo monitoring technique and by cross-transplantation of NGAL−/− kidneys into NGAL+/+ mice and vice versa (Paragas et al. 2011). In a mouse model of ischaemia/reperfusion injury, the administration of a single dose of iron–siderophore-loaded NGAL (holo-NGAL) protected the kidney from ischaemic damage. In this study, it was also shown that holo-NGAL upregulates the renoprotective enzyme heme oxygenase-1 (Mori et al. 2005).
Figure 1.
Sites of origin of biomarkers of acute kidney injury.
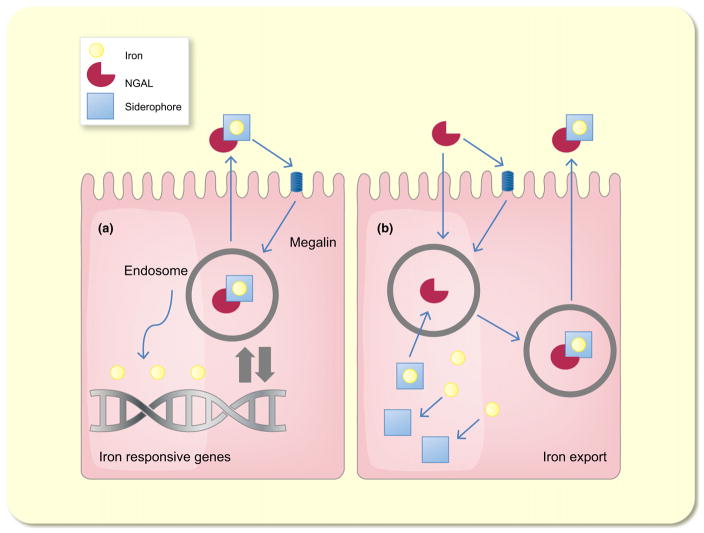
Figure 2.
Schematic model of the functions of neutrophil gelatinase-associated lipocalin (NGAL) (a) Siderophore–iron associated NGAL delivers iron into the cell in a megalin-dependent manner. After the uptake, NGAL traffics into the endosome from where iron is released. This results in a regulation of iron-responsive genes. (b) Siderophore-free NGAL captures siderophore-bound iron and transports it into the extracellular space.
NGAL is the most widely studied biomarker of AKI (Singer et al. 2013, Ho et al. 2015, Zhou et al. 2016). It has shown its performance in various settings such as the prediction of AKI in paediatric (Dent et al. 2007) and adult cardiac surgery patients (Parikh et al. 2011, Prowle et al. 2015), in critically ill patients (de Geus et al. 2011, Cruz et al. 2013), in patients in the emergency room (Nickolas et al. 2008, 2012) as well as in the kidney transplant setting (Pajek et al. 2014, Pianta et al. 2015a, Ramirez-Sandoval et al. 2015). A recent meta-analysis of Ho et al. included 16 studies with a total of 2906 patients investigating urinary NGAL as a biomarker for the prediction of AKI after cardiac surgery in adult patients. The composite area under the curve (AUC) of urinary NGAL was 0.72. Notably, the included studies differed in terms of criteria for AKI (inclusion of urine criteria) and sample collection time (0–24 h after surgery) (Ho et al. 2015).
NGAL is a sensitive gene responding to tubular cell stress and damage via the Toll-like receptor 4 and nuclear factor kappa-light-chain-enhancer of activated B cells pathway triggered by sepsis (Flo et al. 2004). Septic damage to the kidney is the most prevalent cause of admission for AKI from the emergency room (Nickolas et al. 2008). Sepsis is a good example that demonstrates the utility of this biomarker – NGAL can rise days before sepsis causes sCr elevation and the need for dialysis (Parravicini et al. 2010).
NGAL elevates in the setting of stimuli that damage the kidney but not in the setting of rapidly reversible, fluid-sensitive volume depletion. This was shown in emergency room (ER) and in-hospital settings (Nickolas et al. 2008, 2012, Singer et al. 2011). In these studies, clinical criteria have been consulted to distinguish pre-renal from intrinsic renal failure. The AUCs for determining intrinsic AKI ranged from 0.81 to 0.95. However, NGAL was also closely associated with the duration of AKI in these studies, which is consistent with recent work from Parikh’s group demonstrating a short bout of azotaemia (of any change in creatinine) is a demonstration of limited damage (Coca et al. 2010).
NGAL predicted death or dialysis at the time of admission to the ER. This association persisted after stratification according to sCr levels. Patients with NGAL >104 ng mL−1 and sCr >1.4 mg dL−1 demonstrated a 15% incidence of death or dialysis during hospitalization. Elevation of either NGAL or sCr without the other demonstrated a 5% incidence of death or dialysis. The data were reproduced by examining KIM-1 (see below) but not other biomarkers (Nickolas et al. 2012). Moreover, elevated urinary NGAL levels at AKI diagnosis predicted long-term adverse outcomes of end-stage renal disease or death (insert new citation: Singer E, Schrezenmeier EV, Elger A, et al. Urinary NGAL-positive acute kidney injury and poor long-term outcomes in hospitalized patients. Kidney International Reports. In press.
Koyner et al. (2015a) compared the ability of urine output after a standardized furosemide challenge to several AKI biomarkers (FST, urine NGAL, urine IL-18, urine KIM-1, Uromodulin urine Creatinine, urine ACR, FeNa plasma NGAL), measured just before furosemide administration, to predict several clinical outcomes in 77 patients with early AKI. While the FST was superior to all biomarkers in predicting outcomes, among the biomarkers urinary NGAL displayed the best predictive ability (AUC 0.75).
In sum, NGAL is a ‘ready to go gene’ that is rapidly expressed when kidney cells sense stress and/or damage. It is much more sensitive than sCr leading to the following nomenclature: NGAL− sCr− (normal), NGAL+ sCr− (damage to <50% of renal mass or early detection of severe disease), NGAL+ sCr+ (damage to >50% of renal mass) and finally NGAL− sCr+ (no renal stress or damage, but functional impairment consistent with pre-renal azotaemia). These data suggest that the AKI diagnosis based on sCr alone is inadequate and can be fractionated into different patient categories using NGAL. An important caveat is that urinary tract infection (UTI) may in some cases raise levels of urine NGAL, and in this setting, it is recommended to measure plasma NGAL (Devarajan 2007, Schmidt-Ott 2011).
Among all new biomarkers, NGAL is the most widely investigated new biomarker. Different investigators analysed diverse patient populations and determined the performance of NGAL (Singer et al. 2013, Ho et al. 2015, Ramirez-Sandoval et al. 2015). It is clear that NGAL is intensely expressed in a dose-dependent fashion with the severity of kidney disease and is activated at the time of patient presentation, for example in the emergency department (Nickolas et al. 2012). It is also clear that it separates volume depletion and intrinsic damage, two clinically separate entities that have been lumped together in the AKI diagnosis. Progress with this biomarker requires clear cut-off values in different settings [Nickolas suggested 104 ng mL−1 and increasing levels of creatinine, i.e. RIFLE level >R best correlate (Nickolas et al. 2012)]. Reference values for a healthy adult population for urinary NGAL were established, revealing increasing NGAL levels with age and higher NGAL levels in women compared to men (Cullen et al. 2012, Pennemans et al. 2013).
The pathophysiology and the assignment to the kidney have been shown in animal models (Paragas et al. 2011). Compared to other biomarkers (e.g. cell cycle arrest biomarkers,) the relationship to AKI and mechanistic understanding of NGAL is much more advanced.
KIM-1
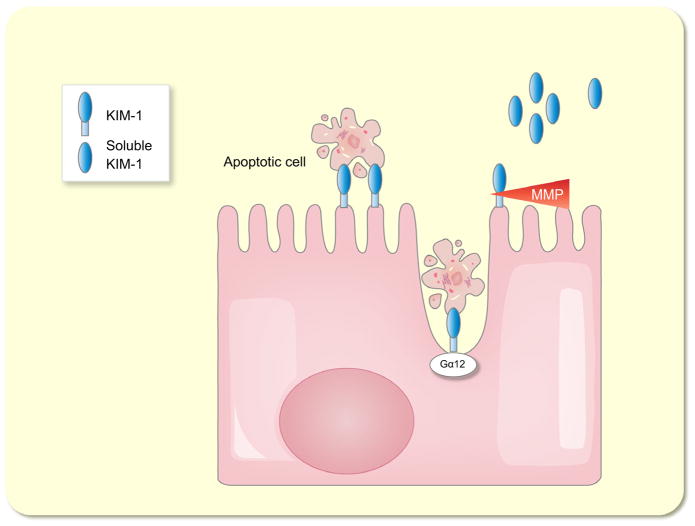
KIM-1 is a 38.7-kDa type I transmembrane glycoprotein with an extracellular immunoglobulin-like domain topping a long mucin-like domain (Ichimura et al. 1998). It is expressed at low levels in the normal kidney as well as in other organs, but its expression is dramatically up-regulated in the kidney post-ischaemia/reperfusion injury in rats (Ichimura et al. 1998) as well as in rodent models of drug-induced AKI (Amin et al. 2004, Prozialeck et al. 2007). The expression is mainly upregulated in proximal tubule cells both in rodents (Ichimura et al. 2004) and in humans (Han et al. 2002). The extracellular domain of KIM-1 is shed from the cell surface by a metalloproteinase-dependent process (Bailly et al. 2002) (Fig. 3). This shedding together with an increased intrarenal synthesis of KIM-1 mRNA and protein is most likely the cause of the increase of KIM-1 in the urine after AKI (Han et al. 2002, Ichimura et al. 2004). It has been proposed that KIM-1 plays an important role in kidney recovery and tubular regeneration because it was shown that it acts as a phosphatidylserine receptor and thereby mediates the phagocytosis of apoptotic bodies and cell debris into cultured renal epithelial cells (Ichimura et al. 2008) (Fig. 3). Mice with a mutation in the KIM-1 mucin domain had greater functional impairment of renal function and a stronger inflammatory response after cisplatin-induced AKI and in ischaemia/reperfusion injury (Yang et al. 2009). Ismail et al. showed that KIM-1-deficient cultured tubular epithelial cells were virtually incapable of taking up apoptotic cells (Ismail et al. 2015a). They also showed that this process is mediated by binding of KIM-1 to the alpha subunit of heterotrimeric G12 protein (Gα12). Just recently, data have been published demonstrating that KIM-1 inhibits Gα12 activation and that endogenous KIM-1 protects mice against renal ischaemia/reperfusion injury (Ismail et al. 2015b) (Fig. 3).
