Abstract
Background
Adverse effects such as fatigue, pain, erythema, nausea and vomiting are commonly known in patients undergoing irradiation (RT) alone or in combination with chemotherapy (RCHT). Patients suffering from these symptoms are limited in their daily life and their quality of life (QOL) is often reduced. As addressed in several trials, acupuncture can cause amelioration of these specific disorders. Especially for pain symptoms, several groups have shown efficacy of acupuncture. To what extent the difference between traditional acupuncture (verum acupuncture) and false acupuncture (sham acupuncture) is in reducing side effects and improvement of QOL is not clear.
Methods/design
ROSETTA is a prospective randomized phase II trial (version 1.0) to examine the efficacy of traditional acupuncture in patients with RT-related side effects. In the experimental (verum) arm (n = 37) an experienced acupuncture-trained person will treat dedicated acupuncture points. In the control (sham) arm (n = 37) sham acupuncture will be performed to provide a blinded comparison of results.
Discussion
This is the first randomized prospective trial to evaluate the effect of traditional acupuncture on RT-related side effects such as fatigue and QOL.
Trial registration
ClinicalTrials.gov, NCT02674646. Registered on 8 December 2015.
Electronic supplementary material
The online version of this article (doi:10.1186/s13063-017-2139-5) contains supplementary material, which is available to authorized users.
Keywords: Acupuncture, Radiotherapy, Fatigue, Quality of life (QOL), Sham-controlled trial
Background
Radiation therapy (RT) is a main pillar in cancer care offering curative treatment in several indications. However, RT-related side effects develop depending on the site treated (e.g. head and neck or prostate etc.), and on individual patients’ predisposition or other factors. One of the main symptoms related to cancer treatment is the development of fatigue, which can evolve during RT and commonly persists some time after completion of treatment. Other side effects, depending on the region treated, include headache, nausea/vomiting, gastrointestinal discomfort, dysuria and others. Fatigue and subsequently a reduction in overall quality of life (QOL) is a main discomforting factor for patients treated with RT; in many patients fatigue is the only side effect observed during RT. Commonly, this leads to a reduction of daily activity and motivation, and can result in severe unhappiness and/or depression. Thus, methods for amelioration of symptoms and especially any possibilities to reduce fatigue and increase QOL are important factors for patients in the oncology setting.
Several means of supportive care to ameliorate patients’ RT-related side effects are described. In general, it has been shown that moderate exercise can improve QOL and reduce fatigue during and after treatment; additionally, light to moderate exercise can lead to improvement in oncological outcome [1, 2]. Other complementary treatments including selenium, mistletoe or several vitamins are controversial due to limited data on significant benefit; depending on the substances’ mechanisms of action, the risk of RT-related side effects may be increased. Complementary medicine such as traditional Chinese medicine (TCM) or other naturopathy concepts are consistently in discussion and are offered by many practitioners on a case-by-case basis.
For acupuncture, which is on pillar amongst others within the TCM concept, there are some data from randomized studies on back pain, headaches and other non-cancer diseases [3–15]. A large German research consortium, the GERAC-Group, has shown that acupuncture can have a significantly beneficial effect on chronic headache, back pain and joint and bone pain due to arthrosis [16–21]. In oncology, some centers have evaluated the effect of acupuncture on RT-related side effects, such as mucositis and dysphagia, and demonstrated significant reduction of symptoms [3, 10, 22–24]. However, most studies included only a small number of patients, and have not evaluated effects on general fatigue, QOL or other RT-related symptoms.
The prospective phase II ROSETTA trial (version 1.0) will include patients with tumors treated with RT in various anatomical regions. In the experimental arm an experienced acupuncture-trained person will treat dedicated traditional acupuncture points. In the control arm control acupuncture will be performed to provide a blinded comparison of results. Sham acupuncture was developed especially for randomized controlled trials as a valid comparator; compared to a pure control arm, patients receive acupuncture with needlepoints, that have no medical function and thus do not have any proven effect on the symptoms addressed [23–29].
