Abstract
Recent studies indicate that C57BL/6J (B6) and FVB inbred mouse strains differ in post-oral fructose conditioning. This was demonstrated by their differential flavor conditioning response to intragastric fructose and their preference for fructose versus a non-nutritive sweetener. The present study extended this analysis to SWR and BALB/c inbred strains which are of interest because they both show robust flavor conditioning responses to fructose. In the first experiment, ad-libitum fed mice were given a series of 2-day, two-bottle preference tests between 8% fructose and a more preferred, but non-nutritive 0.1% sucralose +0.1% saccharin (S + S) solution (tests 1 & 4), and fructose or S + S versus water (tests 2 and 3). In test 1, SWR mice preferred S + S to fructose, and in tests 2 and 3, they preferred both sweeteners to water. In test 4, SWR mice switched their preference and consumed more fructose than S + S. In contrast, ad-libitum fed BALB/c mice strongly preferred S + S to fructose in both tests 1 and 4, although they preferred both sweeteners to water in tests 2 and 3. Food-restricted BALB/c mice also preferred the non-nutritive S + S to fructose in tests 1 and 4. The experience-induced fructose preference reversal observed in SWR, but not BALB/c mice indicates that fructose has a post-oral reinforcing effect in SWR mice as in FVB mice. Because B6 and FVB mice prefer glucose to fructose based on the post-oral actions of the two sugars, the second experiment compared the preferences of SWR and BALB/c mice for 8% glucose and fructose solutions. Ad-libitum fed and food-restricted SWR mice strongly preferred glucose to fructose. In contrast, ad-libitum fed BALB/c mice were indifferent to the sugars, perhaps because of their overall low intakes. Food-restricted BALB/c mice, however, strongly preferred glucose. These findings indicate that SWR and BALB/c mice differ in their preference response to the post-oral actions of fructose.
Keywords: Fructose, Glucose, Sucralose, Saccharin, Learning
1. Introduction
Sugar appetite in rodents depends on both stimulation of oral sweet taste receptors [1] and post-oral sugar sensors [13]. Inbred mouse strains vary in their taste response to sugars and non-nutritive sweeteners, which is attributed, in part, to genetic differences in the T1r3 component of the T1r2/T1r3 sweet taste receptor [9]. Some strains have a “sensitive” form of the receptor which results in increased preferences and intakes of a variety of nutritive and non-nutritive sweet solutions, while other strains have a “sub-sensitive” form of the receptor which produces reduced preferences and intakes of these sweetener solutions, particularly at low concentrations [1]. Sugar intake and preference are also influenced by post-oral nutritive effects via a process referred to as appetition to distinguish it from the satiation process that inhibits sugar intake [11,13]. Post-oral appetition is most clearly demonstrated by the intake and preference-stimulating effects produced by intragastric (IG) sugar infusions in mice and rats [13]. Conceivably, inbred strain variations in sugar preferences may be influenced by strain differences in post-oral appetition as well as by differences in sweet taste sensitivity. Sclafani and Glendinning [17] investigated this possibility in sweet-sensitive C57BL/6J (B6) mice and sub-sensitive 129 mice which differ substantially in their oral intakes of sucrose. Both strains, however, displayed similar post-oral appetition responses to IG sucrose infusions. This and other findings indicate that post-oral sugar appetition is not mediated by gut T1r3 receptors [16].
