Abstract
AIMS
We compared cystatin C in youth with versus without diabetes and determined factors associated with cystatin C in youth with type 1 diabetes (T1D) and type 2 diabetes (T2D).
METHODS
Youth (ages 12–19 years) without diabetes (N=544) were ascertained from the NHANES Study 2000- 2002 and those with T1D (N=977) and T2D (N=168) from the SEARCH for Diabetes in Youth Study. Adjusted means of cystatin C concentrations were compared amongst the 3 groups. Next, we performed multivariable analyses within the T1D and T2D SEARCH samples to determine the association between cystatin C and race, sex, age, diabetes duration, HbA1c, fasting glucose, and BMI.
RESULTS
Adjusted cystatin C concentrations were statistically higher in NHANES (0.85mg/L) than in either the T1D (0.75mg/L) or T2D (0.70mg/L) SEARCH groups (p<0.0001). Fasting glucose was inversely related to cystatin C only in T1D (p<0.001) and BMI positively associated only in T2D (p<0.01) while HbA1c was inversely associated in both groups.
CONCLUSIONS
Cystatin C concentrations are statistically higher in youth without diabetes compared to T1D or T2D, however the clinical relevance of this difference is quite small, especially in T1D. In youth with diabetes, cystatin C varies with BMI and acute and chronic glycemic control, however their effects may be different according to diabetes type.
Keywords: Cystatin C, Type 1 diabetes, Type 2 diabetes, Youth
SECTION 1.0 INTRODUCTION
Diabetes is the leading cause of chronic kidney disease (CKD) in the U.S. and carries significant morbidity and mortality [1]. As glomerular filtration rate (GFR) deteriorates, the risk for end-stage renal disease, cardiovascular events and death increases substantially [2]. Recent data show that the prevalence of type 1 and type 2 diabetes in youth is increasing [3]. Diabetes onset in youth increases the lifetime exposure to the diabetic milieu resulting in a greater risk for developing CKD. Several reports have suggested that type 2 diabetes (T2D) in youth takes a particularly aggressive course, with renal complications beginning earlier than in type 1 diabetes (T1D) [4, 5]. These issues underscore the importance of understanding the natural history of markers of early chronic kidney disease in youth with diabetes.
Serum cystatin C has gained prominence as an endogenous marker of GFR [6]. Cystatin C is a low molecular weight cysteine protease inhibitor, produced by all nucleated cells, freely filtered by the glomerulus and catabolized in the renal tubular cells without reabsorption, and its reciprocal is highly correlated with the GFR [7]. The American Diabetes Association recommends annual GFR measurement in adolescents with diabetes [8]. Numerous studies in adults [9–12] and children [13–15] have demonstrated that serum cystatin C improves estimates of GFR than serum creatinine-based methods alone. Analyses by diabetes status have confirmed that cystatin C improves the accuracy and precision of GFR estimation even in this distinct population [12, 16]
Factors other than GFR can impact cystatin C concentrations, and depending on the population, these factors need to be considered when interpreting cystatin C-based estimated GFR (eGFR). In adults, cystatin C concentration increases with age, inflammation [17] and high-dose glucocorticoid therapy [18] while lower concentrations are seen in hypothyroidism [19]. Data reflecting non-GFR factors that influence cystatin C are limited, especially in youth. The Third National Health and Nutrition Examination Survey (NHANES) III found that cystatin C concentrations were higher in males and decreased with advancing age in children and adolescents [20]. In a study of youth with T1D, cystatin C was shown to be lower with advancing age, female sex, increased body mass index (BMI), increased glycated hemoglobin (HbA1c), and increased C-reactive protein [21] . This is the only report of comparison of cystatin C concentrations in youth with T1D versus youth without diabetes. No difference was found but this has not been confirmed [21]. Factors influencing cystatin C have not been previously reported in youth with T2D. As an important marker of kidney disease, insight to these factors is crucial to better understand the early natural history of cystatin C-based eGFR in youth with diabetes. Our objectives were twofold: 1) to compare the distribution of cystatin C among youth without diabetes versus T1D and T2D; and 2) to determine the factors associated with cystatin C in youth with T1D and T2D.
