Abstract
We determined factors associated with diet quality and assessed the relationship between diet quality, birth weight, and gestational age in a prospective national multicenter cohort study. We evaluated diet quality with the Healthy Eating Index (HEI, scale 0–100) in the third trimester of pregnancy with three 24‐hr multiple‐pass dietary recalls in 266 HIV+ women enrolled in the Pediatric HIV/AIDS Cohort Study. Covariates included demographics, food security, pre‐pregnancy body mass index, HIV disease severity, substance use, and antiretroviral exposures. A two‐stage multivariate process using classification and regression trees (CART) followed by multiple regression described HEI tendencies, controlled possible confounding effects, and examined the association of HEI with birth weight and gestational age. To assess the stability of the CART solution, both the HEI 2005 and 2010 were evaluated. The mean HEI scores were 56.1 and 47.5 for the 2005 and 2010 HEI, respectively. The first‐stage CART analysis examined the relationship between HEI and covariates. Non‐US born versus US‐born mothers had higher HEI scores (15‐point difference, R 2 = 0.28). There was a secondary partition due to alcohol/cigarette/illicit drug usage (3.5‐point difference, R 2 = 0.03) among US‐born women. For the second‐stage CART adjusted multiple regression, birth weight z‐score was positively related to HEI 2005 and 2010 (partial r's > 0.13, P's ≤ 0.0398), but not gestational age (r = 0.00). We conclude that diet quality among HIV+ women is associated with higher birth weight. Despite the influence of a large cultural effect and poor prenatal behaviors, interventions to improve diet in HIV+ women may help to increase birth weight.
Keywords: birth weight, dietary quality, healthy eating index, HIV, pregnancy
1. INTRODUCTION
As the prevalence of HIV‐infected women of childbearing age increases throughout the world, a parallel increase in the number of infants exposed to HIV in utero has ensued (UNAIDS 2013). In developed nations, where there is routine prescription of antiretroviral therapy (ART) to pregnant HIV‐infected women, less than 1% of these infants acquire HIV (CDC, 2006). However, these infants are exposed to an in utero environment unique to HIV‐infected women that may place the infant at risk for low birth weight, prematurity, or other adverse outcomes (Jao et al., 2015; Neri et al., 2013). Substances of abuse, low socioeconomic status, HIV itself, and ART are among factors that may influence risk in pregnancy and are possibly associated with adverse perinatal and infant outcomes (Afran et al., 2014).
Research has indicated that the prenatal nutrient environment is critically important for pregnancy, perinatal, infant, and possibly longer‐term child outcomes (Imdad & Bhutta, 2012). Although there are reported beneficial as well as deleterious effects of dietary micronutrients, minerals, and nutritional supplements (Siegfried, Irlam, Visser, & Rollins, 2012) in HIV‐infected women during pregnancy, very little is known about the associations between dietary quality and the possible association with birth outcomes. Dietary intake as a single concept is difficult to access. Intakes of combinations of foods, and not single nutrients, are generally viewed as better representations of the latent concept of dietary quality and may also help to account for nutrient–nutrient interactions (Liese et al., 2015). One measure of dietary quality that evaluates how well diet conforms to federal dietary recommendations is the Healthy Eating Index (HEI). The HEI composite score and its subscales have been recommended for use in both clinical care and research studies and is a valid measure of diet quality for any population, including pregnant women (Guenther et al., 2013).
Knowledge of what demographics, clinical factors, and lifestyle habits are associated with maternal dietary quality in HIV and, in turn, how maternal dietary quality is associated with birth outcomes has the potential to serve as the basis for effective nutritional interventions in this expanding population. Our objectives are to evaluate dietary quality using the HEI, and to determine demographic and clinical factors, and life style habits associated with HEI scores in a subgroup of HIV‐infected pregnant women enrolled in the multi‐site, national Pediatric HIV/AIDS Cohort Study, Surveillance Monitoring for Antiretroviral Therapy Toxicities (SMARTT) protocol. We then evaluate whether HEI is associated with birth weight and gestational age after controlling for observed confounding effects.
