Abstract
Background
Increase of the expression of γ-globin gene and high production of fetal hemoglobin (HbF) in β-thalassemia patients is widely accepted as associated with a milder or even asymptomatic disease. The search for HbF-associated polymorphisms (such as the XmnI, BCL11A and MYB polymorphisms) has recently gained great attention, in order to stratify β-thalassemia patients with respect to expectancy of the first transfusion, need for annual intake of blood, response to HbF inducers (the most studied of which is hydroxyurea).
Methods
Aγ-globin gene sequencing was performed on genomic DNA isolated from a total of 75 β-thalassemia patients, including 31 β039/β039, 33 β039/β+IVSI-110, 9 β+IVSI-110/β+IVSI-110, one β0IVSI-1/β+IVSI-6 and one β039/β+IVSI-6.
Results
The results show that the rs368698783 polymorphism is present in β-thalassemia patients in the 5’UTR sequence (+25) of the Aγ-globin gene, known to affect the LYAR (human homologue of mouse Ly-1 antibody reactive clone) binding site 5′-GGTTAT-3′. This Aγ(+25 G->A) polymorphism is associated with the Gγ-globin-XmnI polymorphism and both are linked with the β039-globin gene, but not with the β+IVSI-110-globin gene. In agreement with the expectation that this mutation alters the LYAR binding activity, we found that the Aγ(+25 G->A) and Gγ-globin-XmnI polymorphisms are associated with high HbF in erythroid precursor cells isolated from β039/β039 thalassemia patients.
Conclusions
As a potential explanation of our findings, we hypothesize that in β-thalassemia the Gγ-globin-XmnI/Aγ-globin-(G->A) genotype is frequently under genetic linkage with β0-thalassemia mutations, but not with the β+-thalassemia mutation here studied (i.e. β+IVSI-110) and that this genetic combination has been selected within the population of β0-thalassemia patients, due to functional association with high HbF. Here we describe the characterization of the rs368698783 (+25 G->A) polymorphism of the Aγ-globin gene associated in β039 thalassemia patients with high HbF in erythroid precursor cells.
Keywords: β-thalassemia, Fetal hemoglobin, LYAR, Aγ-globin gene polymorphism
Background
The β-thalassemias are relevant hereditary hematological diseases caused by nearly 300 mutations of the β-globin gene, leading to low or absent production of adult β-globin and excess of α-globin content in erythroid cells, causing ineffective erythropoiesis and low or absent production of adult hemoglobin (HbA) [1–5]. Increase of the expression of γ-globin genes and high production of fetal hemoglobin (HbF) in β-thalassemia patients is widely accepted as associated with a milder or even asymptomatic disease [6–8]. In several cases, high HbF expressing β-thalassemia patients do not need transfusion regimen and, consequently, chelation therapy [6–8]. This well recognized finding has prompted researchers to develop efficient HbF inducers for treating β-thalassemia patients expressing low levels of HbF [9–14]. On the other hand, the search for HbF-associated polymorphisms (such as the XmnI, BCL11A and MYB polymorphisms) [15–19] has recently gained great attention, in order to stratify β-thalassemia patients with respect to expectancy of the first transfusion, need for annual intake of blood, response to HbF inducers (the most studied of which is hydroxyurea) [20–22].
In consideration of the fact that several HbF-related polymorphisms probably act in synergy, the interest in finding novel HbF-related genetic biomarkers has remained high. This field of investigation, in addition to a clear interest in diagnostics and prognostics, might bring novel therapeutic options, in the case the polymorphism(s) is (are) associated with novel therapeutic markers. This field of research has identified several direct or indirect transcriptional repressors of γ-globin gene expression such as BCL11A, KLF1, MYB, Oct-1 [16–19].
In a recent paper Ju et al. [23] identified a putative novel nuclear protein repressor of γ-globin gene transcription, LYAR (human homologue of mouse Ly-1 antibody reactive clone). The LYAR DNA-binding motif (GGTTAT) was identified by performing CASTing (cyclic amplification and selection of targets) experiments [23]. Results of EMSA (electrophoretic mobility shift assay) and ChIP (chromatin immunoprecipitation) assays confirmed that LYAR binds a DNA region corresponding to the 5′-untranslated region of the Aγ-globin gene. Ju et al. formally demonstrated that LYAR is a strong repressor of human fetal globin gene expression in both K562 cells and primary human adult erythroid progenitor cells. Interestingly, LYAR was found to directly interact also with the methyltransferase PRMT5 which triggers the histone H4 Arg3 symmetric dimethylation (H4R3me2s) mark. Altogether, these data indicate that LYAR acts as a novel transcription factor that binds the γ-globin gene, and is essential for silencing the γ-globin gene [23].