More recently, Sclafani and co-workers [18] observed a difference in post-oral sugar appetition in B6 and FVB mice, which are both sweet-sensitive strains with high oral intakes of sugar. In this case, the mice were tested with glucose and fructose. Whereas IG glucose infusions stimulated intake of, and preference for, a flavored (CS+) saccharin solution in both strains, IG fructose failed to condition preferences in B6 mice but conditioned significant CS+ preferences in FVB mice. The differential post-oral actions of fructose were also revealed in sugar vs. non-nutritive sweetener choice tests [18,19]. Like B6 mice, naïve FVB mice strongly preferred a 0.1% sucralose +0.1% saccharin (S + S) solution to 8% fructose in an initial 2-day two-bottle test. However, after the mice had separate 2-day choice tests with S + S and fructose versus water, the FVB mice preferred fructose to S + S, whereas the B6 mice continued to prefer S + S to fructose. Taken together, these data indicate that fructose has a post-oral reinforcing action in FVB mice which conditions a preference for the initially less-preferred 8% fructose over 0.1% S + S after separate experience with both sweeteners.
The present experiment extended our analysis of post-oral fructose appetition to SWR and BALB/c inbred mice, which are sweet-sensitive and sub-sensitive strains, respectively [9]. These strains were of interest because in a survey of inbred mouse strains, they both acquired strong preferences for a CS+ flavor added to an 8% fructose +0.2% saccharin solution over a CS− flavored 0.2% saccharin-only solution [7]. In contrast, B6 mice failed to prefer the fructose-paired CS+ flavor. At the time, the fructose-conditioned preference in the SWR and BALB/c mice was attributed to flavor-taste learning reinforced by the sugar’s sweet taste since fructose was known to have little or no post-oral reinforcing actions in B6 mice or Sprague–Dawley rats [12,14,15]. However, in view of the post-oral fructose appetition recently discovered in FVB mice [18], it is possible that the fructose-conditioned flavor preferences observed in SWR and BALB/c mice were due in part to post-oral conditioning in these strains. To evaluate this possibility, Experiment 1 determined the relative preference for fructose and S + S solutions in SWR and BALB/c mice before and after they had separate experience with the two sweeteners. As noted above, unlike B6 mice, FVB mice switch their preference from S + S to fructose after experience with the sweeteners which is indicative of post-oral fructose appetition. In a second experiment we compared the preference for 8% fructose and 8% glucose in the two strains which provides an index of the differential post-oral reinforcing actions of the two sugars.
2. Experiment 1: fructose vs. sucralose + saccharin preferences
2.1. Materials and methods
2.1.1. Animals
Adult male SWR and BALB/c mice obtained from the Jackson Laboratories (Bar Harbor, ME) were adapted to the laboratory for 1 week. The starting body weights of the SWR (25.6 g) and BALB/c mice (25.7 g) were similar. The animals were singly housed in plastic tub cages in a room maintained at 22 °C with a 12:12-h light–dark cycle and given ad libitum access to chow (LabDiet Standard Laboratory Rodent Diet #5001, PMI Nutrition International, Brentwood, MO) and water except where noted. Experimental protocols were approved by the Institutional Animal Care and Use Committee at Queens College and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
2.1.2. Test solutions
Solutions of 8% fructose (Sigma Aldrich Laboratories, St. Louis, MO) and a mixture of 0.1% sucralose (Tate & Lyle, Dayton, OH) and 0.1% saccharin (Sigma Aldrich Laboratories) (S + S) were prepared with tap water on a w/w basis because intakes were measured by weight. The S + S solution was selected based on the finding that B6 mice strongly preferred it to 8% fructose or 8% glucose in 1-min two bottle tests, suggesting that it was “sweeter” than the sugar solutions [19]. The solutions were available through stainless steel sipper spouts attached to 50-ml plastic tubes that were placed on the grid top of the cage and fixed in place with springs. Fluid intakes were measured to the nearest 0.1 g by weighing the drinking bottles on an electronic balance. Spillage in this study was minimal as demonstrated by recording the change in weight of two tubes that were placed on an empty cage.
2.1.3. Procedure
SWR mice (n = 8) and BALB/c mice (n = 10) were given ad-libitum access to chow and two bottles of water for 4 days. They were then given a series of 2-day two-bottle tests as in our prior study [18]: Test 1 (days 1–2): fructose vs. S + S; Test 2 (days 3–4): fructose vs. water; Test 3 (days 5–6): S + S vs. water; Test 4 (days 8–9): fructose vs. S + S. The mice were given water vs. water for one day (day 7) between Tests 3 and 4. The left-right position of the sweetener and water bottles were switched from the first to second day of each test to control for potential position effects.
