Abstract
OBJECTIVE
To identify risk factors predictive of pulmonary embolus (PE) timing after a traumatic injury.
METHODS
One hundred eight traumatic injury patients with a confirmed diagnosis of PE were classified as early PE (≤4 days, n = 54) or late PE (>4 days, n = 54). Independent predictors of early versus late PE were identified using multivariate logistic regression.
RESULTS
Half the PEs were diagnosed ≤4 days of injury. Only long bone fractures independently predicted early PE (odds ratio 2.8; 95% confidence interval, 1.1–7.1). Severe head injuries were associated with late PE (odds ratio 11.1; 95% confidence interval, 3.9–31). Established risk factors such as age did not affect timing.
CONCLUSIONS
Half the PEs were diagnosed ≤4 days after injury. The risk of early PE appeared highest in patients with long bone fractures, and the benefits of immediate prophylaxis may outweigh risks. Patients with severe head injuries appear to have later PE events. Prospective interventional trials in these injury populations are needed.
Keywords: Pulmonary embolus, Trauma, Injury
Venous thromboembolism is a well-known complication in trauma. Patients with multisystem injuries have a greater than 50% risk of developing deep venous thrombosis (DVT), and pulmonary embolism (PE) is a leading cause of death in those surviving longer than 24 hours.1 These events also increase resource utilization associated with diagnostic imaging, monitoring in intensive care units, and treatment with anticoagulation or placement of inferior vena cava (IVC) filters. This has led to the development of clinical management protocols to prevent PE in trauma patients using a combination of anticoagulants and mechanical prophylaxis.2 The early use of heparin-based chemical prophylaxis is most commonly recommended for the prevention of PE in trauma patients.1,2 However, the use of anticoagulants has been associated with an increased risk of hemorrhagic complications.3–5 This risk is particularly concerning in trauma patients in the first few days after injury, especially those with a traumatic brain injury or those with significant hemorrhage or solid organ injuries managed nonoperatively.6 In patients at high risk of bleeding complications who are also considered high risk for PE, IVC filters may be used to prevent PE.7–9 However, IVC filters have also been associated with several complications such as filter migration, vessel and duodenal perforation, and caval thrombosis.7,8,10–12 In addition, their long-term outcomes are largely unknown. To mitigate some of these concerns, retrievable IVC filters have been used increasingly.7,8,11 However, actual filter retrieval rates remain low.8
Recent data suggest that a significant number of PE occur during the critical first few days after injury.9,13 The increasing number of PE diagnoses in the early postinjury period raises a significant clinical dilemma. On the one hand, it argues for the need to initiate chemical prophylaxis within the first 24 hours to 48 hours after injury. By contrast, the first few days are precisely the time when the risk of bleeding complications from heparin-based chemical prophylaxis is highest. Hence, it is critical to identify patients who are at highest risk of PE in the first few days after injury. In such patients, the benefits of using heparin-based chemical prophylaxis or IVC filters may outweigh the risks associated with their use.
Risk factors for PE in injured patients including older age, lower-extremity and pelvic fractures, head and spinal cord injuries, venous injuries, prolonged immobilization, and recent major operative procedures have been well described in several studies.1,14,15 However, patient or injury characteristics that may predispose patients to early occurrence of PE within the first few days after injury have not been described. The purpose of this study was to identify factors associated with the timing of PE after injury. Such information may be used to guide clinical decision making regarding the use of heparin-based chemical prophylaxis or an IVC filter in the early postinjury period. We hypothesized that a large number of PE in trauma patients occur in the first few days after injury and that factors associated with the early occurrence of PE, compared with those that occur later, could be identified.
Methods
Study design
This is a retrospective analysis of all trauma patients admitted to a high-volume, urban level I trauma center during a 4-year period (July 2003–June 2007, n = 17,736). Only patients with a confirmed diagnosis of PE were considered for inclusion in the study (n = 110). The inclusion criteria required a diagnosis of PE confirmed by any one of the following diagnostic modalities: a contrast-enhanced chest computed tomography (CT) scan, a ventilation/perfusion pulmonary nuclear scan, pulmonary angiography, echocardiography, or a pulmonary vascular thrombus discovered at autopsy. There was no uniform screening protocol in place for PE or DVT at our institution during this time period. Workup was initiated on a case-by-case basis upon clinical suspicion of pulmonary embolus. Exclusion criteria consisted of the absence of radiographic imaging confirmation, clinical documentation or autopsy results consistent with a diagnosis of PE, and time to event (PE) greater than 120 days. Patients with time to PE >120 days were excluded (n = 2) to avoid inclusion of patients more representative of a rehabilitation population than an acute trauma admission. The final study population consisted of 108 patients, all of whom had a confirmed occurrence of PE. Based on the sample median of 4 days, the sample distribution (Fig. 1), and also taking into consideration the timing of postinjury hemorrhagic risks, we divided the population into 2 groups. Early PE was defined as an occurrence of PE ≤4 days after injury. Late PE was defined as >4 days after injury.
