Abstract
Youth with family history (FH+) of substance use disorders (SUDs) are at increased risk for developing SUDs. Similarly, childhood attention deficit hyperactivity disorder (ADHD) is considered to be a risk factor for developing SUDs. Recent research has suggested a close association between SUDs and impaired inhibitory control. As such, it is crucial to examine common and distinct neural alterations associated with inhibitory control in these at-risk groups, particularly prior to the initiation of heavy substance use. This paper reviews the functional magnetic resonance imaging (fMRI) literature of inhibitory control in these two at-risk youth populations (FH+ and ADHD), specifically considering studies that used motor response inhibition tasks (Go/No-Go or Stop Signal). Across the selected fMRI studies, we discovered no common alteration in the at-risk groups, but found neural alterations specific to each at-risk group. In FH+ youth and youth who transitioned into heavy substance use, blunted activation in the lateral part of the frontal pole (FP-lat) was most reliably observed. Importantly, longitudinal studies indicate that the blunted FP-lat activation may predict later SUDs, irrespective of the presence of FH+. In regards to ADHD, blunted activation was observed in the right dorsal anterior cingulate cortex (dACC) and left caudate. Of note, similar blunted dACC activation was also reported by one FH+ study, and thus, we cannot preclude a possibility that the right dACC activity may be a potential common alteration in both at-risk groups, particularly given a limited number of FH+ studies in the current review. Research challenges remain, and large-scale, longitudinal efforts will help determine the neurobiological markers predictive of SUDs among at-risk adolescents, including those with FH+, as well as those with ADHD and other psychiatric disorders.
Introduction
Children and adolescents with family history of substance use disorders (SUDs) are at increased risk for developing SUDs as compared to those without such family histories [1•]. Adoption studies highlight a genetic vulnerability demonstrating that, although adopted at birth, children of alcoholic biological parents develop alcohol use disorder (AUD) more frequently than do those of non-alcoholic parents [2]. Critically, environmental factors modulate genetic influences on adolescent substance use (SU), such as parental practice, substance availability, and peer pressure [3]. For example, when youth with family history of SUDs (FH+) receive little parental monitoring, the expression of genetic vulnerabilities enhances risk of SUD [3].
FH+ is not the only risk factor for SUDs. A recent large-scale study-based on the Nationally Comorbidity Survey-Adolescent Supplement has revealed that youth with childhood psychiatric disorders, especially anxiety and behavioral disorders, are also at increased risk for SUDs [4••]. In these psychiatric disorders, which can be genetically inherited in families, the pathway to SUD development differs. For youth with anxiety disorders, the expectation or desire that SU will reduce distress and anxiety may largely contribute to the development of SUDs [5]. In contrast, in youth with behavioral disorders, particularly attention deficit hyperactivity disorders (ADHD), impaired inhibitory control (the ability to withhold or suppress inappropriate, irrelevant, or undesirable actions or thought) may underpin the SUD trajectory [6].
Inhibitory control is a strongly heritable component of higher-order executive functions [7]. A recent meta-analysis has emphasized a close association between impaired inhibitory control (assessed by the Go/No-Go and Stop Signal paradigms) and current SUD symptomologies, except for cannabis use disorder [8••]. However, as described by another review [9•], impaired inhibitory control can be both a risk factor and a consequence of SUDs. If impaired inhibitory control is indeed a risk factor for developing SUDs, it is crucial to understand the neurobiological underpinnings of inhibitory control, prior to the initiation of SU, in youth at risk for SUDs.
Over the past decade, neuroimaging studies have revealed structural and functional deficits in the brain regions (e.g., the prefrontal cortex [PFC]) underlying inhibitory control in individuals with SUDs [10]. Such alterations have also been reported in substance-naive FH+ youth [11••] and youth with ADHD [12,13], indicating that impaired inhibitory control may constitute a common risk factor for SUDs. Of note, neuroimaging studies of anxiety disorders have not focused on inhibitory control, partially because behavioral evidence for inhibitory control deficits remains inconsistent in this clinical population [14].
In this review, we focus primarily on functional magnetic resonance imaging (fMRI) studies, exploring common and distinct neural alterations associated with inhibitory control in two at-risk populations: youth with FH+ and youth with ADHD. Given a relatively limited number of fMRI studies comparing FH+ and FH− youth groups, we also included longitudinal fMRI studies that followed youth who transitioned into heavy substance use and those who remained non-users. We selectively target fMRI studies using the Go/No-Go and/or Stop Signal paradigms—two of the most commonly used inhibitory control paradigms. These tasks assess the ability to withhold a motor response (hereinafter “motor response inhibition”). By limiting the focus of our review to just these two tasks, we aim to mitigate inconsistencies in fMRI results of inhibitory control, which likely stem from the inclusion of several other tasks (e.g., Stroop) that tap different aspects of inhibitory control [11••].
The goal of this review is an enhanced understanding of the neurobiological substrates that underlie motor response inhibition in youth at risk for SUDs (FH+, ADHD), particularly focusing on brain activation patterns prior to the initiation of heavy SU. This focus could eliminate or minimize the effect of elevated SU on fMRI results, potentially identifying neurobiological risk markers of SUDs in at-risk youth populations. Such an understanding could help to develop effective and targeted early-prevention or intervention strategies for SUDs.