The objective of this study was to investigate the presence of genetic variants in β-thalassemia patients potentially affecting the LYAR binding site and the possible association with the most common HbF-associated polymorphism, the XmnI polymorphism [18, 24, 25]. To this aim we focused our attention on β-thalassemic patients from the north-west Mediterranean area, in particular those carrying the β039 and β+IVSI-110 thalassemia mutations, allowing to compare β0- and β+-genotypes. The genomic DNA from these patients was studied by full sequencing of both the Gγ- and Aγ-globin genes.
Methods
Patients
A total of 75 β-thalassemia patients were recruited for this study, including 31 β039/β039, 33 β039/β+IVSI-110, 9 β+IVSI-110/β+IVSI-110, one β0IVSI-1/β+IVSI-6 and one β039/β+IVSI-6 patient. The β-thalassemia patients have been recruited at Ferrara Hospital and Rovigo Hospital. The Declaration of Helsinki was followed for the collection of blood samples from β-thalassemia patients; furthermore, specific approvals by the Ethical Committees of Ferrara Hospital and Rovigo Hospital were obtained. All the β-thalassemia patients duly signed the informed consent form before blood sampling.
Genomic DNA extraction, polymerase chain reaction (PCR) and DNA sequencing
The genomic DNA from β-thalassemia patients was extracted from 500 μL of whole blood using the QIAamp® DNA Blood Mini Kit (Qiagen, Hilden, Germany) as described in Bianchi et al. [15]. PCR amplification of β-, Aγ- or Gγ-globin genes and DNA sequencing methods used in this study have been previously described by Bianchi et al. [15]. The nucleotide sequences of the PCR primers are reported in Table 1. BMR Genomics (Padua, Italy) performed gene sequencing.
Table 1.
PCR primers for amplification of β-, Aγ- and Gγ-globin gene sequences
| Gene | Forward(F)/Reverse(R) | Nucleotide sequence |
|---|---|---|
| β-globin | βF1 | 5′-GTG CCA GAA GAG CCA AGG ACA GG-3′ |
| βR1 | 5′-AGT TCT CAG GAT CCA CGT GCA-3′ | |
| βF2 | 5′-GCC TGG CTC ACC TGG ACA-3′ | |
| βR2 | 5′-GTT GCC CAG GAG CTG TGG-3′ | |
| βF3 | 5′-ACA ATC CAG CTA CCA TTC TGC TTT-3′ | |
| βR3 | 5′-CAC TGA CCT CCC ACA TTC CCT TTT-3′ | |
| Aγ-globin | AγF | 5′-TTT CCT TAG AAA CCA CTG CTA ACT GAA A-3′ |
| AγR | 5′-TTG TGA TAG TAG CCT TGT CCT CCT CT-3′ | |
| Gγ-globin | GγF | 5′-TTC TTA TTT GGA AAC CAA TGC TTA CTA AAT-3′ |
| GγR | 5′-TTG TGA TAG TAG CCT TGT CCT CCT CT-3′ |
Erythroid progenitors (ErPCs) from β-thalassemia patients
The two-phase liquid culture procedure was employed as previously described [26, 27]. The erythroid differentiation status of ErPCs was verified analyzing transferrin receptor (TrfR) and glycophorin A (GYPA) expression by FACS (fluorescence-activated cell sorting) using the BD FACScan™ system (Becton, Dickinson & Company, Franklin Lakes, NJ, USA) and anti-human CD71 (TrfR) FITC-conjugated antibody (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) and anti-human CD235a (GYPA) antibody PE-conjugated (Miltenyi Biotec GmbH) as described elsewhere [28, 29]. Production of hemoglobins was assessed by high performance liquid chromatography (HPLC) as described elsewhere [11, 28].
Statistical analysis
The results reported in this paper are usually presented as average ± SD. The one-way ANOVA (ANalyses Of VAriance between groups) software was used for compare statistical differences between groups. The paired t test of the GraphPad Prism Software was used to obtain the p values. Differences were considered statistically significant when p < 0.05 (*) and highly significant when p < 0.01 (**) [28, 29].