Figure 1.
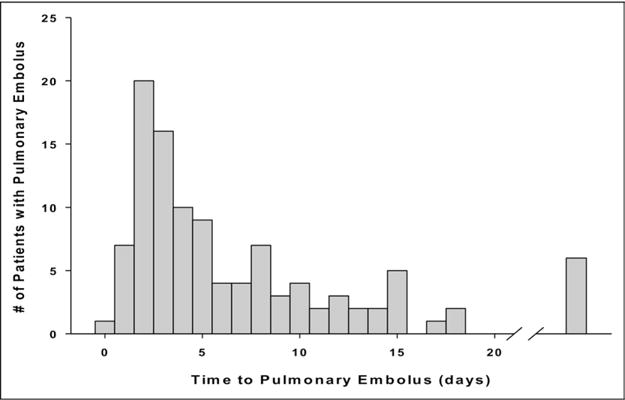
The timing of PE after injury.
Clinical data were obtained from several sources including the trauma registry, pharmacy, radiology, and patient medical databases and consisted of demographics, injury type, injury severity, and survival to discharge. Detailed information was obtained that consisted of timing and method of chemical prophylaxis used, diagnostic modalities, timing of occurrence, presence of known risk factors for PE, and use of IVC filters. Transfusion was defined as the infusion of any blood product including packed red blood cells or fresh frozen plasma within the first 24 hours. Major operative procedures were defined as any intra-abdominal, thoracic, neurosurgical, orthopedic, neck, or vascular procedure requiring general anesthesia. Long bone fractures were defined as fractures involving the humerus, radius, ulna, femur, or tibia.
Statistical analysis
To identify factors associated with the diagnosis of early PE, within the first 4 days, patients in the early PE group were first compared with those in the late PE group using univariate analysis. Continuous variables were summarized as mean ± standard deviation and compared using the Student t test. Categoric variables were summarized as percentages and compared using the Fisher exact statistic. Next, a multivariate logistic regression analysis was performed by using the backward stepwise approach to identify factors that were independently associated with the occurrence of early compared with late PE. All statistical analyses were performed by using SAS version 9.1 (SAS Institute, Inc, Cary, NC). A P value <.05 was considered statistically significant. Institutional review board approval was obtained before the initiation of the study.
Results
The study population consisted of 108 patients with confirmed diagnoses of PE. The patients were relatively young (mean age 41 ± 17 years), mostly male (76%), and severely injured with a median Injury Severity Score of 18. These demographics are consistent with the overall trauma population at our institution. The mechanism of injury was predominantly blunt (vehicular 70%, falls 18%), with only 10% of patients sustaining penetrating injuries.
Timing and characteristics of PE
The timing of the diagnosis of PE was skewed, with one quarter of all PEs diagnosed within 72 hours after injury and half within the first 4 days (Fig. 1). Ninety-five percent of emboli were diagnosed within 20 days of injury. The 6 outliers beyond 20 days were diagnosed between days 24 and 74. All but 10 patients (90%) had documented clinical signs or symptoms concerning for PE at the time of diagnosis. Hypoxia (53%) was the most common indication for diagnostic workup, whereas a contrast-enhanced CT scan (90%) was the primary diagnostic modality used (Table 1). Almost all PEs appeared clinically significant because over 90% of patients were symptomatic, whereas over 75% of all emboli were lodged within a major vessel in the pulmonary circulation (ie, main pulmonary artery, lobar, or a segmental branch) (Table 1). Three PEs were detected incidentally during other studies, and 1 was detected via echocardiography in the operating room in a patient with catastrophic acute right-heart cardiac failure during an orthopedic procedure. This was the only fatality in this series. A review of reports from the trauma registry revealed no PEs were diagnosed solely on autopsy findings.