Because daily fructose and S + S intakes of the BALB/c mice were rather low, which is characteristic of this strain [6,8], a second group of nine BALB/c mice was tested which had restricted access to food to stimulate their sweetener intakes. These mice were given daily chow rations that maintained their body weights at 85–90% of their ad libitum level for two weeks prior to testing, and throughout the four 2-bottle preference test series.
2.1.4. Data analysis
Daily solution intakes were averaged over the 2 days of each test, and sweetener preferences were expressed as percent solution intakes (e.g., fructose intake/total intake × 100). Intakes were analyzed using a mixed model analysis of variance (ANOVA) with test and solution as repeated factors. One ANOVA included results from Tests 1 (naïve mice) and 4 (experienced mice), and evaluated whether relative intakes of fructose and S + S changed across the two tests within groups. A second ANOVA included results from Tests 2 and 3, and compared the intakes of each sweetener vs. water within groups. Percent sweetener intakes within groups were analyzed with t-tests. Additional between groups ANOVAs were performed as described below.
2.2. Results
2.2.1. SWR mice
The SWR mice consumed more S + S than fructose in Test 1, but more fructose than S + S in Test 4, although only the Test 4 difference was significant (Sweetener × Test interaction, (F(1,7) = 94.4, p < 0.0001; Fig. 1A). Seven of the eight mice drank more S + S than fructose in the first test, whereas all 8 mice consumed more fructose than S + S in Test 4. The percent fructose intake increased from 39% in Test 1 to 66% in Test 4 (t(7) = 7.15, p < 0.0001). In Tests 2 and 3, SWR mice consumed more fructose and S + S than water (F(1,7) = 36.0, p < 0.0001) and their sweetener intakes and percent intakes did not differ.
Fig. 1.
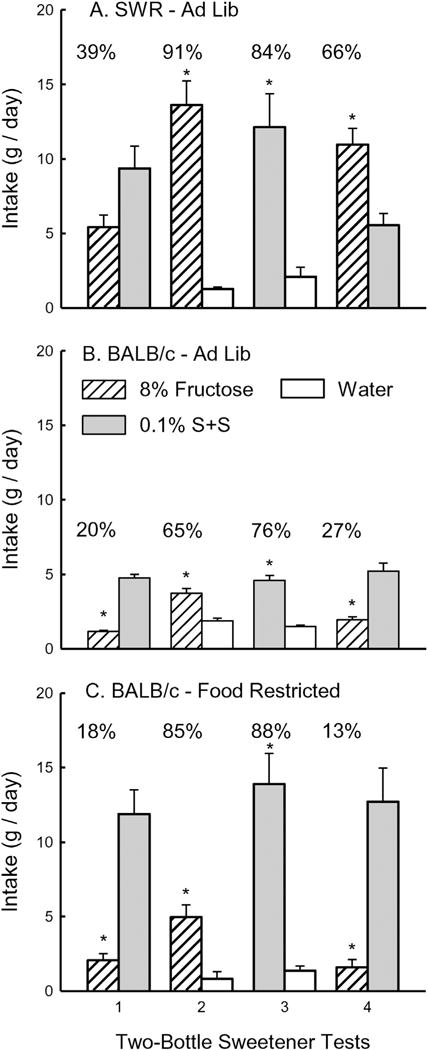
Fructose and sucralose + saccharin preference tests in BALB/c and SWR Mice. Intake (mean + SEM) in 2-day, two-bottle tests with 0.1% sucralose +0.1% saccharin (S + S) vs. 8% fructose (Test 1), fructose vs. water (Test 2), S + S vs. water (Test 3), and S + S vs. fructose (Test 4). A. Mean intakes of ad-libitum fed SWR mice in Tests 1–4. B. Mean intakes of ad-libitum fed BALB/c mice in Tests 1–4. C. Mean intakes of food-restricted BALB/c mice in Tests 1–4. Significant differences (p < 0.05) within each test are denoted by asterisks (*). Numbers atop bars represent mean percent preference for that solution.
