Abstract
Purpose
Lung cancer remains the most common cause of both cancer mortality and brain metastases (BM). The purpose of this study was to assess the effect of gene alterations and tyrosine kinase inhibition (TKI) on median survival (MS) and cause of death (CoD) in patients with BM from lung adenocarcinoma (L-adeno).
Methods
A multi-institutional retrospective database of patients with L-adeno and newly diagnosed BM between 2006 and 2014 was created. Demographics, gene alterations, treatment, MS, and CoD were analyzed. The treatment patterns and outcomes were compared with those in prior trials.
Results
Of 1521 L-adeno patients, 816 (54%) had known alteration status. The gene alteration rates were 29%, 10%, and 26% for EGFR, ALK, and KRAS, respectively. The time from primary diagnosis to BM for EGFR−/+ was 10/15 months (P=.02) and for ALK−/+ was 10/20 months (P<.01), respectively. The MS for the group overall (n=1521) was 15 months. The MS from first treatment for BM for EGFR and ALK−, EGFR+, ALK+ were 14, 23 (P<.01), and 45 (P<.0001) months, respectively. The MS after BM for EGFR+ patients who did/did not receive TKI before BM was 17/30 months (P<.01), respectively, but the risk of death was not statistically different between TKI-naïve patients who did/did not receive TKI after the diagnosis of BM (EGFR/ALK hazard ratios: 1.06 [P=.84]/1.60 [P=.45], respectively). The CoD was nonneurologic in 82% of patients with known CoD.
Conclusion
EGFR and ALK gene alterations are associated with delayed onset of BM and longer MS relative to patients without these alterations. The CoD was overwhelmingly nonneurologic in patients with known CoD.
Introduction
Lung cancer remains the most common cause of cancer and cancer-related mortality in the United States. In 2015, there were an estimated 221,000 new cases of lung cancer diagnosed and 158,000 deaths from this disease (1). A recent population-based analysis in the United States showed that 22% of non-small cell lung cancer (NSCLC) patients have brain metastases at the time of initial diagnosis (2), and brain metastases will develop in an estimated 40% at some point in the course of their disease (3, 4). Histologic data from the population-based analysis showed that 48% of NSCLC patients with brain metastases have adenocarcinoma (2). Before 1990 and the widespread availability of stereotactic radiosurgery, survival after the development of brain metastases was dismal: 2 to 3 months with steroids, 3 to 6 months with treatment (3, 5). In the largest series heretofore reported, our group demonstrated a median survival of 7 months for NSCLC patients treated between 1985 and 2005 (6). Therapeutic options were usually limited to surgery, whole brain radiation therapy, and stereotactic radiosurgery. Chemotherapy has not been an option, primarily because of the lack of effective agents and the blood-brain barrier, which prevents adequate penetration of drug into the brain.
Randomized clinical trials over the past quarter century have yielded 5 important lessons: (1) In patients with a solitary brain metastasis, surgery, when possible, plus whole brain radiation therapy, offers a survival benefit compared with whole brain radiation therapy alone (7); (2) Surgery plus whole brain radiation therapy is preferable to surgery alone for intracranial control and prevention of neurologic death but not for overall survival (8); (3) Whole brain radiation therapy plus stereotactic radiosurgery improves survival compared with whole brain radiation therapy alone for patients with a solitary brain metastasis (9); (4) Stereotactic radiosurgery plus whole brain radiation therapy reduces the rate of subsequent distant brain metastases when compared with stereotactic radiosurgery alone, but that benefit comes with the cost of short-term and long-term toxicity of whole brain radiation therapy and no survival benefit (10, 11); and (5) Cognitive decline has become an important endpoint in brain metastases research (11).
Interpretation of the above literature is confounded by inadequate knowledge of prognostic factors, resulting in suboptimal stratification and reduced statistical power. Indeed, one of the most problematic aspects in the study of patients with brain metastases is the marked clinical (varied diagnoses and nature/extent of prior treatment) and molecular (gene alterations) heterogeneity in this patient population. The discovery of molecular heterogeneity in many cancers led to the development of targeted drug therapies, including tyrosine kinase inhibitors (TKI) for patients whose tumors have epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) gene alterations (12, 13).
