Abstract
Cell culture antiviral experiments were conducted in order to understand the relationship between percentage data generated by plaque reduction (PR) and logarithmic data derived by virus yield reduction (VYR) assays, using three-dimensional MacSynergy II software. The relationship between percentage and logarithmic data has not been investigated previously. Interpretation of drug-drug interactions is based on a Volume of Synergy (VS) calculation, which can be positive (synergy), negative (antagonistic), or neutral (no or minimal interaction). Interactions of two known inhibitors of vaccinia virus replication, cidofovir and 6-azauridine, used in combination by PR assay yielded a Volume of Synergy of 265, indicative of strong synergy. By VYR, the VS value was only 37, or weak synergy using the same criterion, even though profound log10 reductions in virus titer occurred at multiple drug combinations. These results confirm that the differences in VS values is dependent of the measurement scale, and not that the degree of synergy differed between the assays. We propose that for logarithmic data, the calculated VS values will be lower for significant synergy and antagonism and that volumes of >10 μM2log10PFU/ml (or other units such as μM2log10 genomic equivalents/ml or μM2log10 copies/ml) and <−10 μM2log10PFU/ml are likely to be indicative of strong synergy and strong antagonism, respectively. Data presented here show that the interaction of cidofovir and 6-azauridine was strongly synergistic in vitro.
Keywords: drug combination, synergy, vaccinia, cidofovir, 6-azauridine
Various methods have been devised to study and interpret drug-drug interactions. Prior to the advent of computer programs, two-dimensional (2-D) methods were used to approximate the actual three-dimensional (3-D) nature of drug interactions. 2-D methods had their place historically, but 3-D methods have largely replaced them and allow for rigorous analysis of drug-drug interactions over an entire dose-response surface (Prichard and Shipman, 1990). Understanding the shape of the entire 3-D surface is essential to understanding complex drug interactions.
One of the computer software tools developed to evaluate and quantitatively interpret 3-D dose-response surfaces is MacSynergy™ II. This program graphically plots 3-D interactions that fall above or below a neutral surface (baseline). In addition, the program generates an interpretable value referred to as the Volume of Synergy at 95% confidence limits for each set of data or multiple sets of data that are averaged together. For example, this method has been used for interpreting drug-drug interactions for influenza virus infection studies (Ilyushina et al., 2008; Ilyushina et al., 2007; Smee et al., 2009; Smee et al., 2010a; Smee et al., 2010b) using percent mortality data.
A question that has not been addressed since the development of MacSynergy II is how to interpret logarithmic data in comparison to percentage data that is plotted on a linear scale. Percentage data are produced in many assays, such as percentage of viral cytopathology (compared to uninfected cells) or of viral plaques in plaque reduction (PR) assays, or percentage of surviving animals in a group of infected animals. Viral titer data, such as data derived from virus yield reduction (VYR) assays (Tarbet et al., 2014), or of the amount of virus produced in infected animal tissues (Smee et al., 2016), are more appropriately presented on a logarithmic scale. Viral loads determined by qPCR assays are also most appropriately analyzed in logarithmic form (James et al., 2011; Prichard et al., 2011). In the past where analysis of VYR data by MacSynergy II has been performed, the investigators have not generally interpreted the results much beyond declaring interactions as synergistic, antagonistic, or neutral (Tarbet et al., 2012). In contrast, further interpretations of the degree of synergy (or antagonism) have been given for percentage data, such as weak, moderate or strong synergy (or antagonism) (Prichard et al., 1992).
The purpose of the present investigation was to better interpret logarithmic data by MacSynergy II by understanding how the results compared to percentage data. In order to do this, we wanted to use the same virus and cell culture but in two different ways, that would produce both percentage and logarithmic data. Vaccinia virus seemed to be a logical choice of virus, since it is a lytic virus that produces cytopathology and distinct plaques in vitro. Virus yields from the infected cells can readily be quantified by plaque assay. For the present investigation we used the PR and VYR assays as means of deriving percentage and logarithmic data, respectively. This required that we also identify two compounds that would inhibit the virus synergistically when used together in cell culture.