Results
Presence of the rs368698783 (G->A) Aγ-globin gene polymorphism in β-thalassemia patients
In order to verify whether mutations affecting the LYAR-binding site of the Aγ-globin gene are present within our β-thalassemia patient population, sequencing of the Aγ-globin genes was performed using genomic DNA isolated from a total of 75 β-thalassemia patients, including 31 β039/β039, 33 β039/β+IVSI-110, 9 β+IVSI-110/β+IVSI-110, one β0IVSI-1/β+IVSI-6 and one β039/β+IVSI-6 patient. Examples of the sequencing results obtained are shown in Fig. 1a, which indicates that one (G->A) rs368698783 polymorphism was found in position +25 of the Aγ-globin gene, modifying the LYAR-binding sequence from 5′-GGTTAT-3′ to 5′-GATTAT-3′. For this reason, we called this mutation rs368698783 Aγ(+25 G->A) (see its location in Fig. 1b). In the examples reported in Fig. 1a, the representative homozygous (G/G), heterozygous (G/A) and homozygous mutated (A/A) genomic sequences are shown. In addition, as indicated in the representative examples shown in Fig. 1a, the G/G genotype is linked to the XmnI(−/−) haplotype; in contrast the G/A and A/A Aγ(+25) genotypes are linked to XmnI(−/+) and XmnI(+/+) haplotypes, respectively. Figure 1b shows the location of the mutation within the 5’UTR sequence of the Aγ-globin gene and the nucleotide change concerning the 5′-GGTTAT-3′ LYAR binding site proposed by Ju et al. Notably, no other nucleotide variations affecting the LYAR-binding sequence were found in these 75 patients. Moreover, no other mutations were found in the 607 bp and 613 bp sequenced regions of the Aγ-globin and Gγ-globin genes, respectively, with the exception of a 4 bp deletion residing in the promoter region of the Aγ-globin gene (HBG1: g.-225_-222delAGCA) [30], found in three XmnI(−/−), Aγ(+25 G/G) β039/β+IVSI-110 patients. In 16/75 patients (21%) this Aγ(+25 G->A) polymorphism was found in the heterozygous (G/A) state, while the Aγ(+25) homozygous (A/A) state was found only in four patients. While we cannot exclude the presence of other mutations in the Aγ-globin genes of sub populations of β-thalassemia patients, we can conclude that the Aγ(+25 G->A) concerning the rs368698783 polymorphism is the most frequent mutation affecting this Aγ-globin gene region within our population, well representative of the Mediterranean area.
Fig. 1.
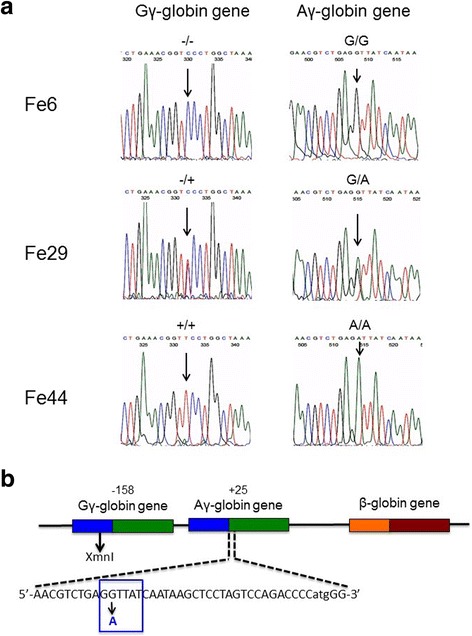
Sequence analysis of selected Aγ- and Gγ-globin genes. a. Right: representative sequence analysis of the Aγ-globin gene surrounding the region involved in LYAR binding site. The +25(G->A) polymorphism is arrowed. This corresponds to the already known rs368698783 polymorphism, which was not analyzed in full detail in the β-thalassemia patient population (including patients carrying β+ and β0 mutations). In the examples depicted the +25 Aγ-globin gene sequence is G/G (Fe6), G/A (Fe29) and A/A (Fe44). Left: the same genomic DNA has been sequenced at the XmnI site, found to be −/− in Fe6, −/+ in Fe29 and +/+ in Fe44 samples. b. Location of the −158 XmnI Gγ-globin and +25 Aγ-globin gene sequences within the Aγ- and Gγ-globin genes
The Aγ(+25 G->A) polymorphism is in complete linkage disequilibrium with the XmnI polymorphism
Table 2 shows that in all the patients analyzed the Aγ(+25 G->A) rs368698783 polymorphism is strictly linked to the Gγ-XmnI polymorphism. In fact all the 55 Gγ-XmnI(−/−) patients were found to be Aγ(+25 G/G). In addition, all the 16 Gγ-XmnI(−/+) patients were found to be Aγ(+25 G/A) and the four Gγ-XmnI(+/+) patients were found to be Aγ(+25 A/A). This very interesting distribution allows to hypothesize that the XmnI polymorphism, when present in this β-thalassemia patient population, is physically linked to the Aγ(+25 G->A) polymorphism.
Table 2.