Table 1.
Diagnosis and location of pulmonary emboli
| N | % | |
|---|---|---|
| Indication | ||
| Hypoxia | 57 | 53 |
| Shortness of breath | 26 | 24 |
| Tachycardia | 10 | 9 |
| Tachypnea | 2 | 2 |
| Chest pain | 2 | 2 |
| Unknown | 8 | 7 |
| Incidental | 3 | 3 |
| Diagnostic modality | ||
| CT angiography—chest | 97 | 90 |
| Ventilation-perfusion scan | 5 | 5 |
| Pulmonary angiography | 2 | 2 |
| CT abdomen/pelvis | 2 | 2 |
| CT aortogram | 1 | 1 |
| Echocardiography | 1 | 1 |
| Location | ||
| Main pulmonary artery | 3 | 3 |
| Lobar | 28 | 26 |
| Segmental | 45 | 42 |
| Subsegmental | 26 | 24 |
| Unclassified | 6 | 6 |
| Total pulmonary emboli | 108 |
Thromboembolic prophylaxis
Thromboembolic prophylaxis was initiated in 80 (74%) patients before the occurrence of PE. A subcutaneous chemical prophylaxis was used in 75 (69%) patients. Of these, unfractionated heparin was administered in 61, whereas 14 patients received low–molecular-weight heparin. Sixty-two patients (57%) had a delay in the initiation of chemical prophylaxis greater than 24 hours. IVC filters were placed in 57 (53%) patients in this study. Of these, 52 patients received an IVC filter after the diagnosis of PE. In 5 patients, IVC filters were placed before the diagnosis of PE. These filters were placed between 3 days and 7 days after injury, and all 5 patients suffered a late PE. It was not clear from the data if this was caused by a late occurrence or a late diagnosis of PE. We did not find a relationship with either the type of chemical prophylaxis administered or the placement of IVC filter before diagnosis with the timing of occurrence of the PE.
Factors associated with early versus late occurrence of PE
As described earlier, the patients were classified as having early versus late PE based on the defined cutoff of 4 days after injury. Univariate analysis identified several significant differences between the 2 groups (Table 2). Patients in the early PE group were more likely to have sustained one or more long bone extremity fractures, were more likely to be admitted to a ward and not an intensive care unit, and were more likely to be females. Patients in the late PE group were more severely injured with a higher Injury Severity Score; were more likely to have sustained a severe head injury (Glasgow Coma Score ≤8, Abbreviated Injury Score [AIS] head region ≥3) or a severe chest injury (AIS chest region ≥3); and were less likely to have undergone a major operative procedure within 48 hours of injury. In addition, patients in the late PE group were more likely to have had a delay in initiating chemical prophylaxis beyond the first 24 hours. Cochran-Armitage testing failed to reveal a significant association or trend between the level of occlusion, a surrogate for emboli size, and timing of PE (P = .12). Only 1 patient received recombinant factor VII, and he sustained a late PE.
Table 2.
Univariate risk factor analysis for the timing of PE
| Predictor | Early PE (%) | Late PE (%) | P value |
|---|---|---|---|
| Age (y, mean) | 42.7 | 40.2 | .44 |
| Sex (male) | 29 (35) | 53 (65) | .066 |
| Mechanism (blunt) | 37 (38) | 60 (62) | .12 |
| ISS (median) | 12 | 26 | .001* |
| Hypotension (SBP <90) | 3 (38) | 5 (62) | 1.00 |
| GCS (≤8) | 5 (19) | 21 (81) | .012* |
| ICU admission | 16 (26) | 46 (74) | <.0001* |
| Number units PRBCs transfused <24 h after injury (mean) | 1.1 | 2.1 | .26 |
| No major operative procedure first 48 h after injury | 11 (26) | 31 (74) | .017* |
| AIS head (≥3) | 3 (8) | 36 (92) | <.0001* |
| AIS chest (≥3) | 13 (28) | 33 (72) | .03* |
| AIS abdomen (≥3) | 5 (28) | 13 (72) | .30 |
| AIS extremity (≥3) | 24 (51) | 23 (49) | .075 |
| Lower-extremity AIS (≥3) | 21 (51) | 20 (49) | .11 |
| ≥1 long bone extremity fracture | 26 (54) | 22 (46) | .018* |
| Pelvic fracture | 13 (38) | 21 (62) | .83 |
| Spine fracture | 5 (24) | 16 (76) | .089 |
| Spinal cord injury | 1 (14) | 6 (86) | .24 |
ISS Injury Severity Score; PRBCs packed red blood cells.
