Approximately 44% of homeless adults are hepatitis C virus (HCV)-infected.1–5 Historically, homeless and marginally housed (HMH) adults have faced barriers to HCV treatment. New, interferon-free therapies have excellent cure rates and improved tolerability, reducing barriers for treatment.6 To our knowledge, no published studies have documented the treatment of HMH populations with these therapies. The Boston Health Care for the Homeless Program (BHCHP) began treating HMH adults with oral agents in 2014.
Methods
We retrospectively describe the experience and outcomes of oral direct acting antiviral agents for HCV in a cohort of HCV-infected HMH adults. The study protocol was approved by the Institutional Review Board at Massachusetts General Hospital and deemed to meet Minimal Risk criteria. Patients received treatment at BHCHP, a federally qualified health center providing integrated primary care services via a patient-centered medical home approach to more than 11 000 individuals in the Boston area annually. Patients were not compensated for their participation. The HCV treatment team (a care coordinator [1.0 full-time equivalent], nurse [0.5 full-time equivalent], and 3 primary care clinicians [1 nurse practitioner, 0.25 full-time equivalent, and 2 primary care physicians—0.1 full-time equivalent combined]) provided care. Patients had an initial evaluation in which HCV testing was performed and a treatment plan was outlined. Patients were selected by clinicians based on an assessment of treatment readiness, which was based on adherence to appointments, urgency for treatment, and team collaborative decision-making. Treatment was covered by Medicare or Medicaid in all but 1 patient. At the time this cohort was treated, Massachusetts Medicaid required patients to have progressed to Metavir stage F2 prior to treatment. There were no formal payer restrictions based on sobriety. Patients received weekly calls from the care coordinator, attended follow-up appointments, and had a visit 12 weeks after treatment.
Study inclusion criteria included the following: the individual (1) must be a BHCHP patient, (2) must be at least 18 years old, (3) must have chronic HCV infection (HCV antibody positive with detectable serum HCV RNA), (4) must be enrolled in HCV treatment with oral antiviral agents between February 2014 and August 2015, and (5) must have completed follow-up by December 31, 2015. The primary outcome was sustained virologic response for 12 weeks (SVR-12) (undetectable HCV RNA ≤ 15 IU/mL 12 weeks after treatment completion). We compared characteristics of the 2 subsets of respondents. Statistical analyses were conducted using STATA software (version 11; StataCorp Inc).
Results
From February 2014 and August 2015, 199 HMH individuals were considered for initiation of oral therapy; 64 were treated in the specified period and included in this analysis, 56 were treated outside the study period, and the rest remain untreated. Forty-nine of the 64 treated patients were male; their mean (SD) age was 55 years (7.7 years). Participant characteristics are shown in the Table, stratified by treatment outcome. All patients completed therapy without subspecialty referral.
Table.
Baseline Characteristics of the Cohort of 64 Hepatitis C Virus (HCV)-Infected Homeless and Marginally Housed Adults Treated With Oral Therapy
| Characteristic | Sustained Virologic Response, No. (%) | |
|---|---|---|
| Not Achieved (n = 2) | Achieved (n = 62) | |
| Age, mean (SD), y | 53.5 (7.8) | 55.5 (7.7) |
| Sex | ||
| Male | 1 (50) | 48 (77) |
| Race | ||
| Nonwhite | 1 (50) | 28 (53) |
| Ethnicity | ||
| Hispanic | 0 | 45 (74) |
| Veteran | 0 | 5 (8) |
| Education | ||
| <High school graduate orGED | 1 (50) | 25 (42) |
| Employment | ||
| Part-time | 2 (100) | 7 (12) |
| Full-time | 0 | 6 (10) |
| Unemployed | 0 | 20 (33) |
| Disability | 0 | 27 (45) |
| Insured | 2 (100) | 60 (97) |
| History of incarceration | 0 | 22 (36) |
| Homeless | 0 | 26 (42) |
| HIV coinfected | 0 | 29 (47) |
| Genotype | ||
| Mixed | 0 | 7 (11) |
| 1 | 1 (50) | 49 (79) |
| 2 | 1 (50) | 3 (5) |
| 4 | 0 | 3 (5) |
| Fibrosis-4 value, mean (SD) | 4.34 | 3.80 (5.84) |
| METAVIR stage | ||
| F0 | 0 | 3 (5) |
| F1 | 0 | 22 (36) |
| F2 | 0 | 9 (14) |
| F3 | 0 | 6 (10) |
| F4 | 2 (100) | 22 (35) |
| Reactive | ||
| Hepatitis Avirus | 1 (100) | 48 (83) |
| Hepatitis B virus | 0 | 29 (49) |
| Alanine aminotransferase level, U/L, mean (SD) | 98.5 (50.5) | 63.5 (6.1) |
| HCV treatment experience | 0 | 12 (19) |
| HCV treatment regimen | ||
| Sofosbuvir-ribavirin | 1 (50) | 3 (5) |
| Simeprevir-sofosbuvir | 0 | 5 (8) |
| Ledipasvir-sofosbuvir | 1 (50) | 49 (79) |
| Ledipasvir-sofosbuvir-ribavirin | 0 | 5 (8) |
| History of IDU | 1 (50) | 46 (84) |
| Used drugs (including marijuana) during HCVtreatment | 0 | 16 (26) |
Abbreviations: GED, General Educational Development test; HIV, human immunodeficiency virus; IDU, injection drug use.