Figure 3.
Schematic model of the functions kidney injury molecule-1 (KIM-1). KIM-1 acts as a phosphatidylserine receptor and binds apoptotic cell bodies. KIM-1 binds to the alpha subunit of heterotrimeric G12 protein (Gα12), thereby mediating phagocytosis of apoptotic cell bodies. The extracellular domain of KIM-1 is shed from the cell surface by a metalloproteinase (MMP)-dependent process.
In comparison with the protective effect of KIM-1 in AKI, it has an unfavourable effect in CKD. Conditional expression of KIM-1 in renal epithelial cells in mice leads to spontaneous and progressive interstitial kidney inflammation and fibrosis (Humphreys et al. 2013). In CKD, KIM-1 is colocalized with areas of inflammation and fibrosis (van Timmeren et al. 2007) and it correlates directly with the degree of interstitial fibrosis in renal allografts before reperfusion (Schröppel et al. 2010). Studies investigating KIM-1 as a biomarker of AKI have overall shown variable results (Hall et al. 2011, Arthur et al. 2014). Combination of KIM-1 with IL-18 achieved an AUC of 0.92 in a study of 32 urine biomarkers in AKI after cardiac surgery and the combination outperformed other biomarkers (Arthur et al. 2014). A detailed summary of available studies in different disease settings was just recently given (Shao et al. 2014, Medić et al. 2015). In addition, KIM-1 might be useful for the detection of nephrotoxicity in pre-clinical and early phase I and II clinical studies (Dieterle et al. 2010, Vaidya et al. 2010). A lateral flow dipstick for KIM-1 has been introduced providing a simplified way of assessing KIM-1 levels (Fuchs et al. 2012). Reference ranges of urinary KIM-1 in healthy individuals were studied, indicating a linear increase of KIM-1 with age and higher KIM-1 values in males (Pennemans et al. 2013).
L-FABP
L-FABP is a 14 kDa protein from the large superfamily of lipid-binding proteins (Tan et al. 2002). The family contains nine members with tissue-specific distribution being named after the tissue where they first have been discovered: liver (L), intestine (I), muscle and heart (H), adipocytes (A), epidermal tissue (E), ileum (IL), brain (B), testis (T) and myelin (M).
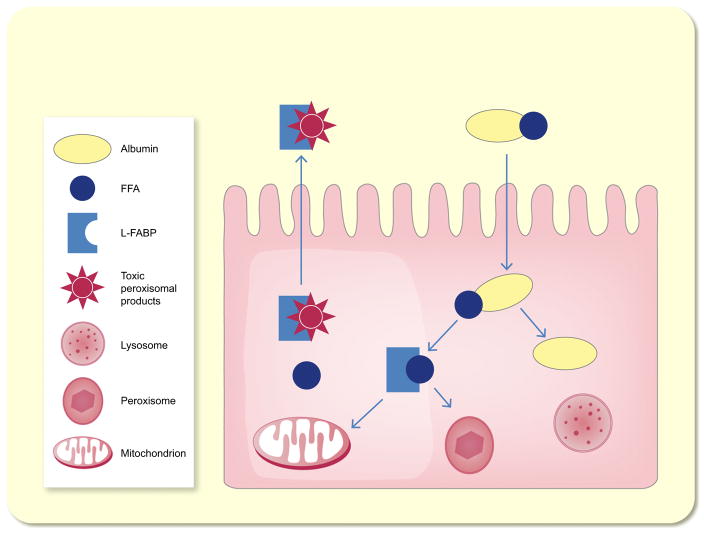
The common function of all members of this family is the regulation of fatty acids uptake and the intracellular transport (Chmurzyńska 2006) (Fig. 4). L-FABP, which is not only expressed in the liver but also in the intestine, stomach, lung and kidney (Smathers & Petersen 2011), binds to fatty acids and transports them to the mitochondria and peroxisomes where β-oxidation takes place providing energy for tubular cells (Sweetser et al. 1987). Besides its transport function, L-FABP also protects cells from oxidative stress induced by H2O2 (Wang et al. 2005). L-FABP expression is inducible by hypoxia because the human L-FABP gene contains a hypoxia-inducible factor 1α response element (Yamamoto et al. 2007, Noiri et al. 2009). In concordance with this finding, the level of L-FABP was shown to directly correlate with ischaemic time in kidney transplant recipients (Yamamoto et al. 2007).
Figure 4.
Schematic model of the functions of liver-type fatty acid-binding protein (L-FABP). L-FABP transports albumin bound free fatty acids (FFA) to mitochondria and peroxisomes to be metabolized. L-FABP is excreted into the tubular lumen together with bound toxic peroxisomal products, which accumulate otherwise.
Within the kidney, it is predominantly located in the proximal tubule and is excreted into the tubular lumen together with bound toxic peroxisomal products, which accumulate otherwise (Maatman et al. 1991, Yamamoto et al. 2007) (Fig. 4). L-FABP is not expressed in mice; therefore, human-L-FABP transgenic mice were used to investigate the role of L-FABP in AKI (Kamijo-Ikemori et al. 2011). These mice showed less histological changes and lower blood urea nitrogen levels after ischaemia and reperfusion (Yamamoto et al. 2007, Noiri et al. 2009). The peroxisome proliferator activated receptor-α (PPAR-α) upregulates L-FABP gene expression (Landrier et al. 2004). Fibrates are known agonists of PPAR-α and were therefore used in models of cisplatin-induced AKI in rodents. In these models, fibrates were able to attenuate AKI (Negishi et al. 2007, 2008). Nevertheless, human observational studies reveal an increased rather than a decreased risk of AKI with fibrate use (Attridge et al. 2012, Zhao et al. 2012).
Studies that investigate L-FABP as predictor of AKI in patients after cardiac surgery have shown AUCs between 0.52 and 0.85 with a composite AUC of 0.72 when summarizing six studies including 1700 patients (Ho et al. 2015). Patients with high L-FABP measured by the time of ICU admission had greater risk for the development of AKI within 1 week (Doi et al. 2011). Therefore, L-FABP can be used to identify patients with a high susceptibility for renal stress. There are no data available on the prediction of long-term mortality or ESRD for L-FABP. A recent review article summarizes available study results on L-FABP in detail (Xu et al. 2015).
IL-18
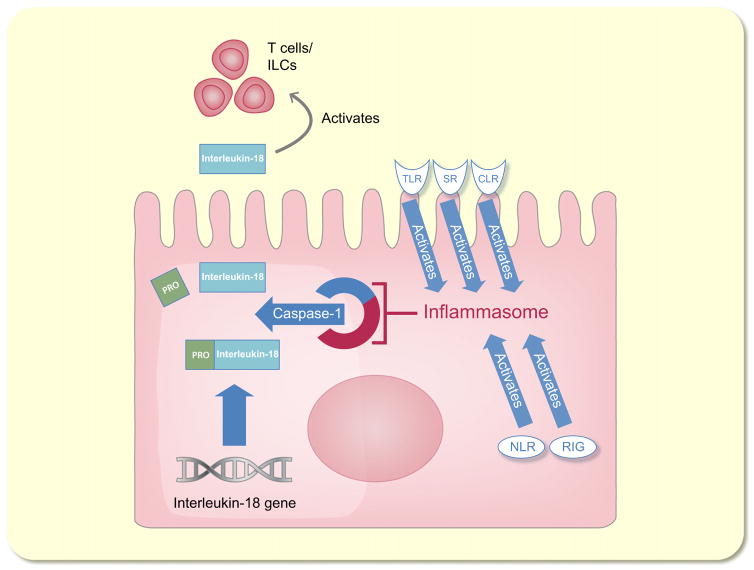
Interleukin-18, also known as interferon-gamma inducing factor, is a 24-kDa cytokine that belongs to the interleukin-1 superfamily (Novick et al. 2013). IL-18 is first synthesized as an inactive precursor without a signal peptide, and it remains intracellular until its cleavage by caspase-1 and its subsequent secretion by monocytes/macrophages (Fantuzzi et al. 1999) (Fig. 5). Caspase-1 acts as a component of the so-called inflammasome, a cytosolic protein complex that mediates cleavage and release of interleukins in response to extrinsic stimuli. Several cell surface receptors such as toll-like receptors (TLRs), retinoic acid-induced gene-like receptors, nucleotide-binding domain-leucine-rich repeat, scavenger receptors and C-type lectins can start the cascade (Chang et al. 2014) (Fig. 5). Cleaved IL-18 exerts a proinflammatory effect by signal transduction through the IL-18 receptor/IL-18 receptor accessory protein heterodimer (Cheung et al. 2005). IL-18 is also produced by the intercalated cells of the collecting ducts in the healthy kidney (Gauer et al. 2007) but appears to be induced more broadly in injured tubular epithelial cells (Franke et al. 2012).