A number of compounds have been discovered that exhibit antiviral activity against vaccinia virus in vitro. Three in particular, cidofovir (De Clercq et al., 1987; Smee et al., 2015), tecovirimat (Jordan et al., 2010; Yang et al., 2005), and brincidofovir (Florescu and Keck, 2014; Quenelle et al., 2007) (an orally active prodrug form of cidofovir), have been considered for human treatment of smallpox and monkeypox virus infections, and two of the compounds have been used to treat complications due to smallpox vaccinations (which employs a live vaccinia virus vaccine) (Lederman et al., 2012). Based on commercial availability, we chose cidofovir as one of the drugs to use in combination to treat vaccinia virus infections in vitro. However, the choice of the compound to combine with cidofovir was not obvious. We first investigated ribavirin, an inhibitor of vaccinia virus (Bougie and Bisaillon, 2004; Smee et al., 2001), but found that the two compounds were just weakly synergistic in combination (D.F. Smee, unpublished). Understanding that cidofovir diphosphate (the antiviral active form of cidofovir that inhibits the viral DNA polymerase (Magee et al., 2008) is a competitive inhibitor of deoxycytidine triphosphate (dCTP) in cells, it was hypothesized that a compound that reduces pyrimidine nucleotide pools may synergize with cidofovir. One such compound, 6-azauridine, an inhibitor of de novo pyrimidine biosynthesis (Handschumacher, 1960; Rada and Dragun, 1977) and of vaccinia virus replication (Rada and Blaskovic, 1966), was evaluated, and we found it to be synergistic when combined with cidofovir. Thus, these two compounds were chosen for the present investigation.
The WR strain of vaccinia virus that was used was obtained from the American Type Culture Collection (ATCC, Manassas, VA). It was propagated in MA-104 cells and titrated by plaque assay in Vero 76 cells. Both cell lines (from ATCC) were derived from African green monkey kidney. Cell culture medium was MEM with 5% fetal bovine serum (FBS).
Plaque reduction (PR) assays were performed in 12-well Corning microplates containing confluent 18 h monolayers of Vero 76 cells. This is possible because plaque sizes at three days are small (≤1 mm diameter). Approximately 80 plaque-forming units (PFU) of vaccinia virus were added to aspirated wells for 1 h, with rocking every 5–10 min to increase the extent of virus adsorption. Virus medium was aspirated from the plates followed by addition of compounds at various concentrations in MEM, 2% FBS and 50 μg/mL gentamicin. Three microwells were used for each concentration (or drug combination) or untreated (virus control) cultures. After 72 h the plates were aspirated dry and fixed with 0.2% crystal violet in 5% buffered formalin for 15 min. The dye solution was removed by pipetting, and the plates were rinsed with water. After air drying, the plaques in each well were counted manually with the aid of a magnifying Plaque Viewer (Bellco, Vineland, NJ). Plaque counts were converted to percentages of the average untreated control wells.
A modification of the above procedure was used for the VYR assay. Approximately 240 PFU of vaccinia virus was rocked onto Vero 76 cells followed 1 h later by drug dilutions. This amount of virus caused nearly 100% cytopathic effect in the wells at 72 h. The plates containing infectious medium were frozen at −80°C for later titration of virus in each well. Later, partly thawed medium in each well (1 mL) was swirled with a micropipet tip to detach and break up the cells. The fluid was collected from each well, using 3 wells per concentration of inhibitor or combination. The samples were each sonicated 1 min, then the samples were individually titrated by plaque assay on fresh monolayers of Vero 76 cells. Virus titers were recorded as log10 PFU/mL.