Indicates P < 0.05.
Multivariate analysis revealed that the only factor independently associated with early PE was the presence of 1 or more long bone fractures (odds ratio 2.8, 95% confidence interval, 1.1–7.1; P < .03, Table 3). By contrast, the only factor independently associated with late PE was presence of a severe head injury defined as AIS head region ≥3 (odds ratio 11.1; 95% confidence interval, 3.9–31.3; P < .0001, Table 3). Direct comparison of the timing of PE in these 2 injury patterns is shown in Figure 2. Other well-established risk factors for PE including age, major operative procedure, pelvic fracture, and spinal cord injury were not independently associated with the timing of PE in multivariate analysis.
Table 3.
Multivariate risk factor analysis for timing of pulmonary embolus
| Outcome | Risk factor | Odds ratio (95% CI) |
P value |
|---|---|---|---|
| Early PE | ≥1 long bone extremity fracture | 2.83 (1.13–7.10) | .024 |
| Late PE | AIS Head ≥3 | 11.09 (3.93–31.31) | <.0001 |
Early PE defined as ≤4 days after a traumatic injury; late PE defined as >4 days after injury.
Figure 2.
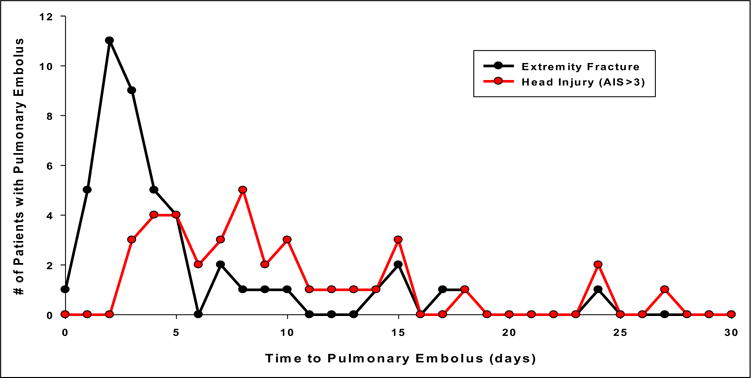
The timing of PE by injury type.
Comments
The findings of this study confirm that pulmonary emboli in trauma patients are occurring early in the postinjury period, with half of them being diagnosed in the first 4 days. This is earlier than has been described previously. Patients with 1 or more long bone fractures who developed PE were most likely to have their occurrence within the first 4 days after injury. In contrast, the timing of PE in patients with severe traumatic brain injury appeared to occur later, after the first 4 days. When these 2 injury populations are directly compared, the early peak in patients with extremity fracture becomes clearly apparent, whereas those with significant head injury occur later (Fig. 2). The risk-benefit of early prophylaxis with heparin to prevent PE versus the risk of worsening intracranial bleeding caused by heparin makes the timing of anticoagulant use controversial. The only alternative prophylactic method is placement of an IVC filter, which carries its own set of risks and long-term outcomes remain uncertain. Our study suggests that if a patient with severe head injury is considered at a significant risk of expansion of intracranial or solid organ bleeding, it may be safe to withhold early placement of an IVC filter and then initiate chemical prophylaxis when the risks of hemorrhagic complications are acceptable.
