Abstract
Maternal smoking during pregnancy (MS) has long-lasting neurobehavioural effects on the offspring. Many MS-associated psychiatric disorders begin or change symptomatology during adolescence, a period of continuous development of the central nervous system. However, the underlying molecular mechanisms are largely unknown. Given that cell adhesion molecules (CAMs) modulate various neurotransmitter systems and are associated with many psychiatric disorders, we hypothesize that CAMs are altered by prenatal treatment of nicotine, the major psychoactive component in tobacco, in adolescent brains. Pregnant Sprague–Dawley rats were treated with nicotine (3 mg/kg.d) or saline via osmotic mini-pumps from gestational days 4 to 18. Female offspring at postnatal day 35 were sacrificed, and several limbic brain regions (the caudate putamen, nucleus accumbens, prefrontal cortex, and amygdala) were dissected for evaluation of gene expression using microarray and quantitative RT–PCR techniques. Various CAMs including neurexin, immunoglobulin, cadherin, and adhesion-GPCR superfamilies, and their intracellular signalling pathways were modified by gestational nicotine treatment (GN). Among the CAM-related pathways, GN has stronger effects on cytoskeleton reorganization pathways than on gene transcription pathways. These effects were highly region dependent, with the caudate putamen showing the greatest vulnerability. Given the important roles of CAMs in neuronal development and synaptic plasticity, our findings suggest that alteration of CAMs contributes to the neurobehavioural deficits associated with MS. Further, our study underscores that low doses of nicotine produce substantial and long-lasting changes in the brain, implying that nicotine replacement therapy during pregnancy may carry many of the same risks to the offspring as MS.
Keywords: Brain development, cell adhesion, fetus, gene expression, gestational nicotine exposure, rat
Introduction
Maternal smoking during pregnancy (MS) produces adverse effects on offspring that persist or emerge after the initial tobacco exposure (Rogers, 2008; Shea & Steiner, 2008). In addition to the MS-linked deficits during the prenatal and neonatal stages (Fantuzzi et al. 2007), MS is significantly associated with neuropsychiatric disorders that emerge or change symptomatology during adolescence, a period of continuous development of the central nervous system (CNS) (Spear, 2000). Children whose mothers smoke during pregnancy are more likely to develop attention deficit hyperactivity disorder (ADHD), conduct disorder, depression, and autism (Indredavik et al. 2007; Weissman et al. 1999). Those exposed to prenatal tobacco also are more vulnerable to various drug addictions (Fergusson et al. 1998; Weissman et al. 1999). Moreover, the intensity of MS is inversely related to offspring intelligence (IQ) and cognitive ability (Batty et al. 2006; Olds et al. 1994). Many of these disorders are thought to be mediated by dysfunction of the limbic system (Drevets et al. 2008; Feltenstein & See, 2008), a collection of brain nuclei that mature during adolescence (Spear, 2000). The delayed onset of MS-related neurobehavioural disorders suggests that alterations during prenatal development manifest only as the limbic circuitry matures.
Animal studies have evaluated the neurochemical mechanisms underlying the effects of prenatal exposure to nicotine, the major psychoactive component of tobacco. Gestational nicotine exposure (GN) modulates cholinergic receptor expression, which remains altered into adolescence (Chen et al. 2005; Tizabi & Perry, 2000). Deficits in monoamine transmission including those of dopamine, norepinephrine, and serotonin also are observed in GN-treated adolescent animals (Kane et al. 2004; Seidler et al. 1992; Xu et al. 2001). Additionally, GN has lasting effects on the glutamate system, producing alterations in AMPA receptor function (Vaglenova et al. 2008). Many of the neurotransmitter systems impacted by GN are regulated at the structural and functional levels by cell adhesion systems (Craig & Kang, 2007; Hulley et al. 1998; Yamagata et al. 2003).
Cell adhesion molecules (CAMs) have broad functions, modulating cell–cell, cell–matrix interactions, and intracellular signal transduction (Juliano, 2002). In the CNS, CAMs such as the cadherins, neurexins, integrin, and immunoglobulin superfamilies have been identified at synapses (Yamagata et al. 2003). Cell adhesion systems play important roles in the development, maturation, and plasticity of the CNS by regulating neuronal migration, neurite outgrowth, axon fasciculations, axon guidance, synaptogenesis in the developing brain, and synaptic formation and function in the mature brain (Sudhof, 2008). Abnormal expression of CAM genes is associated with psychiatric and cognitive disorders such as autism, schizophrenia, bipolar disorder, and Alzheimer’s disease (Liu et al. 2006; Rujescu et al. 2009; Sudhof, 2008). Recent Genome-Wide Association Studies (GWAS) also suggest that CAM genes are related to drug abuse (Li & Burmeister, 2009; Liu et al. 2006). As the prevalence of many of these disorders is increased by MS, it is possible that changes in CAM function underlie the alterations in neurotransmission and the behavioural phenotypes in GN animal models.
In the current study, we have undertaken a systematic evaluation of the relationship between CAM systems and GN in rats. First, we used quantitative real-time PCR to examine the expression pattern of the 29 CAM-related genes that are suggested to play a significant role in drug addiction based on human genetic studies (Li & Burmeister, 2009; Liu et al. 2006) in four limbic brain regions of adolescent female rats subjected to GN. Then, we investigated the regulation pattern of the biochemical pathways related to CAM systems in these brain regions based on microarray data by focusing on most of the genes involved in the system. To our knowledge, this represents the first report that CAMs and CAM-related intracellular signal transduction pathways are significantly modified by GN in limbic brain regions of adolescent female offspring.
Materials and methods
Animals and tissue collection
Sprague–Dawley rats were maintained in a temperature-(21 °C) and humidity-(50%) controlled room on a 12-h light/dark cycle (lights on 07:00 hours) with unlimited access to food and water. Pregnant rats (Charles River, USA) were treated with nicotine or saline as previously described (Park et al. 2006). Each rat was given either nicotine at a concentration of 3 mg/kg.d or saline via an osmotic mini-pump from gestational days 4 to 18. After birth, litters were culled to ten and pups were cross-fostered to drug-naive mothers to minimize the effects of abnormal maternal rearing behaviours. Blood concentrations resulting from this dose of nicotine are equivalent to levels found in humans who smoke about 1.5 packs of cigarettes per day (Matta & Elberger, 2007), approximately (15–45 ng/ml; Benowitz & Jacob, 1984). As previously reported (Franke et al. 2007), GN treatment at this moderate dose did not influence dam weight gain, litter size, or pup weight gain during postnatal development. Pups were weaned at postnatal day 21 (PD 21) and sacrificed at PD 35 via rapid decapitation, and brains were immediately removed. Using a rat brain matrix, 2-mm slices were taken that contained the prefrontal cortex (PFC), caudate putamen (CPu), nucleus accumbens (NAc), and amygdala (Amy), which were identified with reference to a rat brain atlas (Paxinos & Watson, 1998). Using a 1-mm-diameter punch, tissue was collected bilaterally from each brain region from each pup and stored at −80 °C until use. Tissue of ten female pups from different litters was used for microarray with five animals in gestational saline treatment (GS) and GN groups, respectively. To get sufficient mRNA for quantitative real-time polymerase chain reaction (qRT–PCR), total mRNA from each brain region of two animals per litter was combined to yield a total of five litters in each experimental group. All experiments were performed in accordance with the Institutional Animal Care and Use Committee at the University of California, Irvine, and were consistent with Federal guidelines.
Microarray production
A pathway-focused oligoarray designed specifically for drug addiction and brain-related research was used. Briefly, 3565 genes including those implicated in the maintenance of neuronal homeostasis and associated with the neuronal responses to addictive substances were selected on the basis of an earlier version of a pathway-focused cDNA microarray (Konu et al. 2004) and an extensive literature survey. The oligoneucleotide for each gene was designed using OligoWiz (http://www.cbs.dtu.dk/services/OligoWiz/) with a final length of 59.2±3.8 (mean±S.D.), guanine cytosine (GC) content of 0.53±0.05, and Tm 76.4±1.7 °C. Then, the designed oligonucleotides and 10 control clones were synthesized and spotted at a concentration of 40 μM in 3 × SSC and 1.5 M Betaine buffer onto CMT-GAPS II slides (Corning, USA), using OmniGrid MicroArrayer OGR-03 (GeneMachines, USA).
RNA isolation and amplification, cDNA probe synthesis and microarray hybridization
RNA was isolated from each brain region using TRIZol reagent (Invitrogen, USA) according to the manufacturer’s instructions and amplified as described previously for adequate cDNA probe labelling (Gutala et al. 2004; Konu et al. 2004; Li et al. 2004). Briefly, 2 μg total RNA was reverse-transcripted into the first-strand cDNA with an introduction of a T7 promotor region. The RT product was then mixed with 5× second-strand buffer (30 μl), 10 mM dNTP (3 μl), DNA polymerase (4 μl), RNase H (0.5 μl), E. coli DNA ligase (1 μl), and H2O (92.5 μl) and incubated at 16 °C for 3 h to synthesize double-stranded cDNA, which was then amplified using AmpliScribe™ T7 Transcription kits (Epicentre, USA).
cDNA probes were synthesized and hybridized to microarray slides as described previously (Gutala et al. 2004; Li et al. 2004). Briefly, 4 μg of amplified RNA were reverse-transcripted. The product was dissolved in H2O (28 μl) and mixed with 10 × buffer (4 μl), 10 mM dTTP-free dNTP (4 μl), 10 mM dTTP (1 μl), 1 mM cyanine 3-dUTP or cyanine 5-dUTP (2 μl, Enzo, USA), and Klenow fragment (1 μl, 50 units/μl). The mixture was then incubated at 37 °C for 3 h. After purification, cyanine 5-labelled sample cDNA probes were mixed with cyanine 3-labelled control probes and applied in a total of 50 μl volume containing 20 × SSC (7.5 μl), CotI DNA (3 μg), polyA (3 μg), and 10% SDS (0.5 μl). The mixture was applied to the pathway-focused oligonucleotide microarray described above and hybridized overnight at 60 °C. Slides were washed in 1 × SSC and 0.2% SDS at 60 °C for 5 min followed by washing in 0.1 × SSC and 0.2% SDS and in 0.1 × SSC at room temperature for 10 min. Hybridized slides were scanned using the ScanArray Gx microarray scanner, and the intensity of each probe was quantified with the ScanArray Express microarray analysis system (PerkinElmer, USA).