Figure 5.
Schematic model of the functions of interleukin-18 (IL-18). IL-18 is synthesized as an inactive precursor without a signal peptide. It is cleaved by caspase-1, which is a part of the inflammasome. Toll-like receptors (TLRs), retinoic acid-induced gene-like receptors (RIG), nucleotide-binding domain-leucine-rich repeat (NLR), scavenger receptors (SR) and C-type lectins (CLR) can activate the inflammasome. Cleaved IL-18 exerts a proinflammatory effect by activating T cells and innate lymphoid cells.
Because of the pathophysiological plausibility of IL-18 in the development and progression of AKI, which was reported in different rodent animal models, IL-18 was suggested to be a new biomarker in AKI. IL-18-deficient mice are protected from ischemia/reperfusion-induced AKI (Wu et al. 2008). Similarly, caspase-deficient mice, which are not able to cleave IL-18, develop less severe AKI after ischaemia/reperfusion (Melnikov et al. 2001). Inhibition of caspase-1 attenuates glycerol-induced acute renal failure in rats (Homsi et al. 2006). In 1999, a naturally occurring interleukin-18 binding protein was identified and it was shown to abolish IL-18 induction of interferon-gamma (Novick et al. 1999). The administration of this IL-18 binding protein just before ischaemia/reperfusion injury ameliorated kidney damage in rats (Wang et al. 2012). Overall, only few clinical studies have tested the utility of IL-18 as a biomarker for AKI (Lin et al. 2015). The results showed moderate results for AKI in paediatric patients after cardiac surgery (Krawczeski et al. 2011, Zheng et al. 2013), but it failed to show reliable prediction in the general ICU population (Nisula et al. 2015) or in the ER (Nickolas et al. 2012). In a study assaying different biomarkers for the prediction of delayed graft function, urinary IL-18 measured 4 h post-transplantation was among the most promising markers contributing independently to a clinical predictive model (Pianta et al. 2015b). In addition to human studies, several animal models have shown utility of using IL-18 in AKI (Melnikov et al. 2001, Homsi et al. 2006, Wu et al. 2008).
Even if the prognostic and diagnostic value of IL-18 is limited (Zhao et al. 2012, Zheng et al. 2013, Nisula et al. 2015), the use of anti-IL-18 treatment may be a potential future AKI treatment option, which may make urinary IL-18 an important adjunctive biomarker. It would be plausible that patients with high urine IL-18 benefit most from anti-IL-18 treatment, whereas patients with low IL-18 levels do not.
IGFBP7 and TIMP-2 (the so-called cell cycle arrest biomarkers)
Cell cycle arrest in G1 phase may be cellular mechanism to escape from situations when potential DNA damage can occur (Rodier et al. 2007). In the setting of ischaemic or septic kidney injury, renal epithelial cells have been shown to undergo G1 cell cycle arrest (Witzgall et al. 1994, Fraker et al. 1995, Yang et al. 2009). The cyclin-dependent kinase-inhibitor p21 prevents cell cycle progression from G1 to S phase. p21-deficient mice are more susceptible to cisplatinum-induced kidney injury, develop a more severe morphologic damage, and have a higher mortality, suggesting that cell cycle arrest is important in limiting the consequences of AKI (Megyesi et al. 1998).
In 2013, IGFBP7 and TIMP-2 were identified as AKI biomarkers by Kashani et al. in a screen of 340 candidate biomarkers to predict AKI based on creatinine criteria (Kashani et al. 2013). In this study, the product of IGFBP7 and TIMP-2 concentrations was superior to several other biomarkers, which was later validated in additional cohorts (see below). TIMP-2, a 21-kDa protein, is a member of the tissue inhibitor of metalloproteinase (TIMP) family, which are endogenous inhibitors of metalloproteinase activities. IGFBP7, a 29-kDa secreted protein, is known to bind to and inhibit signalling through IGF-1 receptors (Evdokimova et al. 2012).
The cellular sources of IGFBP7 and TIMP-2 in AKI are poorly understood. Increased levels of IGFBP7 mRNA were found in uranyl nitrate-induced acute renal failure in mice (Taulan et al. 2006). Apart from this report and the finding that IGFBP7 and TIMP-2 are enriched in the urine of patients at risk of AKI, the site of synthesis of these molecules in the setting of AKI is unknown. While Kashani et al. speculated that IGFBP7 and TIMP-2 are synthesized by renal tubular cells (Kashani et al. 2013), there is no scientific evidence to support this.
Furthermore, the physiological roles of IGFBP7 and TIMP-2 in the kidney are unknown. Kashani et al. pointed out that IGFBP7 and TIMP2 were known to induce G1 cell cycle arrest and even designated them as ‘cell cycle arrest biomarkers’ (Kashani et al. 2013). Nevertheless, the notion that IGFBP7 or TIMP-2 are associated with cell cycle arrest in the kidney is unsubstantiated by experimental evidence. Data supporting the involvement of TIMP-2 in cell cycle arrest come from an in vitro study in human microvascular cells, not kidney cells (Seo et al. 2006). IGFBP7 has been shown to induce cell cycle arrest in colon cancer cell lines (Ma et al. 2008) and in breast cancer cell lines (Zuo et al. 2012), but not in the kidney.
The diagnostic value of TIMP-2 and IGFBP7 was validated in the Sapphire study (Kashani et al. 2013). In this study, the product of TIMP-2 and IGFBP7 ([TIMP-2]·[IGFBP7]) was superior to seven other biomarkers (urine NGAL, plasma Cystatin C, urine KIM-1, plasma NGAL, urine IL-18, urine pi-GST, urine L-FABP) and to TIMP-2 and IGFBP7 alone in predicting stage 2 or 3 AKI (AUC-receiver operating characteristic (ROC) 0.8). Follow-up studies by the same authors also conducted in the ICU population using [TIMP-2]·[IGFBP7] determined an AUC-ROC of 0.82 for the prediction of stage 2 or 3 AKI (Bihorac et al. 2014) and 0.79 (Hoste et al. 2014), respectively. This indicated that [TIMP-2]·[IGFBP7] are moderate to good biomarkers in predicting severe AKI within 12 h in the ICU setting. Conversely, an independent group of researchers failed to replicate the predictive performance of [TIMP-2]·[IGFBP7] in a similar ICU setting (Bell et al. 2015). [TIMP-2]·[IGFBP7] was also investigated in cardiac surgery patients (Meersch et al. 2014, Pilarczyk et al. 2015, Wetz et al. 2015), in a mixed collective including cardiac and non-cardiac surgery patients (Gunnerson et al. 2016), and in major surgery patients only (Gocze et al. 2015) showing AUCs between 0.7 and 0.85 for the prediction of AKI.
Koyner et al., in a secondary long-term follow-up of the Sapphire study, showed that [TIMP-2]·[IGFBP7] concentrations at the time of ICU admission were predictive of a composite long-term outcome of death of RRT requirement over the next 9 months only in patients who developed AKI, but not in those who did not develop AKI (Koyner et al. 2015b). Currently, no information is available regarding the performance of [TIMP-2]·[IGFBP7] in patients outside the ICU/perioperative setting, nor is it known whether these biomarkers associate with intrinsic injury to the kidney as opposed to pre-renal AKI. In a healthy patient population, [TIMP-2]·[IGFBP7] did not show significant differences between male and female subjects, but a weak inverse correlation with age (Chindarkar et al. 2016).
In sum, data on the so-called cell cycle arrest biomarkers are emerging, but too little is known about their cellular sources and mechanism of action to link them with the pathophysiology of AKI. Given the promising performance of these markers in some clinical studies, research into their pathophysiology will be a major priority.
Calprotectin
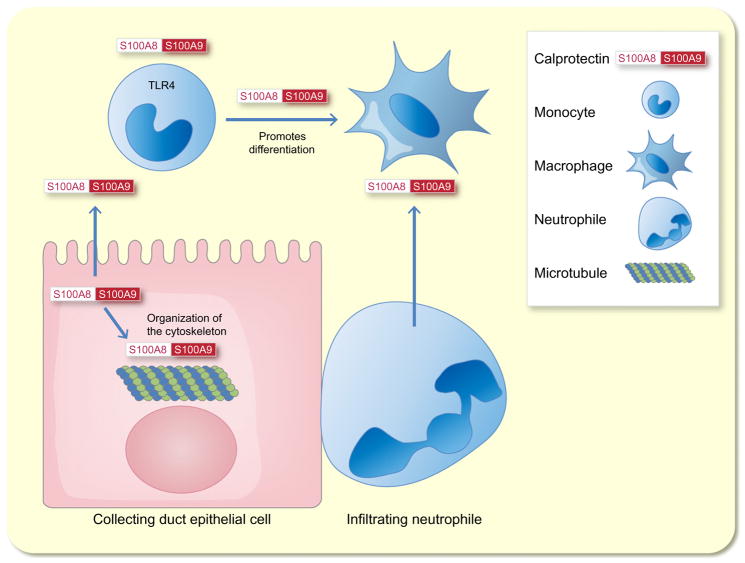
Calprotectin is a 24 kDa heterodimer formed from the two monomers S100A8 (10 835 Da) and S100A9 (13 242 Da) (Stríz & Trebichavský 2004). It has initially been identified as an antimicrobial protein in the cytoplasm of neutrophil granulocytes (Dale et al. 1983). Intracellular calprotectin’s main function is to interact with the cytoskeleton whereas when is secreted by activated immune cells it acts as a danger-associated molecular pattern protein (Ehrchen et al. 2009) (Fig. 6). There is no singular receptor that exerts calprotectin signal transduction (Ehrchen et al. 2009), but it has been shown that S100A8 and S100A9 are endogenous activators of toll-like receptor 4 (Vogl et al. 2007) (Fig. 6).