The enduring debate regarding the role of whole brain radiation therapy, coupled with the heterogeneity of patients with brain metastases, resulted in a quarter-century quandary regarding the management of this common clinical problem. This, in turn, led to extensive efforts to develop prognostic indices to guide clinical decision making and stratification in clinical trials (6, 14, 15). It is now known that outcomes vary widely by diagnosis and diagnosis-specific factors. The graded prognostic assessment for lung cancer (Lung-GPA) demonstrated that the prognostic factors significant for survival include age, Karnofsky performance status (KPS), the presence or absence of extracranial metastases, and the number of brain metastases (6, 15). However, the impact of genetic factors and targeted drugs is not well understood. The purpose of this study was to assess the effect of gene alterations and TKI (gefitinib, erlotinib, and crizotinib only) on survival and cause of death in patients with lung adenocarcinoma and brain metastases.
Methods
A multi-institutional institutional review board—approved retrospective database of 2186 patients with NSCLC and newly diagnosed BM between 2006 and 2014 was created with the use of research electronic data capture (REDCap), browser-based metadata-driven software (16). Clinical factors and gene alteration status for EGFR, ALK, and Kirsten RNA–associated sarcoma (KRAS) were correlated with treatment, survival, and cause of death. This study primarily describes the 1521 NSCLC adenocarcinoma patients; however, the 665 nonadenocarcinoma patients were included only in comparisons with historical cohorts that included all NSCLC subtypes. The most common method of genotyping was polymerase chain reaction, and some institutions used immunohistochemical staining and in more recent years next-generation sequencing.
Group definitions
The adenocarcinoma patients were divided into 5 mutually exclusive subgroups to study genetic mutation effects. Not all patients were tested for all 3 alterations. With few exceptions, EGFR+, ALK+, and KRAS+ alterations were mutually exclusive; thus, any patient with positive test results for an alteration was included in that subgroup regardless of whether he or she was tested for other alterations. Because of mutual exclusivity, a direct comparison of EGFR+/− would be confounded by ALK (only EGFR− patients might be ALK+). Therefore, a reference group was formed that was known to be negative for both EGFR and ALK. Initial analyses showed that KRAS was not associated with outcomes, and inasmuch as KRAS was least commonly tested, negative or untested KRAS patients were allowed in the reference group if they were EGFR and ALK−. The remaining patients were placed in the unknown group.
Statistical analyses
The primary outcome was survival measured from the start of treatment for brain metastases to death. Median survival estimates were calculated by the Kaplan-Meier method. The standard log-rank test was used for pairwise comparisons of survival for each alteration compared with a reference group that was negative for EGFR and ALK and either negative or unknown for KRAS. Within the EGFR+ and the ALK+ groups, log-rank tests were used to compare survival from start of BM treatment between patients who did/did not receive TKI before BM. Because the primary purpose was to describe outcomes, P values were not adjusted for multiple testing. Where noted, multivariable Cox regression was used to assess whether survival differences were independent of established prognostic factors in the Lung-GPA. Two analyses used a time-dependent variable for the initiation of TKI; the first analyzed the effect of TKI before brain metastases on the time to development of brain metastases, and the second analyzed the effect of TKI after brain metastases on survival after brain metastases, excluding patients who received TKI before the start of treatment for brain metastases. The secondary outcomes included cause of death, compared with a χ2 test, and time from primary diagnosis to start of treatment for brain metastases, compared with Wilcoxon rank sum tests. Analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC).
Results
Patients
Table 1 shows patient demographics and survival by gene alteration. Of 2186 NSCLC patients, 1521 (70%) had adenocarcinoma and 816/1521 (54%) had a known gene alteration status for at least 1 of the genes described above. Of those with known alterations, the alteration rates for EGFR, ALK, and KRAS were 29% (n=235), 11% (n=86), and 26% (n=211), respectively. As shown in Table 1, patients with smoking histories of >20 pack-years had low rates of EGFR (4%) and ALK (1%) positivity compared with KRAS (19%). EGFR alterations were more common in women (19%) than in men (12%) and more common in Asians (46%) than in non-Asians (13%) but did not vary by age (~16% in all age groups). Patients under age 50 were more likely to have ALK rearrangements (13%) than were those ages 51 to 60 (7%) or aged >60 (3%). Patients with >3 brain metastases were more likely to have EGFR alterations (25%) or ALK alterations (9%) than were patients with only 1 brain metastasis, of whom 10% were EGFR+ and 4% were ALK+.