Microarray data analysis and Gene Set Enrichment Analysis
After scanning each array, we obtained the raw hybridization intensity of each element and used the background-subtracted median intensity of each spot for further statistical analysis. Two replicates of each gene on a chip were analysed separately. To minimize spot variations and reduce experimental error, we discarded spots that were either over-saturated or poorly expressed (i.e. 5% of the weakest spots in each replicate of an array). We used an intensity-dependent normalization method (locally weighted linear regress; Lowess) to normalize the data for each replicate (Yang et al. 2002). After removing spots with fewer than six valid measurements per experimental group, we averaged two replicates per chip to be used as the measurement of the expression of a gene in a given sample.
Then, a bioinformatics tool, called Gene Set Enrichment Analysis (GSEA; Subramanian et al. 2005) was utilized to determine the pathways showing expression differences in each brain region. GSEA is a bioinformatics tool that computationally identifies whether an a priori-defined set of genes (pathways in our case) shows statistically significant and concordant differences between two biological states. For each predefined gene set (pathway in our case), a Normalized Enrichment Score (NES) is calculated by considering all the gene sets tested and a p value is assigned to determine whether this gene set is statistically enriched in the input genes when compared with random distribution. The pathways included in GSEA database were collected from multiple public domains (e.g. http://www.sigmaaldrich.com/; http://www.biocarta.com; http://www.genome.jp/kegg/). The software and the curated pathway database was downloaded and implemented locally in our laboratory.
For more details about the database, please refer to http://www.broadinstitute.org/gsea/.
Quantitative real-time PCR array
Representative CAMs and key genes in CAM-related intracellular signalling transduction pathways were examined with qRT–PCR using a different set of samples from those in microarray. Primers used in the qRT–PCR array were designed using Primer Express (v. 3.0) software. The sequences were subjected to a BLAST search to ensure specificity of the primers for the target gene and synthesized by Fisher Scientific (USA). All the primers were tested before addition to the qRT–PCR array. The primer sequences are listed in Supplementary Table S1 (available online).
qRT–PCR was conducted as described previously (Gutala et al. 2004; Li et al. 2004). Briefly, RT product was amplified in a volume of 10 μl containing 5 μl 2 × Power SYBR® Green PCR Master Mix (Applied Biosystems, USA), and combined sense and antisense primers (3 μl, final concentration 250 nM) in a 384-well plate using the 7900HT Fast Real Time PCR system (Applied Biosystems). Expressions of all genes were normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and then analysed using a comparative Ct method (Winer et al. 1999). The relative gene expression was compared between GN and GS using the Student’s t test. Genes considered to be significant are those with a p value of <0.05 and fold change of >25%. Because all these genes are from the CAM system and highly functional, we chose not to perform correction for multiple testing in this report. Therefore, it is possible some of the genes identified as significantly expressed may be false positives. However, considering the genes evaluated in our work are so functionally related to each other in the CAM system and many of them showed consistent regulation by GN (see Results section for details), we believe that the conclusions drawn from our data are reasonable and reliable.
Results
Cell adhesion genes were modified by GN
Genes encoding CAMs and their intracellular anchor proteins were modified by GN at the mRNA level in four limbic brain regions of the female adolescent rats (Table 1). Using the qRT–PCR array, we examined 29 cell adhesion-related genes, which fell into six categories: neurexin, immunoglobulin, integrin, cadherin, and adhesion G protein-coupled-receptor (GPCR) superfamilies, with four genes that do not belong to any of the superfamilies grouped into the sixth category. We also included cadherin-associated proteins (catennins), vinculin (Vcl), actinin (Actn1), Fyn proto-oncogene (Fyn), and zyxin (Zyx), which encode intracellular anchoring proteins that connect CAMs to the cytoskeleton. Among the 29 cell adhesion-related genes, we observed 17 significantly modified by GN in one of the four brain regions examined. Three genes, namely, contactin 4 (Cntn4), Down syndrome cell adhesion molecule (Dscam), and latrophilin 3 (Lphn3), were significantly changed in two of the four brain regions. Periostin (Postn), an extracellular CAM, was modified by GN in three brain regions. Most of the affected CAM-related genes were down-regulated, with only Postn exhibiting significant up-regulation in the NAc and PFC.
Table 1.
Gestational nicotine exposure modified mRNA expression of cell adhesion molecules and intracellular anchoring proteins
| Cell adhesion family | Gene symbol | Gene name | Fold change (±S.D.)
|
|||
|---|---|---|---|---|---|---|
| CPu | NAc | PFC | Amy | |||
| Neurexin | Nrxn1a | Neurexin1 | 0.79±0.27 | 1.42±1.15 | 0.94±0.27 | 0.84±0.35 |
| Nrxn2 | Neurexin2 | 0.76±0.11 | 0.78±0.11 | 0.72±0.20 | 0.99±0.65 | |
| Nrxn3a | Neurexin3 | 0.62±0.25 | 1.12±0.83 | 0.75±0.02* | 0.84±0.23 | |
| Nlgn1 | Neuroligin1 | 0.60±0.16* | 1.25±0.84 | 0.84±0.08 | 0.86±0.21 | |
| Immunoglobulin | Ncam1 | Neural cell adhesion molecule 1 | 0.84±0.32 | 0.93±0.14 | 0.96±0.05 | 0.47±0.05** |
| Cntn4a | Contactin 4 | 0.45±0.01*** | 0.67±0.01 | 0.66±0.04** | 1.20±0.23 | |
| Cntn5a | Contactin 5 | 0.72±0.07* | 1.20±0.96 | 0.82±0.67 | 1.06±0.79 | |
| Cntn6a | Contactin 6 | 0.66±0.15* | 1.13±0.30 | 0.74±0.16 | 1.12±0.99 | |
| Dscama | Down syndrome cell adhesion molecule | 0.66±0.17* | 1.46±0.08 | 0.66±0.12* | 0.95±0.43 | |
| Pecam1 | Platelet/endothelial cell adhesion molecule 1 | 0.97±0.45 | 0.94±0.21 | 1.32±0.52 | 0.59±0.03* | |
| Integrin | Itgb1 | Integrin beta 1 | 0.97±0.25 | 1.28±0.49 | 0.99±0.25 | 1.12±1.00 |
| Postn | Periostin, osteoblast specific factor | 0.65±0.03*** | 1.40±0.11* | 1.58±0.42* | 1.00±0.70 | |
| Vclb | Vinculin | 0.74±0.28 | 0.72±0.30 | 1.09±0.03 | 1.22±0.71 | |
| Actn1b | Actinin, alpha 1 | 0.46±0.13* | 0.69±0.27 | 0.95±0.18 | 0.93±0.40 | |
| Zyxb | Zyxin | 0.54±0.62 | 1.09±0.24 | 0.61±0.01 | 0.80±0.07 | |
| Cadherin | Cdh13a | Cadherin 13 | 0.51±0.21* | 0.74±0.11 | 0.94±0.06 | 1.02±0.68 |
| Pcdh9a | Protocadherin 9 | 0.89±0.07 | 0.83±0.17 | 1.15±0.07 | 0.86±0.13 | |
| Ctnna1b | Catenin (cadherin associated protein), alpha 1 | 0.63±0.07** | 1.17±0.42 | 0.73±0.21 | 0.87±0.31 | |
| Ctnna2b | Catenin (cadherin associated protein), alpha 2 | 0.67±0.03*** | 1.22±0.16 | 0.85±0.13 | 1.10±0.92 | |
| Ctnna3b | Catenin (cadherin associated protein), alpha 3 | 0.67±0.20 | 1.32±0.54 | 0.67±0.90 | 1.37±0.56 | |
| Ctnnb1b | Catenin (cadherin associated protein), beta 1 | 0.71±0.05** | 1.08±0.33 | 0.76±0.12 | 1.03±0.65 | |
| Ctnnd2ab | Catenin (cadherin-associated protein), delta 2 | 0.61±0.09** | 1.28±0.13 | 0.67±0.18 | 0.95±0.44 | |
| Vclb | Vinculin | 0.74±0.28 | 0.72±0.30 | 1.09±0.03 | 1.22±0.71 | |
| Actn1b | Actinin, alpha 1 | 0.46±0.13* | 0.69±0.27 | 0.95±0.18 | 0.93±0.40 | |
| Fynb | Fyn proto-oncogene | 0.62±0.10** | 1.13±0.36 | 0.89±0.12 | 0.96±0.82 | |
| Adhesion GPCR | Bai3a | Brain-specific angiogenesis inhibitor 3 | 0.59±0.16* | 0.74±0.27 | 0.96±0.11 | 0.94±0.32 |
| Lphn3a | Latrophilin 3 | 0.66±0.18* | 1.08±0.56 | 1.06±0.17 | 0.62±0.02* | |
| Others | Lrrn1a | Leucine rich repeat neuronal 1 | 0.75±0.18 | 1.33±0.69 | 1.10±0.32 | 0.91±0.25 |
| Ptprda | Receptor-type protein tyrosine phosphatase D | 0.30±0.37 | 0.57±0.11* | 1.69±0.82 | 0.79±0.14 | |
| Csmd1a | CUB and Sushi multiple domains 1 | 0.66±0.04*** | 1.13±0.33 | 0.54±0.05 | 0.96±0.03 | |
| Sgcza | Sarcoglycan zeta | 0.69±0.03 | 0.99±0.17 | 1.46±0.47 | 0.65±0.05** | |
CPu, Caudate putamen; NAc, nucleus accumbens; PFC, prefrontal cortex; Amy, amygdala. GPCR, G protein coupled receptor. Cell adhesion molecules and intracellular anchor proteins were modified by gestational nicotine exposure (GN). Fold change >1 indicates up-regulation of gene expression, whereas fold change <1 indicates down-regulation by GN. Data are from qRT–PCR array.