Methods
Endpoints of the study
In the ROSETTA trial the main endpoint is improvement of QOL and reduction of fatigue. Secondary endpoints are reduction of RT-related side effects such as headache, nausea and skin erythema.
Study design
The ROSETTA trial will evaluate the effect of acupuncture as a complementary treatment parallel to RT. Acupuncture is an established and accepted treatment method, which is indicated especially for pain reduction. The plan for study is to recruit 74 patients. The total study duration is planned to be 24 months.
After inclusion into the study and baseline examination, the patient is randomized into the treatment arm. Randomization is performed under the auspices of the biometrician. The randomization process will be performed with the professional web-based patient randomization service, e.g. at www.randomizer.at. The investigator or an authorized member of the study team will perform data documentation and evaluation.
Depending on the treatment arm either verum or sham acupuncture will be applied [23, 24, 26, 28, 30, 31]. Acupuncture will be performed after treatment bi-weekly during the first week of RT, thereafter weekly until the end of RT. Treatment time in both arms is identical and lies between 20 and 30 minutes. Every patient will be treated according to the treatment arm. Standardized acupuncture needles (sterile needles, e.g. 0.24 × 40 mm) will be used for both treatment types. Verum acupuncture is based on foraminology, the ancient Chinese knowledge of acupuncture points [32]. Control acupuncture is performed on unspecific points with no specific effect according to foraminology.
Patients will receive standardized questionnaires (European Organization for Research and Treatment of Cancer (EORTC) QLQ C-30) before their first, after their fourth and after their last acupuncture treatment. Patients will be questioned by the investigator about their feelings and symptoms and detailed information about their illness. The answers will be documented according to the standardized scoring system (Common Toxicity Criteria for Adverse Events (CTCAE)). The schedule of enrollment, interventions and assessments in the ROSETTA trial is depicted in Fig. 1.
Fig. 1.
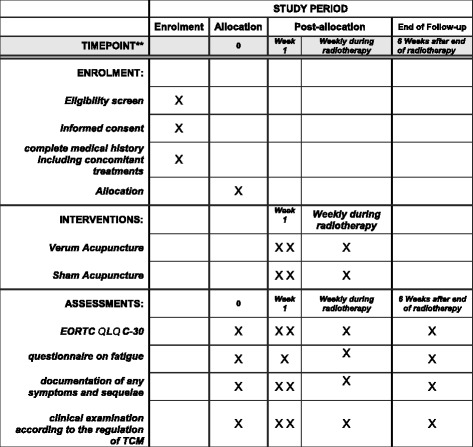
Schedule of enrolment, interventions and assessments in the ROSETTA trial. EORTC European Organization for Research and Treatment of Cancer, QOL quality of life, TCM Traditional Chinese Medicine
Group A: verum acupuncture
Needlepoints
Bilateral PC 6, S 36, L 8, L 9
Unilateral R 4, R 6
Group B: control acupuncture
Needlepoints
Four needles in the medio-axillary line below the 6th rib, bilateral
Two needles unilateral
Baseline examination
After informed consent and inclusion into the study protocol, a baseline examination will be performed. This examination is to be performed within 2 days before initiation of study treatment and includes:
complete medical history including concomitant treatments
clinical examination according to the regulation of TCM
ascertainment of QOL
questionnaire on fatigue
documentation of any symptoms and sequelae prior to radiotherapy
Follow-up examinations during study treatment
After every acupuncture treatment, an examination will be performed. This examination includes:
clinical examination according to the regulation of TCM
ascertainment of QOL
questionnaire on fatigue
documentation of any symptoms and sequelae
Follow up
The final study visit will be 6 weeks after treatment to assess the primary endpoints. After the first 6 weeks, patients will be included into a regular follow-up schedule according to their primary disease and follow-up requirements.