Table 1.
Demographics by gene mutation
| Characteristic | Adeno overall
|
EGFR & ALK−
|
EGFR+
|
ALK+
|
KRAS+
|
Unknown mutation status
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | MS (mo) | N (%) | MS (mo) | N (%) | MS (mo) | N (%) | MS (mo) | N (%) | MS (mo) | N (%) | MS (mo) | |
| Overall | 1521 | 15 | 284 (19) | 14 | 235 (15) | 23 | 86 (6) | 45 | 211 (14) | 12 | 705 (46) | 12 |
| Lung GPA | ||||||||||||
| 0.0–1.0 | 355 | 9 | 69 (19) | 6 | 80 (23) | 18 | 16 (5) | 31 | 36 (10) | 7 | 154 (43) | 9 |
| 1.5–2.0 | 634 | 13 | 109 (17) | 14 | 89 (14) | 23 | 38 (6) | 49 | 93 (15) | 8 | 305 (48) | 10 |
| 2.5–3.0 | 443 | 21 | 84 (19) | 20 | 53 (12) | 35 | 28 (6) | NR | 68 (15) | 25 | 210 (47) | 17 |
| 3.5–4.0 | 89 | 41 | 22 (25) | 41 | 13 (15) | NR | 4 (4) | NR | 14 (16) | 17 | 36 (40) | 37 |
| Cause of death | ||||||||||||
| Non-CNS | 422 | 10 | 89 (21) | 9 | 66 (16) | 15 | 9 (2) | 24 | 64 (15) | 6 | 194 (46) | 10 |
| CNS | 90 | 13 | 27 (30) | 11 | 11 (12) | 18 | 6 (7) | 25 | 15 (17) | 10 | 31 (34) | 21 |
| Unknown | 640 | 10 | 97 (15) | 11 | 79 (12) | 17 | 22 (3) | 13 | 84 (13) | 8 | 358 (55) | 10 |
| Age | ||||||||||||
| <50 | 186 | 21 | 37 (20) | 17 | 32 (17) | 31 | 25 (13) | 33 | 16 (9) | 16 | 76 (41) | 15 |
| 50–60 | 453 | 17 | 87 (19) | 16 | 74 (16) | 23 | 33 (7) | 50 | 66 (15) | 10 | 193 (43) | 13 |
| >60 | 882 | 13 | 160 (18) | 13 | 129 (15) | 20 | 28 (3) | 45 | 129 (15) | 12 | 436 (49) | 11 |
| Sex | ||||||||||||
| Male | 662 | 14 | 136 (21) | 14 | 77 (12) | 23 | 45 (7) | 45 | 88 (13) | 15 | 316 (48) | 11 |
| Female | 851 | 16 | 143 (17) | 17 | 158 (19) | 24 | 41 (5) | 50 | 122 (14) | 11 | 387 (45) | 13 |
| Race | ||||||||||||
| Asian | 112 | 17 | 14 (13) | 3 | 52 (46) | 20 | 6 (5) | 45 | 7 (6) | 7 | 33 (29) | 17 |
| Black or African American | 121 | 15 | 22 (18) | 19 | 17 (14) | 12 | 1 (1) | NR | 19 (16) | 17 | 62 (51) | 11 |
| White | 1154 | 15 | 220 (19) | 14 | 153 (13) | 25 | 67 (6) | 50 | 178 (15) | 12 | 536 (46) | 11 |
| Other | 134 | 17 | 28 (21) | 15 | 13 (10) | 31 | 12 (9) | 31 | 7 (5) | NR | 74 (55) | 15 |
| Tobacco pack-years | ||||||||||||
| None | 346 | 20 | 44 (13) | 10 | 135 (39) | 24 | 61 (18) | 49 | 4 (1) | 7 | 102 (29) | 12 |