Distribution of the G->A Aγ-globin gene polymorphism and association with the XmnI Gγ-globin gene polymorphism in the β-thalassemia patients of this study
| (G->A) mutation Aγ-globin gene | XmnI polymorphism Gγ-globin gene | ||
|---|---|---|---|
| G/G | A/G | A/A | |
| 55 | 0 | 0 | −/− |
| 0 | 16 | 0 | +/− |
| 0 | 0 | 4 | +/+ |
Distribution of the Aγ(+25 G->A) polymorphism within the β039/β039, β039/β+IVSI-110 and β+IVSI-110/β+IVSI-110 thalassemia patients
The results shown in Fig. 2 show that only one of the 9 β+IVSI-110/β+IVSI-110 patients was found to be Gγ-XmnI(−/+) and Aγ(+25 G/A) (11.1%). The other patients were Gγ-XmnI(−/−) and Aγ(+25 G/G) (88.9%). No patients exhibited a Gγ-XmnI(+/+) and Aγ(+25 A/A) combination. By sharp contrast, in the β039/β+IVSI-110 cohort, the Gγ-XmnI(−/+) and Aγ(+25 G/A) patients were found to be 6 (18.2%), 27 being Gγ-XmnI(−/−) and Aγ(+25 G/G) (81.8%). Also in this case no patients exhibited a Gγ-XmnI(+/+) and Aγ(+25 A/A) combination. Finally, in the β039/β039 cohort the Gγ-XmnI(−/+) and Aγ(+25 G/A) patients were found to be 7 (22.6%), 20 being Gγ-XmnI(−/−) and Aγ(+25 G/G) (64.5%). Unlike the β+IVSI-110/β+IVSI-110 and the β039/β+IVSI-110 cohorts, four β039/β039 patients exhibited a Gγ-XmnI(+/+) and Aγ(+25 A/A) combination (12.9%). These data, when analyzed together with the data shown in Table 2, support the hypothesis that the Gγ-XmnI and Aγ(+25 G->A) polymorphisms might be preferentially linked with the β039 thalassemia mutation. To verify this hypothesis the family trees of all the available families with β-thalassemia patients carrying at least one β039-globin gene were analyzed with respect to β-globin genes and Gγ-XmnI and Aγ(+25 G->A) polymorphisms.
Fig. 2.
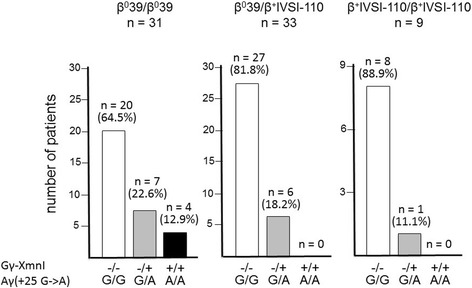
Distribution of the −158 XmnI Gγ-globin and +25 Aγ-globin gene polymorphisms within the studied β-thalassemia patients. The studied 73 patients were divided in β039/β039, β039/β+IVSI-110 and β+IVSI-110/β+IVSI-110 subgroups and the −158 XmnI Gγ-globin and +25 Aγ-globin gene polymorphisms determined by direct sequencing
Association of the β039 thalassemia mutation with Gγ-XmnI and Aγ(+25 G->A) polymorphisms
Figure 3a shows the family trees of a β039/β+IVSI-6, two β039/β039 (out of three present in our cohort) and 5 β039/β+IVSI-110 patients (out of the 6 available). The results obtained firmly demonstrate that in the β039/β+IVSI-6 family (Fe89) the Gγ-XmnI and Aγ(+25 G->A) polymorphisms are linked to the β039 gene (Fig. 3a, upper left side of the panel). In the two families with β039/β039 patients (Fig. 3a, upper middle side of the panel), one β039-globin gene (Fe29) and both β039-globin genes (Fe77) are associated with the Gγ-XmnI and Aγ(+25 G->A) polymorphisms. More importantly, when families with β039/β+IVSI-110 were considered, one (Fe11) was not informative (the genome of the mother was not available), while in patients Fe31, Fe34, Fe88 and Fe91 the β039 genotype was structurally linked to the Gγ-XmnI and Aγ(+25 G->A) polymorphisms combination. These polymorphisms were not associated with the β+IVSI-110 gene, as summarized in Fig. 3b. These data clearly indicate that in the population analyzed the Gγ-XmnI and Aγ(+25 G->A) polymorphisms cosegregate with the β039-globin gene mutation in the majority of the families of compound-heterozygous β0-thalassemia patients.
Fig. 3.