Figure 6.
Schematic model of the functions of calprotectin. The two monomers S100A8 and S100A9 form calprotectin. Intracellular calprotectin’s main function is to interact with the cytoskeleton. When calprotectin is secreted by activated immune cells, it acts as a danger-associated molecular pattern protein. S100A8 and S100A9 are endogenous activators of toll-like receptor 4 (TLR4). This promotes the differentiation of monocytes to macrophages.
Concerning the involvement of calprotectin in renal pathophysiology, it has been shown that renal collecting duct epithelial cells produce S100A8 and S100A9 in a model of kidney injury in response to unilateral ureteral obstruction (UUO) (Fujiu et al. 2011). S100A8 and S100A9 attract CD11b+Ly-6C+ inflammatory monocytes to the kidneys, which then contribute to the cells’ differentiation into M1-type CD11b+F4/80lo cells. These cells promote renal epithelial injury and inflammation. S100A8 and S100A9 are also induced in response to ischaemia reperfusion injury in mice (Dessing et al. 2015). Infiltrating kidney neutrophils are the main source of S100A8/9 in the ischaemic kidney. S100A9-knockout mice, which lack active calprotectin, show an increased transition to renal fibrosis in response to ischaemia reperfusion injury, while the initial renal injury is similar to wild-type mice. This is associated with an enhanced formation of alternatively activated M2 macrophages in the damaged kidney (Dessing et al. 2015).
Ebbing et al. investigated time-dependent changes of calprotectin in patients undergoing nephron-sparing surgery for kidney tumours, which leads to iatrogenic renal ischaemia reperfusion injury due to transient clamping of the renal artery. Calprotectin concentrations started to be significantly increased at the end of the operation (approx. 2 h after ischaemia) and reached maximal levels 48 h post-surgery, with a 69-fold increase over baseline in calprotectin levels. Calprotectin was still significantly increased 5 days after surgery (Ebbing et al. 2016). Elevation of calprotectin has been described in several other diseases such as rheumatoid arthritis (Hammer et al. 2007), inflammatory bowel disease (Foell et al. 2008), myocardial infarction (Altwegg et al. 2007), cancer (Müller et al. 2008) and several others (Seeliger et al. 2003, Brun et al. 2005, Payen et al. 2008). In the clinical interpretation of urinary calprotectin findings, one should be aware that there are two clinical settings other than AKI that lead to an increase of calprotectin: as calprotectin is predominantly derived from neutrophils and monocytes, pyuria substantially increases urinary calprotectin. Moreover, urothelial carcinoma is associated with increased concentrations (Ebbing et al. 2014).
There are three studies investigating the diagnostic accuracy of calprotectin in its ability of distinguishing pre-renal from intrinsic AKI (Heller et al. 2011, Seibert et al. 2013, Chang et al. 2015). Calprotectin showed a very high accuracy in predicting intrinsic AKI with an AUC ranging from 0.92 to 0.97 in these studies. In a study on calprotectin measured in renal allograft recipients, a significant but weak (r = −0.33) inverse association of urinary calprotectin concentrations measured directly after surgery and eGFR 4 weeks after transplantation was observed (Tepel et al. 2014). A recent multi-centre study analysed the diagnostic accuracy of calprotectin in the differentiation of pre-renal and intrinsic acute allograft injury (Seibert et al. 2016). Notably, urinary calprotectin concentrations of subjects with intrinsic AKI were 36 times higher than in pre-renal allograft injury yielding an AUC of 0.94. Immunohistochemical stainings in this study indicated calprotectin was produced primarily by infiltrating inflammatory cells confirming previous observations in the mouse ischaemic kidney (Fig. 6).
Summary and perspectives
A desirable biomarker should be non-invasive, detectable at early stages of the disease and prognostically relevant, but it should also be specific for a tissue type and have a close pathophysiological relation to the disease. Several potential biomarkers for AKI have been introduced, and each of these biomarkers has its advantages and disadvantages (for an overview, see Table 1). Today there is no perfect biomarker of AKI. It seems unlikely that the ‘kidney troponin’ will be found, but this is in part due to the shortcomings and the heterogenous nature of the current definition of AKI, which is itself based on surrogate markers of kidney function.
Table 1.
Overview of origin, physiological function in the kidney, animal models, disadvantages and key clinical studies in different settings of biomarkers of acute kidney injury (AKI)
| NGAL | KIM-1 | L-FABP | IL-18 | IGFBP7 and TIMP-2 | Calprotectin | |
|---|---|---|---|---|---|---|
| Origin | Thick ascending loop of Henle and the intercalated cells of the collecting duct (Schmidt-Ott et al. 2007, Paragas et al. 2014) | Proximal tubule cells (Han et al. 2002, Ichimura et al. 2008) | Proximal tubule cells (Yamamoto et al. 2007) | Collecting duct (Gauer et al. 2007) Tubular epithelial cells (Franke et al. 2012) |
Unknown | Collecting duct and in filtrating immune cells (Fujiu et al. 2011, Seibert et al. 2016) |
| Physiological function in the kidney | Bacteriostatic function in the innate immune system, iron delivery to mammalian cells (Goetz et al. 2002, Flo et al. 2004, Bao et al. 2010) | Tubular regeneration by mediating phagocytosis of apoptotic bodies (Ichimura et al. 2008) | Regulation of fatty acids uptake and the intracellular transport (Chmurzyńska 2006) | Proinflammatory effect (Cheung et al. 2005) | Unknown | Polarization of M2 macrophages, promotion of repair after injury (Dessing et al. 2015) |
| Animal models | Holo-NGAL protects kidney from damage in response to ischaemia reperfusion injury (Mori et al. 2005) | KIM-1 knockout protects against damage in response to ischaemia reperfusion injury (Ismail et al. 2015b) | Human-L-FABP transgenic mice have less damage after ischaemia and reperfusion (Yamamoto et al. 2007) | IL-18-deficient mice are protected from ischaemia reperfusion-induced AKI (Wu et al. 2008) | Unknown | A lack of active calprotectin leads to more fibrosis after inn response to ischaemia reperfusion injury (Dessing et al. 2015) |
| Disadvantages | AKI-independent association with sepsis, CKD, UTI (Devarajan 2007, Schmidt-Ott 2011) | Is induced in various chronic proteinuric, inflammatory diseases (Smith et al. 2006) | Association of L-FABP with anaemia (Imai et al. 2015) | No reliable prediction of AKI | Unclear cellular sources and pathophysiology | Elevated in UTI (Heller et al. 2011) Elevated in urothelial carcinoma (Ebbing et al. 2014) |
| Key clinical studies | ||||||
| Diagnosis of intrinsic AKI | AUC 0.87 (Singer et al. 2011) AUC 0.81 (Nickolas et al. 2012) AUC 0.95 (Nickolas et al. 2008) |
AUC 0.71 (Nickolas et al. 2012) | AUC 0.70 (Nickolas et al. 2012) | AUC 0.64 (Nickolas et al. 2012) | AUC 0.97 (Heller et al. 2011) AUC 0.99 (Seibert et al. 2013) AUC 0.94 (Seibert et al. 2016) AUC 0.94 (Chang et al. 2015) |
|
| Early prediction of AKI | AUC 0.67 (Parikh et al. 2011) AUC 0.72 (Koyner et al. 2010) |
AUC 0.69 (Koyner et al. 2010) AUC 0.71 (Parikh et al. 2013) |
AUC 0.66 (Parikh et al. 2013) AUC 0.69 (Prowle et al. 2015) |
AUC 0.75 (Parikh et al. 2013) AUC 0.55 (Haase et al. 2008) |
AUC 0.85 (Bihorac et al. 2014) AUC 0.80 (Kashani et al. 2013) |
|
| Prediction of in-hospital death | Hall et al. (2011), Singer et al. (2011), Nickolas et al. (2012) | Hall et al. (2011), Gonzalez & Vincent (2012), Nickolas et al. (2012) | Doi et al. (2011), Nickolas et al. (2012) | Doi et al. (2011), Hall et al. (2011) | ||
| Prediction of long-term ESRD/mortality | Bolignano et al. (2009), Ralib et al. (2012), Coca et al. (2014) | Koyner et al. (2015b) | ||||
Among the individual markers, KIM-1 and L-FABP are derived from proximal tubules, NGAL is derived from the distal nephron and collecting duct, while IL-18 and calprotectin are probably largely derived from immune cells infiltrating the injured kidney (Fig. 1). The sources of IGFBP7 and TIMP-2 are currently unknown.
Studies in mice have shown that NGAL, KIM-1, IL-18 and calprotectin participate critically in the pathogenesis of AKI, while IGFBP7 and TIMP2 have not been linked with AKI in model organisms.