Key messages
HIV‐infected pregnant women in the third trimester consume, on average, a low‐quality diet.
HIV‐infected pregnant women who were born outside the continental US consume a diet higher in quality than those born within the continental US.
Use of licit and illicit substances during the third trimester is associated with lower dietary quality in US‐born women.
A higher dietary quality of HIV‐infected pregnant women during the third trimester is associated with higher birth weights of the offspring.
2. PARTICIPANTS AND METHODS
2.1. Subjects
The SMARTT Dynamic cohort is comprised of HIV‐infected pregnant women enrolled during pregnancy or within 1 week of delivery and their newborns from 22 clinical sites across the mainland US and Puerto Rico. The infants are followed prospectively from birth. Enrollment into SMARTT is ongoing since 2007. From 2009 to 2011, we sequentially enrolled HIV‐infected women (R01HD060325) who had enrolled into SMARTT during their second or third trimester and who agreed to provide diet histories, have one blood sample drawn, and whose antiretroviral therapies were known throughout their pregnancy into the Nutrition Sub‐study. Their HIV‐exposed, uninfected infants were enrolled at birth (CDC, 1999). We centrally (University of Miami) administered three 24‐hour dietary recalls in a telephone interview during the third trimester of pregnancy (weeks 28 to 40) and utilized data collected from the parent SMARTT protocol on pregnancy history and birth outcomes. The SMARTT protocol and the Nutrition Sub‐study were approved by the Institutional Review Board at each site, at the University of Miami Human Subjects Research Office and at the Harvard School of Public Health. Informed consent was obtained from the mother for herself and her child.
2.1.1. Exclusion criteria
Fifteen of the 22 clinical sites in the original SMARTT Dynamic cohort agreed to participate in this substudy. A total of 317 pregnant women were initially deemed eligible and consented into the study. Twenty‐six women (8%) dropped out prior to starting the study or were lost to follow‐up. Of the remaining 291 women, 22 (7.5%) completed only one or none of the three dietary recalls (a minimum of two recalls were required for inclusion) (Food and Nutrition Board, 2000; Ludwig, Landy, Kurtz, & Miller, 2013; USDA, 2014). For the 269 remaining women, 233 (86.5%) completed all three dietary recalls. Three women were excluded due to multiple births, change in outpatient status, or a high degree of missing data. Thus, 266 women constituted the sample used for data analysis.
2.2. Study visit measurements
2.2.1. Sociodemographics, clinical history, body measurements, and food assistance
Women were interviewed to obtain information on sociodemographic characteristics and substance use during pregnancy. Data on pregnancy history was abstracted from the medical chart including, but not limited to pregnancy and birth complications, ART use, CD4 percent, and HIV viral load (RNA). Birth weight, method of delivery, and gestational age (calculated from the date of the last menstrual period (LMP) or prenatal ultrasound if LMP was not known) of the infant were collected at the time of delivery. Weight z‐scores for age and sex were calculated using CDC 2000 reference values for infants born at ≥37 weeks gestation and Fenton et al. norms for children born <37 weeks gestation (Fenton & Sauve, 2007). Food security was assessed with the US Household Food Security Survey Module: Six‐Item Short Form (USDA 2000) with one item that was added to check if the participant was receiving a federally supported supplemental assistance program.
2.2.2. 24‐hour dietary recalls
Women were given a prepaid cell phone at the entry visit for the purpose of collecting dietary intake information. The cell phone interviews were conducted centrally at the University of Miami by trained nutritionists (D. N., P. G, and J. V.) during the third trimester of pregnancy. Twenty‐four‐hour dietary recall was obtained following the multiple pass dietary recall method (Moshfegh et al., 2008) and analyzed using the Nutrition Data System for Research (NDSR) version 2010 and 2011 (Nutrition Coordinating Center, University of Minnesota). All foods, beverages, supplements, and nonprescription medications were recorded. We performed three separate 24‐hour dietary recalls with the intent to include two weekdays and one weekend day over a 2‐week period. Recalls were scheduled to be separated by at least 1 day. The reliability of the 24‐hour recall was determined, and any concerns clarified and documented. Recalls were considered unreliable when participants were uncooperative with the interview questions. Those with unreliable data were encouraged to re‐engage, and follow‐up recalls were scheduled. A protocol for quality assurance suggested by NDSR was carried out by a team of three nutritionists, and outlying values for energy, macro‐ and key micronutrients were checked for validity. Data deemed invalid/unreliable were excluded.