It has been well established that trauma patients are at a high risk for developing DVT and PE, especially when specific risk factors are present.14,15 However, little attention has been paid to the timing of PE after traumatic injury. Historically, the timing of PE has been described to occur around 7 days to 10 days after traumatic injury.16 In 1997, Owings et al16 reported a median time to PE of 11 days in patients who received pharmacologic prophylaxis and 6 days in those who did not. However, this study used a broad combination of pulmonary angiography, ventilation-perfusion scans, and autopsy findings for diagnosis of PE. In 2006, Sing et al9 reported a mean time to diagnosis of PE of about 8 days after injury using CT scans. Most recently, Menaker et al13 reported a mean time to PE diagnosis of 12 days, with approximately a third diagnosed in the first 4 days. In our patient population, half of all PEs were diagnosed within the first 4 days after injury. However, this earlier diagnosis of PE after injury does not necessarily indicate that PEs are now occurring earlier than before. This shift likely represents a lower clinical threshold for obtaining noninvasive diagnostic imaging with CT scans, which are more sensitive and specific for diagnosis of PE than previously used ventilation-perfusion nuclear scans.17,18
Generally, it has been thought that injuries, especially orthopedic and neurologic, lead to extended periods of immobility, predisposing to stasis, which then leads to venous thrombosis and subsequent embolism. This purely mechanical explanation was consistent with the timing of PE occurring at 7 days to 10 days. However, there is an emerging body of evidence that suggests that other genetic and molecular pathways also play a role in venous thromboembolism after injury. Both hyper- and hypocoagulable states have been described after traumatic injury. Reported risk factors for hypercoagulability include obesity, extreme hypothermia, age >40 years, spine fractures, and lower-extremity fractures.19–21 Recently, Shreiber et al21 reported that a hypercoagulable state is most prevalent in the first 4 days after injury, especially in female patients. The role played by other thrombophilic factors in the development of DVT and PE in trauma patients, such as proteins C and S, factor V Leiden, G20120A, and other prothrombin gene mutations including antiphospholipid antibodies and anticardiolipin antibodies, remains unknown.1,22–24 Our findings suggest there may also be unrecognized molecular mechanisms associated with fractures that predispose patients to an earlier occurrence of PE. Therefore, it is possible that early PE results from an unrecognized interplay between various genetic-, molecular-, and injury-specific mechanisms. Prolonged immobilization may not be a prerequisite for the occurrence of PE in such a scenario.
To our knowledge, this study is the first attempt to identify risk factors predictive of the timing of PE after traumatic injury. The recognition that PE occurs early after injury creates a dilemma about the timing of initiating prophylaxis of DVT and PE using anticoagulants or IVC filters because they both have inherent risks. Although all trauma patients are at a high risk for PE, the ability to identify factors that may predict when a PE is most likely to occur may allow customization of the timing of initiating prophylaxis for individual patients based on their injury profile. In patients at risk of early PE, the benefits of using heparin-based chemical prophylaxis or IVC filters may outweigh the risks associated with their use. Our analysis suggests that the only independent predictor of early PE was the presence of 1 or more long bone fractures. It is possible that some of the molecular and genetic factors listed previously in the presence of long bone fractures predispose patients to the risk for early PE. Other well-established risk factors for PE in injured patients including age, major operative procedure, pelvic fracture, spine fracture, or spinal cord injury did not affect the timing of PE.
One logical theory that offers an explanation for the early occurrence of PE in patients with long bone fracture is fat emboli from an orthopedic injury or the subsequent operative repair. However, radiologic findings on a CT scan of the chest described with fat emboli syndrome typically lack discrete occlusive emboli. Instead, patients with respiratory distress attributed to fat emboli syndrome manifest CT findings similar to acute respiratory distress syndrome with diffuse “ground glass” type infiltrates.25,26 None of our subjects had clinical or radiographic findings consistent with this syndrome. However, in this study, we are unable to definitively characterize the content or origin of the emboli that were identified.
A somewhat surprising finding of this study was that patients with severe head injuries were more likely to have late rather than early PE. Intuitively, it would seem that these severely injured, immobilized patients would be at risk for early thrombotic complications. However, there are several physiological phenomena, including the well-known coagulopathy associated with traumatic brain injury as well as the more recently described “diffuse coagulopathy of trauma,” that give plausible biological explanations as to why these patients have their PE events later.27–30 Patients with moderate to severe traumatic brain injury are a subgroup of patients in whom the clinical dilemma of using heparin-based prophylaxis is most acute because of the risk of worsening the intracranial bleed and its life-threatening complications. Many centers currently place prophylactic IVC filters in these patients within the first few days after traumatic brain injury.8,9,11 It is an expensive, invasive procedure, with well-known complications and unknown long-term consequences. Our findings suggest that patients with severe head injuries, although at a high risk for developing a PE, may not do so in the first 4 days. Hence, the early placement of prophylactic IVC filters in this patient population may not be necessary. Currently, there is no level I evidence to support recommendations regarding the timing of initiation of pharmacological prophylaxis. Several authors, such as Norwood, argue that the initiation of heparin-based prophylaxis within 24 hours to 48 hours may be safe in selected patients with head injuries, further obviating the need for the use of prophylactic IVC filters in this patient population.31,32
Our study has several limitations and raises a few questions that should be addressed. It is a retrospective analysis of existing data that has inherent limitations and biases, and our results should be considered hypothesis generating. The analysis was limited to well-known clinical risk factors for DVT and PE. We did not have information on other molecular and genetic factors that are associated with thrombophilia. We also did not have consistent data on pneumatic compression utilization, patient body mass index, or the presence of central venous catheters in this dataset and were therefore unable to include these in the analysis. Sample size was relatively small with an extremely low case-fatality rate, which is significantly lower than previously published. We believe this is likely resultant from the increasingly aggressive approach toward PE prophylaxis, diagnosis, and treatment.