Each of the described biomarkers is not entirely specific for AKI. This is reflected by imperfect test characteristics of each biomarker with the best AUC-ROCs ranging between 0.75 and 0.85. The pathophysiologic basis for this lack of sensitivity and specificity is only partially understood. For instance, NGAL, IL-18 and calprotectin are known to be produced in immune cells and display associations with UTIs and sepsis, which are independent from AKI. NGAL, KIM-1 and IL-18 are elevated in patients with CKD. TIMP-2 and IGFBP7 have not been thoroughly tested in settings outside the ICU, and their pathophysiological roles are currently unclear. NGAL and calprotectin are closely associated with intrinsic AKI and display much lower levels in pre-renal AKI, which makes them potentially suitable in the differential diagnosis of patients with established AKI. All new biomarkers have in common that if they are once elevated after AKI they stay elevated for a long time. Therefore, an assignment to a phase of AKI is difficult (Koyner et al. 2012). Notably however, the expression of NGAL, including the length of time of expression, is dose dependent on disease severity so these parameters must be reviewed in greater detail.
Acknowledgments
The work was supported by grants from the Deutsche Forschungsgemeinschaft (Research Unit 1368 to K. M. S.-O. and T. W.). K. M. S.-O. is supported by the Urological Research Foundation (Berlin, Germany). E. Schrezenmeier is supported by the Charité Junior Clinical Scientist Program (Charité-Universitätsmedizin Berlin and the Berlin Institute of Health). We would like to thank the Stiftelsen Nordisk Fysiologi, SNF and the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) for their generous support for the AP Symposium on ‘RENOPROTECTION’.
Footnotes
Conflict of interest
Columbia University has licensed uNGAL for use in the diagnosis of AKI (applies to J. B. and K.M.S.-O.). K. Budde received research funds and/or honoraria from AiCuris, Pfizer, Novartis, Astellas, Roche, Hexal, Bristol-Myers Squibb, Veloxis Pharma, Effimune Pharma and Siemens. Other authors have reported that they have no relationships relevant to the contents of this study to disclose.
References
- Altwegg LA, Neidhart M, Hersberger M, Müller S, Eberli FR, Corti R, Roffi M, Sütsch G, Gay S, von Eckardstein A, Wischnewsky MB, Lüscher TF, Maier W. Myeloid-related protein 8/14 complex is released by monocytes and granulocytes at the site of coronary occlusion: a novel, early, and sensitive marker of acute coronary syndromes. Eur Heart J. 2007;28:941–948. doi: 10.1093/eurheartj/ehm078. [DOI] [PubMed] [Google Scholar]
- Amin RP, Vickers AE, Sistare F, Thompson KL, Roman RJ, Lawton M, Kramer J, Hamadeh HK, Collins J, Grissom S, et al. Identification of putative gene based markers of renal toxicity. Environ Health Perspect. 2004;112:465–479. doi: 10.1289/ehp.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur JM, Hill EG, Alge JL, Lewis EC, Neely BA, Janech MG, Tumlin JA, Chawla LS, Shaw AD SAKInet Investigators. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int. 2014;85:431–438. doi: 10.1038/ki.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attridge RL, Linn WD, Ryan L, Koeller J, Frei CR. Evaluation of the incidence and risk factors for development of fenofibrate-associated nephrotoxicity. J Clin Lipidol. 2012;6:19–26. doi: 10.1016/j.jacl.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, Bonventre JV. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277:39739–39748. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- Bao G, Clifton M, Hoette TM, Mori K, Deng SX, Qiu A, Viltard M, Williams D, Paragas N, Leete T, et al. Iron traffics in circulation bound to a sidero-calin (Ngal)-catechol complex. Nat Chem Biol. 2010;6:602–609. doi: 10.1038/nchembio.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M, Granath F, Mårtensson J, Löfberg E, Ekbom A, Martling CR KING (Karolinska Intensive care Nephrology Group) Cystatin C is correlated with mortality in patients with and without acute kidney injury. Nephrol Dial Transplant. 2009;24:3096–3102. doi: 10.1093/ndt/gfp196. [DOI] [PubMed] [Google Scholar]
- Bell M, Larsson A, Venge P, Bellomo R, Mårtensson J. Assessment of cell-cycle arrest biomarkers to predict early and delayed acute kidney injury. Dis Markers. 2015;2015:158658. doi: 10.1155/2015/158658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihorac A, Yavas S, Subbiah S, Hobson CE, Schold JD, Gabrielli A, Layon AJ, Segal MS. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249:851–858. doi: 10.1097/SLA.0b013e3181a40a0b. [DOI] [PubMed] [Google Scholar]
- Bihorac A, Chawla LS, Shaw AD, Al-Khafaji A, Davison DL, Demuth GE, Fitzgerald R, Gong MN, Graham DD, Gunnerson K, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189:932–939. doi: 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- Bolignano D, Basile G, Parisi P, Coppolino G, Nicocia G, Buemi M. Increased plasma neutrophil gelatinase-associated lipocalin levels predict mortality in elderly patients with chronic heart failure. Rejuvenation Res. 2009;12:7–14. doi: 10.1089/rej.2008.0803. [DOI] [PubMed] [Google Scholar]
- Brun JG, Madland TM, Gran JT, Myklebust G. A longitudinal study of calprotectin in patients with polymyalgia rheumatica or temporal arteritis: relation to disease activity. Scand J Rheumatol. 2005;34:125–128. doi: 10.1080/03009740410009931. [DOI] [PubMed] [Google Scholar]
- Cai L, Rubin J, Han W, Venge P, Xu S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin J Am Soc Nephrol. 2010;5:2229–2235. doi: 10.2215/CJN.00980110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Ko K, Clark MR. The emerging role of the inflammasome in kidney diseases. Curr Opin Nephrol Hypertens. 2014;23:204–210. doi: 10.1097/01.mnh.0000444814.49755.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Yang CH, Yang HY, Chen TH, Lin CY, Chang SW, Chen YT, Hung CC, Fang JT, Yang CW, Chen YC. Urinary biomarkers improve the diagnosis of intrinsic acute kidney injury in coronary care units. Medicine (Baltimore) 2015;94:e1703. doi: 10.1097/MD.0000000000001703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla LS, Amdur RL, Shaw AD, Faselis C, Palant CE, Kimmel PL. Association between AKI and long-term renal and cardiovascular outcomes in United States veterans. Clin J Am Soc Nephrol. 2014a;9:448–456. doi: 10.2215/CJN.02440213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014b;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla LS, Goldstein SL, Kellum JA, Ronco C. Renal angina: concept and development of pretest probability assessment in acute kidney injury. Crit Care. 2015;19:93. doi: 10.1186/s13054-015-0779-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung H, Chen NJ, Cao Z, Ono N, Ohashi PS, Yeh WC. Accessory protein-like is essential for IL-18-mediated signaling. J Immunol. 2005;174:5351–5357. doi: 10.4049/jimmunol.174.9.5351. [DOI] [PubMed] [Google Scholar]
- Chindarkar NS, Chawla LS, Straseski JA, Jortani SA, Uettwiller-Geiger D, Orr RR, Kellum JA, Fitzgerald RL. Reference intervals of urinary acute kidney injury (AKI) markers [IGFBP7]•[TIMP2] in apparently healthy subjects and chronic comorbid subjects without AKI. Clin Chim Acta. 2016;452:32–37. doi: 10.1016/j.cca.2015.10.029. [DOI] [PubMed] [Google Scholar]
- Chmurzyńska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet. 2006;47:39–48. doi: 10.1007/BF03194597. [DOI] [PubMed] [Google Scholar]
- Coca SG, King JT, Rosenthal RA, Perkal MF, Parikh CR. The duration of postoperative acute kidney injury is an additional parameter predicting long-term survival in diabetic veterans. Kidney Int. 2010;78:926–933. doi: 10.1038/ki.2010.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coca SG, Garg AX, Thiessen-Philbrook H, Koyner JL, Patel UD, Krumholz HM, Shlipak MG, Parikh CR, Parikh CR. Urinary biomarkers of AKI and mortality 3 years after cardiac surgery. J Am Soc Nephrol. 2014;25:1063–1071. doi: 10.1681/ASN.2013070742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz DN, Bagshaw SM, Maisel A, Lewington A, Thadhani R, Chakravarthi R, Murray PT, Mehta RL, Chawla LS. Use of biomarkers to assess prognosis and guide management of patients with acute kidney injury. Contrib Nephrol. 2013;182:45–64. doi: 10.1159/000349965. [DOI] [PubMed] [Google Scholar]
- Cullen MR, Murray PT, Fitzgibbon MC. Establishment of a reference interval for urinary neutrophil gelatinase-associated lipocalin. Ann Clin Biochem. 2012;49:190–193. doi: 10.1258/acb.2011.011105. [DOI] [PubMed] [Google Scholar]
- Dale I, Fagerhol MK, Naesgaard I. Purification and partial characterization of a highly immunogenic human leukocyte protein, the L1 antigen. Eur J Biochem. 1983;134:1–6. doi: 10.1111/j.1432-1033.1983.tb07522.x. [DOI] [PubMed] [Google Scholar]