2.3. Healthy Eating Index
The USDA Healthy Eating Index (HEI) scale was used to measure diet quality. Because dietary data were taken during a time when USDA requirements were changing, both the 2005 and 2010 versions of the HEI were analyzed (Guenther et al., 2013; Guenther, Reedy, Krebs‐Smith, & Reeve, 2008; Kennedy, Ohls, Carlson, & Fleming, 1995). Because the 2010 Dietary Guidelines were published along with the revised USDA Food Patterns, the 2005 HEI was updated to the 2010 version in order to capture important changes in diet recommendations (McGuire, 2011). The HEI total score for both versions is the sum of 12 recommendation‐specific component scores (total fruit, whole fruit, total vegetables, green/orange legumes, total grains, whole grains, milk, meat and beans, oils, saturated fat, sodium, and fat/alcohol/sugars) based on the 2005 and 2010 Dietary Guidelines for Americans. Each component ranges from a minimum of 0 to a maximum of 5, 10, or 20 for an overall maximum score of 100. The higher the HEI score, the more it represents a diet quality closer to USDA standards (Kennedy et al., 1995).
Due to the complexity of scoring both the HEI 2005 and 2010 from unedited NDSR output files, a SAS (Cary, NC) program was written to automate the process. (Ludwig et al., 2013).
2.4. Statistical analysis
A combined statistical approach using the techniques of classification and regression trees (CART) and standard multiple regression analysis was used to model the behavioral, demographic, and physiological correlates of the HEI, and subsequently determine the relationship between HEI, birth weight z‐scores, and gestational age. Once the CART solution was obtained, it was superimposed on the birth weight z‐scores and gestational age, and multiple regression was then used to investigate if birth weight z‐scores and gestational age were predominantly a function of the CART‐defined subgroups or HEI. This approach is similar to that proposed by Simpson et al. (Simpson, Gossett, Parker, & Hall, 2003) and Obenchain (Obenchain, 2006) in which the two statistical techniques (i.e., CART and regression) are used in sequence and in parallel to arrive at a more comprehensive multivariate understanding of the possible complexities of moderating, mediating, and confounded effects. Rather than relying on underlying parametric distributional assumptions, the CART technique uses computer‐intensive partitioning and search routines to maximize differences in the dependent variable over the independent effects (Breiman, Olshen, & Stone, 1984). The solution produces a more usable result that suggests prescriptions for targeted rather than global interventions (Kiernan, Kraemer, Winkleby, King, & Taylor, 2001). CART also has distinct advantages over propensity scores for controlling extraneous variation (Westreich, Lessler, & Funk, 2010). The advantages include the following: no assumption of linearity or additivity, the ability to easily handle both continuous and nominal level data, and most importantly, a more decision‐oriented, less abstract solution.
For statistical analysis, HEI component and total scores were averaged over the two to three dietary recalls. Because 86.5% of the subjects had all three recalls, and subjects with only one recall were excluded, HEI mean values were not differentially weighted. Two versions of the HEI were used in the analysis: the original 2005 version, which was current at the time of data collection, and the recently updated 2010 version. The two versions of the HEI also provided a means for sensitivity analysis (Saltelli, Chan, & Scott, 2000) by providing an indication of when an effect may be influenced by the USDA's conceptualization of what constitutes a healthy diet. A variety of other stopping rules were also monitored during CART partitioning. Since 3% is the approximate R 2 value that corresponds to a statistical power of 80% (given a total n of 266), 3% was chosen as one of the stopping rules for the CART analysis. Minimum subgroup sample size for each CART step was set at 30. Additionally, the CART solution was stopped when subsequent partitioning failed to replicate across both versions of the HEI. The strength of candidate variables in the CART solution was also evaluated via multiple random forests and boosted trees (Schwender, Zucknick, Ickstadt, & Bolt, 2004). With the exception of mother's pre‐pregnancy BMI, missing values were sparse. For descriptive purposes, each of the 12 component scores that constitute the total composite sores of the HEI 2005 and 2010 were standardized (i.e., z‐scores) across the 266 subjects and then profiled by terminal leaf groups defined by the final CART solution.