Literature search
To select articles, we first searched previously published meta-analytic reviews of fMRI studies conducted in youth with FH+ (e.g., [11••, 15•]) and those with ADHD (e.g., [12,13]). Additionally, we searched Medline/PubMed for key words such as ‘substance use disorders’; ‘family history’; ‘ADHD’; ‘children’; ‘adolescents’; ‘fMRI’; ‘Go/No-Go’; ‘Stop Signal’; and ‘inhibitory control’. Selected articles had to report studies that (1) used task-based fMRI during either Go/No-Go or the Stop Signal paradigm in youth with FH+; those who transitioned into heavy substance use; or those with ADHD; (2) provided either Talairach or MNI peak coordinates of activated regions/clusters (note that given paucity of studies; manuscripts with uncorrected thresholding were included); and (3) investigated individuals aged ≤20 years old; this is the maximum age used in the Pediatric Imaging; Neurocognition; and Genetics Study; a well-designed large-scale study that investigated brain and cognitive development in adolescents.
The purpose setting the age limit (≤20 years old) is that SU initiation, which occurs during early adolescence, is typically followed by a steep escalation of SU throughout adolescence [16]. This rationale is further strengthened by the clinical observation that early academic and social failures in adolescents with ADHD potentially amplify the risk for antisocial or risk-taking behaviors that are in turn associated with later SUDs [17]. From a neurobiological perspective, adolescence is the period during which the structure and function of the PFC, a core region regulating inhibition [18], undergo rapid maturation [19]. Such developmental changes in the adolescent brain can influence risk-taking behaviors in youth [20].
Tables 1 and 2 in Supplementary materials summarize study fMRI results-based on group comparisons between youth with FH+ and youth with no family history of SUDs (FH−), and those between youth with and without ADHD, respectively. Given a small number of fMRI studies of motor response inhibition for FH+ youth, we also included longitudinal fMRI studies comparing youth who transitioned into heavy substance use to those who remained non-users, in Table 1 in Supplementary materials. In these longitudinal studies, the two youth groups were matched on family history density levels at baseline (the initial time point). Each table includes information regarding study design (cross-sectional or longitudinal), number of participants, age range, task (either Go/No-Go or Stop Signal), task stimulus (e.g., alphabets), task performance, contrasts, statistical analysis (i.e., whole brain or region of interest; p value for cluster-thresholding), and main fMRI findings showing significant group differences (FH+ vs. FH−; those who transitioned into heavy use vs. those who remained non-users; ADHD vs. controls). Although fMRI findings were based on simple group comparisons (i.e., baseline fMRI activations were compared for the longitudinal studies), we also reported findings from “group × time” interactions for the longitudinal studies. Additionally, Table 1 in Supplementary materials includes the definition of family history (or family history density) and SU status (i.e., naïve, minimal), whereas Table 2 in Supplementary materials includes ADHD subtypes.
Crucially, to compare results across all studies, we unified the coordinate systems used to the MNI system (i.e., converting peak Talairach coordinates into MNI ones, using the Yale BioImage Suite Package [21], http://sprout022.sprout.yale.edu/mni2tal/mni2tal.html). Subsequently, each set of peak MNI coordinates was assigned to the corresponding Brodmann Area (BA) using the Yale BioImage Suite Package, and was also relabeled onto the corresponding brain region-based on the Harvard–Oxford Atlas (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases). The latter atlas provides an estimate of the probability that a given voxel in MNI space belongs to a particular region. Our relabeling was based on the highest probability value. However, when the highest and the second highest probability values for a given set of MNI coordinates were close to each other (discrepancy <15%), both regions were reported in the tables.
To explore common and distinct neural alterations in the two at-risk groups, we first determined the frequency of reported activations (i.e., greater and blunted activations relative to controls) in each region/label for each group. Note that for purposes of this review, a region/label in the left hemisphere and its homologue in the right hemisphere are treated as two separate regions/labels. Next, we identified regions/labels, for which a significant activation was reported by ≥2 studies in each group: these regions were considered as loci exhibiting reliable activation in each group. For each of the identified regions, we calculated the ratio – the number of its activations to the total number of activations across all regions – in each group. The calculated ratios were converted in percentages (e.g., the percentage for a given region would be 30% if 3 activations were reported for that region, with a total number of 10 activations across all regions). Finally, among the identified regions in each group, regions reported in both groups and those reported only in each group separately were considered as common and distinct loci of neural alterations, respectively.
Motor response inhibition tasks
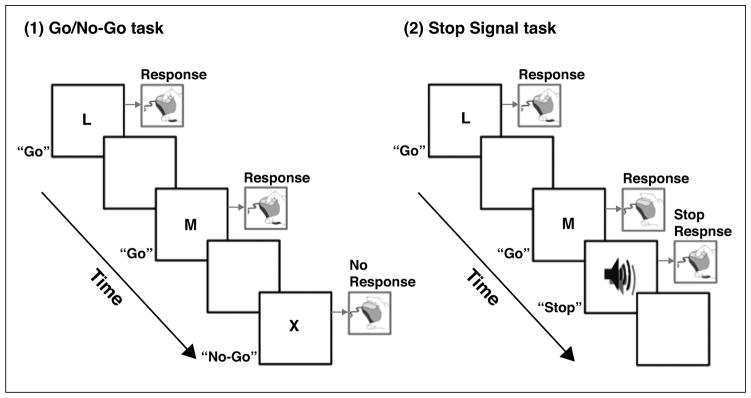
In both the Go/No-Go and Stop Signal paradigms, participants are instructed to withhold a motor response when a No-Go or Stop Signal is presented (Figure 1). In the Go/No-Go task, participants are required to respond (e.g., press the key) only to “Go” stimuli and withhold responses to “No-Go” stimuli. Commission errors (i.e., the inappropriate response to the No-Go stimulus) typically provide the behavioral index of motor response inhibition. In the Stop Signal task, participants are instructed to respond to Go stimuli, but on some trials, the Stop Signal (e.g., a beep) is presented after a Go stimulus with a variable delay, requiring participants to withhold responses. The estimate of time needed to respond to the Stop Signal is referred to as the Stop Signal Reaction Time, which indexes response inhibition. When the Stop process (delay + Stop Signal Reaction Time) terminates before the Go process (Go reaction time), response inhibition is considered successful (i.e., no response is emitted).