Another potential issue is that there was no uniform DVT or PE screening mechanism in place at our institution during the study period, and diagnostic studies for PE were undertaken only in patients with signs or symptoms concerning for clinically significant PE. It is possible that late PE in the head-injured population occurred earlier than when diagnosis was confirmed. However, at our institution, we take an extremely vigilant and aggressive diagnostic approach toward potential PE, especially in obtunded, severely injured patients. Patients without clinical symptoms or significant oxygenation/ventilation issues are unlikely to have suffered a clinically significant PE. Therefore, the combination of these factors should have limited the number of potential delayed diagnoses in this population. Three percent of emboli were identified incidentally, and, therefore, the timing of their occurrence is truly unknown. However, post hoc exclusion of these patients did not significantly change the results of the analysis. The relationship between DVT and PE also has come into question. Recently, it has been proposed that some PE may form de novo in the lungs and are separate entities than distal DVT that subsequently embolize.33 Because there was no DVT screening protocol in place, we were unable to have consistent data evaluating the status of extremity or pelvic DVT and their relation to PE incidence or timing in this study population. It is also possible that DVT occurs early in all patients, but the rate of its propagation and subsequent embolization may vary between patients with different types of injuries.
One final issue is the utilization pattern and efficacy of prophylaxis in these 2 injury populations. The utilization of both fractionated and unfractionated heparin in our study population was representative of the transition at our institution to low–molecular-weight heparin usage during the study period. However, as mentioned in the results, our analysis revealed no association between the type of chemical prophylaxis used and the timing of PE after injury. It is concerning that over two thirds of the patients in our study with PE were receiving heparin-based prophylaxis before their event. Although its presence did not prevent the occurrence of emboli, it is possible that chemical prophylaxis contributed to a lower clot burden in these patients, leading to fewer fatal emboli and our subsequent low case-fatality rate. The fact that many early PEs occurred in less severely injured ward patients with fractures who essentially received immediate postinjury chemical prophylaxis suggests that the efficacy of prophylaxis in this specific injury population may need to be readdressed. We were also not able to determine the efficacy of IVC filters in preventing PE because the study population only included patients who developed a PE. It is possible that IVC filters were placed in patients with traumatic brain injury that prevented PE and hence were not included in our study. This could have the effect of biasing the risk toward late PE in the head-injured population. Five patients who had prophylactic IVC filters placed subsequently had PE events, further calling into question the efficacy of prophylactic filters.
In conclusion, the findings of this study may have significant implications on the timing of PE prophylaxis in trauma patients because half of all PEs occurred within the first 4 days after injury. The presence of 1 or more long bone fractures was associated with the occurrence of PEs in the first 4 days. Hence, the aggressive initiation of chemical or filter prophylaxis may outweigh their concurrent risks. Patients with severe traumatic brain injury remain at a high risk, but the timing of the occurrence of PE appears to be day 5 or later. Our findings show the need for prospective interventional trials of chemical and mechanical prophylaxis in these specific injury populations. For example, trials to evaluate the safety and efficacy of low–molecular-weight prophylaxis early after injury in all patients are needed. If found to be safe and effective, the use of early chemical prophylaxis may render moot the controversy over whether or not IVC filters should be placed in these high-risk patients. For the subset of patients with severe multisystem injuries at risk of bleeding complications with heparin-based prophylaxis, randomized trials of early use of prophylactic IVC filters are needed.
Acknowledgments
Supported by NCRR-NIH Grant Number UL1RR024982, titled, “North and Central Texas Clinical and Translational Science Initiative” (Milton Packer, M.D., P.I.).
Footnotes
Presented at the American College of Surgeons Committee on Trauma National Resident Paper Competition, March 20, 2009, Chicago, IL.