Genes suggested as being associated with drug addiction in human studies.
Genes encode intracellular proteins anchoring cell adhesion molecules.
p<0.05,
p<0.01,
p<0.001 significant difference between GN and control animals.
In the CPu, all 29 genes showed at least a trend for down-regulation by GN, and more genes were significantly changed in this region than in any other examined. Those showing significant down-regulation included Neuroligin 1 (Nlgn1) [0.60±0.16 (fold change ±S.D.); p=0.027] in the neurexin superfamily; Cntn4 (0.45±0.01, p=6.0×10−5), Cntn5 (0.72±0.07, p= 0.024), Cntn6 (0.66±0.15, p=0.035), and Dscam (0.66±0.17, p=0.040) in the immunoglobulin superfamily; cadherin 13 (Cdh13) (0.51±0.21, p=0.013), catenin α1 (Ctnna1) (0.63±0.07, p=1.2×10−3), catenin α2 (Ctnna2) (0.67±0.03, p=1.7×10−4), catenin β1 (Ctnnb1) (0.71±0.05, p=6.5×10−3), catenin δ2 (Ctnnd2) (0.61±0.09, p=2.3×10−3) in the cadherin superfamily; and adhesion GPCRs such as brain-specific angiogenesis inhibitor 3 (Bai3) (0.59±0.16, p=0.037) and Lphn3 (0.66±0.18, p=0.047). Postn (0.65±0.03, p=1.6×10−4) and genes encoding intracellular anchor proteins such as Actn1 (0.46±0.13, p=0.014) and Fyn (0.62±0.10, p=5.4×10−3) were significantly down-regulated. In addition, CUB and Sushi multiple domains 1 (Csmd1), a gene suggested to be involved in drug addiction (Liu et al. 2006) was significantly down-regulated (0.66±0.04, p=3.3×10−4) by GN in the CPu.
In the NAc, there were only two genes significantly regulated by GN. Postn mRNA was 40% up-regulated by GN (1.40±0.11, p=0.033), whereas receptor-type protein tyrosine phosphatase D (Ptprd), a gene suggested to be involved in drug addiction (Liu et al. 2006), was significantly down-regulated (0.57±0.11, p=0.021).
In the PFC, neurexin 3 (Nrxn3) (0.75±0.02, p=0.046), Cntn4 (0.66±0.04, p=3.6×10−3), and Dscam (0.66±0.12, p=0.044) were significantly down-regulated by GN. In contrast, Postn (1.58±0.42, p= 0.029) was significantly up-regulated.
In the Amy, Lphn3 (0.62±0.02, p=0.015), Sarcoglycan zeta (Sgcz) (0.65±0.05, p=7.6×10−3), and two genes in the immunoglobulin superfamily, namely, neural cell adhesion molecule 1 (Ncam1) (0.47±0.11, p=9.5×10−3) and platelet/endothelial cell adhesion molecule 1 (Pecam1) (0.59±0.03, p=0.031), were significantly down-regulated by GN. In contrast, no genes were significantly up-regulated.
Intracellular signalling pathways related to CAMs were changed by gestational nicotine treatment
CAMs not only have adhesive functions that modulate cell–cell and cell–matrix interactions but also transmit signals to the cell interior (Juliano, 2002). CAMs can directly activate MAP kinase cascades and Rho small GTPases and are involved in Wnt/Frizzled pathways (Komiya & Habas, 2008) and signal through G protein-mediated pathways (Bjarnadottir et al. 2007). CAMs can also modulate signal transduction initiated by other receptor types, including GPCRs, growth factor receptors, and Notch receptors (Hu et al. 2006; Juliano, 2002; Maness & Schachner, 2007). To further examine whether GN modified CAM-related intracellular signalling pathways, we searched the annotated database with the GSEA algorithm on the basis of gene ontology (GO) information for all genes included on our microarray chip. Among the overrepresented categories that were significantly associated with GN, we found 2, 9, 7, and 11 major pathways were related to CAMs in the CPu, NAc, PFC, and Amy, respectively (Fig. 1). The CAM-related pathways generally belonged to five groups (Rho small GTPase-related, MAPK-related, GPCR-related signalling, Notch and Wnt/Frizzled, and growth factor-signalling) (Table 2). Rho small GTPase-related pathways (i.e. Rac1, Rho, Cdc42Rac, Epha4, integrin, Akap13) were modified by GN in all four brain regions. In contrast, MAPK-related pathways (i.e. MAPK, P38 MAPK, Cdk5, Pyk2) were modulated by GN in the PFC and Amy, but not in the CPu or NAc. GPCR-related signalling pathways (i.e. Gs, St_G_alpha_i, PLC, and Agpcr), Notch and Wnt/Frizzled pathways, and growth factor-signalling pathways (i.e. Pdgf, Edg1, Egf, Insulin, Met, Igf1, Erbb4) were associated with the treatment in the NAc, PFC, and Amy.
Fig. 1.
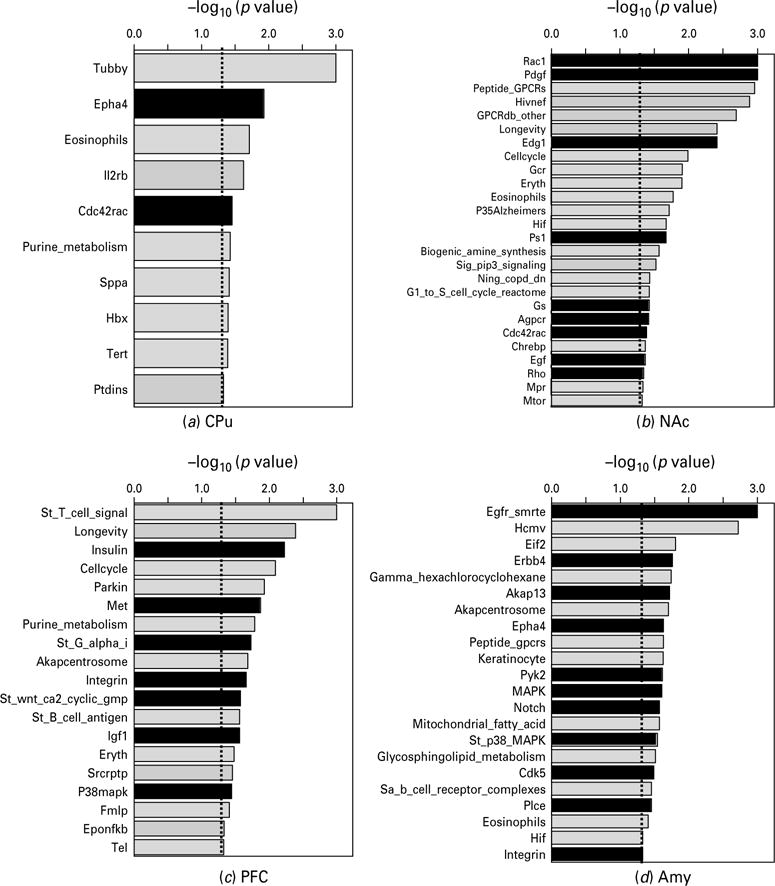
Biological pathways significantly modified by gestational nicotine treatment in the adolescent brain regions analysed by GSEA (nominal p<0.05). For each brain region, the pathways were plotted in descending order of the negative logarithm of their p values at base 10. The biological pathways related to cell adhesion molecules are shown in black columns, whereas others are shown in light grey. For each pathway, a short format of its name in the GSEA database is shown in the figure: for amygdala, Gamma_hexachlorocyclohexane and Mitochondrial_fatty_acid are short formats of Gamma_ hexachlorocyclohexane_degradation and Mitochondrial_fatty_acid_betaoxidation, respectively; for NAc, Sig_pip3_signalling corresponds to Sig_pip3_signalling_in_cardiac_myoctes; for PFC, St_T_cell_signal and St_B_cell_antigen are short formats of St_T_cell_signal_transduction and St_B_cell_antigen_receptor, respectively; for PVN, Oxidative_phosph, Glycerolipid and Sa_B_cell_receptor are short formats of Oxidative_phosphorylation, Glycerolipid_metabolism and Sa_B_cell_receptor_ complexes, respectively; for the other pathways, the word pathway has been omitted from their names.
Table 2.