The data obtained from the assays were plotted in tabular and graphic form. Tabular data were analyzed for synergy by a two-dimensional drug combination index method (Schinazi et al., 1982). By this method, values obtained for drugs in combinations that are lower than mathematically-determined expected values are deemed synergistic. However, with this method there is no interpretation of the degree of synergy obtained. Graphical interpretations of drug-drug interactions for the same data sets as indicated above were made by a three-dimensional method (Prichard and Shipman, 1990) using MacSynergy II software (Prichard et al., 1992). For percentage data, the virus control (VC) and drug combination data from infected cultures were plotted as 100 minus × (where × is the percent plaque count relative to VC). The uninfected cell control (CC) was indicated as 100 minus 0 (since no plaques were present). For logarithmic data, the raw log10 values were plotted, with VC indicated as the average virus titer obtained from that set of data and CC being 0. This enables the program to plot each graph in the correct orientation, and to calculate synergy and antagonism correctly.
General guidelines were established for interpreting the degree of synergy and antagonism for Volume of Synergy Values generated by MacSynergy, as follows: 0 to 25, 25 to 50, 50 to 100, and >100 μm2 unit % calculated values in either a positive or negative direction using MacSynergy software are defined as insignificant synergy or antagonism, minor synergy or antagonism, moderate synergy or antagonism, or strong synergy or antagonism, respectively (Prichard et al., 1992). The interpretation of drug-drug interactions by this method has been based on percentage data (Prichard et al., 1992). Up until now, there have been no general guidelines to help investigators interpret the Volume of Synergy given for logarithmic data.
The results obtained from the PR assays are shown in Table 1. At 64 μM of 6-azauridine, no plaques were present in the wells, regardless of the cidofovir concentration, and very few plaques formed in the presence of 32 μM 6-azauridine. Lower combinations of 6-azauridine combined with various concentrations of cidofovir produced synergistic suppression of viral plaques in the cidofovir concentration range of 16–128 μM. Since this analytical method provides no interpretation of the degree of synergy observed (Schinazi et al., 1982), the same data were plotted and analyzed by MacSynergy II software and analyzed three-dimensionally (Figure 1). Nearly the same region of synergy was evident as was shown in the shaded area of Table 1. The volume of synergy observed by this interaction was 275, which is interpreted as strong synergy.
Table 1.
Effects of 6-azauridine and cidofovir combinations on plaque numbers of vaccinia (WR strain) in Vero 76 cells.
| Cidofovir (μM) | ||||||
|---|---|---|---|---|---|---|
| 6-Azauridine (μM) | 0 | 8 | 16 | 32 | 64 | 128 |
| 64 | 0 ± 0a | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 32 | 1.3 ± 2.3 | 1.87 ± 3.2 | 1.20 ± 2.1 | 0 ± 0b | 0 ± 0 | 0 ± 0 |
| 16 | 29.5 ± 35.2 | 36.6 ± 42.5 | 19.0 ± 20.1 | 4.3 ± 7.5 | 0 ± 0 | 0 ± 0 |
| 8 | 57.6 ± 46.2 | 57.6 ± 46.2 | 46.0 ± 38.2 | 24.2 ± 24.3 | 1.4 ± 2.5 | 0 ± 0 |
| 4 | 85.1 ± 20.4 | 85.1 ± 20.4 | 68.9 ±33.2 | 53.4 ± 33.5 | 11.3 ± 10.0 | 0.10 ± 0.2 |
| 2 | 98.0 ± 3.4 | 98.0 ± 3.4 | 100 ± 0 | 92.1 ± 13.7 | 34.1 ± 27.7 | 0.7 ± 1.2 |
| 0 | 100 ± 0 | 99.9 ± 0.2 | 100 ± 0 | 100 ± 0 | 63.2 ± 16.9 | 5.0 ± 4.6 |
Mean value ± SD (percent of plaque numbers in untreated wells), calculated from 3 independent plaque reduction assays, using three wells in each assay evaluated separately for viral plaque counts. The infectious virus dose for inoculation was an average of 80 PFU per culture for the three experiments, as determined by counts in untreated (virus control) wells.