2.2.2. BALB/c mice
The ad-libitum fed BALB/c mice consumed significantly more S + S than fructose in both Tests 1 and 4 (F(1,9) = 112.7, p < 0.0001), and their percent fructose intakes remained low in both tests (20% and 27%, Fig. 1B). Overall intakes increased from Test 1 to 4 (F(1,9) = 5.8, p < 0.05). In Tests 2 and 3, the mice consumed more fructose and S + S than water (F(1,7) = 36.0, p < 0.0001), and they also consumed more (p < 0.05) S + S than fructose (Sweetener × Test interaction, (F (1,7) = 12.1, p < 0.01). Their percent S + S intake also exceeded that of their percent fructose intake (76% vs. 65%, t(9) = 3.0, p < 0.05).
Similar to the ad-libitum fed mice, the food-restricted BALB/c mice consumed significantly more S + S than fructose in Tests 1 and 4 (F (1,8) = 33.9, p < 0.0001), and their percent fructose intakes were low in both tests (18% and 13%, Fig. 1C). In Tests 2 and 3, the mice consumed more fructose and S + S than water (F(1,8) = 31.3, p < 0.001) and they also consumed much more (p < 0.001) S + S than fructose (Sweetener × Test interaction, (F(1,8) = 45.1, p < 0.001). However, their percent S + S and fructose intakes relative to water did not to differ.
The food-restricted BALB/c mice consumed significantly more fluid than did the ad-libitum fed BALB/c mice in Tests 1 and 4 (F(1,17) = 32.3, p < 0.0001) as well as in Tests 2 and 3 (F(1,17) = 22.2, p < 0.001). In both cases, this was due primarily to the elevated S + S intakes of the food-restricted mice (Sweetener × Group interaction (F (1,17) = 15.4 and 16.1, p < 0.01). The percent sweetener intakes in Tests 2 and 3 of the food-restricted BALB/c mice also exceeded those of the ad-libitum fed BALB/c mice (F(1,17) = 5.5, p < 0.05; Fig. 1). Overall, the ad-libitum fed SWR mice consumed more sweetener than did the ad-libitum fed BALB/c mice in Tests 1 and 4 (F(1,16) = 124.7, p < 0.001) as well as in Tests 2 and 3 (F(1,16) = 31.1, p < 0.001). In contrast, the ad-libitum fed SWR and food-restricted BALB/c mice did not differ in their overall intakes in Tests 1 to 4, but they did differ in their sweetener intakes. Specifically in Test 4, the ad-libitum fed SWR mice consumed more fructose and less S + S than did the food-restricted BALB/c mice (Group × Test × Sweetener interaction, (F(1,16) = 58.3, p < 0.0001).
3. Experiment 2: fructose vs. glucose preferences
Our prior studies of B6 and FVB mice indicated that, while the two strains differed substantially in their post-oral conditioning response to fructose, both strains displayed stronger IG conditioning responses to glucose than to fructose [12,18]. Consistent with this finding, both strains also consumed significantly more glucose than fructose in separate sugar vs. water tests, and strongly preferred glucose to fructose in a 2-day, two-bottle test [18]. In view of these findings, Experiment 2 compared the relative preferences of SWR and BALB/c mice for isocaloric 8% fructose and 8% glucose. The BALB/c and SWR mice were tested under both ad-libitum and food-restricted conditions given the significant effect of food restriction on the sugar intakes and preferences of BALB/c mice observed in the first experiment.