Gestational nicotine exposure modified intracellular signalling pathways related to cell adhesion molecules
| Brain region | Groups of pathways | Gene ontology categories identified by GSEA | No. of genes | NES | Permutated p value |
|---|---|---|---|---|---|
| CPu | Rho small GTPase-related | Epha4 pathway | 10 | −1.71 | 0.0118 |
| Cdc42Rac pathway | 14 | −1.57 | 0.0352 | ||
| NAc | Rho small GTPase-related | Rac1 pathway | 21 | −1.86 | 0.0000 |
| Cdc42Rac pathway | 14 | −1.46 | 0.0410 | ||
| Rho pathway | 26 | −1.45 | 0.0455 | ||
| GPCR-related signalling | Gs pathway | 6 | −1.56 | 0.0377 | |
| Agpcr pathway | 10 | −1.48 | 0.0382 | ||
| Notch and Wnt/Frizzled | Ps1 pathways | 14 | −1.62 | 0.0212 | |
| Growth factor signalling | Pdgf pathway | 26 | −1.82 | 0.0000 | |
| Edg1 pathway | 24 | −1.71 | 0.0039 | ||
| Egf pathway | 26 | −1.45 | 0.0431 | ||
| PFC | Rho small GTPase-related | Integrin pathway | 14 | −1.74 | 0.0221 |
| MAPK-related | p38mapk pathway | 14 | −1.56 | 0.0363 | |
| GPCR-related signalling | St_G_alpha_i pathway | 13 | −1.68 | 0.0187 | |
| Notch and Wnt/Frizzled | St_wnt_ca2_cyclic_gmp | 6 | −1.55 | 0.0266 | |
| Growth factor signalling | Insulin pathway | 10 | −1.78 | 0.0060 | |
| Met pathway | 18 | −1.74 | 0.0135 | ||
| Igf1 pathway | 9 | −1.64 | 0.0277 | ||
| Amy | Rho small GTPase-related | Akap13 pathway | 6 | −1.58 | 0.0190 |
| Epha4 pathway | 10 | −1.56 | 0.0234 | ||
| Integrin pathway | 33 | −1.48 | 0.0469 | ||
| MAPK-related | Pyk2 pathway | 28 | −1.47 | 0.0242 | |
| MAPK pathway | 80 | −1.51 | 0.0246 | ||
| St_p38_MAPK pathway | 32 | −1.54 | 0.0285 | ||
| Cdk5 pathway | 10 | −1.54 | 0.0326 | ||
| GPCR-related signalling | Plce pathway | 10 | 1.61 | 0.0351 | |
| Notch and Wnt/Frizzled | Notch pathway | 6 | 1.54 | 0.0269 | |
| Growth factor signalling | Egfr_smrte pathway | 10 | −1.97 | 0.0000 | |
| Erbb4 pathway | 7 | 1.63 | 0.0175 |
CPu, Caudate putamen; NAc, nucleus accumbens; PFC, prefrontal cortex; Amy, amygdala. NES, normalized enrichment score. The signalling pathways significantly modified by gestational nicotine exposure. Data are from microarray.
To further evaluate CAM-related signalling pathways, representative genes from the microarray with the addition of critical genes were examined by qRT–PCR in all four brain areas. Our results confirmed that genes in the CAM-related pathways were changed by GN in a brain region-dependent manner. Further bioinformatics analyses indicated that these genes generally play important roles in cytoskeleton reorganization (Fig. 2), gene transcription (Fig. 3), or both.
Fig. 2.
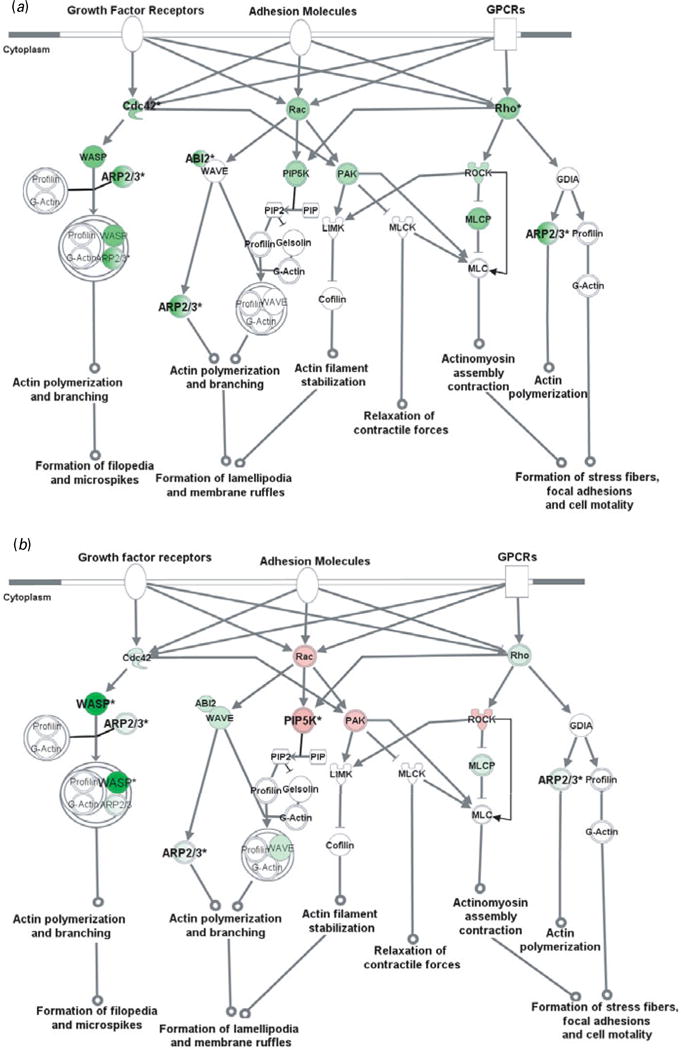
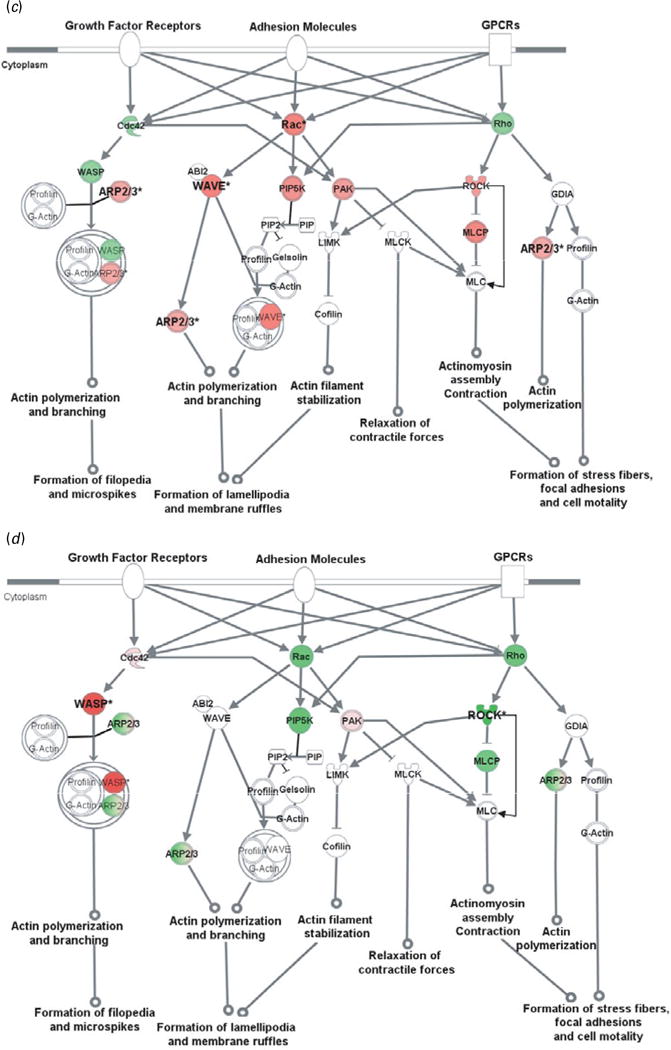
Cell adhesion molecules modulate actin cytoskeleton via Rho small GTPases. Genes in red were up-regulated whereas those in green were down-regulated at the mRNA level by gestation nicotine exposure in (a) the caudate putamen, (b) nucleus accumbens, (c) prefrontal cortex and (d) amygdala. * Significantly modified compared with gestational saline treatment (p<0.05 at least). ABI2, abl-interactor 2; ARP2/3, actin-related protein complex; Cdc42, cell division cycle 42; Cofilin, cofilin 1 (non-muscle); GDIA, Rho GDP dissociation inhibitor (GDI) alpha; Gelsolin, gelsolin (amyloidosis, Finnish type); LIMK, LIM domain kinase 1; MLC, myosin light chain; MLCK, myosin light chain kinase; MLCP, myosin light chain phosphatase; PAK, p21-activated protein kinase 1 (Pak1); PIP, 1-phosphatidyl-1D-myo-inositol 4-phosphate; PIP2, 1-phosphatidyl-1D-myo-inositol 4,5-bisphosphate; PIP5K, 1-phosphatidylinositol-4-phosphate 5-kinase; Rac, ras-related C3 botulinum toxin substrate 1 (Rac1); Rho, ras homolog gene family, member A (RhoA); ROCK, Rho-associated, coiled-coil containing protein kinase 1 (Rock1); WASP, Wiskott–Aldrich syndrome-like (N-WASP); WAVE, WAS protein family.
Fig. 3.