The shading indicates that the combination is synergistic at the concentrations used, as determined by a two-dimensional combination index method (Schinazi et al., 1982).
Figure 1.
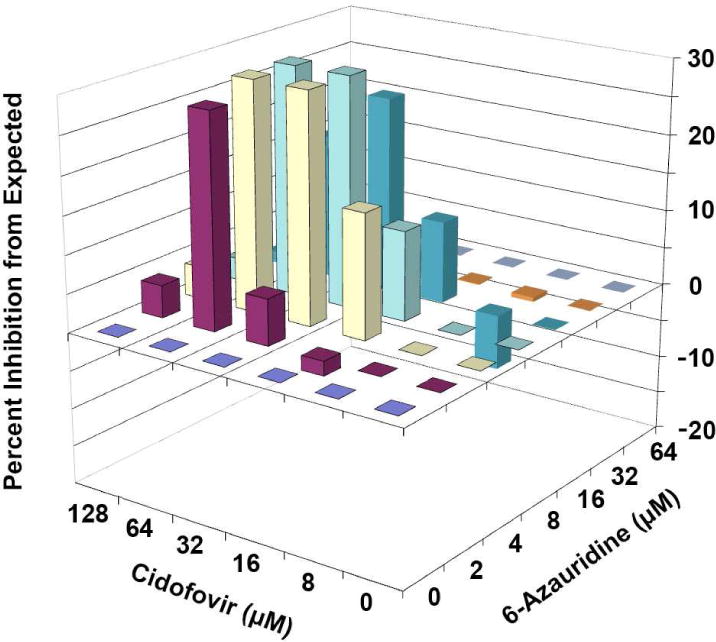
MacSynergy plots of the interaction of cidofovir and 6-azauridine in inhibiting vaccinia virus plaque formation in Vero 76 cells. The expected value (Y axis) for each drug combination is zero, assuming no synergy or antagonism. Values falling above or below the plane represent additive/synergistic or antagonistic interactions, respectively. The Volume of Synergy for this interaction is 275, which is interpreted as strong synergy. Results are from three independently performed plaque reduction assays.
6-Azauridine and cidofovir were evaluated for toxicity in uninfected cell monolayers in 96-well microplates, using the same concentrations (and more) alone and in combination that were used in Figure 1. A neutral red uptake assay was used to quantify cytotoxic effects of the compounds (Smee et al., 2017). 6-Azauridine alone was inhibitory by 35% at 64 μM, whereas uptake inhibition by cidofovir alone at 128 μM was no greater than 5% (Supplemental Figure S1). The combinations of 6-azauridine and cidofovir did not reduce neutral red uptake beyond what occurred with 6-azauridine alone.
During the same time that plaque reduction assays were being conducted, 12-well plates were infected and samples later processed for evaluation of virus yields. Table 2 shows inhibition of virus yields by the compounds used alone and in combination. By the combination index method, synergy was evident with all of the combinations that were tested. The same data were analyzed by MacSynergy II, and inhibition from expected was observed over the entire surface (Figure 2). However, this inhibition was more profound with combinations containing 6-azauridine that were greater than 2 μM. The volume of synergy observed by this interaction was 37, which would be defined as weak synergy using the same interpretive scale as for percentage data.
Table 2.