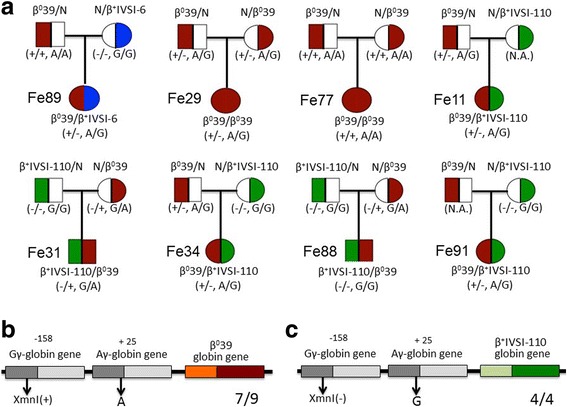
Genetic trees of the informative β-thalassemia families studied. a The analysis of the transmission of the −158 XmnI Gγ-globin and +25 Aγ-globin gene sequences from the parents to the studied β-thalassemia patients allowed to determine the linkage to the β039 mutation. b, c Most frequent XmnI Gγ-globin and +25 Aγ-globin genes linked to β039 (b) and β+IVSI-110 (c) globin genes. The comparative analysis does not include Fe11 because not informative. N.A. = not available
Production of fetal hemoglobin by erythroid precursor cells (ErPCs) from β039/β039 thalassemia patients: Relationship with Gγ-XmnI and Aγ(+25 G->A) polymorphisms
In order to verify possible relationships between the Gγ-XmnI and Aγ(+25 G->A) polymorphisms configuration, 30 available β039/β039 patients were recruited, peripheral blood isolated, erythroid precursor cells (ErPCs) selected and erythropoietin (EPO)-induced as elsewhere reported [27, 28]. After 7 days of EPO treatment, the erythroid differentiation was confirmed by benzidine-staining (looking at hemoglobin production) and FACS analysis of transferrin receptor and glycophorin A expression, as reported elsewhere [28] (data not shown). After demonstration that more than 80% of the cells were benzidine, TrfR and GYPA positive, HPLC analysis was performed to quantify fetal hemoglobin (HbF) production. Figure 4 (panels a and b) shows the representative HPLC profile of two ErPC lysates (arrowed in Fig. 4c), displaying a low (Fig. 4a) and high (Fig. 4b) HbF relative production. All the data obtained are shown in Fig. 4c, in which the ErPCs are stratified with respect to the Gγ-XmnI and Aγ(+25 G->A) polymorphisms configuration. As clearly evident, a significant correlation can be observed between the Gγ-XmnI and Aγ(+25 G->A) polymorphisms configuration and elevated production of HbF. In fact the average HbF production by ErPCs from β039/β039, Gγ-XmnI(+/+) and Aγ(+25 A/A) patients was 66.1 ± 14.1%, a value significantly higher than those found in ErPCs from Gγ-XmnI(−/−) and Aγ(+25 G/G) or Gγ-XmnI(−/+) and Aγ(+25 G/A) patients. This finding suggests that Gγ-XmnI and Aγ(+25 G->A) polymorphisms should be present in both alleles for maximal potentiation of HbF production, even if not “per se” sufficient and probably acting with other “HbF modifiers”, since all the Gγ-XmnI(+/+) and Aγ(+25 A/A) patients did not carry any detectable alteration of the α-globin gene asset. In any case, the cellular system here described might help to dissect genetic control of fetal-hemoglobin persistence and disease phenotypes, especially considering the possibility to access cellular biobanks from β-thalassemia patients stratified with respect to genotype, Gγ-XmnI and Aγ(+25) polymorphisms, enabling cryopreservation and usage of the cryopreserved and thawed cells for molecular biology studies [31].
Fig. 4.
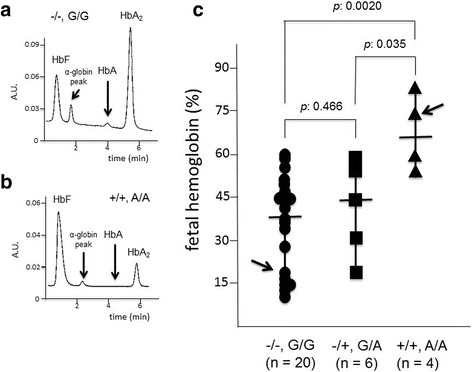
Relationship between the −158 XmnI Gγ-globin and +25 Aγ-globin gene polymorphisms and the level of fetal hemoglobin (HbF) in erythroid precursor cells from β039/β039 thalassemia patients. a, b. Representative HPLC analyses of the cytoplasms isolated from two ErPCs populations, one exhibiting low levels of HbF (a) and the other exhibiting high HbF levels (b) (arrowed in panel c). c HbF levels in ErPCs from 30 β039/β039 thalassemia patients (stratified on the basis of the 158 XmnI Gγ-globin and +25 Aγ-globin gene polymorphisms)
Discussion
Clinical observations have shown that increased levels of fetal hemoglobin (HbF) can ameliorate the severity of the disorders of β-hemoglobin, including β-thalassemia [7]. High HbF levels are associated with transcriptional effects on the γ-globin genes, which are associated with the biological activity of several transcription repressors, including MYB, BCL11A, Oct-1, KLF1 and others [16–19, 31–33]. A recent paper has pointed out the attention on a new putative repressor of the γ-globin gene, LYAR (human homologue of mouse Ly-1 antibody reactive clone), recognizing the Aγ-globin gene sequence 5′-GGTTAT-3′. Interestingly, several alterations within this consensus sequence for LYAR are associated with a decrease binding efficiency [23].
At present, no extensive analysis of this sequence has been reported in β-thalassemia patients; no attempts have been made to verify a possible association with the major HbF associated polymorphism, the Gγ-globin-XmnI; finally, no extensive analysis has been reported on possible linkage with β0- and β+-globin gene mutations.
In this paper we report the sequencing of the Aγ-globin genes performed on genomic DNA isolated from a total of 75 β-thalassemia patients, including 31 β039/β039, 33 β039/β+IVSI-110, 9 β+IVSI-110/β+IVSI-110, one β0IVSI-1/β+IVSI-6 and one β039/β+IVSI-6.