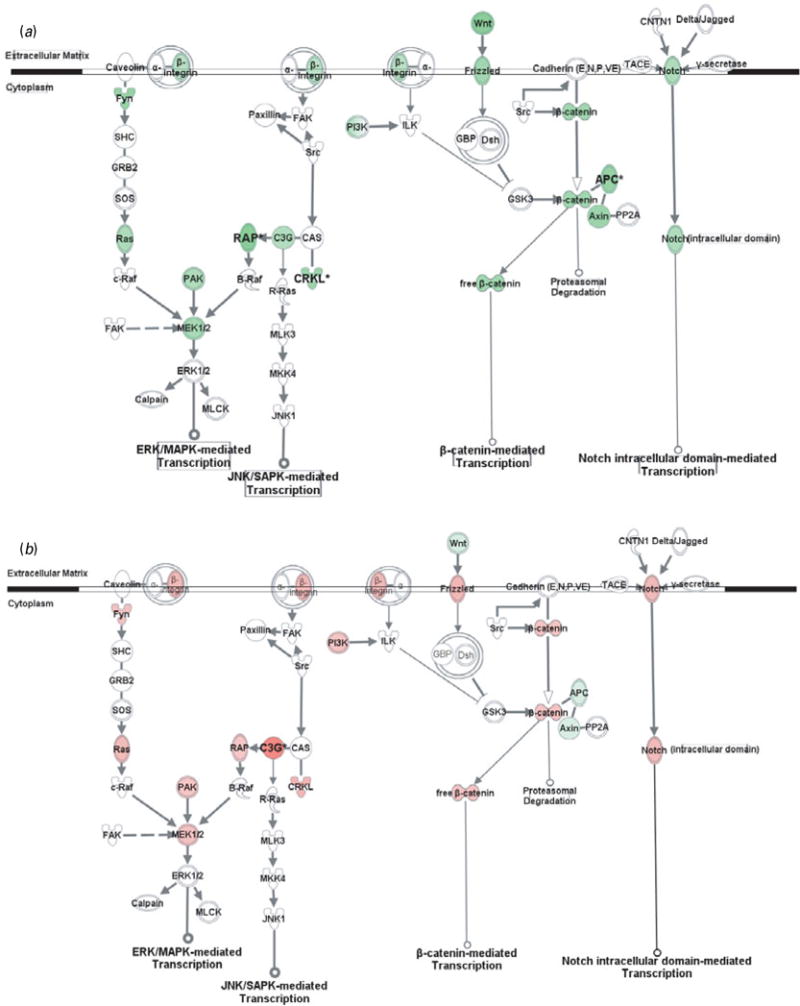
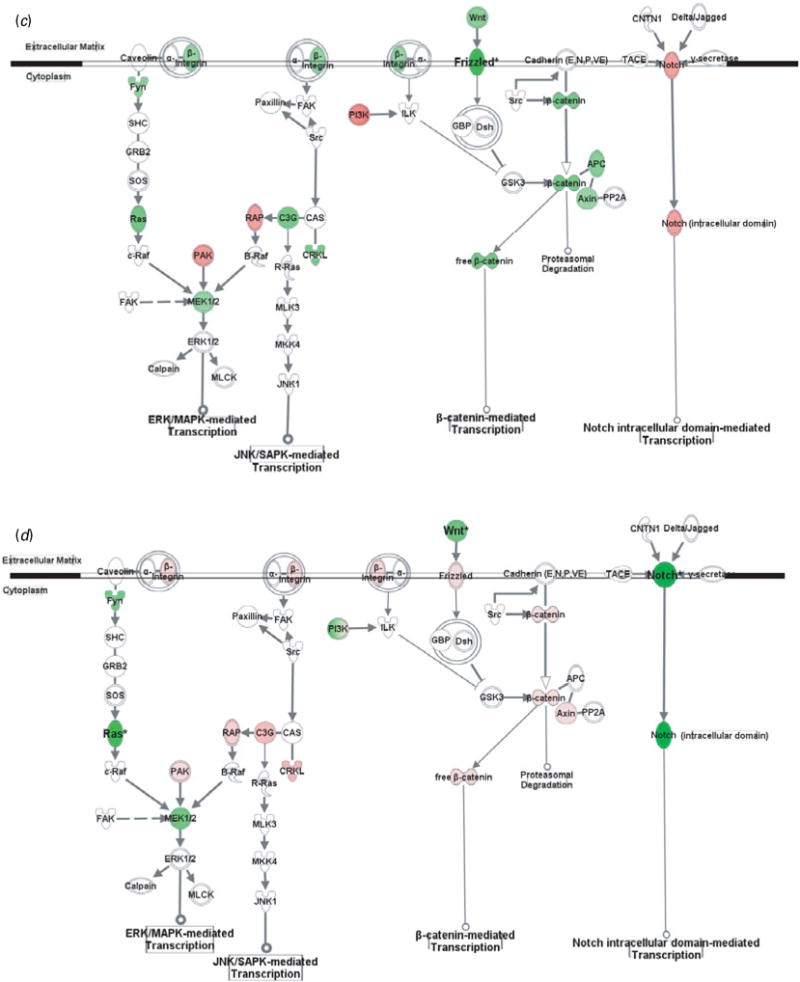
Gene transcription pathways related to cell adhesion molecules. Genes in red were up-regulated whereas those in green were down-regulated at the mRNA level by gestation nicotine exposure in (a) the caudate putamen, (b) nucleus accumbens, (c) prefrontal cortex and (d) amygdala. * Significantly modified compared with gestational saline treatment (p<0.05 at least). APC, adenomatous polyposis coli; Axin, axin 1; B-Raf, v-raf murine sarcoma viral oncogene homolog B1; c-Raf, v-raf-1 murine leukemia viral oncogene homolog 1; C3G, Rap guanine nucleotide exchange factor 1; Calpain, M calpain; CAS, breast cancer anti-estrogen resistance 1; CNTN1, contactin 1; CRKL, v-crk sarcoma virus CT10 oncogene homolog (avian)-like; Delta/Jagged, Notch ligand delta or jagged; Dsh, disheveled; FAK, PTK2 protein tyrosine kinase 2; Frizzled, frizzled homolog 1 (Drosophila); Fyn, FYN oncogene related to SRC, FGR, YES; GBP, frequently rearranged in advanced T-cell lymphomas; GRB2, growth factor receptor-bound protein 2; GSK3, glycogen synthase kinase 3; ILK, integrin-linked kinase; JNK1, mitogen-activated protein kinase 8; MKK4, mitogen-activated protein kinase kinase 4; MLCK, myosin light chain kinase; MLK3, mitogen-activated protein kinase kinase kinase 11; Notch, Notch gene homolog 1 (Notch 1); PAK, p21-activated protein kinase 1 (Pak1); PI3K, phosphatidylinositol 3-kinase; PP2A, protein phosphatase type 2a; R-Ras, related RAS viral (r-ras) oncogene homolog; RAP, RAS-related protein 1a; Ras, Harvey rat sarcoma viral (v-Ha-ras) oncogene homolog; SHC, SHC (Src homology 2 domain containing) transforming protein 1; SOS, son of sevenless homolog; Src, v-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (avian); TACE, ADAM metallopeptidase domain 17; Wnt, wingless-type MMTV integration site family, member 1 (Wnt1); β-catenin, cadherin-associated protein, beta.
Actin cytoskeleton modified by Rho small GTPases
Rho small GTPase-related pathways were modified by GN in all brain regions examined, and one of the principal functions of Rho small GTPases is to modulate the actin cytoskeleton. We therefore examined cytoskeleton reorganization using Rho small GTPase-related pathways as an example. Our data showed that GN modified key genes in these pathways, which implies a critical effect of GN on cytoskeleton reorganization (Fig. 2).
In the CPu, all of the genes examined were down-regulated with significance for Cdc42 (0.61±0.07, p=2.5×10−3), Rho family member A (RhoA) (0.61±0.06, p=3.3×10−3), abl-interactor 2 (Abl2) (0.58±0.16, p=0.034), and subunits of actin-related protein complex (Arp2/3). Although most genes also showed a trend for down-regulation in the NAc, Rac1, p21-activated protein kinase 1 (Pak1), and Rho-associated, coiled-coil-containing protein kinase 1 (Rock1) showed a trend for up-regulation with significance for 1-phosphatidylinositol-4-phosphate 5– kinase (Pip5k) (1.34±0.05, p=4.2×10−4). In contrast, most genes in the PFC were up-regulated with significance for Rac1 (1.52±0.47, p=0.033), WAS protein family, Wave3 (1.36±0.11, p=7.1×10−3), and ARP complex. In the Amy, Wiskott–Aldrich syndrome-like (N-Wasp) was significantly up-regulated (2.84±0.30; p=0.010) whereas Rock1 was down-regulated (0.59± 0.21, p=0.047).
The Wasp/Arp2/3 complex is an important downstream effector of Rho small GTPases that plays a critical role in actin branching and extension, and probably serves important roles in neurite extension and dendritic spine formation (Takenawa & Suetsugu, 2007). We selectively examined N-Wasp, Wave2, and Wave3 in the Wasp family and Arp subunits (Arpc1b, Arpc3, Arpc4, Actr2, Actr3). These genes were significantly modified by GN in a region-dependent way (Table 3). N-Wasp expression was dramatically decreased 94% by GN treatment in the NAc (p=2.7×10−3) but increased 2.84-fold in the Amy (p=0.010). Wave3 was 1.36-fold up-regulated only in the PFC (p=7.1×10−3). In contrast, Wave2 did not show significant change in any brain region. For the Arp2/3 complex, each subunit with the exception of Actr3 was significantly changed in at least one brain region.
Table 3.
Gestational nicotine exposure-modified gene expression in Wiskott–Aldrich syndrome protein family (WASP) and actin-related protein 2/3 complex (ARP2/3)
| Gene symbol | Gene name | Fold change (mean±S.D.)
|
||||
|---|---|---|---|---|---|---|
| CPu | NAc | PFC | Amy | |||
| WASP | Nwasp | Wiskott–Aldrich syndrome-like (human) | 0.47±0.05 | 0.06±0.02** | 0.94±0.32 | 2.84±0.30* |
| Wave2 | WAS protein family, member 2 | 0.78±0.28 | 0.85±0.35 | 1.01±0.13 | 0.95±0.02 | |
| Wave3 | WAS protein family, member 3 | 0.81±0.32 | 0.86±0.24 | 1.36±0.11** | 1.22±0.26 | |
| ARP2/3 | Arpc1b | Actin related protein 2/3 complex, subunit 1B | 0.69±0.06* | 0.9±0.41 | 1.27±0.13* | 1.25±0.09 |
| Arpc3 | Actin related protein 2/3 complex, subunit 3 | 0.62±0.02*** | 0.82±0.02 | 1.06±0.03* | 0.86±0.17 | |
| Arpc4 | Actin related protein 2/3 complex, subunit 4 | 0.65±0.18 | 0.62±0.05** | 1.11±0.08 | 0.95±0.23 | |
| Actr2 | ARP2 actin-related protein 2 homolog (yeast) | 0.34±0.24* | 0.72±0.19 | 1.02±0.02 | 0.9±0.19 | |
| Actr3 | ARP3 actin-related protein 3 homolog (yeast) | 0.66±0.17 | 0.68±0.05 | 0.8±0.23 | 0.26±0.19 | |
CPu, Caudate putamen; NAc, nucleus accumbens; PFC, prefrontal cortex; Amy, amygdala.
The effect of gestational nicotine exposure on mRNA expression of Wiskott–Aldrich syndrome protein family and actin-related protein 2/3 complex. Data are from PCR array.
p<0.05,
p<0.01,
p<0.001 significantly modified by gestational nicotine exposure compared with gestational saline-treated animals.