Effects of 6-azauridine and cidofovir combinations on yield of vaccinia (WR strain) virus from Vero 76 cells.
| Cidofovir (μM) | ||||||
|---|---|---|---|---|---|---|
| 6-Azauridine (μM) | 0 | 8 | 16 | 32 | 64 | 128 |
| 32 | 5.76 ± 0.25 | 3.88 ± 0.88a,b | 3.50 ± 0.88 | 2.62 ± 0.35 | 2.38 ± 0.05 | 2.11 ± 0.21 |
| 16 | 6.88 ± 0.69 | 4.91 ± 0.62 | 4.28 ± 0.09 | 2.76 ± 0.51 | 2.46 ± 0.28 | 2.13 ± 0.04 |
| 8 | 6.94 ± 0.20 | 5.85 ± 0.02 | 5.13 ± 0.31 | 3.87 ± 0.03 | 3.05 ± 0.42 | 2.31 ± 0.29 |
| 4 | 7.08 ± 0.84 | 6.26 ± 0.42 | 5.66 ± 0.32 | 4.64 ± 0.44 | 3.37 ± 0.13 | 2.31 ± 0.07 |
| 2 | 7.65 ± 0.54 | 6.82 ± 0.27 | 6.54 ± 0.07 | 5.73 ± 0.02 | 4.02 ± 0.01 | 2.70 ± 0.34 |
| 0 | 7.66 ± 0.15 | 7.11 ± 0.27 | 6.70 ± 0.20 | 6.08 ± 0.01 | 4.52 ± 0.38 | 3.81 ± 0.50 |
Mean value ± SD (Log10 PFU/mL), calculated from 2 independent virus yield reduction assays, using three wells in each assay evaluated separately for virus titer determinations.). The infectious virus dose for inoculation was approximately 240 PFU per culture in each assay.
The shading indicates that the combination is synergistic at the concentrations used, as determined by a two-dimensional method (Schinazi et al., 1982).
Figure 2.
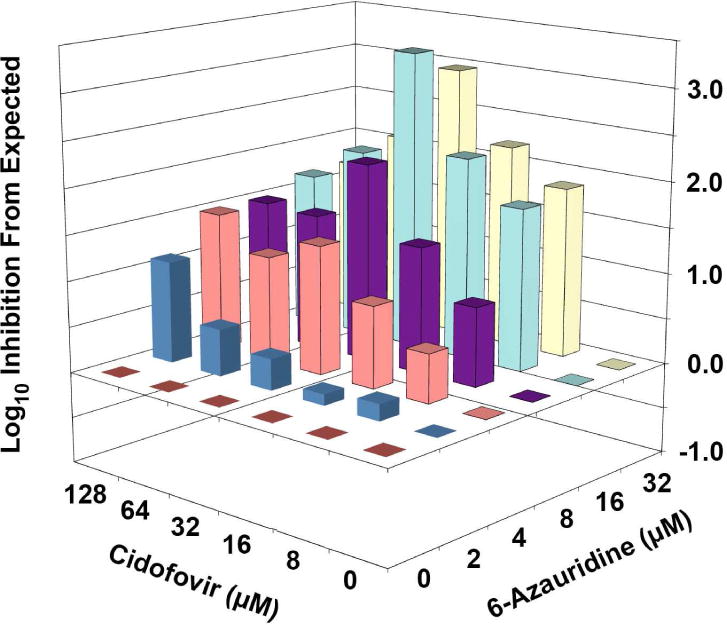
MacSynergy plots of the interaction of cidofovir and 6-azauridine in inhibiting vaccinia virus yield in Vero 76 cells. The expected value (Y axis) for each drug combination is zero, assuming no synergy or antagonism. Values falling above or below the plane represent additive/synergistic or antagonistic interactions, respectively. The Volume of Synergy for this interaction is 37, which would be considered minor synergy using the same scale as for percentage data, or strong synergy based on the new interpretation for logarithmic data proposed in this report. Results are from two independently performed virus yield reduction assays.
The experimental designs for these studies involved the same cells and virus, but different assay methods (PR versus VYR) were employed in order to compare the relationship between percentage versus logarithmic data. The two vaccinia virus assay systems were chosen because of ease of quantitation and for consistency of the results. Using PR and VYR methodologies, there was disparity in Volume of Synergy values (275 versus 37, respectively). However, the data suggested that strong synergy should be evident for both assays. The results suggest further that the Volume of Synergy cannot be directly compared with data using linear and logarithmic data. Essentially the Volume of Synergy calculation is a summation of values above the plane (for synergy) or below the plane (for antagonism), and the degree of the volume is based on the scale. Percentage and logarithmic scales differ by a factor of 10.