- Dent CL, Ma Q, Dastrala S, Bennett M, Mitsnefes MM, Barasch J, Devarajan P. Plasma neutrophil gelatinase-associated lipocalin predicts acute kidney injury, morbidity and mortality after pediatric cardiac surgery: a prospective uncontrolled cohort study. Crit Care. 2007;11:R127. doi: 10.1186/cc6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessing MC, Tammaro A, Pulskens WP, Teske GJ, Butter LM, Claessen N, van Eijk M, van der Poll T, Vogl T, Roth J, Florquin S, Leemans JC. The calcium-binding protein complex S100A8/A9 has a crucial role in controlling macrophage-mediated renal repair following ischemia/reperfusion. Kidney Int. 2015;87:85–94. doi: 10.1038/ki.2014.216. [DOI] [PubMed] [Google Scholar]
- Devarajan P. Emerging biomarkers of acute kidney injury. Contrib Nephrol. 2007;156:203–212. doi: 10.1159/000102085. [DOI] [PubMed] [Google Scholar]
- Dieterle F, Sistare F, Goodsaid F, Papaluca M, Ozer JS, Webb CP, Baer W, Senagore A, Schipper MJ, Vonderscher J, et al. Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol. 2010;28:455–462. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- Doi K, Negishi K, Ishizu T, Katagiri D, Fujita T, Matsubara T, Yahagi N, Sugaya T, Noiri E. Evaluation of new acute kidney injury biomarkers in a mixed intensive care unit. Crit Care Med. 2011;39:2464–2469. doi: 10.1097/CCM.0b013e318225761a. [DOI] [PubMed] [Google Scholar]
- Ebbing J, Mathia S, Seibert FS, Pagonas N, Bauer F, Erber B, Günzel K, Kilic E, Kempkensteffen C, Miller K, Bachmann A, Rosenberger C, Zidek W, Westhoff TH. Urinary calprotectin: a new diagnostic marker in urothelial carcinoma of the bladder. World J Urol. 2014;32:1485–1492. doi: 10.1007/s00345-013-1227-8. [DOI] [PubMed] [Google Scholar]
- Ebbing J, Seibert FS, Pagonas N, Bauer F, Miller K, Kempkensteffen C, Günzel K, Bachmann A, Seifert HH, Rentsch CA, Ardelt P, Wetterauer C, Amico P, Babel N, Westhoff TH. Dynamics of urinary calprotectin after renal ischaemia. PLoS One. 2016;11:e0146395. doi: 10.1371/journal.pone.0146395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86:557–566. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- Evdokimova V, Tognon CE, Benatar T, Yang W, Krutikov K, Pollak M, Sorensen PHB, Seth A. IGFBP7 binds to the IGF-1 receptor and blocks its activation by insulin-like growth factors. Sci Signal. 2012;5:ra92. doi: 10.1126/scisignal.2003184. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Reed DA, Dinarello CA. IL-12-induced IFN-gamma is dependent on caspase-1 processing of the IL-18 precursor. J Clin Invest. 1999;104:761–767. doi: 10.1172/JCI7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–2256. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- Foell D, Wittkowski H, Ren Z, Turton J, Pang G, Daebritz J, Ehrchen J, Heidemann J, Borody T, Roth J, Clancy R. Phagocyte-specific S100 proteins are released from affected mucosa and promote immune responses during inflammatory bowel disease. J Pathol. 2008;216:183–192. doi: 10.1002/path.2394. [DOI] [PubMed] [Google Scholar]
- Fraker PJ, King LE, Lill-Elghanian D, Telford WG. Quantification of apoptotic events in pure and heterogeneous populations of cells using the flow cytometer. Methods Cell Biol. 1995;46:57–76. doi: 10.1016/s0091-679x(08)61924-x. [DOI] [PubMed] [Google Scholar]
- Franke EI, Vanderbrink BA, Hile KL, Zhang H, Cain A, Matsui F, Meldrum KK. Renal IL-18 production is macrophage independent during obstructive injury. PLoS One. 2012;7:e47417. doi: 10.1371/journal.pone.0047417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs TC, Frick K, Emde B, Czasch S, von Landenberg F, Hewitt P. Evaluation of novel acute urinary rat kidney toxicity biomarker for subacute toxicity studies in preclinical trials. Toxicol Pathol. 2012;40:1031–1048. doi: 10.1177/0192623312444618. [DOI] [PubMed] [Google Scholar]
- Fujiu K, Manabe I, Nagai R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest. 2011;121:3425–3441. doi: 10.1172/JCI57582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauer S, Sichler O, Obermüller N, Holzmann Y, Kiss E, Sobkowiak E, Pfeilschifter J, Geiger H, Mühl H, Hauser IA. IL-18 is expressed in the intercalated cell of human kidney. Kidney Int. 2007;72:1081–1087. doi: 10.1038/sj.ki.5002473. [DOI] [PubMed] [Google Scholar]
- de Geus HRH, Bakker J, Lesaffre EMEH, le Noble JLML. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am J Respir Crit Care Med. 2011;183:907–914. doi: 10.1164/rccm.200908-1214OC. [DOI] [PubMed] [Google Scholar]
- Gocze I, Koch M, Renner P, Zeman F, Graf BM, Dahlke MH, Nerlich M, Schlitt HJ, Kellum JA, Bein T. Urinary biomarkers TIMP-2 and IGFBP7 early predict acute kidney injury after major surgery. PLoS One. 2015;10:e0120863. doi: 10.1371/journal.pone.0120863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez F, Vincent F. Biomarkers for acute kidney injury in critically ill patients. Minerva Anestesiol. 2012;78:1394–1403. [PubMed] [Google Scholar]
- Gunnerson KJ, Shaw AD, Chawla LS, Bihorac A, Al-Khafaji A, Kashani K, Lissauer M, Shi J, Walker MG, Kellum JA Sapphire Topaz Investigators. TIMP2•IGFBP7 biomarker panel accurately predicts acute kidney injury in high-risk surgical patients. J Trauma Acute Care Surg. 2016;80:243–249. doi: 10.1097/TA.0000000000000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase M, Bellomo R, Story D, Davenport P, Haase-Fielitz A. Urinary interleukin-18 does not predict acute kidney injury after adult cardiac surgery: a prospective observational cohort study. Crit Care. 2008;12:R96. doi: 10.1186/cc6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall IE, Coca SG, Perazella MA, Eko UU, Luciano RL, Peter PR, Han WK, Parikh CR. Risk of poor outcomes with novel and traditional biomarkers at clinical AKI diagnosis. Clin J Am Soc Nephrol. 2011;6:2740–2749. doi: 10.2215/CJN.04960511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer HB, Odegard S, Fagerhol MK, Landewé R, van der Heijde D, Uhlig T, Mowinckel P, Kvien TK. Calprotectin (a major leucocyte protein) is strongly and independently correlated with joint inflammation and damage in rheumatoid arthritis. Ann Rheum Dis. 2007;66:1093–1097. doi: 10.1136/ard.2006.064741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- Heller F, Frischmann S, Grünbaum M, Zidek W, Westhoff TH. Urinary calprotectin and the distinction between prerenal and intrinsic acute kidney injury. Clin J Am Soc Nephrol. 2011;6:2347–2355. doi: 10.2215/CJN.02490311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J, Tangri N, Komenda P, Kaushal A, Sood M, Brar R, Gill K, Walker S, MacDonald K, Hiebert BM, Arora RC, Rigatto C. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis. 2015;66:993–1005. doi: 10.1053/j.ajkd.2015.06.018. [DOI] [PubMed] [Google Scholar]
- Homsi E, Janino P, de Faria JBL. Role of caspases on cell death, inflammation, and cell cycle in glycerol-induced acute renal failure. Kidney Int. 2006;69:1385–1392. doi: 10.1038/sj.ki.5000315. [DOI] [PubMed] [Google Scholar]
- Hoste EAJ, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoste EAJ, McCullough PA, Kashani K, Chawla LS, Joannidis M, Shaw AD, Feldkamp T, Uettwiller-Geiger DL, McCarthy P, Shi J, Walker MG, Kellum JA Sapphire Investigators. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant. 2014;29:2054–2061. doi: 10.1093/ndt/gfu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Xu F, Sabbisetti V, Grgic I, Movahedi Naini S, Wang N, Chen G, Xiao S, Patel D, Henderson JM, et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest. 2013;123:4023–4035. doi: 10.1172/JCI45361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hvidberg V, Jacobsen C, Strong RK, Cowland JB, Moestrup SK, Borregaard N. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett. 2005;579:773–777. doi: 10.1016/j.febslet.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–63. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Asseldonk EJPV, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai N, Yasuda T, Kamijo-Ikemori A, Shibagaki Y, Kimura K. Distinct roles of urinary liver-type fatty acid-binding protein in non-diabetic patients with anemia. PLoS One. 2015;10:e0126990. doi: 10.1371/journal.pone.0126990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail O, Zhang X, Bonventre JV, Gunaratnam L. G protein, 12 (Gα12) is a negative regulator of kidney injury molecule-1-mediated efferocytosis. Am J Physiol Renal Physiol. 2015a doi: 10.1152/ajprenal.00169.2015. ajprenal.00169.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail OZ, Zhang X, Wei J, Haig A, Denker BM, Suri RS, Sener A, Gunaratnam L. Kidney injury molecule-1 protects against Gα12 activation and tissue damage in renal ischemia-reperfusion injury. Am J Pathol. 2015b;185:1207–1215. doi: 10.1016/j.ajpath.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2:356–365. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- Kamijo-Ikemori A, Sugaya T, Matsui K, Yokoyama T, Kimura K. Roles of human liver type fatty acid binding protein in kidney disease clarified using hL-FABP chromosomal transgenic mice. Nephrology. 2011;16:539–544. doi: 10.1111/j.1440-1797.2011.01469.x. [DOI] [PubMed] [Google Scholar]
- Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw SM, Bell M, Bihorac A, Birkhahn R, Cely CM, Chawla LS, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi: 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum JA, Bellomo R, Ronco C. Kidney attack. JAMA. 2012;307:2265–2266. doi: 10.1001/jama.2012.4315. [DOI] [PubMed] [Google Scholar]