All statistical calculations were performed using SAS (version 9.3) and SAS‐JMP (version Pro 11) statistical software (SAS Institute, Cary, NC). The CART solution used by SAS/JMP handles missing data with a built‐in internal imputation algorithm.
3. RESULTS
3.1. Sociodemographic and clinical characteristics
The geographical distribution of the cohort is given in Table 1. Sample‐wide descriptive statistics for all the variables used in the statistical modeling are given in Table 2. The majority (72%) of the women were Black, and 77% of the women were born within the United States with most achieving some high school education. Most women had fairly good HIV control with 84% having undetectable viral load (<400 copies/mL) in the third trimester. Thirty‐five percent were on ART throughout their pregnancy, and others started ART in the first and second trimester (64% of the cohort). Most women received nutritional aid through the WIC program (78%); however, 62% of the cohort met the criteria for food insecurity.
Table 1.
Geographical area and study site
| Study site | N (percent) | |
|---|---|---|
| Illinois | ||
| University of Illinois, Chicago | 18 (7) | |
| Florida | ||
| Children's Diagnostic & Research Center, Fort Lauderdale | 13 (5) | |
| University of Miami, Miami | 28 (11) | |
| New York/New Jersey | ||
| New York University, NY | 7 (2) | |
| SUNY Downstate, Brooklyn, NY | 11 (4) | |
| Bronx Lebanon Hospital, NY | 40 (15) | |
| University of Medicine and Dentistry, Newark, NJ | 10 (4) | |
| Puerto Rico | ||
| San Juan Research Hospital, San Juan | 13 (5) | |
| University of Puerto Rico, San Juan | 10 (4) | |
| Southern United States | ||
| University of Alabama, Birmingham | 19 (7) | |
| St. Jude Children's Hospital, Memphis, TN | 22 (8) | |
| Baylor University, Waco, TX | 23 (9) | |
| Tulane University; New Orleans, LA | 9 (3) | |
| Western United States | ||
| University of Southern California, Los Angeles | 25 (9) | |
| Children's Hospital Colorado, Aurora | 18 (7) | |
| Total | 266 (100) | |
Table 2.
Subjects' clinical characteristics and birth outcomes of their offspring
| Maternal characteristics | Mean (SD) or N (percent) |
|---|---|
| Age at delivery (years) (n = 266) | 30 (6.2) |
| Race, Black | 183 (72) |
| Ethnicity, Hispanic | 89 (33) |
| Born in the United States, Yes | 205 (77) |
| Education, highest year (n = 263) | 11.8 (2.2) |
| Pre‐pregnancy BMI (kg/m2) (n = 215) | 29.3 (8.3) |
| Alcohol, tobacco, illicit drug use during pregnancy | |
| None reported | 189 (73) |
| Tobacco use | 53 (20) |
| Alcohol use | 29 (11) |
| Illicit drugs | 24 (9) |
| HIV‐specific characteristics | Mean (SD) or N (percent) |
| CD4 count, absolute (cells/mm3) (n = 262) | 512.2 (248.1) |
| Viral load, log10 (n = 265) | 2.00 (0.89)a |
| Trimester started cART | |
| Before pregnancy | 89 (35) |
| First trimester | 51 (20) |
| Second trimester | 98 (39) |
| Third trimester | 13 (5) |
| Tenofovir, third trimester, Yes | 126 (48) |
| Protease inhibitor, third trimester, Yes | 230 (86) |
| Dietary outcomes | Mean (SD) or N (percent) |
| Receipt of WIC, Yes | 208 (78) |
| Food insecure, Yes | 165 (62) |
| 2005 HEI score (n = 266) | 56.1 (11.5) |
| 2010 HEI score (n = 266) | 47.5 (12.9) |
| Birth outcomes | Mean (SD) |
| Birth weight, z‐score (n = 263) | −0.47 (0.90) |
| Gestational age, weeks (n = 264) | 37.8 (1.6) |
Note. BMI = body mass index; ART = combination antiretroviral therapy; WIC = women, infants and children; HEI = Healthy Eating Index.