References
- 1.Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133(6 suppl):381S–453. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 2.Rogers FB, Cipolle MD, Velmahos G, et al. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group. J Trauma. 2002;53:142–64. doi: 10.1097/00005373-200207000-00032. [DOI] [PubMed] [Google Scholar]
- 3.Friis E, Hørby J, Sørensen LT, et al. Thromboembolic prophylaxis as a risk factor for postoperative complications after breast cancer surgery. World J Surg. 2004;28:540–3. doi: 10.1007/s00268-004-7223-9. [DOI] [PubMed] [Google Scholar]
- 4.Ginzburg E, Cohn SM, Lopez J, et al. Randomized clinical trial of intermittent pneumatic compression and low molecular weight heparin in trauma. Br J Surg. 2003;90:1338–44. doi: 10.1002/bjs.4309. [DOI] [PubMed] [Google Scholar]
- 5.Green D, Lee MY, Ito VY, et al. Fixed-vs adjusted-dose heparin in the prophylaxis of thromboembolism in spinal cord injury. J Am Med Assoc. 1988;260:1255–8. [PubMed] [Google Scholar]
- 6.Sauaia A, Moore FA, Moore EE, et al. Epidemiology of trauma deaths: a reassessment. J Trauma. 1995;38:185–93. doi: 10.1097/00005373-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Antevil JL, Sise MJ, Sack DI, et al. Retrievable vena cava filters for preventing pulmonary embolism in trauma patients: a cautionary tale. J Trauma. 2006;60:35–40. doi: 10.1097/01.ta.0000197607.23019.ab. [DOI] [PubMed] [Google Scholar]
- 8.Karmy-Jones R, Jurkovich GJ, Velmahos GC, et al. Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study. J Trauma. 2007;62:17–24. doi: 10.1097/TA.0b013e31802dd72a. [DOI] [PubMed] [Google Scholar]
- 9.Sing RF, Camp SM, Heniford BT, et al. Timing of pulmonary emboli after trauma: implications for retrievable vena cava filters. J Trauma. 2006;60:732–4. doi: 10.1097/01.ta.0000210285.22571.66. [DOI] [PubMed] [Google Scholar]
- 10.Adair JD, Harvey KP, Mahmood A. Inferior vena cava filter migration to right ventricle with destruction of tricuspid valve: a case report. J Trauma. 2008;64:509–11. doi: 10.1097/TA.0b013e318058251c. [DOI] [PubMed] [Google Scholar]
- 11.Hermsen JL, Ibele AR, Faucher LD, et al. Retrievable inferior vena cava filters in high-risk trauma and surgical patients: factors influencing successful removal. World J Surg. 2008;32:1444–9. doi: 10.1007/s00268-007-9462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahrer A, Zippel D, Garniek A, et al. Retrievable vena cava filters in major trauma patients: prevalence of thrombus within the filter. Cardiovasc Interv Radiol. 2008;31:785–9. doi: 10.1007/s00270-008-9294-8. [DOI] [PubMed] [Google Scholar]
- 13.Menaker J, Stein DM, Scalea TM. Incidence of early pulmonary embolism after injury. J Trauma. 2007;63:620–4. doi: 10.1097/TA.0b013e31812f60aa. [DOI] [PubMed] [Google Scholar]
- 14.Geerts WH, Code KI, Jay RM, et al. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331:1601–6. doi: 10.1056/NEJM199412153312401. [DOI] [PubMed] [Google Scholar]
- 15.Knudson MM, Ikossi DG, Khaw L, et al. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004;240:490–6. doi: 10.1097/01.sla.0000137138.40116.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owings JT, Kraut E, Battistella F, et al. Timing of the occurrence of pulmonary embolism in trauma patients. Arch Surg. 1997;132:862–6. doi: 10.1001/archsurg.1997.01430320064010. [DOI] [PubMed] [Google Scholar]
- 17.Kim KI, Muller NL, Mayo JR. Clinically suspected pulmonary embolism: utility of spiral CT. Radiology. 1999;210:693–7. doi: 10.1148/radiology.210.3.r99mr01693. [DOI] [PubMed] [Google Scholar]