| ≤ 10 | 129 | 20 | 20 (16) | 23 | 42 (33) | 20 | 10 (8) | 20 | 16 (12) | 15 | 41 (32) | 12 |
| 11–20 | 149 | 14 | 37 (25) | 14 | 17 (11) | 18 | 4 (3) | 18 | 25 (17) | 11 | 66 (44) | 13 |
| >20 | 812 | 13 | 169 (21) | 14 | 29 (4) | 23 | 8 (1) | 50 | 158 (19) | 13 | 448 (55) | 12 |
| Histopathologic grade | ||||||||||||
| GX | 80 | 15 | 11 (14) | 16 | 13 (16) | 22 | 2 (3) | NR | 7 (9) | 10 | 47 (59) | 15 |
| G1 | 36 | 18 | 6 (17) | 28 | 7 (19) | 8 | 2 (6) | NR | 4 (11) | 15 | 17 (47) | 16 |
| G2 | 287 | 19 | 56 (20) | 17 | 58 (20) | 23 | 11 (4) | NR | 42 (15) | 19 | 120 (42) | 17 |
| G3 | 626 | 14 | 136 (22) | 16 | 84 (13) | 28 | 32 (5) | 32 | 79 (13) | 12 | 295 (47) | 11 |
| G4 | 8 | 12 | 2 (25) | NR | 0 | NR | 1 (13) | 24 | 1 (13) | 6 | 4 (50) | 12 |
| KPS | ||||||||||||
| <70 | 123 | 6 | 23 (19) | 9 | 17 (14) | 4 | 6 (5) | NR | 9 (7) | 10 | 68 (55) | 6 |
| 70–80 | 751 | 13 | 132 (18) | 14 | 102 (14) | 20 | 29 (4) | 31 | 100 (13) | 10 | 388 (52) | 11 |
| 90–100 | 607 | 19 | 119 (20) | 16 | 108 (18) | 31 | 49 (8) | 55 | 98 (16) | 16 | 233 (38) | 15 |
| No. of brain metastases | ||||||||||||
| 1 | 650 | 16 | 124 (19) | 22 | 68 (10) | 34 | 25 (4) | NR | 94 (14) | 11 | 339 (52) | 13 |
| 2–3 | 469 | 15 | 86 (18) | 13 | 72 (15) | 26 | 26 (6) | 45 | 72 (15) | 17 | 213 (45) | 11 |
| >3 | 367 | 14 | 69 (19) | 7 | 90 (25) | 19 | 33 (9) | 31 | 43 (12) | 10 | 132 (36) | 11 |
| Volume of brain metastases | ||||||||||||
| n/a | 638 | 13 | 140 (22) | 11 | 116 (18) | 20 | 29 (5) | 32 | 115 (18) | 10 | 238 (37) | 11 |
| <1.1 cc | 552 | 18 | 96 (17) | 17 | 98 (18) | 26 | 46 (8) | 55 | 65 (12) | 13 | 247 (45) | 13 |
| 1.1–5.1 cc | 192 | 15 | 32 (17) | 12 | 12 (6) | 30 | 9 (5) | 37 | 20 (10) | 13 | 119 (62) | 14 |
| 5.1–10 cc | 65 | 15 | 10 (15) | 34 | 5 (8) | 8 | 0 | NR | 6 (9) | 20 | 44 (68) | 14 |
| >10 cc | 74 | 16 | 6 (8) | NR | 4 (5) | NR | 2 (3) | 3 | 5 (7) | NR | 57 (77) | 11 |
Abbreviations: GPA = graded prognostic assessment; MS = median survival (months); NR = not reached.
Percentages (%) are within the row subgroup. For example, 235/1521 = 15% of patients were EGFR+, and 41/851 = 5% of women were ALK+.