SECTION 2.0 SUBJECTS, MATERIALS AND METHODS
2.1: Population without self-reported diabetes: NHANES
Continuous NHANES is a cross-sectional epidemiologic study of a nationally representative sample of the non-institutionalized civilian U.S. population conducted in two-year cycles starting in 1999. This analysis includes NHANES 2001–2002 participants aged 12–19 years, without self-reported diabetes and with measured serum cystatin C (n = 544). NHANES instruments, methods and measures have been described previously (http://www.cdc.gov/nchs/about/major/nhanes/datalink.htm). Weight and height were recorded and BMI was calculated. Blood pressure (BP) was measured in the seated position after a 5-minute rest period. HbA1c was measured by boronate-affinity high-performance liquid chromatography (Primus Corp, Kansas City, MO). Cystatin C was measured in a random 25% sample of NHANES participants aged 12–59 years (http://www.cdc.gov/nchs/nhanes/nhanes2001-2002/SSCYST_B.htm).
Cystatin C was measured in 2006 using a Siemens automated particle-enhanced nephelometric assay on serum samples collected from NHANES participants in 2001–2002, stored at −70°C. In 2011, it was reported that the calibration of the assay for cystatin C had drifted during the 2006–2010 period such that there was a 15% decrease in cystatin C values reported after 2008 [22] . To correct for this drift in the NHANES data, equations were developed to standardize the cystatin C results by making them traceable to the International Federation of Clinical Chemistry (IFCC) Standard ERM DA 471 [23]. The equation is as follows: Adjusted cystatin C= [(raw cystatin C)−0.12]×1.12. Since the cystatin C assay was run in 2006, we followed the recommended standardization to make it comparable to the IFCC standard.
2.2 Population with diagnosed diabetes: SEARCH
SEARCH is a multi-center, longitudinal study of youth with type 1 and type 2 diabetes, diagnosed prior to the age of 20 years. A detailed description of SEARCH methodology is published elsewhere [24]. Briefly, incident cases of diabetes from 2002–2010 were identified through a reporting network of clinics, healthcare providers, school nurses and diabetes educators. Study sites were located in geographically defined populations in Ohio, Washington, South Carolina and Colorado and among health plan enrollees in Hawaii (Hawaii Medical Service Association, Med-Quest, Kaiser Permanente Hawaii) and in southern California (Kaiser Permanente). Patients were invited for an in-person visit at baseline and follow up visits at 12, 24 and 60 months. In this analysis we included participants with an in-person visit and with measured serum cystatin C between July 2002 and September 2010 (n=1145). If participants had more than one visit during this time interval, we used the earliest visit. Diabetes “type” was defined by the healthcare provider’s diagnosis. We excluded 9 participants with “other” forms of diabetes that were not classified as T1D or T2D (4 with monogenic diabetes, 1 hybrid, 4 unknown type).
2.3 Measurements
During the in-person visit, height and weight were measured and used to calculate BMI (kg/m2), which was then converted to z-scores using the standard Centers for Disease Control and Prevention (CDC) approach [25]. BP was obtained in the seated position after at least a 5-minute rest period. BP Z-scores were calculated by adjusting for age, sex and height based on CDC growth references for 2000 [25]. Fasting blood samples were drawn at a time of metabolic stability when the participants were free of diabetic ketoacidosis or infection, for determination of glucose, HbA1c and cystatin C. Urinary creatinine was measured by the Jaffe method using Roche reagent on the Roche Modular P autoanalyzer on random spot urine samples. Urine albumin was measured in the same samples immunochemically using Siemens reagent on a Siemens BNII nephelometer and the urine albumin/creatinine ratio was calculated. Samples were processed locally and shipped within 24 hours to the central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories). HbA1c was measured by non-porous ion-exchange high-performance liquid chromatography on a TOSOH G8 dedicated analyzer (TOSOH Biosciences Inc., South San Francisco, CA). Cystatin C was measured using Siemens reagent on a BNII nephelometer (Siemens Healthcare Diagnostics). SEARCH noted the same assay drift as previously described [22], however, there appeared to be two somewhat distinct drift periods that diverged in mid-2008. Using Deming regression models [26], two independent calibration equations were provided, making them traceable to the IFCC Standard (see Supplemental Data 1 for further details).
For assays performed in years prior to 5/1/2008 the equation was:
For assays performed between 5/1/2008–4/7/2014 the equation was:
2.4 Statistical Analyses
All summary measures from the NHANES data are reported after incorporating the specific sample weights that account for the probability of having a cystatin C measurement and the complex survey design. For continuous measures, two sample t-tests were used to compare SEARCH T1D and T2D with NHANES. Chi-square tests were used for categorical measures to compare SEARCH T1D and T2D with NHANES. Cystatin C measures were evaluated overall, by diabetes type, and subcategorized by age (12–14, 15–19), sex, and race/ethnicity. The distribution of cystatin C was confirmed to approximate a normal distribution. Analysis of covariance (ANCOVA) models were used to estimate the mean cystatin C concentration after adjusting for age, sex, and race/ethnicity in both NHANES and SEARCH studies. These adjusted means were then compared using an inverse-variance weighted test to evaluate differences between the studies and by diabetes type.