Viral load Log 10 was positively skewed, median = 1.68, interquartile range = 0.34
3.2. Healthy Eating Index and correlates
The overall mean 2005 and 2010 HEI scores were 56.1 (SD = 11.5, range = 31.4 to 88.5) and 47.5 (SD = 12.9, range = 22.5 to 85.1), respectively. Figure 1 depicts the final CART result. Mothers who were born outside the US had the highest HEI scores of 67.3 (2005) and 59.9 (2010), and an approximate 14‐ to 16‐point improvement in their HEI scores compared with US‐born mothers. The first split in the CART solution was large and accounted for 28% of the total variance in HEI. Among mothers born in the US, reported use of alcohol, tobacco, or illicit drugs was associated with a decrement in HEI scores by approximately 3.5 units (3% additional variance explained). Reported alcohol, tobacco, or illicit drug usage within mothers born in the US resulted in HEI 2005 and 2010 scores of 49.4 and 40.4, respectively. Except for a difference in scaling (mean offset HEI 2005–2010 = 8.6 units, SD = 4.5), between HEI 2005 and HEI 2010, the partition history was identical for both versions of the HEI. Additional partitioning (including study site) did not meet the threshold level of 3% explained variance, and/or the solutions for HEI 2005 did not correspond with that of HEI 2010. Furthermore, bootstrapped random forests and boosted trees indicated that further splits beyond these first two partitions were unstable.
Figure 1.
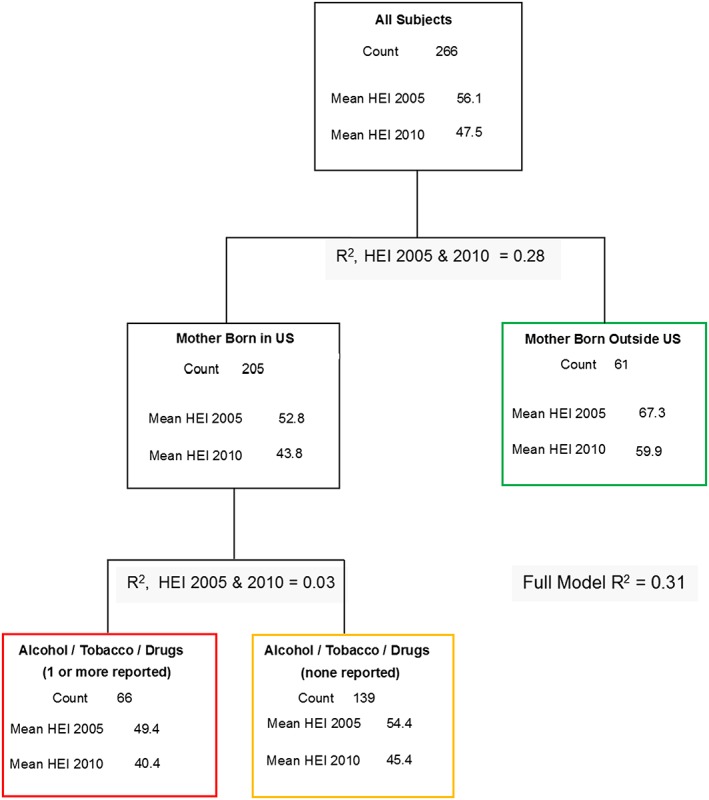
Classification and regression tree solution for the prediction of the Healthy Eating Index (HEI 2005 and 2010). Terminating leafs are color‐coded. Bolded HEI means are terminating leaf estimates
For descriptive purposes, and to provide a bit more detail associated with the CART result, a profiling by terminal leaf classification of the 12 mean component scores that constitute both the HEI 2005 and 2010 (post standardization) is given in Figure 2. As expected, the results reflect the proportional effects observed for the R 2 values associated with the more reliable HEI total scores. Although there were a few instances in which a difference in a component score by terminal leaf grouping varied compared with the HEI composite terminal leaf solution (i.e., total grains, meat and beans, oils (HEI 2005); total protein, fatty acids (HEI 2010)), an overall large height difference was seen in the profile of the non‐US‐born mothers compared with US‐born mothers. Smaller but consistent overall profile height differences were also seen between US‐born mothers who reported using no alcohol, tobacco, or illicit drugs compared with those reporting the use of one or more substances.