General criteria for patient selection
All patients treated with RT or radiochemotherapy (RCHT) can be included in the trial if fulfilling the inclusion and exclusion criteria.
Inclusion criteria
Patients meeting all of the following criteria will be considered for the trial:
treatment with radiotherapy
age ≥ 18 years
ability of subject to understand the nature and individual consequences of the clinical trial
written informed consent (must be available before enrolment in the trial)
Exclusion criteria
Patients presenting with any of the following criteria will not be included in the trial:
any contraindication to acupuncture
known coagulopathy or anticoagulation therapy with bleeding time > 4 minutes, thrombocyte count < 50 000/μl
missing compliance
skin disease in the region of the acupuncture points
refusal of the patient to take part in the study
participation in another clinical study or observation period of competing trials
Statistical considerations
The study is powered to show that continuous acupuncture during RT can have a positive effect on QOL. ROSETTA is a prospective randomized trial comparing traditional (verum) acupuncture to control (sham) acupuncture. Randomization will be performed using automated information technology (IT)-based randomization, and patients will be allocated to groups by the Study Center within the Department of Radiation Oncology, Technische Universität München (TUM). Additionally, the study will evaluate whether acupuncture can reduce QOL-related side effects such as fatigue, headache, nausea etc.
All patient data on symptoms, side effects and QOL according to the QOL questionnaires will be documented in our dedicated study database. Evaluation of the primary endpoint (QOL) will be performed using the Wilcoxon test (U test) and the evaluation tool provided by the EORTC. The secondary endpoints (fatigue, headache, nausea etc.) will be evaluated using comparable statistical calculations. The interpretation of the data will be purely descriptive.
The sample size calculation is based on the Wilcoxon test and the primary endpoint. A sample size of 37 patients per group (i.e. 74 patients in total) is associated with power of 80% to detect a difference between the two groups (with two-sided α = 5%) using the Wilcoxon test, with a probability of 69% that an increase in QOL can be achieved when comparing the two groups 8 (d.h. P(x < y).
Data management
In the ROSETTA trial the EORTC QLQ-C30 will be used to document the QOL of patients during treatment. Data on side effects and symptoms during RT will be documented on dedicated case report forms (CRFs) designed for the trial and documented in the institutional database. All interventions and every other medical treatment will be recorded. The data will be collected in accordance with all Data Protection Regulations in the MiRO Database of the Department of Radiation Oncology, TUM.
Discussion
Several studies have shown that traditional acupuncture can alleviate clinical symptoms: In Germany, especially for back pain, a multicenter study group has shown solid data in this regard and provided a strong basis for the use of acupuncture in this regard [16–21].
In oncology, a main focus of acupuncture treatment centers is to reduce RT-related side effects of either chemotherapy or RT. Meng et al. showed that acupuncture can prevent RT-related xerostomia in patients with nasopharyngeal cancer [7, 8]; Lu et al. focused on dysphagia in patients treated with RCHT for tumors of the head and neck, and observed a substantial effect of acupuncture [33]. A comprehensive review on acupuncture in cancer treatment illustrated that there have been 41 randomized controlled trials involving eight different symptoms, such as pain, nausea, hot flashes, fatigue, xerostomia, prolonged postoperative ileus, anxiety/mood disorders and sleep disturbance [34]. Cochrane criteria were used to classify the studies for risk of bias. Only a few studies were identified to be free of risk of bias, or to have a low risk of bias. Thus, more trials focusing on clear questions and applying valid control arms are needed to further clarify the role of acupuncture in oncology care.
To address these questions, the ROSETTA trial was set up as a prospective randomized controlled trial. In the control arm, sham-acupuncture points not associated with any function in the doctrine of TCM are applied (sham acupuncture). This concept has been applied in different trials, in patients with and without cancer [3, 7, 8, 23, 24, 33, 35, 36]. Compared to a pure non-treatment controlled trial, the bias of no treatment is not included in this trial concept. In the experimental arm, valid acupuncture points are used, which are known to reduce the associated side effects (verum acupuncture). Therefore, the ROSETTA trial will help to clarify the assumed benefit of acupuncture in terms of fatigue and QOL in the oncology setting in patients treated with RT Additional file 1.