To investigate the effect of clinical characteristics on cystatin C, by diabetes type within SEARCH, several approaches were used. Specifically, analyses were performed separately for T1D and T2D participants. For each group, three model selection approaches were used considering the variables: sex, race (black vs. other), age at diagnosis, duration of diabetes, blood glucose, HbA1c, and BMI Z-score. These approaches included a stepwise selection method, a backwards selection method and a LASSO (least absolute shrinkage and selection operator) approach [27]. For the two selection approaches, variables were selected (or retained) using a p-value of 0.1 or less at each iteration to create the final models, and for the LASSO approach the Schwarz Bayesian information criteria [28] with 30 selection steps was used to identify the best model. Each of the “best” models were compared among the three methods and if they were in agreement then the model that was produced using a selection method would be used. If there were differences among the “best” models, then additional diagnostics would be used to determine the preferred final model, specifically, the correlation among potential predictors was examined to determine if there was evidence of collinearity among any predictors in the final model and if so then a model removing the least significant variable that had a collinear relationship with other variables was removed. The resulting adjusted means for T1D and T2D were compared using an inverse-variance weighted test. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Section 3.0 RESULTS
3.1 Distribution of cystatin C in youth with and without diabetes
A total of 1145 cystatin C measures from SEARCH (977 T1D and 168 T2D) collected between July 2002 and September 2010 in participants aged 12–19 years were compared to 544 measures from NHANES participants aged 12–19 years without diagnosed diabetes. Demographic and clinical characteristics of NHANES versus SEARCH participants, stratified by diabetes type, are outlined in Table 1. There are clear differences amongst the three groups with respect to almost all characteristics including age, sex and race/ethnicity.
Table 1.
Demographic and clinical characteristics of participants ages 12–19 years with cystatin C (adjusted for age, sex and race/ethnicity) from the SEARCH for Diabetes in Youth Study, stratified by diabetes type, and NHANES 2001–2002 without diagnosed diabetes.
| Clinical characteristics | SEARCH Type 1* N=977 Mean (SE) |
SEARCH Type 2* N=168 Mean (SE) |
NHANES 2001–2002** N=544 Mean (SE) |
|---|---|---|---|
| Age, years | 14.9 (0.07)$& | 16.3 (0.17)$# | 15.5(0.12)#& |
| Diabetes Duration | 3.6 (0.07)& | 2.8 (0.2)# | NA |
| Sex, % Male | 51.3& | 36.3$# | 54.5& |
| Race/Ethnicity (%) | $& | $# | #& |
| White | 76.8 | 19.6 | 62.8 |
| Black | 9.2 | 45.8 | 13.8 |
| Hispanic | 10.2 | 25.6 | 16.8 |
| Other | 3.8 | 8.9 | 6.6 |
| SBP z-score | −0.5 (0.03)& | 0.3 (0.08) $# | −0.3 (0.07)& |
| DBP z-score | 0.1 (0.03) $& | 0.6 (0.07) $# | −0.4 (0.07)#& |
| BMI z-score | 0.7 (0.03) $& | 2.1 (0.05) $# | 0.5(0.06) # & |
| Fasting glucose, mg/dL | 191.7 (2.9) | 169.3 (6.8) | NA |
| HbA1c, % | 8.5 (0.06) $ | 7.9 (0.2) $ | 5.2 (0.01) # & |
| (IFCC units) | (69 mmol/mol) | (63 mmol/mol) | (33 mmol/mol) |
| Albumin/Creatinine ratio (µg/mg)@ | 6.6 (4.4, 12.1) | 7.2 (4.3, 18.8)$ | 6.3 (4.2, 11.2)& |
| Adjusted Cystatin C, mg/L^ | 0.75 (0.004)$& | 0.70 (0.01)$# | 0.85 (0.01)#& |
Restricted to age 12–19, Cystatin C measured, and Type1 or Type 2 Diabetes.