CAM-related gene transcription pathways
In addition to modulating cytoskeleton reorganization, CAMs regulate gene expression via various intracellular pathways. Although CAMs also interact with growth factor receptors and GPCRs, and subsequently modulate gene transcription, we showed only pathways directly related to CAMs, including MAPK-mediated, β-catenin-mediated, and Notch-mediated transcription (Fig. 2).
Genes in the CPu showed at least a trend for down-regulation with significance for RAS-related protein 1a (Rap1a; 0.52±0.18, p=0.023), v-crk sarcoma virus CT10 oncogene homolog (avian)-like (Crkl; 0.67±0.11, p=9.0×10−3), and adenomatous polyposis coli (Apc; 0.64±0.07, p=3.9×10−3). In contrast, most genes in the NAc showed a trend for up-regulation, with significance for Rap guanine nucleotide exchange factor 1 (C3g; 2.31±0.17, p=0.014). Frizzled homolog 1 was significantly down-regulated in the PFC (Frizzled; 0.57±0.18, p=0.030), whereas wingless-type MMTV integration site family, member 1 (Wnt1; 0.70±0.02, p=0.011) and gene homolog 1 (Notch 1; 0.55±0.04, p=1.9×10−3) were significantly down-regulated in the Amy.
Discussion
These data suggest broad effects of GN on the cell adhesion system which modified genes in the neurexin, immunoglobulin, cadherin, and adhesion GPCR superfamilies in four limbic regions. In addition, GN indirectly regulates the integrin system by altering periostin, an extracellular integrin binding partner (Kudo et al. 2007), as well as the intracellular anchoring proteins actinin and vinculin.
Our data also suggest that critical CAM downstream pathways were significantly altered by GN. Although these pathways are highly interconnected and have complicated intracellular functions, CAM signal transduction generally causes cytoskeleton reorganization and gene transcription. GN modified more genes in cytoskeleton reorganization-related pathways than in gene transcription-related pathways, suggesting enhanced interaction of GN with the CAM system in cytoskeleton reorganization. Since the CAM-related genes were evaluated only at the mRNA level, future studies will be needed to assess their regulation at the protein level.
The regional heterogeneity of GN-induced alterations in CAM gene expression and their related pathways within the limbic system is striking. Much is known regarding the roles of the CPu, NAc, PFC, and Amy in the neural circuitry implicated in neurobehavioural disorders. The present data provide compelling evidence for regionally selective vulnerability to GN in the adolescent limbic system, with important implications for the aetiology of MS-linked deficits. On the other hand, given that these brain regions closely interact with each other, alterations in one brain region may also indirectly change the functions of others, leading to abnormal functions of the whole limbic system.
CPu
GN modified more genes in the CPu than in any other region. Remarkably, all affected genes were down-regulated, suggesting that GN negatively regulates CAMs in this region and that the CPu may be particularly vulnerable to the effects of GN.
The CPu regulates motor control, procedural learning, and memory (Herrero et al. 2002; Squire et al. 1993), and aberrant CPu processing has been linked to psychiatric disorders such as ADHD (Vaidya & Stollstorff, 2008), autism (Stanfield et al. 2008), and addiction (Hyman et al. 2006). Several CAMs down-regulated by GN in this region have been implicated in these same disorders. For example, Cdh13 and Ctnna contain clusters of single nucleotide polymorphisms (SNPs) associated with ADHD (Lesch et al. 2008). Moreover, both Nlgn1 down-regulation and loss of function of Cntn4 have been linked to autism (Roohi et al. 2009; Ylisaukko-oja et al. 2005). GN effects on the CPu may relate to the link between MS and ADHD and autism (Hultman et al. 2002; Linnet et al. 2003).
Many of the altered CAMs in the CPu have been associated with addiction, including Bai3, Lphn3, and Csmd1, whose mechanisms are not known (Liu et al. 2006). Further inquiry into the molecular function of these genes in addiction-related regions is needed. In addition, catenin β -like 1 (CTNNBL1) is associated with obesity (Liu et al. 2006). Thus, altered CAM gene expression in the CPu may cause abnormal sensitivity to natural reward and vulnerability to addiction. Indeed, GN-treated adolescent rats exhibit abnormal responses to food and addictive drugs (Franke et al. 2007, 2008; Levin et al. 2006). Data from this model are consistent with clinical studies linking MS to obesity and addiction in the offspring (Kandel et al. 1994; Oken et al. 2008).
Animal studies of CAM function provide a more mechanistic framework for understanding how CAM alterations contribute to the behavioural and neurochemical phenotypes in the GN model. Many of the CAMs down-regulated by GN in the adolescent CPu are crucial for excitatory synaptic morphology and function, and their interaction with modulatory neurotransmitter systems. For example, catenins promote formation of dendritic spines and excitatory synapses (Arikkath, 2009). Neuroligin 1 (Nlgn 1), located mainly at glutamatergic synapses, modulates synaptic assembly (Graf et al. 2004; Nam & Chen, 2005) and glutamate release (Futai et al. 2007). Both Dscam and actinin contribute to synaptic plasticity by recruitment and clustering of glutamate receptors (Cabello et al. 2007; Li et al. 2009; Schulz et al. 2004). Thus, reduction of these transcripts in the GN-treated CPu may alter dendritic spines and excitatory synapses. Fyn, a protein tyrosine kinase, is particularly important in glutamate-dopamine cross-talk, modulating redistribution of NMDA receptor in a D1 receptor-dependent way (Dunah et al. 2004). Behavioural testing of GN-treated adolescent animals suggests that glutamate– dopamine interactions are altered, as GN-treated, but not normal, adolescent animals exhibit behavioural sensitization to cocaine (Franke et al. 2007).
Pathway analysis also confirmed that GN modulates CAM-related pathways in the CPu. GN decreased Rho GTPase-related pathways and reduced expression of ARP2/3, a complex that regulates dendritic spine and excitatory synapse formation (Wegner et al. 2008). These data further suggest that GN alters excitatory synapse formation in the CPu.
NAc
The NAc, involved in motivational control and reward (Ikemoto, 2007), showed few GN-induced alterations in CAMs. Given that this region is regulated by inputs from both the PFC and the Amy (Berendse et al. 1992; Kelley et al. 1982), NAc may be indirectly influenced by GN-induced alterations in other limbic structures.
Pathway analysis showed that several pathways, including GPCR-related, growth factor signalling, and Notch and Wnt/Frizzled, were down-regulated by GN. These alterations suggest that CAM-initiated signal transduction is modified by GN in the NAc in spite of normal CAM transcript. As with the CPu, GN down-regulated Rho small GTPase-related pathways, specifically reducing expression of the Nwasp transcript, a brain-specific regulator of the ARP2/3 complex (Wegner et al. 2008). Loss of function of Nwasp significantly decreases dendritic spine density and the number of excitatory synapses (Wegner et al. 2008). Thus, GN may reduce excitatory synapses in the ventral striatum while compromising structural and functional aspects of glutamatergic signalling in the dorsal striatum.
PFC
The PFC serves an executive and decision-making role (Arnsten, 1997; Osada et al. 2008) and regulates limbic system activity via projections to the CPu, NAc, and Amy (Berendse et al. 1992). Some of the changes in CAMs in the PFC were similar to those of striatal regions, including down-regulation of Dscam and contactin 4. The contactin system not only regulates neuronal interactions but also contributes to axonal myelination (Boyle et al. 2001; Tait et al. 2000). Given that the adolescent PFC matures substantially with myelination-induced increases in white matter (Huttenlocher, 1979; Sowell et al. 2001), down-regulation of contactins by GN might disturb normal development. Neurexin 3 (Nrxn3), down-regulated only in the PFC, has been linked to alcohol, nicotine, and opiate addiction (Bierut et al. 2007; Hishimoto et al. 2007; Lachman et al. 2007; Li & Burmeister, 2009). Animal studies suggest that Nrxn3 plays a preferential role in GABAergic synapse formation and function (Craig & Kang, 2007). During adolescence, the function and regulation of GABAergic interneurons in the PFC continue to mature (Tseng & O’Donnell, 2007). Given that abnormal myelination and GABA signalling in the PFC is observed in various neuropsychiatric disorders (Feng, 2008; Lewis et al. 1999; Steketee, 2005), reduction of Nrxn3 and contactins may link the cognitive and neurobehavioural disorders (Fergusson et al. 1998; Weissman et al. 1999).
Pathway analysis further revealed GN-induced alterations in the PFC. GN down-regulated pathways related to GPCR, growth factor, MAPK, Notch, and Wnt/Frizzled signalling. During adolescence, the PFC undergoes extensive synaptic pruning, which refines the circuitry to produce adult-like executive function (Spear, 2000). In contrast to striatal regions, GN up-regulated genes in the WASP/ARP2/3 family, which may reflect resistance to excitatory pruning. This idea is supported by our observed finding of a decrease in cell death pathways in the PFC of GN-treated adolescents (data not shown).
Amy
The Amy is an important mediator of the stress response, fear and anxiety-like behaviour, and emotional learning (Herman et al. 1996; Koob, 1999), and provides input to the PFC and NAc (Cunningham et al. 2002; Kelley et al. 1982). GN altered a unique set of CAMs in the Amy. Ncam1, down-regulated nearly 50%, and plays a particularly important role in emotional behaviour, with its genetic deletion impairing Amy-dependent fear conditioning (Stork et al. 2000). GN reduction of Ncam1 suggests that emotional behaviours are altered in this model, which could provide a mechanism for the mood disorders linked to MS (Fergusson et al. 1998; Weissman et al. 1999). Recently, enhancement of fear conditioning has been reported in mice after maternal nicotine consumption in drinking water (Paz et al. 2007).