3.1. MATERIALS and methods
The ad-libitum fed SWR (n = 7) and BALB/c (n = 9) mice from Experiment 1 were used except for one mouse from each strain that died after the first experiment. The mice were maintained on ad-libitum food and given a series of 2-day, two-bottle tests as follows: Test 1 (days 1–2) 8% fructose vs. water, Test 2 (days 3–4) 8% glucose vs. water, Test 3 (days 6–7) 8% fructose vs. glucose. The mice were given two-bottle access to water only on day 5 between tests 2 and 3. The mice were then food-restricted as in the first experiment and given the same series of preferences tests described above. The left-right position of the sugars was switched from the first to second day of each test to control for potential position effects.
3.2. Results
3.2.1. SWR mice
The SWR mice consumed much more sugar than water in Tests 1 and 2, and therefore an ANOVA was performed only on the sugar intake data (Fig. 2). When tested under ad-libitum food and food-restriction conditions, the SWR mice consumed significantly (p < 0.01) more glucose than fructose (F(1,6) = 45.6, p < 0.001) in the sugar vs. water tests (Tests 1 and 2). Percent glucose intakes were also higher than percent fructose intakes in both food availability conditions (F(1,6) = 5.7, p < 0.055). In addition, SWR mice consumed more glucose under food restriction than with ad-libitum food, while fructose intakes did not vary with deprivation state (State × Sugar interaction, F(1,6) = 5.6, P < 0.056). In Test 3, SWR mice in ad-libitum and food-restriction states consumed substantially more glucose than fructose (F(1,6) = 37.6, p < 0.001), but they did not differ in their absolute or percent sugar intakes (Fig. 2).
Fig. 2.

Fructose and Glucose preference tests in SWR Mice. Intake (mean, +SEM) in 2-day, two-bottle tests with 8% fructose vs. water (Test 1), 8% glucose vs. water (Test 2), and 8% glucose vs. 8% fructose (Test 3) in SWR mice. A. Mean intakes of SWR mice fed ad-libitum in Tests 1–3. B. Mean intakes of food-restricted SWR mice in Tests 1–3. Significant differences (p < 0.05) within each test are denoted by asterisks (*). Numbers atop bars represent mean percent preference for that solution.
3.2.2. BALB/c mice
Overall, BALB/c mice consumed more sugar under food restriction than ad-libitum feeding states in Tests 1 and 2 (F(1,8) = 9.5, p < 0.01), and they tended to consume more glucose than fructose, but this difference was not significant (Fig. 3). However, percent glucose intakes exceeded percent fructose intakes in both feeding states (F(1,8) = 5.6, p < 0.05). In Test 3, BALB mice drank significantly more glucose than fructose under food restriction, while they drank similar amounts of the two sugars with ad-libitum feeding (State × Sugar interaction, (F(1,8) = 8.1, p < 0.05)). In addition, more glucose was consumed in the food-restricted state than in the ad-libitum state (p < 0.01), whereas fructose intakes did not differ as a function of food availability. Consequently, the percent glucose intakes were higher in the food-restricted relative to the ad-libitum state (75% vs. 51%, t(8) = 3.56, p < 0.01).
Fig. 3.

Fructose and Glucose preference tests in BALB/c Mice. Intake (mean, +SEM) of 2-day, two-bottle tests with 8% fructose vs. water (Test 1), 8% glucose vs. water (Test 2), and 8% glucose vs. 8% fructose (Test 3) in BALB/c mice. A. Mean intakes of BALB/c mice fed ad-libitum in Tests 1–3. B. Mean intakes of food-restricted BALB/c mice in Tests 1–3. Significant differences (p < 0.05) within each test are denoted by asterisks (*). Numbers atop bars represent mean percent preference for that solution.