Figure 2.

Mean HEI 2005 (Panel A) and 2010 (Panel B) component score profiling by final CART terminal leaf classification. Component scores were standardized (i.e., z‐scores) over the entire sample mean and standard deviation so that the 12 individual component scores which makeup the HEI 2005 and HEI 2010 could be plotted using a common y axis
3.3. Healthy Eating Index and birth weight z‐scores and gestational ages
Subjects were grouped by the three terminating leafs (i.e., branch ends) of the CART result. Side‐by‐side box plots of birth weight z‐scores and gestational age by the terminating leaf classification are presented in Figure 3. Although the effect was somewhat small (R 2 = 0.025, (F (2, 260) = 3.26, P = 0.0397), mean birth weight z‐scores were highest among mothers born outside the US (−0.276), followed by US‐born mothers with no reported alcohol, tobacco, or illicit drug usage (−0.450). Reported alcohol, tobacco, or illicit drug use amount US‐born mothers resulted in the lowest mean birth weight z‐scores (−0.681). Mean gestational age was virtually constant (38 weeks) across the three terminating leaf classifications (F (2,261) = 1.25, P = 0.2871).
Figure 3.

Side‐by‐side quantile box plots of birth weight z‐scores (Panel A) and gestational age (Panel B) by terminating leaf classification. Means are connected with a dashed line. Reference lines are given for zero z‐score and 37 weeks gestation. Percentage less than 37 weeks gestational age equaled 17% (44/264). Box plots are color coded to terminating leafs (see Figure 1)
In an effort to separate the confounded result of HEI and terminating leaf classification with respect to the prediction of birth weight z‐scores, the two independent variables were considered simultaneously in a multiple regression model (i.e., covariance). The adjusted (i.e., partial) correlations between HEI and birth weight z‐scores were 0.13 (t (259) = 2.07, P = 0.0398) and 0.14 (t (259) = 2.29, P = 0.0228) for HEI 2005 and HEI 2010, respectively. In the simultaneous model, which includes both effects, the contribution of terminating leaf classification was negligible (F's (2,259) ≤ 0.8795, P's ≥ 0.4162), suggesting, at least empirically, that birth weight z‐scores share variation with HEI that is not associated with terminal leaf classification (i.e., location of mothers' birth and/or reported alcohol, tobacco, or illicit drug use).
The multiple regression step was repeated using gestational age as the dependent effect. In the simultaneous model, which included the terminating leaf classification as the covariate, the adjusted correlation between HEI 2005 and gestational age was 0.04 (t (260) = 0.58, P = 0.5591). For HEI 2010, the adjusted correlation was zero.
It should be noted that study center site (collapsed to geographical location to avoid sparse subgroupings) was also included in the CART solution, but did not influence the partitioning. The result indicated that the observed effect of diet on birth weight was not dependent on geographical location. In the second stage of the analysis (i.e., least squares), no additional interactions were examined because this would be somewhat redundant with the CART result. It was also felt that in an observational study of this type, examination of interactive components at the second stage of the analysis without firm theoretical justification would increase the study wide TYPE I Error rate.
4. DISCUSSION
We evaluated dietary quality by calculating the 2005 and 2010 HEI using the multiple pass dietary recall methodology in a multicenter, national sample of 266 HIV‐infected women in their third trimester of pregnancy. Overall average HEI score in our cohort was similar to the psychometric means that were found when the HEI scales were originally developed (Kennedy et al., 1995), yet these means are lower than what is desired for a healthy diet (Guenther et al., 2014). Women who were born in the US and, among them, those who reported using alcohol, tobacco, or illicit drugs during pregnancy, were more likely to have a lower 2005 and 2010 HEI than mothers without these characteristics. Higher HEI scores were associated with higher birth weight z‐scores, but were not associated with gestational age. No HIV‐specific factors or other sociodemographic factors were associated with the HEI or HEI's association with birth weight or gestational age.