Figure 1.
Schematic diagrams illustrating the Go/No-Go task and the Stop Signal task.
In neuroimaging research, regional brain activations associated with motor response inhibition are assessed by specific contrasts, most commonly the correct “No-Go minus Go” (or “No-Go minus Rest”) trials on the Go/No-Go task and the correct “Stop minus Go” trials on the Stop Signal task. These contrasts activate a distributed array of similar brain regions [22], particularly frontal regions (e.g., inferior frontal gyrus and insula). In regards to the current review, reduced activation in prefrontal circuits during response inhibition is consistently found in individuals with SUDs [10] and those with ADHD [12].
Typical development of motor response inhibition
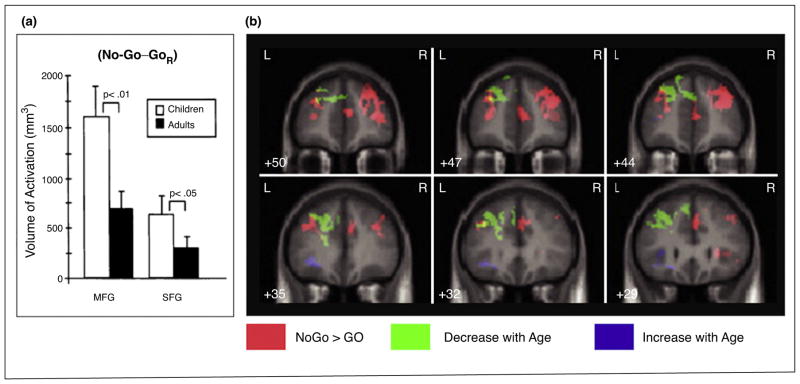
Inhibitory control functions, including motor response inhibition, improve with age; better performance is observed in reduced reaction times and commission errors [23]. Similarly, patterns of brain activation during motor response inhibition (e.g., No-Go minus Go) exhibit developmental changes. Across Go/No-Go fMRI studies in non-clinical populations, age-related decreases in the PFC, including the middle frontal gyrus and superior frontal gyrus, have been consistently reported, as shown in Figure 2A [24]. Moreover, Tamm et al. [23] have demonstrated developmental changes in prefrontal activation during the Go/No-Go task in adolescents (age range: 8–20 years): age-related decreases in the medial and lateral parts of the left frontal pole (FP), extending into the superior frontal gyrus, but age-related increases in the left inferior frontal gyrus/insula. For the equivalent contrast on the Stop Signal task (correct Stop minus correct Go), age-related decreases in activation were found in the supragenual anterior cingulate cortex in individuals aged 9–30 years [25]. The greater prefrontal activation, particularly in the FP, observed in children as compared to adults, may reflect an increased involvement of effortful inhibitory control, which lessens as inhibitory control becomes more automatic/less effortful with age. Taken together, these previous findings could indicate that the developmental shift to lesser FP activation and greater activation in the ventral parts of the PFC (e.g., inferior frontal gyrus) during motor response inhibition characterize a typical developmental pattern.
Figure 2.
A. The volume of activation associated with the Go/No-Go task in children and adults. The bilateral middle frontal gyrus (MFG) and superior frontal gyrus (SFG) showed greater volumes of activation in children (age range: 7–12 years) than adults (age range: 21–24 years) to the No-Go minus Go contrast. From Casey et al. [24]. B. Developmental shifts in the blood oxygen level dependent activation associated with the Go/No-Go task. Activation of regions in pink demonstrates the No-Go minus Go contrast. Activation of regions in green, including the left frontal pole/superior frontal gyrus (MNI -14 45 51: BA8), decreases with age (age range: 8–20 years), whereas activation of regions in purple, including the left inferior frontal gyrus and insula (MNI -34 12 6: BA13) increases with age. Modified from Tamm et al. [23].
Motor response inhibition in FH+
Definitions of FH+ vary, but previous studies have largely defined FH+ as having at least one biological parent and two (or more) second-degree relatives diagnosed with SUDs. In contrast, individuals with FH− have no familial SUDs in first or second-degree relatives [15]. In addition to this categorical, dichotomous definition (FH+ vs. FH−), a continuous measure of family history density can index the degree of FH+: each parent with a history of SUDs contributes to 0.5, and each grandparent with SUD history adds 0.25 to the score (range 0–2). Using either of these definitions, we found five fMRI studies [26,27,28••,29,30••] (i.e., all of them used cluster-thresholding) that examined motor response inhibition in youth with FH+, two of which were longitudinal studies that had both baseline and follow-up scans in youth who transitioned into heavy substance use. It is worth mentioning that the majority of these fMRI studies (Table 1 in Supplementary materials) examined AUD, which is not surprising given that alcohol is one of the most widely available and used substances during adolescence (http://www.samhsa.gov/disorders/substance-use). This dominant pattern in the selected FH+ studies may also reflect an urgent need to further understand AUD in adolescence.