SI conversion factor: To convert alanine aminotransferase to microkatals per liter, multiply by 0.0167.
Ninety-seven percent of patients (62 of 64) in this cohort achieved SVR-12. Treatment outcomes by treatment regimen are shown in the Figure. Only 13% reported more than 3 missed doses.
Figure. Treatment Outcomes Stratified by Regimen.
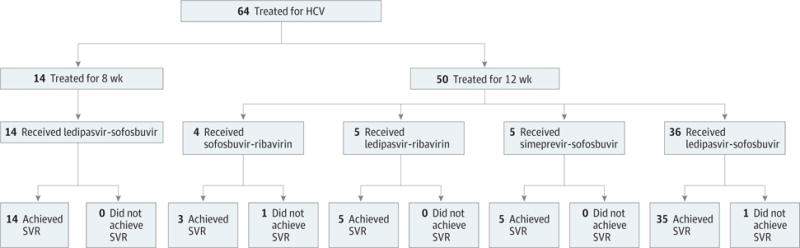
HCV indicates hepatitis C virus; SVR, sustained virologic response.
Two patients did not achieve SVR-12. Patient 1 was genotype 2 with cirrhosis, treatment naïve, HCV-monoinfected, and received only 12 weeks of sofosbuvir-ribavirin. The patient reported no missed doses of treatment, had an undetectable viral load at the end of treatment, but had a detectable viral load 12 weeks later. Patient 2 was genotype 1 with cirrhosis, treatment naïve, HCV-monoinfected, and was treated with ledi-pasvir-sofosbuvir for 12 weeks and missed 1 dose. The patient had a detectable viral load at end of treatment and resistance testing demonstrated H58P and L31V mutations.
Discussion
In this cohort of HCV-infected HMH adults, excellent responses to community-based treatment within a primary care program were observed with a SVR rate of 97% across genotypes and oral therapies. Viral resistance and treatment duration contributed to treatment failure in the 2 patients with treatment failure. These findings demonstrate that with a dedicated program for treating HCV in HMH adults in the primary care setting, it is possible to achieve outcomes similar to those of clinical trials and other cohorts despite significant additional barriers and competing priorities to health care faced by this population.
Acknowledgments
Funding/Support: This work was supported by the National Institute on Drug Abuse at the National Institutes of Health (grants 5T32DA013911-15 and 1R25DA037190-01 to Dr Barocas) and (R01DA031059 and P30DA040500 to Dr Linas).
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Barocas had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Barocas, Beiser, O’Connell, Linas.
Acquisition, analysis, or interpretation of data: Barocas, Beiser, León, Gaeta.
Drafting of the manuscript: Barocas.
Critical revision of the manuscript for important intellectual content: Barocas, Beiser, León, Gaeta, O’Connell, Linas.
Statistical analysis: Barocas.
Obtained funding: Barocas, Gaeta.
Administrative, technical, or material support: Barocas, Beiser, Gaeta, Linas.
Study supervision: León, Gaeta, O’Connell, Linas.
Conflict of Interest Disclosures: None reported.
Additional Contributions: We thank Molly Ingemi and Lena Cardoso of BHCHP, and Paul McCabe of Quinnipiac University School of Medicine for their contributions to this project, which included data extraction review. No compensation was received.
References
- 1.Beijer U, Wolf A, Fazel S. Prevalence of tuberculosis, hepatitis C virus, and HIV in homeless people: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(11):859–870. doi: 10.1016/S1473-3099(12)70177-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharel M, Lin W-C, Zhang J, O’Connell E, Taube R, Clark RE. Health care utilization patterns of homeless individuals in Boston: preparing for Medicaid expansion under the Affordable Care Act. Am J Public Health. 2013;103(suppl 2):S311–S317. doi: 10.2105/AJPH.2013.301421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinbaum C, Lyerla R, Margolis HS, Centers for Disease Control and Prevention Prevention and control of infections with hepatitis viruses in correctional settings. MMWR Recomm Rep. 2003;52(RR-1):1–36. [PubMed] [Google Scholar]
- 4.Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002;36(5 suppl 1):S30–S34. doi: 10.1053/jhep.2002.36791. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51(3):729–733. doi: 10.1002/hep.23561. [DOI] [PubMed] [Google Scholar]
- 6.Vermehren J, Peiffer K-H, Welsch C, et al. The efficacy and safety of direct acting antiviral treatment and clinical significance of drug-drug interactions in elderly patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2016;44(8):856–865. doi: 10.1111/apt.13769. [DOI] [PubMed] [Google Scholar]