Status of the trial
Recruitment into the ROSETTA trial is ongoing at the time of submission.
Acknowledgement
The study is supported by the Roman Herzog Cancer Center (RHCCC) at the Klinikum rechts der Isar, Onkologisches Zentrum (OZ).
Funding
The study is funded in the framework of the Roman Herzog Cancer Center (RHCCC) at the Klinikum rechts der Isar, Onkologisches Zentrum (OZ), Department of Radiation Oncology. No specific funding number is present.
Availability of data and materials
All data are collected at the study center and can be made available as necessary.
Abbreviations
- CAM
Complementary and alternative medicine
- EORTC
European Organization for Research and Treatment of Cancer
- QOL
Quality of life
- RCHT
Radiochemotherapy
- RT
Radiotherapy, radiation therapy
- TCM
Traditional Chinese medicine, TUM, Technische Universität München
Additional file
SPIRIT 2013 Checklist: Recommended items to address in a clinical trial protocol and related documents. (DOC 110 kb)
Authors’ contributions
SEC, SS and RA designed the study protocol. SEC obtained the ethical approval. KK helped in writing the protocol and setting up treatment procedures. RA and SEC will include patients into the trial, treat them according to the protocol and document and evaluate the data. TB participated in the design of the study and performed the statistical calculations for the trial. TB, KK and SS will help analyze and evaluate the data and write the final manuscript at the end of the study. SEC and RA drafted the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study was evaluated and approved by the Ethics Committee of the Klinikum rechts der Isar, Technische Universität München (TUM), vote Nr. 512/15 s. Written informed consent is obtained by all patients prior to inclusion into the trial.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13063-017-2139-5) contains supplementary material, which is available to authorized users.
Contributor Information
Rebecca Asadpour, Email: rebecca.asadpour@gmx.de.
Kerstin A. Kessel, Email: kerstin.kessel@tum.de
Tom Bruckner, Email: Tom.bruckner@imbi.med.uni-heidelberg.de.
Serkan Sertel, Email: praxis@prof-sertel.de.
Stephanie E. Combs, Phone: +49-89-4140-4501, Email: stephanie.combs@tum.de
References
- 1.Levin GT, Greenwood KM, Singh F, Tsoi D, Newton RU. Exercise Improves Physical Function and Mental Health of Brain Cancer Survivors: Two Exploratory Case Studies. Integr Cancer Ther. 2016;15(2):190-6. doi:10.1177/1534735415600068. Epub 2015 Aug 14. [DOI] [PMC free article] [PubMed]
- 2.Nilsen TS, Thorsen L, Fosså SD, Wiig M, Kirkegaard C, Skovlund E, Benestad HB, Raastad T. Effects of strength training on muscle cellular outcomes in prostate cancer patients on androgen deprivation therapy. Scand J Med Sci Sports. 2016;26(9):1026-35. doi:10.1111/sms.12543. Epub 2015 Aug 17. [DOI] [PubMed]
- 3.Enblom A, et al. Acupuncture compared with placebo acupuncture in radiotherapy-induced nausea–a randomized controlled study. Ann Oncol. 2012;23(5):1353–61. doi: 10.1093/annonc/mdr402. [DOI] [PubMed] [Google Scholar]
- 4.Enblom A, et al. The nonpenetrating telescopic sham needle may blind patients with different characteristics and experiences when treated by several therapists. Evid Based Complement Alternat Med. 2011;2011:185034. doi: 10.1155/2011/185034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enblom A, et al. Getting the grip on nonspecific treatment effects: emesis in patients randomized to acupuncture or sham compared to patients receiving standard care. PLoS One. 2011;6(3):e14766. doi: 10.1371/journal.pone.0014766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enblom A, et al. Pilot testing of methods for evaluation of acupuncture for emesis during radiotherapy: a randomised single subject experimental design. Acupunct Med. 2011;29(2):94–102. doi: 10.1136/aim.2010.003384. [DOI] [PubMed] [Google Scholar]