The major results of this paper are the following: (a) a G->A mutation at the level of the rs368698783 polymorphism is present in β-thalassemia patients in the 5’UTR sequence (+25) of the Aγ-globin gene, affecting the LYAR binding site 5′-GGTTAT-3′ sequence (Fig. 1); (b) no other mutations of the LYAR binding site were found; (c) this Aγ(+25 G->A) polymorphism is in complete linkage disequilibrium with a promoter variant of the Gγ-globin-gene (the XmnI polymorphism, rs7482144, C->T); (d) the Aγ(+25 G->A) and Gγ-globin-XmnI polymorphisms are linked with the β039-globin gene, but not with the β+IVSI-110-globin gene (Figs. 2 and 3). Further genetic analysis in different β-thalassemia patient population is necessary (a) to extend this specific finding to other β0-thalassemia mutations and (b) to verify the link of Aγ(+25 G->A) and Gγ-globin-XmnI (C->T) polymorphisms with the β039-globin gene in a statistically more significant number of patients.
Conclusions
It is interesting to note that the Aγ(+25 G->A) rs368698783 polymorphism is expected to deeply alter the LYAR binding activity, thereby activating the Aγ-globin gene [23]. One possibility, which deserves to be verified in further studies, is that rs368698783, rather than the XmnI polymorphism, could be the physiologically (and even clinically) active variant in hemoglobinopathy patients carrying haplotypes including the XmnI(+) allele.
In respect to this point, our last conclusion is that the Aγ(+25 G->A) and Gγ-globin-XmnI polymorphisms might be associated with high HbF in erythroid precursor cells isolated from the β039/β039 thalassemia patients (Fig. 4), in agreement with several studies suggesting the association between XmnI polymorphism and high HbF production [18, 24, 34, 35].
On the other hand, as a potential explanation of our findings, we hypothesize that in β-thalassemia the Gγ-globin-XmnI/Aγ-globin-(G->A) genotype is frequently under genetic linkage with β0-thalassemia mutations, but not with the β+-thalassemia mutation here studied (i.e. β+IVSI-110). One hypothesis is the very interesting possibility that this genetic combination has been selected within the population of the β0-thalassemia patients, due to its functional association with high HbF.
Acknowledgements
The Ferrara Association for the Fight against Thalassemia (ALT) is deeply acknowledged for help in patients recruitment. This paper is dedicated to the memory of our friend and colleague Chiara Gemmo.
Funding
This study was supported by UE THALAMOSS Project (Thalassemia Modular Stratification System for Personalized Therapy of Beta-Thalassemia; n. 306,201-FP7-HEALTH-2012-INNOVATION-1). Roberto Gambari is funded by Fondazione Cariparo (Cassa di Risparmio di Padova e Rovigo), CIB (Consorzio Interuniversitario per le Biotecnologie), and Telethon (contract GGP10124). This research activity has been also supported by Associazione Veneta per la Lotta alla Talassemia (AVLT), Rovigo.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BCL11A
B-cell lymphoma/leukemia 11A
- EPO
erythropoietin
- ErPCs
erythroid precursor cells
- HbA
adult hemoglobin A
- HbA2
adult hemoglobin A2
- HbF
fetal hemoglobin
- HPLC
high performance liquid chromatography
- LYAR
human homologue of mouse Ly-1 antibody reactive clone
Authors’ contributions
RG, GB and NB conceived and designed the experiments. GB performed DNA sequencing and was responsible for the design and interpretation of the relative data; CZ performed the analysis of Aγ(+25 G->A) and Gγ-globin-XmnI polymorphisms; NB and LCC performed the cultures of erythroid precursor cells and the FACS analyses; MRG was responsible of the patient’s recruitment at Ferrara Hospital; FC was responsible of the patient’s recruitment at Rovigo Hospital; GM performed all the PCR reactions and the relative characterization by agarose gel electrophoresis; MB extracted genomic DNA; IL and AF performed the HPLC analysis; AF performed the statistical analyses; RG, GB, NB and AF wrote the paper. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The collection and processing of the human biological samples for this research were carried out by the Ethics Committee of Ferrara District, number 06/2013 (approved on June 20, 2013), and by CESC (Ethics Committee of Rovigo and Verona Districts), number 36056 (approved on August 5, 2014). The study complies with the Declaration of Helsinki, the principles of Good Clinical Practice and all further applicable regulations. All samples of peripheral blood have been obtained after written documentation of informed consent from patient or legal representative. Copies of the consents have been collected for archiving by the “Day Hospital Talassemici”, Divisione Pediatrica of Hospital S. Anna, Ferrara, Italy and by the Department of Transfusional Medicine - ULSS 18, Rovigo, Italy.
Consent for publication
All the subjects involved in the present study gave their consent to publish the data obtained.