Effect of gene alteration status on survival and cause of death
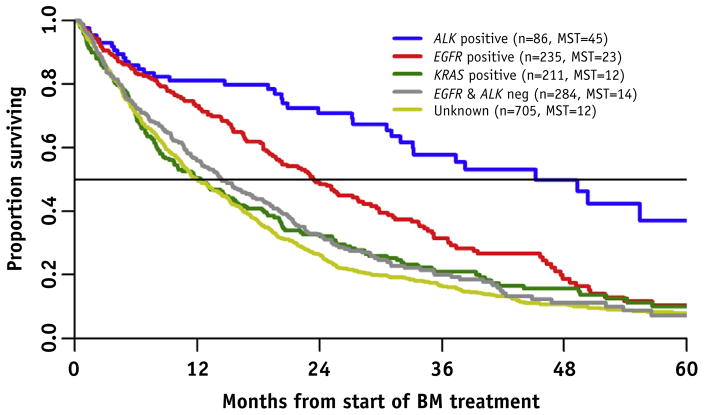
Table 1 and Figure 1 compare survival by gene alteration. The median survival for all adenocarcinoma was 15 months. The median survival for EGFR and ALK−, EGFR+, ALK +, KRAS +, and unknown was 14, 23 (P<.01), 45 (P<.0001), 12 (P=.84), and 12 (P=.12) months, respectively (P values compared with EGFR and ALK−, unadjusted for other factors). The survival advantage of EGFR and ALK alterations persisted regardless of age, performance status, extracranial metastases, or number of brain metastases (Lung-GPA). There was no difference in the time from primary diagnosis to brain metastases or median survival in KRAS+ versus EGFR/ALK/KRAS− or untested patients. The cause of death was reported for 512/1152 (44%) of deceased patients with lung adenocarcinoma (Table 1); 422/512 (82%) died of nonneurologic causes, and this did not vary by gene alteration.
Fig. 1.
Overall survival by gene alteration. The unknown group included 177 patients who had a negative test result for EGFR or ALK but were not tested for both. With the use of multiple Cox regression to adjust for Lung-GPA prognosis, year of BM, and smoking history, ALK+ (P<.0001) and EGFR+ (P=.001) patients had longer overall survival than did EGFR/ALK− patients. KRAS+ was not significantly different (P=.45) from EGFR/ALK−. Abbreviations: ALK = anaplastic lymphoma kinase; BM = brain metastases; EFGR = epidermal growth factor receptor; KRAS = Kirsten RNA–associated sarcoma; MST = median survival time.
Effect of tyrosine kinase inhibition
Time from primary diagnosis to brain metastases
The effect of TKI before brain metastases was measured by the time from initial primary diagnosis to brain metastases, gene status, and receipt of TKI (Table 2). The times from primary diagnosis to brain metastases for EGFR−/+ and ALK−/+ patients were 10/15 (P=.02) and 10/20 (P<.01) months, respectively. A time-dependent analysis of patients who did and did not receive TKI before the diagnosis of brain metastases showed a hazard ratio (HR) of 1.17 (95% confidence interval [CI] 0.86–1.59; P=.31; n=74/161) and 2.90 (95% CI 1.73–4.87; P<.01; n=33/53) for EGFR+ and ALK+ patients, respectively. This means that in EGFR and ALK+ patients, there was no increase in the time from primary diagnosis to brain metastases between those treated and not treated with TKI before the diagnosis of brain metastases.
Table 2.
Median survival and time from primary diagnosis to brain metastasis by gene status and tyrosine kinase inhibition
| Factor | n (%) | MS, mo | IQR, mo | P values vs EGFR & ALK−;* vs no TKI† | Mean time: primary diagnosis to BM, mo | P value vs EGFR & ALK−‡ |
|---|---|---|---|---|---|---|
| Adenocarcinoma overall | 1521 | 15 | 14–17 | 16 | ||
| Alteration status unknown | 705 (46) | 12 | 5–25 | .12* | 19 | .08‡ |
| Alteration status known | 816 (54) | 19 | 7–40 | 13 | ||
| EGFR & ALK− | 284 (35) | 14 | 10 | ref* | 10 | ref‡ |
| EGFR+ | 235 (29) | 23 | 20–28 | <.01* | 15 | .02‡ |
| No TKI before BM | 161 (69) | 30 | 12–47 | ref† | ||
| Received TKI before BM | 74 (31) | 17 | 9–26 | <.01† | ||
| ALK+ | 86 (10) | 45 | 32–62 | <.01* | 20 | <.01‡ |
| No TKI before BM | 53 (61) | 45 | 31-NR | ref† | ||
| Received TKI before BM | 33 (39) | NR | 5-NR | .43† | ||
| KRAS+ | 211 (26) | 12 | 5–32 | .84* | 13 | .25‡ |
Abbreviations: MS = median survival; NR = not reached; ref = reference; TKI = tyrosine kinase inhibition.
Percentages (%) are within the subgroup whose total is above and 1 column left. For example, 211/816 = 26% of patients with known alterations were KRAS+, and 161/235 = 69% of patients who were EGFR+ did not receive TKI before BM.