Restricted to age 12–19, Cystatin C measured, and non-diabetic; NHANES values reported incorporate the sampling weights for the complex survey design. SBP Systolic blood pressure; DBP Diastolic blood pressure;
median (25th, 75th percentiles) -Tests performed on log transformed values as data were not normally distributed;
P<0.001 compared with NHANES
P<0.001 compared with SEARCH Type 1
P<0.001 compared with SEARCH Type 2
Adjusted for age, sex, race/ethnicity
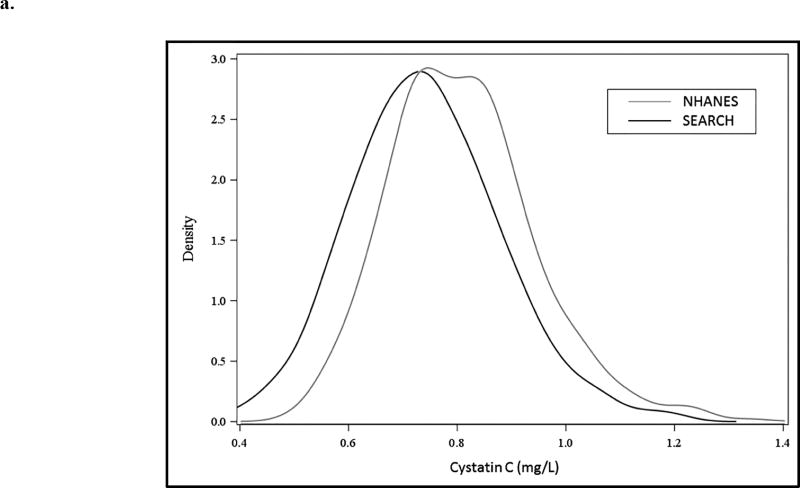
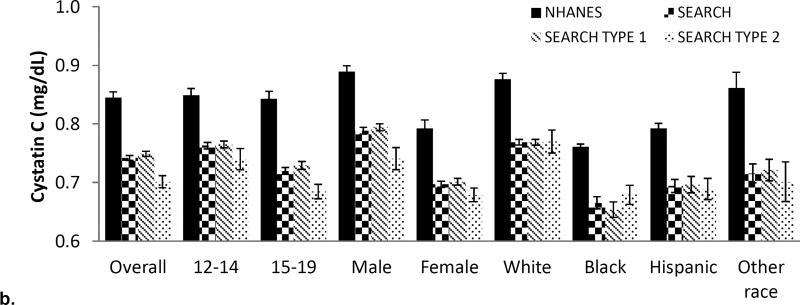
The distribution of raw cystatin C is depicted in Figure 1a with mean cystatin C being higher in the NHANES group compared with SEARCH. Figure 1b depicts the distribution of cystatin C values in NHANES and SEARCH (overall and by diabetes type), subcategorized by age, sex and race/ethnicity. Serum cystatin C was higher in NHANES compared with SEARCH across all strata of age, sex and race/ethnicity. Younger participants, males, and non-Hispanic whites had a higher cystatin C in both youth with and without diabetes compared to older participants, females, and black, Hispanic, and other race/ethnicity youth (numeric data can be found in Supplemental Table 1).
Figure 1. Distribution of cystatin C in SEARCH and NHANES (a) Density plot across the spectrum of cystatin C and (b) according to diabetes type, sex and race/ethnicity.
Overall and for all subgroups: differences between NHANES and SEARCH Type 1 are significant (p<0.001); differences between NHANES and SEARCH Type 2 are significant (p<0.001);
Overall cystatin C differences between SEARCH type 1 and type 2 are significant (P<0.001) but differences in subgroups between SEARCH Type 1 and Type 2 are not significant at the <0.001 significance level
After adjusting for age, sex and race/ethnicity, the estimated mean (SE) of cystatin C was 0.85 mg/L (0.009) in NHANES; 0.75 mg/L (0.004) in the SEARCH type 1 diabetes stratum and 0.70 mg/L (0.009) in the SEARCH type 2 diabetes stratum. Each pairwise comparison was statistically significantly different (p<0.0001).
3.2 Factors associated with cystatin C concentrations in youth with diabetes in SEARCH
Results from stepwise regression modeling are illustrated in Table 2. All three model selection approaches (stepwise, backwards and LASSO) arrived at the same “best” model for T1D. For T2D, the stepwise and backwards selection approaches included sex, race, age at diagnosis, duration of diabetes, HbA1c (%), and BMI Z-score while the LASSO approach suggested these 6 variables and fasting glucose. The p-value for fasting glucose in the model suggested by the LASSO method was 0.37. Furthermore, when the correlation matrix among these variables was calculated, we recognized that the correlation between fasting glucose and Hba1c (%) in T2D was 0.81 (p<0.0001) suggesting that these two variables were highly collinear. Therefore, the final model chosen for T2D was the model determined by the stepwise (and backwards) selection procedures and fasting glucose was removed. Fasting glucose was only significant in the group with T1D in the final model.. For every 100 mg/dL increase in fasting blood glucose, cystatin C decreased by 0.03 mg/L. In contrast, BMI-Z score was significant only in the T2D group, with a 0.04 mg/L increase in cystatin C for each unit increase in BMI Z-score. The HbA1c was independently associated with cystatin C in both T1D and T2D with a decrease in cystatin C of approximately 0.02 mg/L per unit increase in HbA1c.