Across these studies, while task performance (e.g., commission errors, reaction times) was comparable between the FH+ and FH− groups (and also between those who transitioned into heavy use [“Future-User” in Table 1 in Supplementary materials] and those who remained non-users), significant group differences in brain activations were reported. As shown in Figure 3, blunted activity in the ventral part of the FP has been most frequently reported in FH+ relative to FH− (for comparisons between “Future-Users” and non-users, baseline activations were examined). Given that the FP, corresponding to BA10, is the largest single architectonic area in the human brain, we divided it into three subregions (lateral, medial, and orbital) according to recent parcellation diffusion tensor imaging results [31]. This analysis-based on the FP subregions revealed that the lateral FP (FP-lat) was consistently the most reported subregion; the left FP-lat was reported by three studies and the right FP-lat by two studies; the left medial FP (FP-med) and the right FP-med were each reported by one study. Additionally, three studies reported blunted activation in subcortical regions in FH+, though the anatomical location reported differed across these studies (the right thalamus, left putamen, left pallidum). No study has reported greater activations in FH+ (or greater baseline activations in Future-Users) for the “No-Go minus Go” contrast.
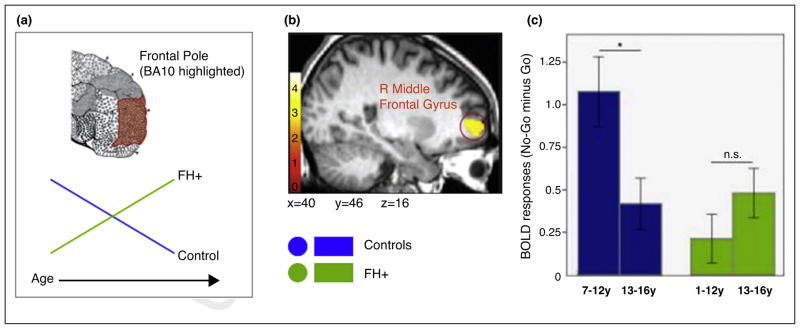
Figure 3.
A. The schematic diagram illustrating the developmental shift in the frontal pole (FP) activation (BA10 highlighted) during the Go/No-Go task. The green and blue lines represent individuals with FH+ and controls, respectively: B. The blood oxygen level dependent activation associated with the “No-Go minus Go” contrast in the right lateral FP (FP-lat) that exhibits “age × FH status” interaction. Note that originally this region was labeled as “R Middle Frontal Gyrus”, but we relabeled it as the right FP-lat based on both the Harvard–Oxford Atlas and the FP subregions defined by Liu et al. [31]. C. The bar graph showing the effect of “age × FH status” interaction on the right FP-lat activation. The blunted activation at baseline (age range: 7–12 years) increased with age in the FH+ group (right, green bars), but the opposite pattern of developmental shift characterized controls (left, blue bars). From Hardee et al. [28••].
Interestingly, the FP-lat activation during motor response inhibition seems to undergo differential developmental trajectories in FH+ and FH−. For example, youth with FH+ (of AUD) exhibited blunted activation in the right FP-lat at baseline (7–12 years) and an age-related increase (13–16 years) in the same region (Figure 3C, right) [28]. This FP-lat activation pattern is opposite to that reported for the typically developing youth, as shown by this study (Figure 3C, left) [28], and supported by others (e.g., [23]). A similar developmental dissociation for FP-lat activation has been reported in youth who transitioned into heavy drinking and those who remained non-drinkers [26,27]. For example, in the future drinking group, bilateral FP-lat activation during motor response inhibition was blunted at baseline (11–16 years) but increased with age (14–19 years). This pattern was different from the expected age-related decrease observed in the non-drinking group [27]. Critically, in these studies, family history density of AUD was matched between the two groups, indicating that the observed FP-lat activation pattern may not be driven by FH+ status.
Our findings from both FH+ youth and those who transitioned into heavy drinking indicate that blunted FP-lat activation prior to the initiation of SU may serve as a neurobiological risk marker that predicts the development of SUDs, rather than solely characterizing FH+. We speculate that heavy alcohol use and other environmental factors (e.g., stress [3]) may have contributed to the increased FP-lat activation in the future drinking group. Future studies using a longitudinal approach could help demarcate potential mechanisms underlying predictive associations between blunted baseline FP-lat activation and future SUDs.
Motor response inhibition in ADHD
ADHD is characterized by developmentally inappropriate inattentiveness, impulsivity, and hyperactivity. For diagnostic purposes, its symptoms must have been present before the age of 12. ADHD is particularly of interest to SUD research considering common behavioral impairments (including deficits in inhibitory control) and dopaminergic abnormalities [32]. Impaired inhibitory control is considered to be a core deficit in the hyperactive/impulsive and combined (with both inattentive and hyperactive/impulsive symptoms) subtypes of ADHD, rather than in the primarily inattentive subtype of ADHD [33].
Across the selected eight ADHD studies [34–41], two studies reported that ADHD youth exhibited blunted activation in the right dorsal anterior cingulate cortex (dACC), extending into the paracingulate cortex (note that one additional study reported the left dACC reduction). Similarly, two studies reported blunted activation in the left caudate (one study reported the left sided reduction, and another study reported the bilateral caudate reduction) (Table 2 in Supplementary materials). This pattern of reduced activations remains even after excluding studies without using cluster-thresholding, and is largely consistent with recent meta-analytic reviews [12,13].