Forty-five patients had invalid dates and were excluded for metastasis time evaluation.
P values are from log-rank tests comparing survival distributions between each alteration group—EGFR+, ALK+, KRAS+, and unknown—and the EFGR & ALK− group as reference.
P values are from log-rank tests comparing survival distributions between each TKI group, within EGFR+ and ALK+ subgroups, respectively.
P values are from Wilcoxon rank sum tests comparing the time from primary diagnosis to BM between each alteration group and the EGFR & ALK− group as reference. TKI could be initiated at any time between primary diagnosis and BM, so time from primary diagnosis to BM was compared for TKI with time-dependent analyses (results not presented in table).
Survival after brain metastases
The effect of TKI after brain metastases was measured by survival. The median survival after brain metastases for EGFR+ and ALK+ patients treated/not treated with TKI before the diagnosis of brain metastases was 17/30 months (P<.01; n=74/161) and (median survival not yet reached)/45 months (P=.43; n=33/53), respectively, but a time-dependent analysis of TKI after brain metastases showed no survival benefit in EGFR+ (HR 1.06; 95% CI 0.62–1.80; P=.84; n=122/39; TKI before brain metastases excluded) or ALK+ patients (HR 1.60; 95% CI 0.47–5.42; P=.45; n=39/14). This means that in EGFR+ patients, survival was improved only for TKI-naïve patients relative to patients who previously received and failed TKI (by the development of brain metastases).
Multivariable analysis of risk of death and median survival by treatment and gene status
Table 3 shows a multivariable analysis of risk of death and median survival by treatment and gene status with comparison to our prior report (4) as a historical control. In a comparison of the periods 1985 to 2005 and 2006 to 2014, the percentage of patients treated with SRS alone increased from 22% to 50%, whereas the percentage of patients treated with whole brain radiation therapy alone decreased from 42% to 22%. Only 13% underwent surgery. Patients with the better prognosis (higher lung GPA) tended to receive more treatment. In addition to the primary treatments described in Table 3, salvage treatment (most commonly stereotactic radiosurgery) was delivered in 87% of patients.
Table 3.
Multivariable analysis of risk of death and median survival by treatment
| Histology | Treatment
|
|||||
|---|---|---|---|---|---|---|
| WBRT | SRS | WBRT + SRS | S + SRS | S + WBRT | S + WBRT + SRS | |
| Historical control, NSCLC (n=1833) | ||||||
| Risk of death (HR) | 1.00 | 0.62 | 0.54 | 0.48 | 0.48 | 0.39 |
| 95% CI | 0.51–0.74 | 0.46–0.64 | 0.34–0.68 | 0.40–0.57 | 0.27–0.55 | |
| P | <.01 | <.01 | <.01 | <.01 | <.01 | |
| Median survival | 4 | 10 | 13 | 12 | 12 | 12 |
| n (%) | 768 (42%) | 395 (22%) | 339 (18%) | 58 (3%) | 212 (12%) | 61 (3%) |
| Mean GPA | 1.9 | 2.2 | 2.3 | 2.2 | 2.6 | 2.4 |
| Adenocarcinoma overall (n=1521) | ||||||
| Risk of death (HR) | 1.00 | 1.08 | 1.20 | 0.66 | 0.78 | 0.79 |
| 95% CI | 0.92–1.27 | 0.94–1.54 | 0.50–0.88 | 0.58–1.06 | 0.40–1.58 | |
| P | .35 | .15 | <.01 | .11 | .51 | |
| Median survival | 13 | 14 | 10 | 32 | 20 | 20 |
| n (%) | 342 (22%) | 767 (50%) | 139 (9%) | 114 (7%) | 76 (5%) | 13 (1%) |
| Mean GPA | 1.5 | 2.1 | 1.8 | 2.3 | 2.2 | 2.6 |
Abbreviations: GPA = graded prognostic assessment; HR = hazard ratio; NR = not reached; NSCLC = non-small cell lung cancer; S = surgery; SRS = stereotactic radiosurgery; WBRT = whole brain radiation therapy.
Seventy patients had incomplete treatment data and were excluded from the columns in this table. Within each subgroup, risk of death HR, 95% CI, and P values are compared with patients treated with WBRT alone (HR, 1.0), using multiple Cox regression, adjusted for GPA. Median survival is given in months and is based on the Kaplan-Meier method, unadjusted for other factors.