Table 2.
Multivariable Model with independent predictors of cystatin C in type 1 and type 2 diabetes in SEARCH
| Type 1 N=924 Beta (95% CI) |
P-value | Type 2 N=162 Beta (95% CI) |
P-value | |
|---|---|---|---|---|
| Sex (male vs female) | 0.089 (0.073, 0.105) | <0.0001 | 0.075 (0.037, 0.113) | 0.0001 |
| Race (black vs other) | −0.056 (−0.084, −0.028) | <0.0001 | −0.036 (−0.073, 0.0002) | 0.0514 |
| Age at Diagnosis (years) | −0.012 (−0.016, −0.008) | <0.0001 | −0.016 (−0.025, −0.007) | 0.0009 |
| Duration of DM (years) | −0.012 (−0.017, −0.007) | <0.0001 | −0.012 (−0.023, −0.002) | 0.0243 |
| HbA1c (%) | −0.015 (−0.019, −0.010) | <0.0001 | −0.019 (−0.025, −0.012) | <0.0001 |
| Fasting Glucose (mg/dl) | −0.0003 (−0.0004, −0.0002) | <0.0001 | NS | |
| BMI Z-score | NS | 0.038 (0.011, 0.065) | 0.0058 |
There are 2 models above, one for each group of youth (SEARCH type 1 and type 2 diabetes). A forward selection model was used, adding the most significant predictor with a p-value of 0.1 or less at each iteration. Predictors with cells shaded were not included in the final model for that particular group of individuals.
For the categorical variables, the reference groups are female and other race. NS= not significant
Section 4.0 DISCUSSION
We found cystatin C concentrations to be statistically lower in youth with versus without diabetes; however, the magnitude of difference was quite small especially when viewed from the perspective of the variability of cystatin C measurements [29]. While NHANES and SEARCH both used a Siemens assay, the coefficient of variation for this instrument in different laboratories is 8%. The percent difference between the NHANES and SEARCH T1D sample was 12% and the difference between NHANES and SEARCH T2D sample was 18%. A study of people with T1D versus controls with cystatin C assay performed in a single laboratory, found no clinical or statistical difference in the unadjusted cystatin C concentration, regardless of diabetes status. [21] While our results would suggest a small difference in cystatin C concentration between type 1 diabetes versus non-diabetic youth, this difference is probably not clinically meaningful when considering the bias introduced by inter-laboratory variation. The difference in cystatin C concentration between the SEARCH T2D versus NHANES groups, however, was larger and likely does represent a true difference. Our study is the first to examine the distribution of cystatin C between T2D and non-diabetic youth and our findings warrant further investigation to both corroborate this result and help determine the source of this difference.
We also found a statistically significant difference in adjusted cystatin C according to diabetes type, with cystatin C performed in the same laboratory. The difference was small and may not be clinically significant. While the SEARCH assays were all performed in the same laboratory, the percent difference between the T1D and T2D samples was 12% and day to day intra-individual variability of cystatin C can be as high as 8% [30]. Further studies will be necessary to determine whether there is a consistent difference between diabetes types.
Consistent with prior studies, we found that cystatin C concentrations were slightly lower in females compared with males,[20, 21] and higher in non-Hispanic Whites compared with other races/ethnicities.[31]. There was an inverse relationship between age and cystatin C concentration, with lower cystatin C in 15 – 19-year-old participants compared with 12 – 14-year-old participants, regardless of diabetes type. Our findings are discordant with Bokenkamp et al. [32] who reported that cystatin C did not vary by age in children without kidney disease. However, that study had a smaller number of patients in the 15–18 year age group (less than 30 vs 258 in our study). Our findings are concordant with the report of Groesbeck et al. [20] using NHANES data and Maahs et al. [21] which studied individuals with type 1 diabetes. Based on our data, holding all other variables constant, a 19-year-old with type 2 diabetes would have a cystatin C concentration 0.112 mg/L lower than a 12-year-old with type 2 diabetes. Given the small effect size (beta value) for age cited by Groesbeck et al. (0.009), Maahs et al. (0.02), and our study (Table 2), further work is required to establish whether this finding is clinically significant.