Interestingly, the two studies examining medication-naïve ADHD youth have reported ADHD-related hypoactivations in the ventral part of the PFC within and adjacent to the orbitofrontal cortex. Although the exact locations (i.e., regions/labels) differ in these studies, this observation indicates that dysfunctions in the orbitofrontal cortex and adjacent regions may be inherent neural abnormalities in ADHD youth, independent of treatment. In addition to hypoactivations in the ventral PFC, hyperactivations were noted in an array of regions, although no region was reported by ≥2 studies, indicating the need for further investigation.
To date, neither prospective nor longitudinal studies have examined whether ADHD-specific activations prior to the initiation of SU predict later SUDs. Thus, possible predictive associations between ADHD biomarkers and the development of SUDs during adolescence remain to be explored. One study in adult cannabis users with a childhood diagnosis of ADHD has demonstrated blunted fronto-parietal activation during motor response inhibition (Go/No-Go), irrespective of cannabis use history (four groups were examined: childhood ADHD with and without cannabis use; no childhood ADHD with and without cannabis use) [42•]. This retrospective finding, together with previous fMRI findings from youth with ADHD (Table 2 in Supplementary materials), suggests that blunted fronto-parietal activation during motor response inhibition may be characteristic of ADHD, irrespective of age or SUDs. However, given that cannabis use disorder, unlike other SUDs (e.g., alcohol, cocaine), is not specifically associated with deficits in response inhibition [8], it is vital to longitudinally examine a potential interaction effect of “childhood ADHD × the use of other substances” on brain activation during motor response inhibition.
Common activations in FH+ and ADHD
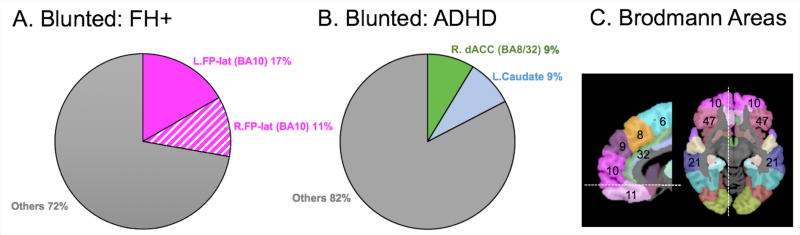
Figure 4 illustrates the percentage of regions/labels showing blunted activations that were reported by ≥ 2 studies in each at-risk group: A and B for blunted activations in youth with FH+ (including those who transitioned into substance use) and those with ADHD, respectively. Figure 4C depicts some Brodmann Areas (BAs) relevant to the results in this review. There seems to be no common alterations in FH+ and ADHD groups. Notably, the left FP-lat, a region reported by three FH+ studies, was not reported by any of the selected ADHD studies, although one ADHD study reported blunted activation in the right FP-lat and the orbital part of the right FP. Although family history of SUDs or current SU levels are likely to influence fMRI activation during motor response inhibition, as indicated by the current FH+ findings and others [10], the majority of the selected ADHD studies did not provide this critical information. Particularly when considering high risks of SUDs in ADHD youth, it is crucial to consider FH and current SU in this clinical population. Further research needs to longitudinally examine young children with ADHD and follow them into late adolescence and early adulthood, during which the risk of SUDs is escalated [20].
Figure 4.
A. Percentages of regions exhibiting blunted activation in FH+ youth. B. Percentages of regions exhibiting blunted activation in ADHD youth. To calculate the percentages, we used the following steps: (1) to determine the frequency of reported activations (i.e., greater and blunted activations relative to controls) in each region/label for each group; (2) to identify regions/labels, for which a significant activation was reported by ≥2 studies in each group; (3) To calculate the ratio – the number of the activations for each identified region to the total number of the reported activations across all regions reported – for each group; (4) to convert the calculated ratios into the percentages. No consistent pattern (i.e., reported by ≥2 studies) for greater activation was observed for either FH+ or ADHD youth. C. Brodmann Area (BA). BA numbers are shown in the corresponding frontal and temporal regions. R. = Right; L. = Left; FP-lat = Frontal Pole lateral; dACC = dorsal Anterior Cingulate Cortex.
Of note, we did not find any significant alterations in regions known to underlie motor response inhibition (e.g., inferior frontal gyrus, insula). This negative result may be attributed to our focus on group comparisons “prior to the heavy SU”. For example, when comparing youth with heavy SU to those with light SU, blunted activations in the inferior frontal gyrus and insula during motor response inhibition have been reported [43], indicating that these regions’ activations may capture neurobiological alterations associated with inhibitory control in the current SUDs, rather than those predicting subsequent SUDs. However, these regions’ activations during motor response inhibition undergo developmental changes (i. e., age-related increase) in normally developing children [23]. This may explain the inconsistent results for the inferior frontal gyrus opercularis in the current work (i.e., one study showed an ADHD-related increase in children ages 6–10, whereas another showed an ADHD-related reduction in children ages 9–16). Future research with large-scale, longitudinal examinations on these known regions for motor response inhibition in the at-risk groups could provide valuable insights into developmental pathways linking initial neurobiological risks to subsequent SUDs.