Discussion
These data illuminate both contemporary and historical questions regarding the treatment of lung cancer patients with brain metastases.
Were EGFR and ALK alterations associated with delayed development of brain metastases? Yes. In comparison with EFGR/ALK− patients, the mean time from primary diagnosis to brain metastases lengthened from 10 to 15 months (P=.02) and 20 months (P<.01) in EGFR and ALK+ patients, respectively;
Were KRAS alterations associated with shorter time from primary diagnosis to brain metastases? No. There was no difference in the time from primary diagnosis to brain metastases or median survival in KRAS+ versus EGFR/ALK/KRAS− patients.
Were EGFR and ALK alterations associated with improved survival after the diagnosis of brain metastases? Yes. In comparison with EGFR/ALK− patients, median survival improved from 14 to 23 months (P<.01) and 45 months (P<.0001) in EGFR and ALK+ patients, respectively. The interquartile ranges (25th–75th percentile) for EGFR/ALK+ were remarkable: 20 to 28 months and 32 to 62 months, respectively.
Did KRAS+ patients do worse? No. There was no significant difference in median survival between KRAS+ and EGFR/ALK/KRAS− or unknown patients.
Was prior treatment with TKI associated with longer time from primary diagnosis to brain metastases? No. Although Heon et al (17) suggested a chemopreventive effect with lower rates of brain metastases in EGFR+ patients treated with TKI compared with chemotherapy, our data show that TKI before the diagnosis of brain metastases did not prolong the time from primary diagnosis to brain metastases in comparison with patients not receiving TKI (HR for development of brain metastases was 1.17 and 2.90 for EGFR and ALK+ patients, respectively).
Was TKI associated with improved survival after the diagnosis of brain metastases? Patients who did not receive TKI before the diagnosis of brain metastases (TKI-naïve) survived longer after the diagnosis of brain metastases than did those who had already failed TKI (by the development of brain metastases). These findings are also consistent with the findings of others (18, 19) who found a survival benefit for TKI after the diagnosis of BM only in patients who had not previously received that treatment at that time. Furthermore, randomized studies in the literature suggests increased toxicity from combining TKI with radiation therapy in albeit unselected NSCLC patients (20–22).
Did cause of death vary by gene alteration? No, not significantly. The cause of death was nonneurologic in 82% of known patients but reported in only 44% of patients in this dataset, and this did not vary by gene alteration. The cause of death, however, can be confounded by institutional variation in how cause is assigned, particularly in retrospective studies. Interestingly, a recent autopsy study of 100 patients who died of lung cancer showed that only 3 patients (3%) died of brain metastases (23). The data on cause of death and the clinical and genetic heterogeneity of this patient population suggests that any future survival benefit in brain metastases patients will likely depend on improvement in systemic therapies.
What was the effect of smoking on gene alterations? Pack-years varied directly with KRAS and indirectly with EGFR and ALK alterations.
Was the higher rate of EGFR alterations in women and Asians a result of hormonal or genetic differences? No, not necessarily. Although our data generated these hypotheses, these observations may have been confounded by differences in smoking because smokers are less likely to be EGFR+: 27% of women had never smoked versus 17% of men, and 64% of Asians had never smoked versus 19% of whites.
What were the weaknesses of this study? (1) Selection bias exists in all retrospective series and should be interpreted accordingly; (2) Gene alteration status was unknown for 46% of patients, although this was due to clinical testing protocols; and (3) We have no data on second-generation or third-generation TKI.
Did these data confirm the prognostic factors in the GPA? Yes. All of the component factors of the Lung-GPA (age, KPS, extracranial metastases, and number of BM) were again confirmed. Also, EGFR/ALK alterations were prognostic, independently of the existing Lung-GPA factors.
Were EGFR/ALK alterations prognostic factors independently of other prognostic factors and treatment? Yes.