GN also caused alterations in CAM-related pathways in the Amy, with mixed effects on signal transduction pathways. Similar to the PFC, some members of the WASP/ARP2/3 family were up-regulated, suggesting positive regulation of spine formation. The Amy also undergoes significant synaptic pruning in adolescence (Zehr et al. 2006), which may be altered by GN treatment.
Conclusions
The present study has suggested that CAMs and their intracellular signal transduction pathways are modified in GN-treated adolescent female rats, although the genes were only examined at the mRNA level. Importantly, these changes are region-specific in the limbic system, which provide a novel framework for viewing GN-induced alterations at the neuro-chemical and behavioural levels (Fig. 4). Specifically, in striatal regions, CAMs related to glutamate synapse structure and function are down-regulated, with the CPu showing the greatest vulnerability. Conversely, in the PFC, CAMs related to GABAergic synapse formation appear to be compromised, while pruning of excitatory synapses are impaired. In both the PFC and the CPu, CAMs related to myelination are also down-regulated, suggesting a defect in glia–neuron interactions. In the Amy, CAMs related to emotional learning and memory are altered, and synaptic pruning of excitatory synapses may also be modified.
Fig. 4.
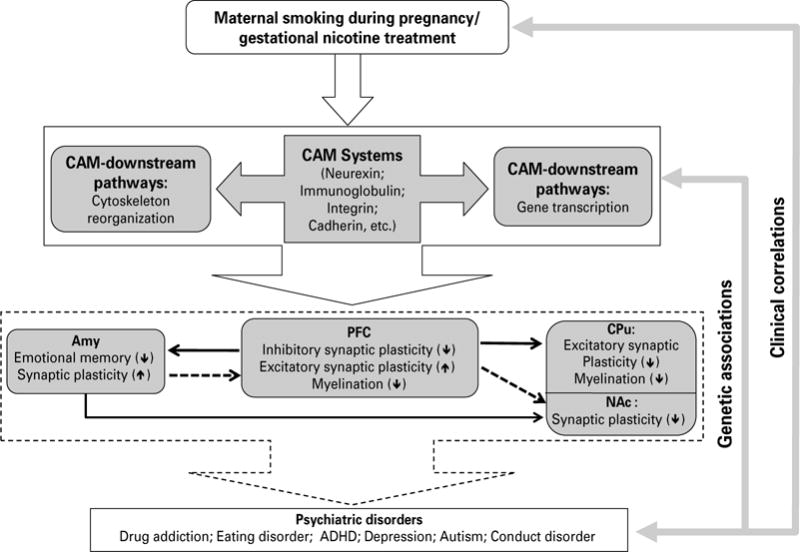
A proposed neuronal mechanism underlying the neurobehavioural effects of maternal smoking during pregnancy on the adolescent offspring via cell adhesion systems. Animal model of gestational nicotine treatment (GN), which mimics human maternal smoking during pregnancy, showed significant alterations of cell adhesion molecules (CAMs), and CAM downstream signalling pathways, including cytoskeleton reorganization-related and transcription-related pathways. The effects of GN were heterogeneous among the limbic brain regions examined. Given that CAM systems play important roles in synaptic plasticity and myelination, the potential effects of altered CAM systems on the prefrontal cortex (PFC), amygdala (Amy), caudate putamen (CPu), and nucleus accumbens (NAc) were hypothesized. The dotted lines between each brain region indicate connections actively maturing in adolescence and solid lines are connections thought to be mature by adolescence. Up- or down-regulation on each neuronal process is shown by ↑ or ↓, respectively. Given the obvious neurobehavioural consequences of maternal smoking, and strong genetic associations between CAM-related genes and neuropsychiatric disorders, the alterations of CAM systems by GN in the limbic brain regions suggest a new mechanism underling MS-linked neurobehavioural deficits.
This circuitry has been highly implicated in the MS-linked neurobehavioural disorders that are observed clinically in adolescents. The late onset of these deficits probably relates to the substantial maturation of the limbic system during adolescence. Alterations of CAMs at this critical age may disturb the development of the limbic system and therefore suggest a neuronal mechanism underlying MS-linked psychiatric disorders. Further, the present study underscores that low doses of nicotine produce substantial and long-lasting changes in the brain, suggesting that nicotine replacement therapy during pregnancy may carry many of the same risks to the offspring as MS.
Supplementary Material
Acknowledgments
This project was in part supported by NIH grant DA-13783 to Ming D. Li and DA-10612 to Frances M. Leslie. The authors thank Dr David L. Bronson for his excellent editing of this manuscript. The authors also thank Celina Mojica and Susan McQuown for their technical assistance.
Footnotes
Note
Supplementary material accompanies this paper on the Journal’s website (http://journals.cambridge.org/pnp). (For an example of the melt curves for four representative genes relative to that for GAPDH, see Supplementary Figure S1, available online.)
Statement of Interest
None.
References
- Arikkath J. Regulation of dendrite and spine morphogenesis and plasticity by catenins. Molecular Neurobiology. 2009;40:46–54. doi: 10.1007/s12035-009-8068-x. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine regulation of the prefrontal cortex. Journal Psychopharmacology. 1997;11:151–162. doi: 10.1177/026988119701100208. [DOI] [PubMed] [Google Scholar]
- Batty GD, Der G, Deary IJ. Effect of maternal smoking during pregnancy on offspring’s cognitive ability: empirical evidence for complete confounding in the US national longitudinal survey of youth. Pediatrics. 2006;118:943–950. doi: 10.1542/peds.2006-0168. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., III Daily intake of nicotine during cigarette smoking. Clinical Pharmacology & Therapeutics. 1984;35:499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. Journal of Comparative Neurology. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Madden PA, Breslau N, Johnson EO, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Human Molecular Genetics. 2007;16:24–35. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnadottir TK, Fredriksson R, Schioth HB. The adhesion GPCRs: a unique family of G protein-coupled receptors with important roles in both central and peripheral tissues. Cellular Molecular Life Sciences. 2007;64:2104–2119. doi: 10.1007/s00018-007-7067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle ME, Berglund EO, Murai KK, Weber L, et al. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- Cabello N, Remelli R, Canela L, Soriguera A, et al. Actin-binding protein alpha-actinin-1 interacts with the metabotropic glutamate receptor type 5b and modulates the cell surface expression and function of the receptor. Journal of Biological Chemistry. 2007;282:12143–12153. doi: 10.1074/jbc.M608880200. [DOI] [PubMed] [Google Scholar]
- Chen H, Parker SL, Matta SG, Sharp BM. Gestational nicotine exposure reduces nicotinic cholinergic receptor (nAChR) expression in dopaminergic brain regions of adolescent rats. European Journal of Neuroscience. 2005;22:380–388. doi: 10.1111/j.1460-9568.2005.04229.x. [DOI] [PubMed] [Google Scholar]
- Craig AM, Kang Y. Neurexin-neuroligin signaling in synapse development. Current Opinion in Neurobiology. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. Journal of Comparative Neurology. 2002;453:116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure & Function. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Sirianni AC, Fienberg AA, Bastia E, et al. Dopamine D1-dependent trafficking of striatal N-methyl-D-aspartate glutamate receptors requires Fyn protein tyrosine kinase but not DARPP-32. Molecular Pharmacology. 2004;65:121–129. doi: 10.1124/mol.65.1.121. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Aggazzotti G, Righi E, Facchinetti F, et al. Preterm delivery and exposure to active and passive smoking during pregnancy: a case-control study from Italy. Paediatric and Perinatal Epidemiology. 2007;21:194–200. doi: 10.1111/j.1365-3016.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. British Journal of Pharmacology. 2008;154:261–274. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. Convergence and divergence in the etiology of myelin impairment in psychiatric disorders and drug addiction. Neurochemical Research. 2008;33:1940–1949. doi: 10.1007/s11064-008-9693-x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Woodward LJ, Horwood LJ. Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Archives of General Psychiatry. 1998;55:721–727. doi: 10.1001/archpsyc.55.8.721. [DOI] [PubMed] [Google Scholar]
- Franke RM, Belluzzi JD, Leslie FM. Gestational exposure to nicotine and monoamine oxidase inhibitors influences cocaine-induced locomotion in adolescent rats. Psychopharmacology (Berlin) 2007;195:117–124. doi: 10.1007/s00213-007-0876-y. [DOI] [PubMed] [Google Scholar]
- Franke RM, Park M, Belluzzi JD, Leslie FM. Prenatal nicotine exposure changes natural and drug-induced reinforcement in adolescent male rats. European Journal of Neuroscience. 2008;27:2952–2961. doi: 10.1111/j.1460-9568.2008.06253.x. [DOI] [PubMed] [Google Scholar]
- Futai K, Kim MJ, Hashikawa T, Scheiffele P, et al. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nature Neuroscience. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, et al. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutala R, Wang J, Kadapakkam S, Hwang Y, et al. Microarray analysis of ethanol-treated cortical neurons reveals disruption of genes related to the ubiquitin-proteasome pathway and protein synthesis. Alcoholism: Clinical and Experimental Research. 2004;28:1779–1788. doi: 10.1097/01.alc.0000148117.17707.b4. [DOI] [PubMed] [Google Scholar]
- Herman JP, Prewitt CM, Cullinan WE. Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Critical Reviews in Neurobiology. 1996;10:371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50. [DOI] [PubMed] [Google Scholar]
- Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Child’s Nervous System. 2002;18:386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Hishimoto A, Liu QR, Drgon T, Pletnikova O, et al. Neurexin 3 polymorphisms are associated with alcohol dependence and altered expression of specific isoforms. Human Molecular Genetics. 2007;16:2880–2891. doi: 10.1093/hmg/ddm247. [DOI] [PubMed] [Google Scholar]
- Hu QD, Ma QH, Gennarini G, Xiao ZC. Cross-talk between F3/contactin and Notch at axoglial interface: a role in oligodendrocyte development. Developmental Neuroscience. 2006;28:25–33. doi: 10.1159/000090750. [DOI] [PubMed] [Google Scholar]