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weatherall DJ. Phenotype-genotype relationships in monogenic disease: lessons from the thalassaemias. Nat Rev Genet. 2001;2:245–255. doi: 10.1038/35066048. [DOI] [PubMed] [Google Scholar]
- 2.Weatherall DJ. Pathophysiology of thalassaemia. Baillieres Best Pract Res Clin Haematol. 1998;11:127–146. doi: 10.1016/S0950-3536(98)80072-3. [DOI] [PubMed] [Google Scholar]
- 3.Giardine B, Borg J, Viennas E, Pavlidis C, Moradkhani K, Joly P, et al. Updates of the HbVar database of human hemoglobin variants and thalassemia mutations. Nucleic Acids Res. 2014;42(Database issue):D1063–D1069. doi: 10.1093/nar/gkt911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao A, Galanello R. Beta-thalassemia. Genet Med. 2010;12:61–76. doi: 10.1097/GIM.0b013e3181cd68ed. [DOI] [PubMed] [Google Scholar]
- 5.Old JM. Screening and genetic diagnosis of haemoglobin disorders. Blood Rev. 2003;17:43–53. doi: 10.1016/S0268-960X(02)00061-9. [DOI] [PubMed] [Google Scholar]
- 6.Atweh G, Fathallah HV. Pharmacologic induction of fetal hemoglobin production. Hematol Oncol Clin North Am. 2010;24:1131–1144. doi: 10.1016/j.hoc.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Gambari R, Fibach E. Medicinal chemistry of fetal hemoglobin inducers for treatment of beta-thalassemia. Curr Med Chem. 2007;14:199–212. doi: 10.2174/092986707779313318. [DOI] [PubMed] [Google Scholar]
- 8.Quek L, Thein SL. Molecular therapies in beta-thalassaemia. Br J Haematol. 2007;136:353–365. doi: 10.1111/j.1365-2141.2006.06408.x. [DOI] [PubMed] [Google Scholar]
- 9.Finotti A, Gambari R. Recent trends for novel options in experimental biological therapy of β-thalassemia. Expert Opin Biol Ther. 2014;14:1443–1454. doi: 10.1517/14712598.2014.927434. [DOI] [PubMed] [Google Scholar]
- 10.Gambari R. Alternative options for DNA-based experimental therapy of β-thalassemia. Expert Opin Biol Ther. 2012;12:443–462. doi: 10.1517/14712598.2012.665047. [DOI] [PubMed] [Google Scholar]
- 11.Fibach E, Bianchi N, Borgatti M, Prus E, Gambari R. Mithramycin induces fetal hemoglobin production in normal and thalassemic human erythroid precursor cells. Blood. 2003;102:1276–1281. doi: 10.1182/blood-2002-10-3096. [DOI] [PubMed] [Google Scholar]
- 12.Fibach E, Bianchi N, Borgatti M, Zuccato C, Finotti A, Lampronti I, et al. Effects of rapamycin on accumulation of alpha-, beta- and gamma-globin mRNAs in erythroid precursor cells from beta-thalassaemia patients. Eur J Haematol. 2006;77:437–441. doi: 10.1111/j.1600-0609.2006.00731.x. [DOI] [PubMed] [Google Scholar]
- 13.Perrine SP, Pace BS, Faller DV. Targeted fetal hemoglobin induction for treatment of beta hemoglobinopathies. Hematol Oncol Clin North Am. 2014;28:233–248. doi: 10.1016/j.hoc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Lampronti I, Bianchi N, Zuccato C, Dall'acqua F, Vedaldi D, Viola G, et al. Increase in gamma-globin mRNA content in human erythroid cells treated with angelicin analogs. Int J Hematol. 2009;90:318–327. doi: 10.1007/s12185-009-0422-2. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi N, Cosenza LC, Lampronti I, Finotti A, Breveglieri G, Zuccato C, et al. Structural and functional insights on an uncharacterized Aγ-Globin-gene polymorphism present in four β0-Thalassemia families with high fetal hemoglobin levels. Mol Diagn Ther. 2016;20:161–173. doi: 10.1007/s40291-016-0187-2. [DOI] [PubMed] [Google Scholar]
- 16.Thein SL. The emerging role of fetal hemoglobin induction in non-transfusion-dependent thalassemia. Blood Rev. 2012;26(Suppl 1):S35–S39. doi: 10.1016/S0268-960X(12)70011-5. [DOI] [PubMed] [Google Scholar]
- 17.Thein SL, Menzel S, Lathrop M, Garner C. Control of fetal hemoglobin: new insights emerging from genomics and clinical implications. Hum Mol Genet. 2009;18(R2):R216–R223. doi: 10.1093/hmg/ddp401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen TK, Joly P, Bardel C, Moulsma M, Bonello-Palot N, Francina A. The XmnI (G)gamma polymorphism influences hemoglobin F synthesis contrary to BCL11A and HBS1L-MYB SNPs in a cohort of 57 beta-thalassemia intermedia patients. Blood Cells Mol Dis. 2010;45:124–127. doi: 10.1016/j.bcmd.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Trakarnsanga K, Wilson MC, Lau W, Singleton BK, Parsons SF, Sakuntanaga P, et al. Induction of adult levels of β-globin in human erythroid cells that intrinsically express embryonic or fetal globin by transduction with KLF1 and BCL11A-XL. Haematologica. 