Overall, SWR mice consumed much more sugar than did BALB/c mice under ad-libitum and food-restricted conditions in Tests 1 to 3 (F(1,14) = 216.6, p < 0.0001). The ad-libitum fed SWR mice also displayed greater preferences for fructose and glucose over water than did the ad-libitum fed BALB/c mice (fructose: 88% vs. 68%; glucose: 93% vs. 75%, F(1,14) = 6.0, p < 0.05). In addition, the ad-libitum fed SWR mice displayed a greater preference for glucose over fructose in Test 3 than did the ad libitum fed BALB/c mice (87% vs. 51%, t(14) = 3.50, p < 0.01). In contrast, the food-restricted SWR and BALB/c mice did not significantly differ in their preferences for fructose and glucose over water in Tests 1 and 2 (fructose: 88% vs. 71%, glucose 98% vs. 82%) or their preference for glucose over fructose in Test 3 (78% vs. 75%).
4. Discussion
Experiment 1 investigated whether fructose has post-oral reinforcing actions in SWR and BALB/c mice as it does in FVB mice, but not B6 mice [18]. This outcome seemed plausible given the finding that SWR and BALB/c, but not B6 mice acquired significant preferences for a flavor mixed into a fructose + saccharin solution over a flavor mixed into a less preferred saccharin-only solution [7]. The finding that SWR mice reversed their initial preference for non-nutritive S + S over fructose in Test 1 to a fructose preference over S + S in Test 4 after separate experience with the two sweeteners in Tests 2 and 3 strongly indicates that fructose exerts post-oral reinforcing actions in this inbred strain. We previously observed a similar S + S to fructose preference shift in FVB mice exposed to the same test experience [18]. In addition, IG fructose infusions were found to condition flavor preferences in FVB mice. In contrast, B6 mice failed to develop a preference for fructose over S + S, although they strongly preferred glucose to S + S consistent with the post-oral reinforcing effect of glucose, but not fructose, observed in this strain [19].
In contrast to ad-libitum fed SWR mice, ad-libitum fed BALB/c mice did not reverse their preference for S + S over fructose, but rather displayed strong S + S preferences in both Tests 1 and 4. Because the ad-libitum fed BALB/c mice consumed less than half as much sweetener as did the SWR mice in the 2-day tests, it was possible that their low intakes provided insufficient post-oral feedback to enhance their fructose preference. We therefore tested a separate group of food-restricted BALB/c mice which consumed significantly more of the sweeteners than did the ad-libitum fed BALB/c mice. Yet they also did not reverse their preference for S + S over fructose. Their failure to develop a fructose preference is particularly noteworthy given their need for the energy provided by the sugar but not the S + S. We previously observed that food-restricted B6 mice also failed to develop a preference for nutritive fructose over non-nutritive S + S [19]. These findings challenge the notion that the energy value of sugars accounts for their preference over non-nutritive sweeteners ([2]; see also [19]).
In our earlier inbred mouse survey of sugar conditioning [7], we assumed that the fructose-conditioned flavor preference was due to the sweet taste of the sugar rather than a post-oral reinforcing action given the inability of IG fructose infusions to induce a flavor preference in B6 mice. The finding of fructose-conditioned preferences in sweet-sub-sensitive BALB/c mice (and other strains), but not sweet-sensitive B6 mice seemed inconsistent with this interpretation [7]. The B6 mice, unlike BALB/c mice, consumed more flavored saccharin than flavored fructose + saccharin solution during training, which suggested that the enhanced saccharin preference of B6 mice, relative to BALB/c mice, may have contributed to their failure to acquire a fructose-based flavor preference. However, this interpretation was questioned by the finding that SWR mice also consumed considerably more saccharin than fructose + saccharin during training yet acquired a preference for the CS+/fructose solution. That fructose has post-oral reinforcing actions in SWR, but not B6 mice appears to explain why SWR, but not B6 mice developed a fructose-based flavor preference in the earlier study [7]. The present findings, however, fail to explain why BALB/c, but not B6, mice acquired a fructose preference.