Distinct activations specific to FH+
As mentioned above, the blunted activation in the FP-lat is specific to FH+ youth and youth who transitioned into heavy substance use. The FP-lat is involved in cognitive branching, the ability to hold in mind one goal while performing concurrent subgoals [44]. Importantly, this cognitive function is required for appropriate motor response inhibition in the Go/No-Go and Stop Signal tasks. In the selected FH+ studies, the performance on motor response inhibition was statistically comparable between FH+ and FH− groups (and also between future substance users and future non-users). However, the presence of neural alteration in the FP-lat, prior to the heavy SU, indicates that vulnerability of this brain region may predict the development of SUDs.
It is worth mentioning that in fMRI studies using tasks that assess domains other than inhibition (e.g., monetary tasks), the FP-lat has rarely been reported as a locus of abnormalities in both FH+ youth and those who transitioned into heavy substance use. This indicates that the prediction capacity of FP-lat activation may be specific to motor response inhibition. Yet, this region’s activation may be informative in different stages on the path to the development of SUDs in FH+ individual; Kareken et al. [45] has demonstrated that the right FP-lat activation during the Signal Stop task at the placebo condition (i.e. saline infusion) was significantly decreased at the alcohol infusion condition in FH− AUD adults, but that this shift in the FP-lat activation pattern was absent in FH+ AUD adults (a statistically non-significant increase observed in this group). These findings suggest that less susceptibility to (or greater resistance to) the effect of alcohol exposure in FP-lat activity may characterize FH+. Longitudinal fMRI investigations on FH+ individuals, specifically before, during, and after the development of SUD, merit further research.
Distinct activations specific to ADHD
Among the regions reported (Table 2 in Supplementary materials), a consistent pattern was observed in the right dACC and left caudate, which is in line with previous meta-analysis findings in ADHD [12,13]. In the fMRI literature, the dACC is thought to play a central role in cognitive control functions, including response inhibition and error detection [46], and is sensitive to the effects of stimulant medications (e.g., methylphenidate) in children with ADHD [47]. Moreover, abnormal activation in this region has been reported in individuals with other psychiatric disorders, such as schizophrenia, some of which are comorbid with SUD [48]. Importantly, blunted dACC activation is observed in those with SUDs [10], and also was reported by one of the selected FH+ study included in the current review [28]. Particularly given a limited number of the selected Fh+ studies, we cannot rule out the possibility that the dACC may be commonly dysfunctional in both at-risk populations.
Interestingly, unlike FH+ youth, ADHD youth (relative to controls) exhibited atypically increased activations in a distributed array of regions, although its pattern was not consistent (i.e., not reported by ≥2 studies in this group). Of note, one study reported ADHD-related increase in the posterior part of the middle temporal gyrus in the left hemisphere. Another study reported increase in the homologue region in the right hemisphere. The observed increase in these clusters and others may reflect a compensatory mechanism for inefficient response inhibition in ADHD individuals, as previously suggested [40]. Consistent with this suggestion, in our review, some studies reported lower accuracy in youth with ADHD relative to those without ADHD, indicating impaired behavioral response inhibition, and the need to deploy compensatory mechanisms in ADHD youth.
Limitations
The current review has several limitations. First, and most notably, relative to studies in ADHD youth, the number of the selected studies that examined FH+ youth was limited. Second, the sample size of the selected FH+ and ADHD studies was relatively small. Third, statistical approaches (e.g., cluster-thresholding) varied across the studies. Fourth, our analysis focused on peak coordinates, as well as their corresponding BAs and brain regions/labels (based on the Harvard–Oxford atlas). This approach is unable to fully describe the spatial extent of volumes activated across different studies, thus our findings could be biased towards larger brain regions, although we divided the biggest region, FP, into three subregions. Fifth, the information about SU status in ADHD youth was scarce in the selected ADHD studies, and only a few FH+ studies reported ADHD diagnosis. Finally, the ADHD studies included differential ratios of ADHD subtypes, which may exert differential impact on brain activation patterns. For example, the literature indicates that inattentive symptoms are not associated with SU in adolescents [49•], and also that individuals with predominantly inattentive subtype, relative to those with other ADHD subtypes, may have differential inhibitory control deficits (and thus dissimilar underlying neural mechanisms for inhibitory control) [50]. Large-scale, longitudinal examinations, such as those planned for the Adolescent Brain and Cognitive Development (ABCD) study (http://abcdstudy.org/), could aid in overcoming these limitations, which may impact sensitivity and reliability of the combined/aggregated fMRI results. The ABCD study will administer a wide range of behavioral and neuroimaging measures to approximately 10 000 children (starting at the ages of 9 and 10 years), following them into early adulthood, and thus potentially opening a highly promising avenue to understand and treat SUDs.
Conclusions
The current literature review aims to identify common and distinct alterations in fMRI activity during motor response inhibition in two at-risk groups for SUDs, youth with FH+ (including youth who transitioned into heavy substance use) and youth with ADHD. We highlight distinct patterns in each group (e.g., the FP-lat for FH +; the right dACC and left caudate for ADHD). There appears to be no common alteration between these two at-risk groups for SUDs, but a small number of the selected studies does not preclude a possibility of common alterations (e.g., dACC). Importantly, blunted activation in the FP-lat, prior to the heavy initiation of SU, could serve as a neurobiological risk marker that predicts later SUDs, irrespective of the presence of FH+. The potential value of this finding needs to be longitudinally explored, preferably in youth with ADHD and some of whom would transition into heavy substance use. Given the increased risks of SUDs in youth with ADHD, it is crucial to monitor developmental shifts in activations during motor response inhibition in this population, particularly targeting the dACC and the caudate, regions that exhibited consistent ADHD-specific alterations (i.e., blunted activation) in the current review. Research challenges remain in the field, but large-scale, longitudinal efforts, such as the ABCD study, will help to identify the neurobiological markers that predict SUDs in at-risk adolescents, including those with FH+, as well as those with ADHD and other psychiatric disorders.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse (grant number 1U01DA041174-01 to R.Z.G). We thank Ms. Jameson Mitchell for proofreading the manuscript.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cobeha.2016.12.006.