Place of birth (non‐US born vs US born) was the strongest predictor of higher HEI (more than 15 points higher) in our cohort and accounted for 28% of the variance in our model. Individuals who move to the US can acculturate into unhealthy dietary practices of Americans (Satia‐Abouta, Patterson, Neuhouser, & Elder, 2002). These unhealthy dietary practices may contribute to obesity and cardiovascular disease in those who move to the US from other countries (Rosenthal, 2014). However, those who move to the US from other countries may also hold on to the traditional diets of their home country to maintain ethnic and cultural identity (Brussaard, van Erp‐Baart, Brants, Hulshof, & Lowik, 2001; Nicolaou, van Dam, & Stronks, 2006; Pomerleau et al., 1998a, 1998b; Pomerleau, Ostbye, & Bright‐See, 1998a, 1998b; Vyas et al., 2003), and their diets are often more “healthful” than the traditional American diet, similar to what we found in this study as well as reported by others (Dixon, Sundquist, & Winkleby, 2000; Dubowitz, Subramanian, Acevedo‐Garcia, Osypuk, & Peterson, 2008; Duffey, Gordon‐Larsen, Ayala, & Popkin, 2008, December; Harley & Eskenazi, 2006). Thus, promotion and maintenance of cultural identity through diet seems beneficial.
We also found smoking and the use of alcohol or illicit drugs were associated with a 3.5‐point decrease in HEI among women born in the US. Only 8% of the women born outside of the US reported using alcohol, tobacco, or drugs compared with 34% of US‐born women. Adolescents and young adults from high‐resource countries smoke, consume more fat (Burke et al., 1997), soft drinks, (Kvaavik, Andersen, & Klepp, 2005) and fast food, (Larson, Story, Perry, Neumark‐Sztainer, & Hannan, 2007) and less fruits and vegetables (Baer Wilson & Nietert, 2002; Kvaavik et al., 2005). Also, alcohol is positively associated with fat intake (Burke et al., 1997). Smoking and alcohol use are modifiable behaviors that are associated with fetal growth retardation and other adverse birth outcomes (Brooke, Anderson, Bland, Peacock, & Stewart, 1989). These behaviors may affect birth outcomes independently, or indirectly through modification of the mother's diet. Because some HIV‐infected women are at risk for substance use (Purohit, Rapaka, & Shurtleff, 2010), it is important to understand that these behaviors can contribute to lower dietary quality, which may eventually translate into lower birth weight of their offspring.
There are a number of other factors that could potentially place HIV‐infected pregnant women at risk for poor dietary quality that we may not have been able to account for because our cohort was fairly homogeneous in race and income (i.e., low‐income Blacks). HIV‐infected women often come from disadvantaged backgrounds. Dietary quality tracks with level of income, education, and maternal age that could, in turn, be associated with the cost of healthier foods (Dammann & Smith, 2009; Lukmanji, Hertzmark, Spiegelman, & Fawzi, 2013; Rifas‐Shiman, Rich‐Edwards, Kleinman, Oken, & Gillman, 2009). Antiretroviral exposure can cause nausea and may influence dietary quality. HIV infection itself is a chronic inflammatory state (Miller et al., 2010) that can lead to primary anorexia (Braun & Marks, 2010). However, in our study, we did not find that dietary quality was associated with any HIV‐specific factors.