It is concluded that these data address the fundamental questions discussed above regarding EGFR, ALK, and KRAS alterations in patients with lung adenocarcinoma with brain metastases. EGFR and ALK alterations were associated with longer time from primary diagnosis to brain metastases and with improved survival after the diagnosis of BM, in comparison with EGFR/ALK− patients, regardless of other prognostic factors and treatment. In EGFR and ALK+ patients, there was no difference in the time from primary diagnosis to brain metastases between those treated and not treated with TKI before the diagnosis of brain metastases. In EGFR+ patients, the median survival was longer for TKI-naïve patients than in patients who had previously received and failed that treatment (by the development of brain metastases). The development of a Lung-GPA-2016 incorporating gene alterations is ongoing.
Summary.
This retrospective study of patients with lung adenocarcinoma and newly diagnosed brain metastases (BM) was designed to determine the effect of gene alterations and tyrosine kinase inhibition on survival. EGFR and ALK gene alterations are associated with delayed onset of BM and longer survival relative to patients without these alterations.
Acknowledgments
Supported by National Institutes of Health (NIH) Grant No. UL1TR000114 from the National Center for Advancing Translational Sciences (NCATS); study data were collected and managed with REDCap electronic data capture tools hosted at the University of Minnesota; and by NIH Grant No. P30 CA77598 with use of the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota, and the NCATS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
The authors thank Susan Lowry of the Biostatistical Design and Analysis Center, Clinical and Translational Science Institute, University of Minnesota, for database support and management.
Footnotes
Presented at the 57th Annual Meeting of the American Society of Radiation Oncology, October 18–21, 2015, San Antonio, TX, and at the Best of ASTRO Meeting, November 13, 2015, San Diego, CA.
Conflict of interest: none.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Villano JL, Durbin EB, Normandeau C, et al. Incidence of brain metastasis at initial presentation of lung cancer. Neuro Oncol. 2015;17:122–128. doi: 10.1093/neuonc/nou099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathoo N, Chahlavi A, Barnett GH, et al. Pathobiology of brain metastases. J Clin Pathol. 2005;58:237–242. doi: 10.1136/jcp.2003.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen PY, Loeffler JS. Brain metastases. Curr Treat Options Oncol. 2000;1:447–458. doi: 10.1007/s11864-000-0072-3. [DOI] [PubMed] [Google Scholar]
- 5.Park DM, Posner JB. Management of intracranial metastases: History. In: Sawaya R, editor. Intracranial metastases: Current management strategies. Oxford, England: Blackwell Publishing Ltd; 2004. pp. 3–19. [Google Scholar]
- 6.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2011;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 8.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 9.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 10.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 11.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomized controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 12.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EUR-TAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 13.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 14.Gaspar LE, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 15.Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heon S, Yeap BY, Lindeman NI, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2012;18:4406–4414. doi: 10.1158/1078-0432.CCR-12-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mak KS, Gainor JF, Niemierko A, et al. Significance of targeted therapy and genetic alterations in EGFR, ALK, or KRAS on survival in patients with non-small cell lung cancer treated with radiotherapy for brain metastases. Neuro Oncol. 2015;17:296–302. doi: 10.1093/neuonc/nou146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johung KL, Yeh N, Desai NB, et al. Extended survival and prognostic factors for patients with ALK-rearranged non-small cell lung cancer and brain metastasis. J Clin Oncol. 2015;33:1–7. doi: 10.1200/JCO.2015.62.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sperduto PW, Wang M, Robins HI, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolamide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. 2013;85:1312–1318. doi: 10.1016/j.ijrobp.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperduto PW, Shanley R, Luo X. Secondary analysis of RTOG 9508, a phase III randomized trial of whole brain radiation therapy (WBRT) versus WBRT plus stereotactic radiosurgery (SRS) in patients with 1–3 brain metastases; post-stratified by the graded prognostic assessment (GPA) Int J Radiat Oncol Biol Phys. 2014;90:526–531. doi: 10.1016/j.ijrobp.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoyama H, Tago M, Shirato H. Stereotactic radiosurgery with or without whole-brain radiotherapy for brain metastases: Secondary analysis of the JROSG 99-1 randomized clinical trial. JAMA Oncol. 2015;1:457–464. doi: 10.1001/jamaoncol.2015.1145. [DOI] [PubMed] [Google Scholar]
- 23.Nichols L, Saunders R, Knollman FD. Causes of death of patients with lung cancer. Arch Pathol Lab Med. 2012;136:1552–1557. doi: 10.5858/arpa.2011-0521-OA. [DOI] [PubMed] [Google Scholar]