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, Raman J, Jeevanandam V, O’Connor MF, Devarajan P, Bonventre JV, Murray PT. Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol. 2010;5:2154–2165. doi: 10.2215/CJN.00740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, Patel UD, Shlipak MG, Parikh CR TRIBE-AKI Consortium. Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol. 2012;23:905–914. doi: 10.1681/ASN.2011090907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyner JL, Davison DL, Brasha-Mitchell E, Chalikonda DM, Arthur JM, Shaw AD, Tumlin JA, Trevino SA, Bennett MR, Kimmel PL, Seneff MG, Chawla LS. Furosemide stress test and biomarkers for the prediction of AKI severity. J Am Soc Nephrol. 2015a;26:2023–2031. doi: 10.1681/ASN.2014060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyner JL, Shaw AD, Chawla LS, Hoste EAJ, Bihorac A, Kashani K, Haase M, Shi J, Kellum JA Sapphire Investigators. Tissue inhibitor metalloproteinase-2 (TIMP-2)•IGF-binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol. 2015b;26:1747–1754. doi: 10.1681/ASN.2014060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczeski CD, Goldstein SL, Woo JG, Wang Y, Piyaphanee N, Ma Q, Bennett M, Devarajan P. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol. 2011;58:2301–2309. doi: 10.1016/j.jacc.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrier JF, Thomas C, Grober J, Duez H, Percevault F, Souidi M, Linard C, Staels B, Besnard P. Statin induction of liver fatty acid-binding protein (L-FABP) gene expression is peroxisome proliferator-activated receptor-alpha-dependent. J Biol Chem. 2004;279:45512–45518. doi: 10.1074/jbc.M407461200. [DOI] [PubMed] [Google Scholar]
- Lin X, Yuan J, Zhao Y, Zha Y. Urine interleukin-18 in prediction of acute kidney injury: a systemic review and meta-analysis. J Nephrol. 2015;28:7–16. doi: 10.1007/s40620-014-0113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Lu B, Ruan W, Wang H, Lin J, Hu H, Deng H, Huang Q, Lai M. Tumor suppressor gene insulin-like growth factor binding protein-related protein 1 (IGFBP-rP1) induces senescence-like growth arrest in colorectal cancer cells. Exp Mol Pathol. 2008;85:141–145. doi: 10.1016/j.yexmp.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Maatman RG, Van Kuppevelt TH, Veerkamp JH. Two types of fatty acid-binding protein in human kidney. Isolation, characterization and localization. Biochem J. 1991;273:759–766. doi: 10.1042/bj2730759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medić B, Rovčanin B, Basta Jovanović G, Radojević-Škodrić S, Prostran M. Kidney injury molecule-1 and cardiovascular diseases: from basic science to clinical practice. Biomed Res Int. 2015;2015:854070. doi: 10.1155/2015/854070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meersch M, Schmidt C, Van Aken H, Martens S, Rossaint J, Singbartl K, Görlich D, Kellum JA, Zarbock A. Urinary TIMP-2 and IGFBP7 as early biomarkers of acute kidney injury and renal recovery following cardiac surgery. PLoS One. 2014;9:e93460. doi: 10.1371/journal.pone.0093460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megyesi J, Safirstein RL, Price PM. Induction of p21WAF1/CIP1/SDI1 in kidney tubule cells affects the course of cisplatin-induced acute renal failure. J Clin Invest. 1998;101:777–782. doi: 10.1172/JCI1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM. Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol. 2002;13:1350–1357. doi: 10.1097/01.asn.0000014692.19351.52. [DOI] [PubMed] [Google Scholar]
- Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107:1145–1152. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, Barasch J, Devarajan P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- Mishra J, Mori K, Ma Q, Kelly C, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller H, Haug U, Rothenbacher D, Stegmaier C, Brenner H. Evaluation of serum and urinary myeloid related protein-14 as a marker for early detection of prostate cancer. J Urol. 2008;180:1309–1312. doi: 10.1016/j.juro.2008.06.025. discussion 1312–1313. [DOI] [PubMed] [Google Scholar]
- Murugan R, Karajala-Subramanyam V, Lee M, Yende S, Kong L, Carter M, Angus DC, Kellum JA Genetic and Inflammatory Markers of Sepsis (GenIMS) Investigators. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77:527–535. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Heart, Lung and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- Negishi K, Noiri E, Sugaya T, Li S, Megyesi J, Nagothu K, Portilla D. A role of liver fatty acid-binding protein in cisplatin-induced acute renal failure. Kidney Int. 2007;72:348–358. doi: 10.1038/sj.ki.5002304. [DOI] [PubMed] [Google Scholar]
- Negishi K, Noiri E, Maeda R, Portilla D, Sugaya T, Fujita T. Renal L-type fatty acid-binding protein mediates the bezafibrate reduction of cisplatin-induced acute kidney injury. Kidney Int. 2008;73:1374–1384. doi: 10.1038/ki.2008.106. [DOI] [PubMed] [Google Scholar]
- Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, Elger A, Maarouf O, Sola-Del Valle DA, O’Rourke M, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59:246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisula S, Yang R, Poukkanen M, Vaara ST, Kaukonen KM, Tallgren M, Haapio M, Tenhunen J, Korhonen AM, Pettilä V FINNAKI Study Group. Predictive value of urine interleukin-18 in the evolution and outcome of acute kidney injury in critically ill adult patients. Br J Anaesth. 2015;114:460–468. doi: 10.1093/bja/aeu382. [DOI] [PubMed] [Google Scholar]
- Noiri E, Doi K, Negishi K, Tanaka T, Hamasaki Y, Fujita T, Portilla D, Sugaya T. Urinary fatty acid-binding protein 1: an early predictive biomarker of kidney injury. Am J Physiol Renal Physiol. 2009;296:F669–79. doi: 10.1152/ajprenal.90513.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- Novick D, Kim S, Kaplanski G, Dinarello CA. Interleukin-18, more than a Th1 cytokine. Semin Immunol. 2013;25:439–448. doi: 10.1016/j.smim.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Pajek J, Škoberne A, Šosterič K, Adlešič B, Leskošek B, Bučar Pajek M, Osredkar J, Lindič J. Non-inferiority of creatinine excretion rate to urinary L-FABP and NGAL as predictors of early renal allograft function. BMC Nephrol. 2014;5:50. doi: 10.1186/1471-2369-15-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med. 2011;17:216–222. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paragas N, Kulkarni R, Werth M, Schmidt-Ott KM, Forster C, Deng R, Zhang Q, Singer E, Klose AD, Shen TH, et al. α-Intercalated cells defend the urinary system from bacterial infection. J Clin Invest. 2014;124:2963–2976. doi: 10.1172/JCI71630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX TRIBE-AKI Consortium. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh CR, Thiessen-Philbrook H, Garg AX, Kadiyala D, Shlipak MG, Koyner JL, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Coca SG. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery.) Clin J Am Soc Nephrol. 2013;8:1079–1088. doi: 10.2215/CJN.10971012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parravicini E, Nemerofsky SL, Michelson KA, Huynh TK, Sise ME, Bateman DA, Lorenz JM, Barasch JM. Urinary neutrophil gelatinase-associated lipocalin is a promising biomarker for late onset culture-positive sepsis in very low birth weight infants. Pediatr Res. 2010;67:636–640. doi: 10.1203/PDR.0b013e3181da75c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payen D, Lukaszewicz AC, Belikova I, Faivre V, Gelin C, Russwurm S, Launay JM, Sevenet N. Gene profiling in human blood leucocytes during recovery from septic shock. Intensive Care Med. 2008;34:1371–1376. doi: 10.1007/s00134-008-1048-1. [DOI] [PubMed] [Google Scholar]
- Pennemans V, Rigo JM, Faes C, Reynders C, Penders J, Swennen Q. Establishment of reference values for novel urinary biomarkers for renal damage in the healthy population: are age and gender an issue? Clin Chem Lab Med. 2013;51:1795–1802. doi: 10.1515/cclm-2013-0157. [DOI] [PubMed] [Google Scholar]
- Pianta TJ, Peake PW, Pickering JW, Kelleher M, Buckley NA, Endre ZH. Clusterin in kidney transplantation: novel biomarkers versus serum creatinine for early prediction of delayed graft function. Transplantation. 2015a;99:171–179. doi: 10.1097/TP.0000000000000256. [DOI] [PubMed] [Google Scholar]
- Pianta TJ, Peake PW, Pickering JW, Kelleher M, Buckley NA, Endre ZH. Evaluation of biomarkers of cell cycle arrest and inflammation in prediction of dialysis or recovery after kidney transplantation. Transpl Int. 2015b;28:1392–1404. doi: 10.1111/tri.12636. [DOI] [PubMed] [Google Scholar]