- Hulley P, Schachner M, Lubbert H. L1 neural cell adhesion molecule is a survival factor for fetal dopaminergic neurons. Journal of Neuroscience Research. 1998;53:129–134. doi: 10.1002/(SICI)1097-4547(19980715)53:2<129::AID-JNR1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Hultman CM, Sparen P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology. 2002;13:417–423. doi: 10.1097/00001648-200207000-00009. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex – developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Research Reviews. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indredavik MS, Brubakk AM, Romundstad P, Vik T. Prenatal smoking exposure and psychiatric symptoms in adolescence. Acta Paediatrica. 2007;96:377–382. doi: 10.1111/j.1651-2227.2006.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annual Review of Pharmacology and Toxicology. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- Kandel DB, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. American Journal of Public Health. 1994;84:1407–1413. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane VB, Fu Y, Matta SG, Sharp BM. Gestational nicotine exposure attenuates nicotine-stimulated dopamine release in the nucleus accumbens shell of adolescent Lewis rats. Journal of Pharmacology and Experimental Therapeutics. 2004;308:521–528. doi: 10.1124/jpet.103.059899. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJ. The amygdalostriatal projection in the rat – an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konu O, Xu X, Ma JZ, Kane J, et al. Application of a customized pathway-focused microarray for gene expression profiling of cellular homeostasis upon exposure to nicotine in PC12 cells. Brain Research. Molecular Brain Research. 2004;121:102–113. doi: 10.1016/j.molbrainres.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Annals of the New York Academy of Sciences. 1999;877:445–460. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Siriwardena BS, Hatano H, Ogawa I, et al. Periostin: novel diagnostic and therapeutic target for cancer. Histology and Histopathology. 2007;22:1167–1174. doi: 10.14670/HH-22.1167. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Fann CS, Bartzis M, Evgrafov OV, et al. Genomewide suggestive linkage of opioid dependence to chromosome 14q. Human Molecular Genetics. 2007;16:1327–1334. doi: 10.1093/hmg/ddm081. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Timmesfeld N, Renner TJ, Halperin R, et al. Molecular genetics of adult ADHD: converging evidence from genome-wide association and extended pedigree linkage studies. Journal of Neural Transmission. 2008;115:1573–1585. doi: 10.1007/s00702-008-0119-3. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence S, Petro A, Horton K, et al. Increased nicotine self-administration following prenatal exposure in female rats. Pharmacology, Biochemistry and Behavior. 2006;85:669–674. doi: 10.1016/j.pbb.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, et al. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biological Psychiatry. 1999;46:616–626. doi: 10.1016/s0006-3223(99)00061-x. [DOI] [PubMed] [Google Scholar]
- Li HL, Huang BS, Vishwasrao H, Sutedja N, et al. Dscam mediates remodeling of glutamate receptors in Aplysia during de novo and learning-related synapse formation. Neuron. 2009;61:527–540. doi: 10.1016/j.neuron.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Burmeister M. New insights into the genetics of addiction. Nature Reviews Genetics. 2009;10:225–231. doi: 10.1038/nrg2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Kane JK, Wang J, Ma JZ. Time-dependent changes in transcriptional profiles within five rat brain regions in response to nicotine treatment. Brain Research. Molecular Brain Research. 2004;132:168–180. doi: 10.1016/j.molbrainres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. American Journal of Psychiatry. 2003;160:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- Liu QR, Drgon T, Johnson C, Walther D, et al. Addiction molecular genetics: 639,401 SNP whole genome association identifies many ‘cell adhesion’ genes. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics. 2006;141B:918–925. doi: 10.1002/ajmg.b.30436. [DOI] [PubMed] [Google Scholar]
- Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nature Neuroscience. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- Matta SG, Elberger AJ. Combined exposure to nicotine and ethanol throughout full gestation results in enhanced acquisition of nicotine self-administration in young adult rat offspring. Psychopharmacology (Berlin) 2007;193:199–213. doi: 10.1007/s00213-007-0767-2. [DOI] [PubMed] [Google Scholar]
- Nam CI, Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proceedings of the National Academy of Sciences USA. 2005;102:6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. International Journal of Obesity (London) 2008;32:201–210. doi: 10.1038/sj.ijo.0803760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds DL, Henderson CR, Jr, Tatelbaum R. Intellectual impairment in children of women who smoke cigarettes during pregnancy. Pediatrics. 1994;93:221–227. [PubMed] [Google Scholar]
- Osada T, Adachi Y, Kimura HM, Miyashita Y. Towards understanding of the cortical network underlying associative memory. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 2008;363:2187–2199. doi: 10.1098/rstb.2008.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Loughlin SE, Leslie FM. Gestational nicotine-induced changes in adolescent neuronal activity. Brain Research. 2006;1094:119–126. doi: 10.1016/j.brainres.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Paz R, Barsness B, Martenson T, Tanner D, et al. Behavioral teratogenicity induced by nonforced maternal nicotine consumption. Neuropsychopharmacology. 2007;32:693–699. doi: 10.1038/sj.npp.1301066. [DOI] [PubMed] [Google Scholar]
- Rogers JM. Tobacco and pregnancy: overview of exposures and effects. Birth Defects Research, Part C: Embryo Today. 2008;84:1–15. doi: 10.1002/bdrc.20119. [DOI] [PubMed] [Google Scholar]
- Roohi J, Montagna C, Tegay DH, Palmer LE, et al. Disruption of contactin 4 in three subjects with autism spectrum disorder. Journal of Medical Genetics. 2009;46:176–182. doi: 10.1136/jmg.2008.057505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujescu D, Ingason A, Cichon S, Pietilainen OP, et al. Disruption of the neurexin 1 gene is associated with schizophrenia. Human Molecular Genetics. 2009;18:988–996. doi: 10.1093/hmg/ddn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TW, Nakagawa T, Licznerski P, Pawlak V, et al. Actin/alpha-actinin-dependent transport of AMPA receptors in dendritic spines: role of the PDZ-LIM protein RIL. Journal of Neuroscience. 2004;24:8584–8594. doi: 10.1523/JNEUROSCI.2100-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler FJ, Levin ED, Lappi SE, Slotkin TA. Fetal nicotine exposure ablates the ability of postnatal nicotine challenge to release norepinephrine from rat brain regions. Brain Research. Developmental Brain Research. 1992;69:288–291. doi: 10.1016/0165-3806(92)90170-2. [DOI] [PubMed] [Google Scholar]
- Shea AK, Steiner M. Cigarette smoking during pregnancy. Nicotine & Tobacco Research. 2008;10:267–278. doi: 10.1080/14622200701825908. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. Journal of Neuroscience. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Squire LR, Knowlton B, Musen G. The structure and organization of memory. Annual Review of Psychology. 1993;44:453–495. doi: 10.1146/annurev.ps.44.020193.002321. [DOI] [PubMed] [Google Scholar]
- Stanfield AC, McIntosh AM, Spencer MD, Philip R, et al. Towards a neuroanatomy of autism: a systematic review and meta-analysis of structural magnetic resonance imaging studies. European Psychiatry. 2008;23:289–299. doi: 10.1016/j.eurpsy.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Steketee JD. Cortical mechanisms of cocaine sensitization. Critical Reviews in Neurobiology. 2005;17:69–86. doi: 10.1615/critrevneurobiol.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- Stork O, Welzl H, Wolfer D, Schuster T, et al. Recovery of emotional behaviour in neural cell adhesion molecule (NCAM) null mutant mice through transgenic expression of NCAM180. European Journal of Neuroscience. 2000;12:3291–3306. doi: 10.1046/j.1460-9568.2000.00197.x. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait S, Gunn-Moore F, Collinson JM, Huang J, et al. An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axo-glial junction. Journal of Cell Biology. 2000;150:657–666. doi: 10.1083/jcb.150.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nature Reviews. Molecular Cell Biology. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Perry DC. Prenatal nicotine exposure is associated with an increase in [125I]epibatidine binding in discrete cortical regions in rats. Pharmacology Biochemistry and Behavior. 2000;67:319–323. doi: 10.1016/s0091-3057(00)00379-8. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O’Donnell P. Dopamine modulation of prefrontal cortical interneurons changes during adolescence. Cerebral Cortex. 2007;17:1235–1240. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaglenova J, Parameshwaran K, Suppiramaniam V, Breese CR, et al. Long-lasting teratogenic effects of nicotine on cognition: gender specificity and role of AMPA receptor function. Neurobiology of Learning and Memory. 2008;90:527–536. doi: 10.1016/j.nlm.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Stollstorff M. Cognitive neuroscience of Attention Deficit Hyperactivity Disorder: current status and working hypotheses. Developmental Disabilities Research Reviews. 2008;14:261–267. doi: 10.1002/ddrr.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner AM, Nebhan CA, Hu L, Majumdar D, et al. N-wasp and the arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. Journal of Biological Chemistry. 2008;283:15912–15920. doi: 10.1074/jbc.M801555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38:892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- Winer J, Jung CK, Shackel I, Williams PM. Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Analytical Biochemistry. 1999;270:41–49. doi: 10.1006/abio.1999.4085. [DOI] [PubMed] [Google Scholar]
- Xu Z, Seidler FJ, Ali SF, Slikker W, Jr, et al. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Research. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Current Opinion in Cell Biology. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Yang YH, Dudoit S, Luu P, Lin DM, et al. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Research. 2002;30:e15. doi: 10.1093/nar/30.4.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylisaukko-oja T, Rehnstrom K, Auranen M, Vanhala R, et al. Analysis of four neuroligin genes as candidates for autism. European Journal of Human Genetics. 2005;13:1285–1292. doi: 10.1038/sj.ejhg.5201474. [DOI] [PubMed] [Google Scholar]
- Zehr JL, Todd BJ, Schulz KM, McCarthy MM, et al. Dendritic pruning of the medial amygdala during pubertal development of the male Syrian hamster. Journal of Neurobiology. 2006;66:578–590. doi: 10.1002/neu.20251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