Hemoglobin A1c was inversely associated with cystatin C in both T1D and T2D. Whether this is due to a decrease in cystatin C production versus rise in cystatin C clearance would need to be assessed with mechanistic and measured GFR studies. The magnitude of effect was also quite small, so the clinical relevance is unclear. In contrast, there was an association of fasting glucose with cystatin C only in T1D. The T2D group had a lower distribution of glucose compared to the T1D group and may have been under the threshold at which glucose can impact GFR, as has been previously demonstrated [33]. The impact of acute glycemia on cystatin C and GFR measures in patients with diabetes is generally underappreciated. Cherney et al. reported the ability of cystatin C to detect acute changes in measured GFR under hyperglycemic conditions in uncomplicated T1D [33]. Moreover, cystatin C based eGFR was superior to creatinine based eGFR, in detecting these changes, suggesting that GFR estimated by cystatin C may be preferable in diabetic persons [33] . Subsequently, Maahs et al. demonstrated that correction for eGFR using simultaneous glucose and cystatin C measures significantly improved the accuracy and precision of cystatin C based eGFR methods [34]. Measured GFR was not performed in the SEARCH study and so we cannot conclude whether the association of fasting glucose and cystatin C in T1D was due to differences in GFR.
We found that BMI was a statistically significant predictor of cystatin C in participants with T2D but not T1D. The distribution of BMI was much higher in the T2D versus T1D sample which could have resulted in the difference in association. Other studies of type 1 diabetic cohorts have also found a lack of association between BMI and cystatin C [21]. Studies in nondiabetic children and adolescents have had conflicting findings regarding the association between BMI and cystatin C [20, 35]. In adults, one study found an increasing prevalence of elevated serum cystatin C (>99th percentile) with increasing BMI [36]. These authors postulated a role for cystatin C in adipogenesis as a possible cause for the association. Another study found cystatin C concentrations to be higher in obese versus non-obese adults and also found cystatin C mRNA in adipose cells to be twice that in non-adipose cells [37].
Our study has several limitations. Given the cross-sectional study design, we cannot make any causal inferences. NHANES and SEARCH are very distinct study designs, and despite our attempts to control for population differences, we cannot exclude the possibility of unmeasured differences that may have biased our comparisons. Moreover, the cystatin C assay was performed in different laboratories for NHANES and SEARCH and a significant inter-laboratory variability exists for cystatin C despite attempts at standardization. Neither NHANES nor SEARCH had measured GFR to decipher whether differences in cystatin C is due to differences in kidney function.
Strengths of our study include the large sample size and ethnic and geographic diversity of the SEARCH cohort, allowing for generalizability of our findings. Inclusion of both type 1 and type 2 diabetes in the SEARCH cohort allows direct comparison between diabetes types, which may be important to markers of kidney function such as cystatin C. The increasing incidence of type 2 diabetes in youth lends great importance to the study of this population at high risk for future complications.
In conclusion, cystatin C concentrations appear to be slightly lower in youth with diabetes (especially in T2D) than nondiabetic youth and there may be differences according to diabetes type. Acute and chronic glycemia and BMI are characteristics which may mediate some of these distinctions in cystatin C. Whether these phenomenon are due to differences in cystatin C metabolism or are a reflection of differences in GFR are an important future direction of study given the recent emphasis on using cystatin C to establish eGFR longitudinally in individuals with diabetes and other chronic diseases.
Supplementary Material
HIGHLIGHTS.
Serum cystatin C levels are statistically higher in youth without diabetes compared to youth with type 1 or type 2 diabetes of short duration. This difference may not be clinically significant.
Factors associated with serum cystatin C levels in youth with diabetes include age, sex, race and HbA1c.
BMI is associated with serum cystatin C levels only in youth with type 2 diabetes.
Fasting glucose level is associated with serum cystatin C levels only in youth with type 1 diabetes.
Acknowledgments
The SEARCH for Diabetes in Youth Study is indebted to the many youth and their families, and their health care providers, whose participation made this study possible.
Grant Support: SEARCH for Diabetes in Youth is funded by the Centers for Disease Control and Prevention (PA numbers 00097, DP-05-069, and DP-10-001) and supported by the National Institute of Diabetes and Digestive and Kidney Diseases.