Given that B6 mice in general are more sensitive to sweeteners than BALB/c mice [4,8,9], the role of sweet taste sensitivity in fructose conditioning is questionable. Other data indicate that T1r3 sweetener sensitivity does not fully account for the hedonic or motivational response of inbred mice to sugars. For example, in short-term lick tests, which are thought to reflect taste hedonics, sweet sub-sensitive 129 mice licked at lower rates for dilute sucrose solutions than did sweet-sensitive B6 and SWR mice, but 129 mice licked as much or more for concentrated sucrose solutions as did B6 and SWR mice [4]. Also, whereas 129 mice licked less on a progressive ratio schedule for a 4% sucrose solution than did B6 mice, they actually licked more for a 16% sucrose solution than did the B6 mice [10]. These and other findings [3] indicate that multiple genetic factors contribute to variations in sugar intake, preference and motivation in inbred mouse strains. Thus, genetic and phenotypic differences other than those related to sweet taste sensitivity may be responsible for the differential flavor conditioning displayed by BALB/c and B6 mice in our prior study. Note that BALB/c mice also displayed a stronger sucrose-conditioned preference than did B6 mice (86% vs. 66%; [7]), indicating that the strains differ in their response to sugars that have post-oral reinforcing actions as well. Future studies should compare flavor conditioning in BALB/c and B6 mice using other nutritive and non-nutritive solutions to further characterize the differences in flavor/sugar learning in these strains.
Further work is also required to explain why the post-oral actions of fructose are reinforcing in some inbred mouse strains (FVB, SWR) but not others (B6, BALB/c). Recent sugar conditioning studies in B6 mice indicate that intestinal glucose transporters/sensors SGLT1 and SGLT3 have an important role in glucose conditioning in B6 mice [20]. These “transceptors,” which do not bind to fructose, presumably mediate glucose conditioning in FVB and SWR mice that also display fructose conditioning. Consistent with this view, our studies of sugar conditioning in FVB mice revealed that glucose was more potent than fructose in conditioning flavor preferences and had a more rapid time course [18]. The present finding that SWR and food-restricted BALB/c mice, like FVB and B6 mice, strongly prefer glucose to fructose in direct choice tests also suggests that different physiological processes mediate post-oral fructose and glucose reinforcement in these strains. The availability of inbred strains that differ in their post-oral conditioning response to fructose should facilitate the study of post-oral fructose reinforcement. Finally, the present findings indicating that SWR and BALB/c mice differ in their post-oral conditioning response to fructose are relevant to our recent report of differential actions of dopamine and opioid antagonist drugs on fructose-conditioned flavor preferences in the two strains [5].
HIGHLIGHTS.
SWR and BALB/c mice initially prefer sucralose + saccharin to fructose solutions.
SWR mice reverse this preference following experience with the two solutions.
Ad-libitum fed and food restricted BALB/c mice fail to reverse this preference.
Ad-libitum fed and food-restricted SWR mice prefer glucose to fructose solutions.
Food-restricted, not ad-libitum fed BALB/c mice display this glucose preference.
Acknowledgments
This research was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases grant DK031135 to AS and PSC/CUNY grants 41-62438, 42-336 and 43-232 to RJB. The authors thank Karen Ackroff for her helpful comments on this manuscript.