- Pilarczyk K, Edayadiyil-Dudasova M, Wendt D, Demircioglu E, Benedik J, Dohle DS, Jakob H, Dusse F. Urinary [TIMP-2]*[IGFBP7] for early prediction of acute kidney injury after coronary artery bypass surgery. Ann Intensive Care. 2015;99:171–179. doi: 10.1186/s13613-015-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prowle JR, Calzavacca P, Licari E, Ligabo EV, Echeverri JE, Bagshaw SM, Haase-Fielitz A, Haase M, Ostland V, Noiri E, Westerman M, Devarajan P, Bellomo R. Combination of biomarkers for diagnosis of acute kidney injury after cardiopulmonary bypass. Ren Fail. 2015;37:408–416. doi: 10.3109/0886022X.2014.1001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Vaidya VS, Liu J, Waalkes MP, Edwards JR, Lamar PC, Bernard AM, Dumont X, Bonventre JV. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007;72:985–993. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralib AM, Pickering JW, Shaw GM, Devarajan P, Edelstein CL, Bonventre JV, Endre ZH. Test characteristics of urinary biomarkers depend on quantitation method in acute kidney injury. J Am Soc Nephrol. 2012;23:322–333. doi: 10.1681/ASN.2011040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Sandoval JC, Herrington W, Morales-Buenrostro LE. Neutrophil gelatinase-associated lipocalin in kidney transplantation: a review. Transplant Rev (Orlando) 2015;29:139–144. doi: 10.1016/j.trre.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Rodier F, Campisi J, Bhaumik D. Two faces of p53: aging and tumor suppression. Nucleic Acids Res. 2007;35:7475–7484. doi: 10.1093/nar/gkm744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco C, Kellum JA, Haase M. Subclinical AKI is still AKI. Crit Care. 2012;16:313. doi: 10.1186/cc11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Ott KM. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury–where do we stand today? Nephrol Dial Transplant. 2011;26:762–764. doi: 10.1093/ndt/gfr006. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–413. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- Schröppel B, Krüger B, Walsh L, Yeung M, Harris S, Garrison K, Himmelfarb J, Lerner SM, Bromberg JS, Zhang PL, et al. Tubular expression of KIM-1 does not predict delayed function after transplantation. J Am Soc Nephrol. 2010;21:536–542. doi: 10.1681/ASN.2009040390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeliger S, Vogl T, Engels IH, Schröder JM, Sorg C, Sunderkötter C, Roth J. Expression of calcium-binding proteins MRP8 and MRP14 in inflammatory muscle diseases. Am J Pathol. 2003;163:947–956. doi: 10.1016/S0002-9440(10)63454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert FS, Pagonas N, Arndt R, Heller F, Dragun D, Persson P, Schmidt-Ott K, Zidek W, Westhoff TH. Calprotectin and neutrophil gelatinase-associated lipocalin in the differentiation of pre-renal and intrinsic acute kidney injury. Acta Physiol (Oxf) 2013;207:700–708. doi: 10.1111/apha.12064. [DOI] [PubMed] [Google Scholar]
- Seibert FS, Rosenberger C, Mathia S, Arndt R, Arns W, Andrea H, Pagonas N, Bauer F, Zidek W, Westhoff TH. Urinary calprotectin differentiates between prerenal and intrinsic acute renal allograft failure. Transplantation. 2016 doi: 10.1097/TP.0000000000001124. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Seo DW, Li H, Qu CK, Oh J, Kim YS, Diaz T, Wei B, Han JW, Stetler-Stevenson WG. Shp-1 mediates the antiproliferative activity of tissue inhibitor of metalloproteinase-2 in human microvascular endothelial cells. J Biol Chem. 2006;281:3711–3721. doi: 10.1074/jbc.M509932200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Tian L, Xu W, Zhang Z, Wang C, Qi C, Ni Z, Mou S. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: a meta-analysis. PLoS One. 2014;9:e84131. doi: 10.1371/journal.pone.0084131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer E, Elger A, Elitok S, Kettritz R, Nickolas TL, Barasch J, Luft FC, Schmidt-Ott KM. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int. 2011;80:405–414. doi: 10.1038/ki.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer E, Markó L, Paragas N, Barasch J, Dragun D, Müller DN, Budde K, Schmidt-Ott KM. Neutrophil gelatinase-associated lipocalin: pathophysiology and clinical applications. Acta Physiol (Oxf) 2013;207:663–672. doi: 10.1111/apha.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smathers RL, Petersen DR. The human fatty acid-binding protein family: evolutionary divergences and functions. Hum Genomics. 2011;5:170–191. doi: 10.1186/1479-7364-5-3-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz HM. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- Stríz I, Trebichavský I. Calprotectin – a pleiotropic molecule in acute and chronic inflammation. Physiol Res. 2004;53:245–253. [PubMed] [Google Scholar]
- Supavekin S, Zhang W, Kucherlapati R, Kaskel FJ, Moore LC, Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63:1714–1724. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- Sweetser DA, Heuckeroth RO, Gordon JI. The metabolic significance of mammalian fatty-acid-binding proteins: abundant proteins in search of a function. Annu Rev Nutr. 1987;7:337–359. doi: 10.1146/annurev.nu.07.070187.002005. [DOI] [PubMed] [Google Scholar]
- Tan NS, Shaw NS, Vinckenbosch N, Liu P, Yasmin R, Desvergne B, Wahli W, Noy N. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol Cell Biol. 2002;22:5114–5127. doi: 10.1128/MCB.22.14.5114-5127.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taulan M, Paquet F, Argiles A, Demaille J, Romey MC. Comprehensive analysis of the renal transcriptional response to acute uranyl nitrate exposure. BMC Genom. 2006;7:2. doi: 10.1186/1471-2164-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepel M, Borst C, Bistrup C, Marcussen N, Pagonas N, Seibert FS, Arndt R, Zidek W, Westhoff TH. Urinary calprotectin and posttransplant renal allograft injury. PLoS One. 2014;9:e113006. doi: 10.1371/journal.pone.0113006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJL, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol. 2007;212:209–217. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MAD, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- Waikar SS, Betensky RA, Bonventre JV. Creatinine as the gold standard for kidney injury biomarker studies? Nephrol Dial Transplant. 2009;24:3263–3265. doi: 10.1093/ndt/gfp428. [DOI] [PubMed] [Google Scholar]
- Wang G, Gong Y, Anderson J, Sun D, Minuk G, Roberts MS, Burczynski FJ. Antioxidative function of L-FABP in L-FABP stably transfected chang liver cells. Hepatology. 2005;42:871–879. doi: 10.1002/hep.20857. [DOI] [PubMed] [Google Scholar]
- Wang J, Long Q, Zhang W, Chen N. Protective effects of exogenous interleukin 18-binding protein in a rat model of acute renal ischemia-reperfusion injury. Shock. 2012;37:333–340. doi: 10.1097/SHK.0b013e318240bdc8. [DOI] [PubMed] [Google Scholar]
- Wetz AJ, Richardt EM, Wand S, Kunze N, Schotola H, Quintel M, Bräuer A, Moerer O. Quantification of urinary TIMP-2 and IGFBP-7: an adequate diagnostic test to predict acute kidney injury after cardiac surgery? Crit Care. 2015;19:3. doi: 10.1186/s13054-014-0717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93:2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Craft ML, Wang P, Wyburn KR, Chen G, Ma J, Hambly B, Chadban SJ. IL-18 contributes to renal damage after ischemia-reperfusion. J Am Soc Nephrol. 2008;19:2331–2341. doi: 10.1681/ASN.2008020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu VC, Wu CH, Huang TM, Wang CY, Lai CF, Shiao CC, Chang CH, Lin SL, Chen YY, Chen YM, et al. Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25:595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Xie Y, Shao X, Ni Z, Mou S. L-FABP: a novel biomarker of kidney disease. Clin Chim Acta. 2015;445:85–90. doi: 10.1016/j.cca.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Noiri E, Ono Y, Doi K, Negishi K, Kamijo A, Kimura K, Fujita T, Kinukawa T, Taniguchi H, Nakamura K, Goto M, Shinozaki N, Ohshima S, Sugaya T. Renal L-type fatty acid–binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894–2902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R, Barasch J. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10:1045–1056. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- Yang QH, Liu DW, Long Y, Liu HZ, Chai WZ, Wang XT. Acute renal failure during sepsis: potential role of cell cycle regulation. J Infect. 2009;58:459–464. doi: 10.1016/j.jinf.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Zhao YY, Weir MA, Manno M, Cordy P, Gomes T, Hackam DG, Juurlink DN, Mamdani M, Moist L, Parikh CR, Paterson JM, Wald R, Yao Z, Garg AX. New fibrate use and acute renal outcomes in elderly adults: a population-based study. Ann Intern Med. 2012;156:560–569. doi: 10.7326/0003-4819-156-8-201204170-00003. [DOI] [PubMed] [Google Scholar]
- Zheng J, Xiao Y, Yao Y, Xu G, Li C, Zhang Q, Li H, Han L. Comparison of urinary biomarkers for early detection of acute kidney injury after cardiopulmonary bypass surgery in infants and young children. Pediatr Cardiol. 2013;34:880–886. doi: 10.1007/s00246-012-0563-6. [DOI] [PubMed] [Google Scholar]
- Zhou F, Luo Q, Wang L, Han L. Diagnostic value of neutrophil gelatinase-associated lipocalin for early diagnosis of cardiac surgery-associated acute kidney injury: a meta-analysis. Eur J Cardiothorac Surg. 2016;49:746–755. doi: 10.1093/ejcts/ezv199. [DOI] [PubMed] [Google Scholar]
- Zuo S, Liu C, Wang J, Wang F, Xu W, Cui S, Yuan L, Chen X, Fan W, Cui M, Song G. IGFBP-rP1 induces p21 expression through a p53-independent pathway, leading to cellular senescence of MCF-7 breast cancer cells. J Cancer Res Clin Oncol. 2012;138:1045–1055. doi: 10.1007/s00432-012-1153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]