- 18.Schultz DJ, Brasel KJ, Washington L, et al. Incidence of asymptomatic pulmonary embolism in moderately to severely injured trauma patients. J Trauma. 2004;56:727–31. doi: 10.1097/01.ta.0000119687.23542.ec. [DOI] [PubMed] [Google Scholar]
- 19.Ferraro FJ, Jr, Spillert CR, Swan KG, et al. Cold-induced hypercoagulability in vitro: a trauma connection? Am Surg. 1992;58:355–7. [PubMed] [Google Scholar]
- 20.Meissner MH, Chandler WL, Elliott JS. Venous thromboembolism in trauma: a local manifestation of systemic hypercoagulability? J Trauma. 2003;54:224–31. doi: 10.1097/01.TA.0000046253.33495.70. [DOI] [PubMed] [Google Scholar]
- 21.Schreiber MA, Differding J, Thorborg P, et al. Hypercoagulability is most prevalent early after injury and in female patients. J Trauma. 2005;58:475–80. doi: 10.1097/01.ta.0000153938.77777.26. [DOI] [PubMed] [Google Scholar]
- 22.Rodeghiero F, Tosetto A. Activated protein C resistance and factor V Leiden mutation are independent risk factors for venous thromboembolism. Ann Intern Med. 1999;130:643–50. doi: 10.7326/0003-4819-130-8-199904200-00004. [DOI] [PubMed] [Google Scholar]
- 23.Pasquier E, Amiral J, de Saint Martin L, et al. A cross sectional study of antiphospholipid-protein antibodies in patients with venous thromboembolism. Thromb Haemost. 2001;86:538–42. [PubMed] [Google Scholar]
- 24.Kovac M, Mitic G, Mikovic Z, et al. Type and location of venous thromboembolism in carriers of factor V Leiden or prothrombin G20210A mutation versus patients with no mutation. Clin Appl Thromb/Hemost. 2008;16:66–70. doi: 10.1177/1076029608320721. [DOI] [PubMed] [Google Scholar]
- 25.Malagari K, Economopoulos N, Stoupis C, et al. High-resolution CT findings in mild pulmonary fat embolism. Chest. 2003;123:1196–201. doi: 10.1378/chest.123.4.1196. [DOI] [PubMed] [Google Scholar]
- 26.Arakawa H, Yamada H, Kurihara Y, et al. Nonspecific interstitial pneumonia associated with polymyositis and dermatomyositis: serial high-resolution CT findings and functional correlation. Chest. 2003;123:1096–103. doi: 10.1378/chest.123.4.1096. [DOI] [PubMed] [Google Scholar]
- 27.Cohen MJ, Brohi K, Ganter MT, et al. Early coagulopathy after traumatic brain injury: the role of hypoperfusion and the protein C pathway. J Trauma. 2007;63:1254–61. doi: 10.1097/TA.0b013e318156ee4c. [DOI] [PubMed] [Google Scholar]
- 28.Hulka F, Mullins RJ, Frank EH. Blunt brain injury activates the coagulation process. Arch Surg. 1996;131:923–7. doi: 10.1001/archsurg.1996.01430210021004. [DOI] [PubMed] [Google Scholar]
- 29.Stein SC, Chen XH, Sinson GP, et al. Intravascular coagulation: a major secondary insult in nonfatal traumatic brain injury. J Neurosurg. 2002;97:1373–7. doi: 10.3171/jns.2002.97.6.1373. [DOI] [PubMed] [Google Scholar]
- 30.Zehtabchi S, Soghoian S, Liu Y, et al. The association of coagulopathy and traumatic brain injury in patients with isolated head injury. Resuscitation. 2008;76:52–6. doi: 10.1016/j.resuscitation.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 31.Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. V. Deep vein thrombosis prophylaxis. J Neurotrauma. 2007;24(suppl 1):S32–6. doi: 10.1089/neu.2007.9991. [DOI] [PubMed] [Google Scholar]
- 32.Norwood SH, McAuley CE, Berne JD, et al. Prospective evaluation of the safety of enoxaparin prophylaxis for venous thromboembolism in patients with intracranial hemorrhagic injuries. Arch Surg. 2002;137:696–701. doi: 10.1001/archsurg.137.6.696. [DOI] [PubMed] [Google Scholar]
- 33.Velmahos GC, Spaniolas K, Tabbara M, et al. Pulmonary embolism and deep venous thrombosis in trauma: are they related? Arch Surg. 2009;144:928–32. doi: 10.1001/archsurg.2009.97. [DOI] [PubMed] [Google Scholar]