References
- 1.Bachmanov AA, Beauchamp GK. Taste receptor genes. Annu Rev Nutr. 2007;27:389–414. doi: 10.1146/annurev.nutr.26.061505.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beeler JA, McCutcheon JE, Cao ZF, Murakami M, Alexander E, Roitman MF, Zhuang X. Taste uncoupled from nutrition fails to sustain the reinforcing properties of food. Eur J Neurol. 2012;36:2533–2546. doi: 10.1111/j.1460-9568.2012.08167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boughter JD, Jr, Bachmanov A. Behavioral genetics and taste. BMC Neurosci. 2007;8:S3. doi: 10.1186/1471-2202-8-S3-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glendinning JI, Chyou S, Lin I, Onishi M, Patel P, Zheng KH. Initial licking responses of mice to sweeteners: effects of Tas1R3 polymorphisms. Chem Senses. 2005;30:601–614. doi: 10.1093/chemse/bji054. [DOI] [PubMed] [Google Scholar]
- 5.Kraft TT, Yakubov Y, Huang D, Fitzgerald G, Natanova E, Sclafani A, Bodnar RJ. Dopamine D1 and opioid receptor antagonists differentially reduce the acquisition and expression of fructose-conditioned flavor preferences in BALB/c and SWR mice. Physiol Behav. 2015;151:213–220. doi: 10.1016/j.physbeh.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis SR, Ahmed S, Dym C, Khaimova E, Kest B, Bodnar RJ. Inbred mouse strain survey of sucrose intake. Physiol Behav. 2005;85:546–556. doi: 10.1016/j.physbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Pinhas A, Aviel M, Koen M, Gurgov S, Acosta V, Israel M, Kakuriev L, Guskova L, Fuzailov I, Touzani K, Sclafani A, Bodnar RJ. Strain differences in sucrose- and fructose-conditioned flavor preferences in mice. Physiol Behav. 2012;105:451–459. doi: 10.1016/j.physbeh.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramirez I, Fuller JL. Genetic influence on water and sweetened water consumption in mice. Physiol Behav. 1976;16:163–168. doi: 10.1016/0031-9384(76)90300-0. [DOI] [PubMed] [Google Scholar]
- 9.Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24:938–946. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sclafani A. Sucrose motivation in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice measured by progressive ratio licking. Physiol Behav. 2006;87:734–744. doi: 10.1016/j.physbeh.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Sclafani A. Gut-brain nutrient signaling: appetition vs. satiation. Appetite. 2013;71:454–458. doi: 10.1016/j.appet.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sclafani A, Ackroff K. Flavor preferences conditioned by intragastric glucose but not fructose or galactose in C57BL/6J mice. Physiol Behav. 2012;106:457–461. doi: 10.1016/j.physbeh.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sclafani A, Ackroff K. The role of gut nutrient sensing in stimulating appetite and conditioning food preferences. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1119–R1133. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. Intragastric glucose but not fructose conditions robust flavor preferences in rats. Am J Physiol Regul Integr Comp Physiol. 1993;265:R320–R325. doi: 10.1152/ajpregu.1993.265.2.R320. [DOI] [PubMed] [Google Scholar]
- 15.Sclafani A, Fanizza LJ, Azzara AV. Conditioned flavor avoidance, preference and indifference produced by intragastric infusions of galactose, glucose and fructose in rats. Physiol Behav. 1999;67:227–234. doi: 10.1016/s0031-9384(99)00053-0. [DOI] [PubMed] [Google Scholar]
- 16.Sclafani A, Glass DS, Margolskee RF, Glendinning JI. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1643–R1650. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sclafani A, Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav. 2003;79:783–788. doi: 10.1016/s0031-9384(03)00174-4. [DOI] [PubMed] [Google Scholar]
- 18.Sclafani A, Zukerman S, Ackroff K. Fructose and glucose conditioned preferences in FVB mice: strain differences in post-oral sugar appetition. Am J Physiol Regul Integr Comp Physiol. 2014;307:R1448–R1457. doi: 10.1152/ajpregu.00312.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sclafani A, Zukerman S, Ackroff K. Post-oral glucose sensing, not caloric content, determines sugar reward in C57BL/6J mice. Chem Senses. 2015;40:245–258. doi: 10.1093/chemse/bjv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zukerman S, Ackroff K, Sclafani A. Post-oral appetite stimulation by sugars and non-metabolizable sugar analogs. Am J Physiol Regul Integr Comp Physiol. 2013;305:R840–R853. doi: 10.1152/ajpregu.00297.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