2014;99:1677–1685. doi: 10.3324/haematol.2014.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danjou F, Francavilla M, Anni F, Satta S, Demartis FR, Perseu L, et al. A genetic score for the prediction of beta-thalassemia severity. Haematologica. 2015;100:452–457. doi: 10.3324/haematol.2014.113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badens C, Joly P, Agouti I, Thuret I, Gonnet K, Fattoum S, et al. Variants in genetic modifiers of β-thalassemia can help to predict the major or intermedia type of the disease. Haematologica. 2011;96:1712–1714. doi: 10.3324/haematol.2011.046748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banan M, Bayat H, Azarkeivan A, Mohammadparast S, Kamali K, Farashi S. The XmnI and BCL11A single nucleotide polymorphisms may help predict hydroxyurea response in Iranian β-thalassemia patients. Hemoglobin. 2012;36:371–380. doi: 10.3109/03630269.2012.691147. [DOI] [PubMed] [Google Scholar]
- 23.Ju J, Wang Y, Liu R, Zhang Y, Xu Z, Wang Y, et al. Human fetal globin gene expression is regulated by LYAR. Nucleic Acids Res. 2014;42:9740–9752. doi: 10.1093/nar/gku718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerdpoo S, Limweeraprajak E, Tatu T. Effect of Swiss-type heterocellular HPFH from XmnI-Gγ and HBBP1 polymorphisms on HbF, HbE, MCV and MCH levels in Thai HbE carriers. Int J Hematol. 2014;99:338–344. doi: 10.1007/s12185-014-1516-z. [DOI] [PubMed] [Google Scholar]
- 25.Roy P, Bhattacharya G, Mandal A, Dasgupta UB, Banerjee D, Chandra S, et al. Influence of BCL11A, HBS1L-MYB, HBBP1 single nucleotide polymorphisms and the HBG2 XmnI polymorphism On Hb F levels. Hemoglobin. 2012;36:592–9. doi: 10.3109/03630269.2012.735626. [DOI] [PubMed] [Google Scholar]
- 26.Fibach E, Manor D, Oppenheim A, Rachmilewitz EA. Proliferation and maturation of human erythroid progenitors in liquid culture. Blood. 1989;73:100–103. [PubMed] [Google Scholar]
- 27.Fibach E, Kollia P, Schechter AN, Noguchi CT, Rodgers GP. Hemin-induced acceleration of hemoglobin production in immature cultured erythroid cells: preferential enhancement of fetal hemoglobin. Blood. 1995;85:2967–2974. [PubMed] [Google Scholar]
- 28.Bianchi N, Finotti A, Ferracin M, Zuccato C, Breveglieri G, Brognara E, et al. Increase of microRNA-210, decrease of raptor gene expression and alteration of mammalian target of Rapamycin regulated proteins following Mithramycin treatment of human Erythroid cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0121567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finotti A, Bianchi N, Fabbri E, Borgatti M, Breveglieri G, Gasparello J, et al. Erythroid induction of K562 cells treated with mithramycin is associated with inhibition of raptor gene transcription and mammalian target of rapamycin complex 1 (mTORC1) functions. Pharmacol Res. 2015;91:57–68. doi: 10.1016/j.phrs.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coleman MB, Steinberg MH, Adams JG. A four-base deletion 5′ to the Aγ-globin gene is a common polymorphism. Blood. 1991;78:2473–2474. [PubMed] [Google Scholar]
- 31.Cosenza LC, Breda L, Breveglieri G, Zuccato C, Finotti A, Lampronti I, et al. A validated cellular biobank for β-thalassemia. J Transl Med. 2016;14:255–65. doi: 10.1186/s12967-016-1016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sankaran VG, Menne TF, Xu J, Akie TE, Lettre G, Van Handel B, et al. Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science. 2008;322:1839–1842. doi: 10.1126/science.1165409. [DOI] [PubMed] [Google Scholar]
- 33.Sankaran VG. Targeted therapeutic strategies for fetal hemoglobin induction. Hematology Am Soc Hematol Educ Program. 2011;2011:459–465. doi: 10.1182/asheducation-2011.1.459. [DOI] [PubMed] [Google Scholar]
- 34.Motovali-Bashi M, Ghasemi T. Role of XmnIgG polymorphism in Hydroxyurea treatment and fetal hemoglobin level at Isfahanian intermediate β-Thalassemia patients. Iran Biomed J. 2015;19:177–182. doi: 10.7508/ibj.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pereira C, Relvas L, Bento C, Abade A, Ribeiro ML, Manco L. Polymorphic variations influencing fetal hemoglobin levels: association study in beta-thalassemia carriers and in normal individuals of Portuguese origin. Blood Cells Mol Dis. 2015;54:315–320. doi: 10.1016/j.bcmd.2015.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