- 7.Meng Z, et al. Randomized controlled trial of acupuncture for prevention of radiation-induced xerostomia among patients with nasopharyngeal carcinoma. Cancer. 2012;118(13):3337–44. doi: 10.1002/cncr.26550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng Z, et al. Sham-controlled, randomised, feasibility trial of acupuncture for prevention of radiation-induced xerostomia among patients with nasopharyngeal carcinoma. Eur J Cancer. 2012;48(11):1692–9. doi: 10.1016/j.ejca.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monson K, et al. Group recruitment sessions enhance patient understanding in a small multi-centre phase III clinical trial. Contemp Clin Trials. 2012;33(2):286–90. doi: 10.1016/j.cct.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Simcock R, et al. ARIX: a randomised trial of acupuncture v oral care sessions in patients with chronic xerostomia following treatment of head and neck cancer. Ann Oncol. 2013;24(3):776–83. doi: 10.1093/annonc/mds515. [DOI] [PubMed] [Google Scholar]
- 11.Vickers AJ. Statistical reanalysis of four recent randomized trials of acupuncture for pain using analysis of covariance. Clin J Pain. 2004;20(5):319–23. doi: 10.1097/00002508-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Vickers AJ, et al. Acupuncture for chronic headache in primary care: large, pragmatic, randomised trial. BMJ. 2004;328(7442):744. doi: 10.1136/bmj.38029.421863.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vickers AJ, et al. Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis. Health Technol Assess. 2004;8(48):iii. doi: 10.3310/hta8480. [DOI] [PubMed] [Google Scholar]
- 14.Vickers AJ, et al. Acupuncture for postchemotherapy fatigue: a phase II study. J Clin Oncol. 2004;22(9):1731–5. doi: 10.1200/JCO.2004.04.102. [DOI] [PubMed] [Google Scholar]
- 15.Wonderling D, et al. Cost effectiveness analysis of a randomised trial of acupuncture for chronic headache in primary care. BMJ. 2004;328(7442):747. doi: 10.1136/bmj.38033.896505.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endres HG, et al. [German Acupuncture Trials (gerac) address problems of methodology associated with acupuncture studies] Schmerz. 2005;19(3):201–4. doi: 10.1007/s00482-004-0345-z. [DOI] [PubMed] [Google Scholar]
- 17.Haake M, et al. German Acupuncture Trials (GERAC) for chronic low back pain: randomized, multicenter, blinded, parallel-group trial with 3 groups. Arch Intern Med. 2007;167(17):1892–8. doi: 10.1001/Archinte.167.17.1892. [DOI] [PubMed] [Google Scholar]
- 18.Haake M, et al. The German multicenter, randomized, partially blinded, prospective trial of acupuncture for chronic low-back pain: a preliminary report on the rationale and design of the trial. J Altern Complement Med. 2003;9(5):763–70. doi: 10.1089/107555303322524616. [DOI] [PubMed] [Google Scholar]
- 19.Haake M, et al. Acupuncture in chronic back pain. Background, development and design of the German Acupuncture Trial (gerac-cLBP) Z Orthop Ihre Grenzgeb. 2003;141(1):6–10. [PubMed] [Google Scholar]
- 20.Molsberger A, et al. Acupuncture in diseases of the locomotor system. Status of research and clinical applications. Orthopade. 2002;31(6):536–43. doi: 10.1007/s00132-002-0339-4. [DOI] [PubMed] [Google Scholar]
- 21.Molsberger AF, et al. Designing an acupuncture study: II. The nationwide, randomized, controlled German acupuncture trials on low-back pain and gonarthrosis. J Altern Complement Med. 2006;12(8):733–42. doi: 10.1089/acm.2006.12.733. [DOI] [PubMed] [Google Scholar]