Footnotes
Conflict of interest
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1•.Mellentin AI, et al. The risk of offspring developing substance use disorders when exposed to one versus two parent(s) with alcohol use disorder: a nationwide, register-based cohort study. J Psychiatr Res. 2016;80:52–58. doi: 10.1016/j.jpsychires.2016.06.001. This population-based cohort study investigates whether exposure to one vs. two parent(s) with alcohol use disorder (AUD) would increase the risk of offspring developing AUD. The risk is additive for offspring exposed to double parental AUD, providing important implications for clinical assessment and intervention strategies for these at-risk populations. [DOI] [PubMed] [Google Scholar]
- 2.Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103:1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- 3.Meyers JL, Dick DM. Genetic and environmental risk factors for adolescent-onset substance use disorders. Child Adolesc Psychiatr Clin N Am. 2010;19:465–477. doi: 10.1016/j.chc.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Conway KP, et al. Association of lifetime mental disorders and subsequent alcohol and illicit drug use: results from the national comorbidity survey-adolescent supplement. J Am Acad Child Adolesc Psychiatry. 2016;55:280–288. doi: 10.1016/j.jaac.2016.01.006. The findings from this large-scale cross-sectional study (i.e., adolescents of age 13 to 18 in US) provide the first evidence that prior mental disorders, particularly anxiety and behavioral disorders, represent risk factors for the transition from nonuse to use, and the progression to drug-and alcohol-related problems. [DOI] [PubMed] [Google Scholar]
- 5.Hussong AM, et al. An internalizing pathway to alcohol use and disorder. Psychol Addict Behav. 2011;25:390–404. doi: 10.1037/a0024519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SS, et al. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schachar RJ, et al. Heritability of response inhibition in children. J Int Neuropsychol Soc. 2011;17:238–247. doi: 10.1017/S1355617710001463. [DOI] [PubMed] [Google Scholar]
- 8••.Smith JL, et al. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend. 2014;145:1–33. doi: 10.1016/j.drugalcdep.2014.08.009. This meta-analysis study emphasizes that substance use disorders and addiction-like behavioral disorders (e.g., cocaine, alcohol, tobacco) are associated with impairments in inhibitory control, measured by two experimental paradigms, the Go/NoGo task and the Stop-Signal Task. [DOI] [PubMed] [Google Scholar]
- 9•.Lopez-Caneda E, et al. Impact of alcohol use on inhibitory control (and vice versa) during adolescence and young adulthood: a review. Alcohol Alcohol. 2014;49:173–181. doi: 10.1093/alcalc/agt168. The review examines the changes on brain development and inhibitory function during adolescence, as well as the relationship between inhibitory control and alcohol use at this early age. Poor inhibitory control can be both the cause and the consequence of excessive alcohol use. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Heitzeg MM, et al. Neuroimaging risk markers for substance abuse: recent findings on inhibitory control and reward system functioning. Curr Addict Rep. 2015;2:91–103. doi: 10.1007/s40429-015-0048-9. This comprehensive review summarizes recent findings from fMRI studies that investigated risk markers for substance use disorders (SUD) in children, adolescents, and emerging adults, specifically in the domains of inhibitory control and reward responsiveness. The convergent findings suggest that the association between SUD risk and neurofunctions may be moderated by age, gender, and history of substance use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortese S, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hart H, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 14.Oosterlaan J, Sergeant JA. Inhibition in ADHD, aggressive, and anxious children: a biologically based model of child psychopathology. J Abnorm Child Psychol. 1996;24:19–36. doi: 10.1007/BF01448371. [DOI] [PubMed] [Google Scholar]
- 15•.Cservenka A. Neurobiological phenotypes associated with a family history of alcoholism. Drug Alcohol Depend. 2016;158:8–21. doi: 10.1016/j.drugalcdep.2015.10.021. This review provides an overview of functional alterations associated with inhibitory control and working memory in youth and adults with family history of alcohol use disorder (AUD), relative to those without such history. The authors suggest that neural markers of executive functioning may be related to increased vulnerability for developing AUDs in individuals with family history of AUD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wells JE, et al. Onset and lifetime use of drugs in New Zealand: results from Te Rau Hinengaro: the New Zealand Mental Health Survey 2003–2004. Drug Alcohol Rev. 2009;28:166–174. doi: 10.1111/j.1465-3362.2008.00043.x. [DOI] [PubMed] [Google Scholar]
- 17.Zametkin AJ, Ernst M. Problems in the management of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340:40–46. doi: 10.1056/NEJM199901073400107. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui SV, et al. Neuropsychology of prefrontal cortex. Indian J Psychiatry. 2008;50:202–208. doi: 10.4103/0019-5545.43634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson SB, Blum RW, Giedd JN. Adolescent maturity and the brain: the promise and pitfalls of neuroscience research in adolescent health policy. J Adolesc Health. 2009;45:216–221. doi: 10.1016/j.jadohealth.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lacadie CM, et al. More accurate Talairach coordinates for neuroimaging using non-linear registration. Neuroimage. 2008;42:717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright L, et al. Response inhibition and psychopathology: a meta-analysis of go/no-go task performance. J Abnorm Psychol. 2014;123:429–439. doi: 10.1037/a0036295. [DOI] [PubMed] [Google Scholar]