Previous reports using viral loads determined by qPCR assays also yielded Volumes of Synergy that were similar in magnitude to those generated by the logarithmic VYR assays. Combinations of CMX001 and acyclovir yielded reproducible synergy against HSV-1 and HSV-2 of 23 ± 2.2 and 16 ± 3.1 μM2log10 genomic equivalents, respectively. Further, these values were reproducible in three separate experiments and were confirmed in animal models of infection (Prichard et al., 2011). A second report described the antagonism of the antiviral activity of ganciclovir against cytomegalovirus by cyclopropavir and maribavir, a known inhibitor of the virus UL97 protein kinase that is required for the metabolic activation of ganciclovir (Prichard, 2009). In this report, the Volume of Synergy for the combination of ganciclovir and maribavir was −12 ± 0.57 μM2log10 genomic equivalents of cytomegalovirus as the average of three independent experiments (James et al., 2011). A similar value of −9.2 ± 0.99 μM2log10 genomic equivalents was obtained with the combination of ganciclovir and cyclopropavir, which also appears to inhibit UL97 kinase activity. Both these reports confirm the biologically significant levels of synergy and antagonism occur with logarithmic endpoints at volumes of synergy that are much lower than the previous guidelines would suggest. These data taken together with the data from vaccinia virus presented here suggest that this relationship appears to hold for three different viruses and two different logarithmic endpoint values.
Clearly the guidelines for the interpretation of synergy and antagonism need to be different for data generated on a logarithmic scale. We propose here that for logarithmic data a value of >10 μM2log10PFU/ml is likely to be indicative of strong synergy. Similarly, a value of <−10 μM2log10PFU/ml would likely indicate strong antagonism. Alternatively, the Volume of Synergy value obtained from logarithmic data can be multiplied by 10, then directly related to the interpretive scale used for percentage data. Repeated measurements of synergy volumes in separate experiments can also be helpful in assessing the biological and statistical significance of synergy or antagonism.
As a side issue to this research, we determined that 6-azauridine would combine with cidofovir in a synergistic manner to inhibit vaccinia virus replication. The practical utility of this discovery is low, because 6-azauridine has not been shown to inhibit virus replication in animal models. Thus, it would not yield a greater survival benefit to use the two compounds in combination in vivo. There are other inhibitors of pyrimidine biosynthesis that should work similarly to 6-azauridine in vitro (because they inhibit the same cellular pathway) in combination with cidofovir, and may also have in vivo activity.
In conclusion, we used vaccinia virus cell culture assays for determining the relationship between linear and logarithmic data by three-dimensional analysis using MacSynergy II software. From this investigation we propose that Volume of Synergy values obtained from logarithmic data are clearly lower than volumes obtained from linear data and suggest general guidelines for interpreting the data.
Supplementary Material
Highlights.
In vitro antiviral studies were performed with cidofovir and 6-azauridine in combination against vaccinia virus.
Plaque reduction (percentage) and virus yield reduction (logarithmic) methods were used for determination of synergy.
Drug interactions were interpreted as strong and weak synergy using percentage and logarithmic data, respectively.
Discrepancy between the two assays was deemed due to scale, since percentage data plot 10-fold higher than logarithmic.
An interpretation of synergy was derived for logarithmic data based on Volume of Synergy.