Site Contract Numbers: Kaiser Permanente Southern California (U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado Denver (U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1), Kuakini Medical Center (U58CCU919256 and U01 DP000245), Children’s Hospital Medical Center (Cincinnati) (U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U48/CCU419249, U01 DP000254, and U18DP002708), University of Washington School of Medicine (U58/CCU019235-4, U01 DP000244, and U18DP002710-01), Wake Forest University School of Medicine (U48/CCU919219, U01 DP000250, and 200-2010-35171).
The authors wish to acknowledge the involvement of General Clinical Research Centers (GCRC) at the South Carolina Clinical & Translational Research (SCTR) Institute, at the Medical University of South Carolina (NIH/NCRR Grant number UL1RR029882); Seattle Children’s Hospital (NIH CTSA Grant UL1 TR00423 of the University of Washington); University of Colorado Pediatric Clinical and Translational Research Center (CTRC) (Grant Number UL1 TR000154) and the Barbara Davis Center at the University of Colorado at Denver (DERC NIH P30 DK57516); and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8 UL1 TR000077; and the Children with Medical Handicaps program managed by the Ohio Department of Health.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
The authors acknowledge the contributions of Desmond E. Williams, MD, PhD, Henry S. Kahn, MD, Bernice Moore, MBA, Edward W. Gregg, PhD from the Centers for Disease Control and Prevention.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention and the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ETHICAL APPROVAL
All procedures performed involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
References
- 1.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, et al. US renal data system 2015 annual data report: Epidemiology of kidney disease in the united states. Am J Kidney Dis. 2016;67:A7–8. doi: 10.1053/j.ajkd.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, et al. ADVANCE Collaborative Group. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–21. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, et al. SEARCH for Diabetes in Youth Study. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–86. [Google Scholar]
- 4.Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care. 2014;37:436–43. doi: 10.2337/dc13-0954. [DOI] [PubMed] [Google Scholar]
- 5.TODAY Study Group. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: The TODAY clinical trial. Diabetes Care. 2013;36:1735–41. doi: 10.2337/dc12-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nilsson-Ehle P, Grubb A. New markers for the determination of GFR: Iohexol clearance and cystatin C serum concentration. Kidney Int Suppl. 1994;47:S17–9. [PubMed] [Google Scholar]
- 7.Grubb A. Diagnostic value of analysis of cystatin C and protein HC in biological fluids. Clin Nephrol. 1992;38(Suppl 1):S20–7. [PubMed] [Google Scholar]
- 8.American Diabetes Association. Foundations of care and comprehensive medical evaluation. Diabetes Care. 2016;39:S23–S35. doi: 10.2337/dc16-S006. [DOI] [PubMed] [Google Scholar]
- 9.Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, et al. Serum cystatin C: A replacement for creatinine as a biochemical marker of GFR. Kidney Int Suppl. 1994;47:S20–1. [PubMed] [Google Scholar]
- 10.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 11.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan L, Inker LA, Rossert J, Froissart M, Rossing P, Mauer M, et al. Glomerular filtration rate estimation using cystatin C alone or combined with creatinine as a confirmatory test. Nephrol Dial Transplant. 2014;29:1195–203. doi: 10.1093/ndt/gft509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Byrd D, Brodehl J. Cystatin C--a new marker of glomerular filtration rate in children independent of age and height. Pediatrics. 1998;101:875–81. doi: 10.1542/peds.101.5.875. [DOI] [PubMed] [Google Scholar]
- 14.Zaffanello M, Franchini M, Fanos V. Is serum cystatin-C a suitable marker of renal function in children? Ann Clin Lab Sci. 2007;37:233–40. [PubMed] [Google Scholar]