- 22.Enblom A, et al. One third of patients with radiotherapy-induced nausea consider their antiemetic treatment insufficient. Support Care Cancer. 2009;17(1):23–32. doi: 10.1007/s00520-008-0445-x. [DOI] [PubMed] [Google Scholar]
- 23.Sertel S, et al. Acupuncture for nasal congestion: a prospective, randomized, double-blind, placebo-controlled clinical pilot study. Am J Rhinol Allergy. 2009;23(6):e23–8. doi: 10.2500/ajra.2009.23.3380. [DOI] [PubMed] [Google Scholar]
- 24.Sertel S, et al. Additional use of acupuncture to NSAID effectively reduces post-tonsillectomy pain. Eur Arch Otorhinolaryngol. 2009;266(6):919–25. doi: 10.1007/s00405-008-0851-1. [DOI] [PubMed] [Google Scholar]
- 25.Karner M, et al. Objectifying specific and nonspecific effects of acupuncture: a double-blinded randomised trial in osteoarthritis of the knee. Evid Based Complement Alternat Med. 2013;2013:427265. doi: 10.1155/2013/427265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maimer A, et al. Objectifying acupuncture effects by lung function and numeric rating scale in patients undergoing heart surgery. Evid Based Complement Alternat Med. 2013;2013:219817. doi: 10.1155/2013/219817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matos LC, et al. Qigong as a traditional vegetative biofeedback therapy: long-term conditioning of physiological mind-body effects. Biomed Res Int. 2015;2015:531789. doi: 10.1155/2015/531789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pais I, et al. Effects of acupuncture on leucopenia, neutropenia, NK, and B cells in cancer patients: a randomized pilot study. Evid Based Complement Alternat Med. 2014;2014:217397. doi: 10.1155/2014/217397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schroder S, et al. Acupuncture treatment improves nerve conduction in peripheral neuropathy. Eur J Neurol. 2007;14(3):276–81. doi: 10.1111/j.1468-1331.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- 30.Hauer K, et al. Stimulation of acupoint ST-34 acutely improves gait performance in geriatric patients during rehabilitation: a randomized controlled trial. Arch Phys Med Rehabil. 2011;92(1):7–14. doi: 10.1016/j.apmr.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 31.Sousa CM, et al. Effects of self-administered exercises based on Tuina techniques on musculoskeletal disorders of professional orchestra musicians: a randomized controlled trial. J Integr Med. 2015;13(5):314–8. doi: 10.1016/S2095-4964(15)60194-7. [DOI] [PubMed] [Google Scholar]
- 32.Porkert M, Hempen C-H. Classical acupuncture: the standard textbook. 1995. Phainon Ed. and Media, Acta Medicinae Sinensis.
- 33.Lu W, et al. Acupuncture for dysphagia after chemoradiation in head and neck cancer: rationale and design of a randomized, sham-controlled trial. Contemp Clin Trials. 2012;33(4):700–11. doi: 10.1016/j.cct.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia MK, et al. Systematic review of acupuncture in cancer care: a synthesis of the evidence. J Clin Oncol. 2013;31(7):952–60. doi: 10.1200/JCO.2012.43.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albrecht T, et al. Measurable impact of acupuncture on mucosal swelling of inferior turbinates: a prospective, randomized, controlled study. Acta Otolaryngol. 2015;135(2):169–76. doi: 10.3109/00016489.2014.973533. [DOI] [PubMed] [Google Scholar]
- 36.Enblom A, et al. Can individuals identify if needling was performed with an acupuncture needle or a non-penetrating sham needle? Complement Ther Med. 2008;16(5):288–94. doi: 10.1016/j.ctim.2008.02.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are collected at the study center and can be made available as necessary.