Acknowledgments
This project was funded in part with Federal funds from the Virology Branch, Division of Microbiology and Infectious Diseases, National Institute of Allergy and infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN272201100019I. The opinions expressed herein are those of the authors and not of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bougie I, Bisaillon M. The broad spectrum antiviral nucleoside ribavirin as a substrate for a viral RNA capping enzyme. J Biol Chem. 2004;279:22124–22130. doi: 10.1074/jbc.M400908200. [DOI] [PubMed] [Google Scholar]
- De Clercq E, Sakuma T, Baba M, Pauwels R, Balzarini J, Rosenberg I, Holy A. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res. 1987;8:261–272. doi: 10.1016/s0166-3542(87)80004-9. [DOI] [PubMed] [Google Scholar]
- Florescu DF, Keck MA. Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev Anti Infect Ther. 2014;12:1171–1178. doi: 10.1586/14787210.2014.948847. [DOI] [PubMed] [Google Scholar]
- Handschumacher RE. Orotidylic acid decarboxylase: inhibition studies with azauridine 5′-phosphate. J Biol Chem. 1960;235:2917–2919. [PubMed] [Google Scholar]
- Ilyushina NA, Hay A, Yilmaz N, Boon AC, Webster RG, Govorkova EA. Oseltamivir-ribavirin combination therapy for highly pathogenic H5N1 influenza virus infection in mice. Antimicrob Agents Chemother. 2008;52:3889–3897. doi: 10.1128/AAC.01579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyushina NA, Hoffmann E, Salomon R, Webster RG, Govorkova EA. Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antivir Ther. 2007;12:363–370. [PubMed] [Google Scholar]
- James SH, Hartline CB, Harden EA, Driebe EM, Schupp JM, Engelthaler DM, Keim PS, Bowlin TL, Kern ER, Prichard MN. Cyclopropavir inhibits the normal function of the human cytomegalovirus UL97 kinase. Antimicrob Agents Chemother. 2011;55:4682–4691. doi: 10.1128/AAC.00571-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R, Leeds JM, Tyavanagimatt S, Hruby DE. Development of ST-246® for Treatment of Poxvirus Infections. Viruses. 2010;2:2409–2435. doi: 10.3390/v2112409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman ER, Davidson W, Groff HL, Smith SK, Warkentien T, Li Y, Wilkins KA, Karem KL, Akondy RS, Ahmed R, Frace M, Shieh WJ, Zaki S, Hruby DE, Painter WP, Bergman KL, Cohen JI, Damon IK. Progressive vaccinia: case description and laboratory-guided therapy with vaccinia immune globulin, ST-246, and CMX001. J Infect Dis. 2012;206:1372–1385. doi: 10.1093/infdis/jis510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee WC, Aldern KA, Hostetler KY, Evans DH. Cidofovir and (S)-9-[3-hydroxy-(2-phosphonomethoxy)propyl]adenine are highly effective inhibitors of vaccinia virus DNA polymerase when incorporated into the template strand. Antimicrob Agents Chemother. 2008;52:586–597. doi: 10.1128/AAC.01172-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard MN. Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir. Rev Med Virol. 2009;19:215–229. doi: 10.1002/rmv.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard MN, Aseltine KR, Shipman C., Jr . MacSynergy™ II. University of Michigan; Ann Arbor: 1992. [Google Scholar]
- Prichard MN, Kern ER, Hartline CB, Lanier ER, Quenelle DC. CMX001 potentiates the efficacy of acyclovir in herpes simplex virus infections. Antimicrob Agents Chemother. 2011;55:4728–4734. doi: 10.1128/AAC.00545-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard MN, Shipman C., Jr A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 1990;14:181–205. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