- 15.Schwartz GJ, Schneider MF, Maier PS, Moxey-Mims M, Dharnidharka VR, Warady BA, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82:445–53. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Zinman B, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group. Longitudinal changes in estimated and measured GFR in type 1 diabetes. J Am Soc Nephrol. 2014;25:810–8. doi: 10.1681/ASN.2013050557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lertnawapan R, Bian A, Rho YH, Raggi P, Oeser A, Solus JF, et al. Cystatin C is associated with inflammation but not atherosclerosis in systemic lupus erythematosus. Lupus. 2012;21:279–87. doi: 10.1177/0961203311425527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, Grubb A. Cystatin C as a marker of GFR--history, indications, and future research. Clin Biochem. 2005;38:1–8. doi: 10.1016/j.clinbiochem.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Fricker M, Wiesli P, Brandle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003;63:1944–47. doi: 10.1046/j.1523-1755.2003.00925.x. [DOI] [PubMed] [Google Scholar]
- 20.Groesbeck D, Kottgen A, Parekh R, Selvin E, Schwartz GJ, Coresh J, et al. Age, gender, and race effects on cystatin C levels in US adolescents. Clin J Am Soc Nephrol. 2008;3:1777–85. doi: 10.2215/CJN.00840208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maahs DM, Prentice N, McFann K, Snell-Bergeon JK, Jalal D, Bishop FK, et al. Age and sex influence cystatin C in adolescents with and without type 1 diabetes. Diabetes Care. 2011;34:2360–62. doi: 10.2337/dc11-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsson A, Hansson LO, Flodin M, Katz R, Shlipak MG. Calibration of the siemens cystatin C immunoassay has changed over time. Clin Chem. 2011;57:777–8. doi: 10.1373/clinchem.2010.159848. [DOI] [PubMed] [Google Scholar]
- 23.Selvin E, Juraschek SP, Eckfeldt J, Levey AS, Inker LA, Coresh J. Calibration of cystatin C in the national health and nutrition examination surveys (NHANES) Am J Kidney Dis. 2013;61:353–4. doi: 10.1053/j.ajkd.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.SEARCH Study Group. SEARCH for diabetes in youth: A multicenter study of the prevalence, incidence and classification of diabetes mellitus in youth. Control Clin Trials. 2004;25:458–71. doi: 10.1016/j.cct.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 26.Deming WE. Statistical adjustment of data. wiley; NY: 1943. (dover publications edition, 1985) [Google Scholar]
- 27.Tibshirani R. Regression shrinkage and selection via the lasso: a retrospective. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2011;73:273–82. [Google Scholar]
- 28.Schwarz G. Estimating the Dimension of a Model. The Annals of Statistics. 1978;6(2):461–4. [Google Scholar]
- 29.Eckfeldt JH, Karger AB, Miller WG, Rynders GP, Inker LA. Performance in Measurement of Serum Cystatin C by Laboratories Participating in the College of American Pathologists 2014 CYS Survey. Arch Pathol Lab Med. 2015;139:888–93. doi: 10.5858/arpa.2014-0427-CP. [DOI] [PubMed] [Google Scholar]
- 30.Selvin E, Juraschek SP, Eckfeldt J, Levey AS, Inker LA, Coresh J. Within-person variability in kidney measures. Am J Kidney Dis. 2013;61:716–22. doi: 10.1053/j.ajkd.2012.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kottgen A, Selvin E, Stevens LA, Levey AS, Van Lente F, Coresh J. Serum cystatin C in the united states: The third national health and nutrition examination survey (NHANES III) Am J Kidney Dis. 2008;51:385–94. doi: 10.1053/j.ajkd.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Bokenkamp A, Domanetzki M, Zinck R, Schumann G, Brodehl J. Reference values for cystatin C serum concentrations in children. Pediatr Nephrol. 1998;12:125–9. doi: 10.1007/s004670050419. [DOI] [PubMed] [Google Scholar]
- 33.Cherney DZ, Sochett EB, Dekker MG, Perkins BA. Ability of cystatin C to detect acute changes in glomerular filtration rate provoked by hyperglycaemia in uncomplicated type 1 diabetes. Diabet Med. 2010;27:1358–65. doi: 10.1111/j.1464-5491.2010.03121.x. [DOI] [PubMed] [Google Scholar]
- 34.Maahs DM, Bushman L, Kerr B, Ellis SL, Pyle L, McFann K, et al. A practical method to measure GFR in people with type 1 diabetes. J Diabetes Complications. 2014;28:667–73. doi: 10.1016/j.jdiacomp.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Marmarinos A, Garoufi A, Panagoulia A, Dimou S, Drakatos A, Paraskakis I, et al. Cystatin-C levels in healthy children and adolescents: Influence of age, gender, body mass index and blood pressure. Clin Biochem. 2016;49:150–3. doi: 10.1016/j.clinbiochem.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Muntner P, Winston J, Uribarri J, Mann D, Fox CS. Overweight, obesity, and elevated serum cystatin C levels in adults in the united states. Am J Med. 2008;121:341–8. doi: 10.1016/j.amjmed.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naour N, Fellahi S, Renucci JF, Poitou C, Roault C, Basdevant A, et al. Potential contribution of adipose tissue to elevated serum cystatin C in human obesity. Obesity (Silver Spring) 2009;17(12):2121–6. doi: 10.1038/oby.2009.96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.