- 23.Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Casey BJ, et al. A developmental functional MRI study of prefrontal activation during performance of a Go-No-Go task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- 25.Cohen JR, et al. Decoding developmental differences and individual variability in response inhibition through predictive analyses across individuals. Front Hum Neurosci. 2010;4:47. doi: 10.3389/fnhum.2010.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norman AL, et al. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wetherill RR, et al. A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology (Berl) 2013;230:663–671. doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Hardee JE, et al. Development of impulse control circuitry in children of alcoholics. Biol Psychiatry. 2014;76:708–716. doi: 10.1016/j.biopsych.2014.03.005. This longitudinal fMRI study investigates neural correlates of response inhibition (i.e., the Go/NoGo task) in children with family history of alcohol use disorder (AUD), who exhibit atypically blunted activations in the cuadate and middle frontal gyrus. These activations undergo atypical developmental trajectories in this population, and thus may contribute to subsequent substance use problems. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acheson A, et al. Increased forebrain activations in youths with family histories of alcohol and other substance use disorders performing a Go/NoGo task. Alcohol Clin Exp Res. 2014;38:2944–2951. doi: 10.1111/acer.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Heitzeg MM, et al. Left middle frontal gyrus response to inhibitory errors in children prospectively predicts early problem substance use. Drug Alcohol Depend. 2014;141:51–57. doi: 10.1016/j.drugalcdep.2014.05.002. This fMRI study demonstrate that blunted activation in the left middle frontal gyrus during the Go/NoGo task at the age of 9–12 significantly predicts subsequent substance use disorder (SUD). The finding allows for the characterization of early neurobiological risk markers for SUD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, et al. Connectivity-based parcellation of the human frontal pole with diffusion tensor imaging. J Neurosci. 2013;33:6782–6790. doi: 10.1523/JNEUROSCI.4882-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volkow ND, et al. Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1410–1415. doi: 10.1016/j.biopsych.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Nigg JT. Is ADHD a disinhibitory disorder? Psychol Bull. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- 34.Tamm L, et al. Event-related FMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- 35.Durston S, et al. Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60:1062–1070. doi: 10.1016/j.biopsych.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 36.Booth JR, et al. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD) J Child Psychol Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- 37.Suskauer SJ, et al. Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: differences in activation associated with response inhibition but not habitual motor response. J Cogn Neurosci. 2008;20:478–493. doi: 10.1162/jocn.2008.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Passarotti AM, Sweeney JA, Pavuluri MN. Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. J Int Neuropsychol Soc. 2010;16:106–117. doi: 10.1017/S1355617709991019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubia K, et al. Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Hum Brain Mapp. 2010;31:287–299. doi: 10.1002/hbm.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma J, et al. Compensatory brain activation in children with attention deficit/hyperactivity disorder during a simplified Go/No-go task. J Neural Transm (Vienna) 2012;119:613–619. doi: 10.1007/s00702-011-0744-0. [DOI] [PubMed] [Google Scholar]
- 41.Durston S, et al. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- 42•.Rasmussen J, et al. ADHD and cannabis use in young adults examined using fMRI of a Go/NoGo task. Brain Imaging Behav. 2015 doi: 10.1007/s11682-015-9438-9. This study evaluates main and interaction effects of childhood ADHD diagnosis and cannabis uses on behavioral and fMRI profiles during the Go/NoGo task. ADHD participants have impaired response inhibition combined with less fronto-parietal/striatal activity, regardless of cannabis use history. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feldstein Ewing SW, Houck JM, Bryan AD. Neural activation during response inhibition is associated with adolescents’ frequency of risky sex and substance use. Addict Behav. 2015;44:80–87. doi: 10.1016/j.addbeh.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dreher JC, et al. Damage to the fronto-polar cortex is associated with impaired multitasking. PLoS One. 2008;3:e3227. doi: 10.1371/journal.pone.0003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kareken DA, et al. Family history of alcoholism interacts with alcohol to affect brain regions involved in behavioral inhibition. Psychopharmacology (Berl) 2013;228:335–345. doi: 10.1007/s00213-013-3038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garavan H, et al. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- 47.Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand. 2012;125:114–126. doi: 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- 48.Volkow ND. Substance use disorders in schizophrenia–clinical implications of comorbidity. Schizophr Bull. 2009;35:469–472. doi: 10.1093/schbul/sbp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Molina BS, Pelham WE., Jr Attention-deficit/hyperactivity disorder and risk of substance use disorder: developmental considerations, potential pathways, and opportunities for research. Annu Rev Clin Psychol. 2014;10:607–639. doi: 10.1146/annurev-clinpsy-032813-153722. This review covers the current knowledge on characteristics of ADHD and ADHD-related risk of substance use disorder. The authors suggest that research should move beyond deficits in standard executive functions to deficits in the interface between cognitive control, reward, and motivation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adams ZW, et al. Inhibitory functioning across ADHD subtypes: recent findings, clinical implications, and future directions. Dev Disabil Res Rev. 2008;14:268–275. doi: 10.1002/ddrr.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.