- Quenelle DC, Prichard MN, Keith KA, Hruby DE, Jordan R, Painter GR, Robertson A, Kern ER. Synergistic efficacy of the combination of ST-246 with CMX001 against orthopoxviruses. Antimicrob Agents Chemother. 2007;51:4118–4124. doi: 10.1128/AAC.00762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada B, Blaskovic D. Some characteristics of the effects of 6-azauridine on vaccinia virus multiplication, in comparison with those of 5-iododeoxyuridine. Acta Virol. 1966;10:1–9. [PubMed] [Google Scholar]
- Rada B, Dragun M. Antiviral action and selectivity of 6-azauridine. Ann N Y Acad Sci. 1977;284:410–417. doi: 10.1111/j.1749-6632.1977.tb21977.x. [DOI] [PubMed] [Google Scholar]
- Schinazi RF, Peters J, Williams CC, Chance D, Nahmias AJ. Effect of combinations of acyclovir with vidarabine or its 5′-monophosphate on herpes simplex viruses in cell culture and in mice. Antimicrob Agents Chemother. 1982;22:499–507. doi: 10.1128/aac.22.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Barnard DL, Jones SM. Activities of JNJ63623872 and oseltamivir against influenza A H1N1pdm and H3N2 virus infections in mice. Antiviral Res. 2016;136:45–50. doi: 10.1016/j.antiviral.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Smee DF, Bray M, Huggins JW. Antiviral activity and mode of action studies of ribavirin and mycophenolic acid against orthopoxviruses in vitro. Antivir Chem Chemother. 2001;12:327–335. doi: 10.1177/095632020101200602. [DOI] [PubMed] [Google Scholar]
- Smee DF, Dagley A, Downs B, Hagloch J, Tarbet EB. Enhanced efficacy of cidofovir combined with vaccinia immune globulin in treating progressive cutaneous vaccinia virus infections in immunosuppressed hairless mice. Antimicrob Agents Chemother. 2015;59:520–526. doi: 10.1128/AAC.04289-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Hurst BL, Evans WJ, Clyde N, Wright S, Peterson C, Jung KH, Day CW. Evaluation of cell viability dyes in antiviral assays with RNA viruses that exhibit different cytopathogenic properties. J Virol Methods. 2017;246:51–57. doi: 10.1016/j.jviromet.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Hurst BL, Wong MH, Bailey KW, Morrey JD. Effects of double combinations of amantadine, oseltamivir, and ribavirin on influenza A (H5N1) virus infections in cell culture and in mice. Antimicrob Agents Chemother. 2009;53:2120–2128. doi: 10.1128/AAC.01012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Hurst BL, Wong MH, Bailey KW, Tarbet EB, Morrey JD, Furuta Y. Effects of the combination of favipiravir (T-705) and oseltamivir on influenza A virus infections in mice. Antimicrob Agents Chemother. 2010a;54:126–133. doi: 10.1128/AAC.00933-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Hurst BL, Wong MH, Tarbet EB, Babu YS, Klumpp K, Morrey JD. Combinations of oseltamivir and peramivir for the treatment of influenza A (H1N1) virus infections in cell culture and in mice. Antiviral Res. 2010b;88:38–44. doi: 10.1016/j.antiviral.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbet EB, Maekawa M, Furuta Y, Babu YS, Morrey JD, Smee DF. Combinations of favipiravir and peramivir for the treatment of pandemic influenza A/California/04/2009 (H1N1) virus infections in mice. Antiviral Res. 2012;94:103–110. doi: 10.1016/j.antiviral.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbet EB, Vollmer AH, Hurst BL, Barnard DL, Furuta Y, Smee DF. In vitro activity of favipiravir and neuraminidase inhibitor combinations against oseltamivir-sensitive and oseltamivir-resistant pandemic influenza A (H1N1) virus. Arch Virol. 2014;159:1279–1291. doi: 10.1007/s00705-013-1922-1. [DOI] [PubMed] [Google Scholar]
- Yang G, Pevear DC, Davies MH, Collett MS, Bailey T, Rippen S, Barone L, Burns C, Rhodes G, Tohan S, Huggins JW, Baker RO, Buller RL, Touchette E, Waller K, Schriewer J, Neyts J, DeClercq E, Jones K, Hruby D, Jordan R. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J Virol. 2005;79:13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