HIV‐exposed, but uninfected infants have lower birth weights than non‐exposed infants (Neri et al., 2013). We found higher HEI scores in the third trimester, a potentially modifiable factor, were associated with higher birth weight z‐scores, but not gestational ages. Published studies are conflicted regarding the associations of diet and birth weights. In the US Infant Feeding Practices Study II of 893 women, the HEI‐P (2005) (HEI modified for pregnancy) was not associated with birth weight, gestational age, or early infant growth, but was associated with other maternal characteristics such as race, smoking, and pre‐pregnancy weight (Poon, Yeung, Boghossian, Albert, & Zhang, 2013). In Project Viva, a prospective cohort of 1,777 pregnant US women, there was a nonsignificant lowering of risk of small for gestational age (SGA) per 5‐point increase using a modified version of the HEI (Rifas‐Shiman et al., 2009). Knudsen used factor analysis to evaluate dietary quality in over 44,000 Danish pregnant women and found there was a lower odds (0.74 [95% CI 0.64, 0.86]) of having an SGA infant when women ate a “health‐conscious diet” in pregnancy (Knudsen, Orozova‐Bekkevold, Mikkelsen, Wolff, & Olsen, 2008). Comparable findings were reported among 700 Spanish women (Rodriguez‐Bernal et al., 2010) and in a group of Nordic women using a similar dietary quality scale (Hillesund, Bere, Haugen, & Overby, 2014). Although our findings are not unique to pregnant women, this is the first time that dietary quality has been linked to birth weight z‐scores among HIV‐exposed infants, a group known to have lower birth weights than non‐HIV‐exposed infants (Neri et al., 2013).
This is one of the first studies to evaluate dietary quality among a national, multi‐centered sample of HIV‐infected pregnant women. There are several strengths of this study. We sampled HIV‐infected women over a broad geographical range of the US and our results should be generalizable because the demographics of our sample are similar to what is reported nationally in the US (http://www.CDC.gov/HIV/statistics/overview/ataglance.html). We also increased the reliability of the HEI scores by using more than one recall (Landy, Kurtz, Miller, & Ludwig, 2013; Ludwig et al., 2013; The Healthy Eating Index 1995), and we used dedicated cell phones to collect information that we believe decreased the dietary recall nonresponse rate. We also calculated HEI scores through NDSR 24‐hour multiple pass recall methodology that is more detailed than the typical 24‐hour recall method. However, caution must be taken in generalizing our findings to the effects of diet earlier in pregnancy as we collected diet information only in the third trimester. Thus, early pregnancy exposures and premature birth outcomes were not evaluated. Although we tried to account for variables that would affect maternal diet and birth weight, there is a possibility that there are unmeasured clinical or social covariates, such as maternal depression or poverty that could also influence our outcomes. Lastly, although self‐report dietary recall methodology contains biases that are difficult to avoid, we had a large sample, used multiple recalls, while standardizing, and centralizing our recall procedures.
In summary, we found dietary quality, as measured by the HEI, was low and comparable with the original scaling system (Kennedy et al., 1995). Birthplace, as a potential surrogate for cultural identity, was strongly associated with HEI. Illicit and unhealthy substance use was also a factor, although to a lesser extent. Optimization of maternal diet in pregnancy may help mitigate the effects of HIV exposure and other demographic and treatment factors that are associated with birth weights of infants of HIV‐infected women (Jao et al., 2015).
ACKNOWLEDGMENTS
We thank the women and their infants for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS.
SOURCE OF FUNDING
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co‐funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (R01HD060325, Principal Investigators Tracie L. Miller and Denise Jacobson; 1RO1HL095127, Principal Investigator Tracie L. Miller; HD052102, 3 U01 HD052102‐05S1, 3 U01 HD052102‐06S3, Principal Investigator: George Seage; Project Director: Julie Alperen; and the Tulane University School of Medicine, HD052104, 3U01 HD052104‐06S1, Principal Investigator: Russell Van Dyke; Co‐Principal Investigator: Kenneth Rich; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
TLM designed and conducted research, wrote the manuscript, and had primary responsibility for final content. DLJ designed and conducted research, wrote the manuscript, and had primary responsibility for final content. GS designed and conducted research, wrote the manuscript, and had primary responsibility for final content. DN designed and conducted research, wrote the manuscript, and had primary responsibility for final content. JK‐V designed and conducted research, wrote the manuscript, and had primary responsibility for final content. PG designed and conducted research, wrote the manuscript, and had primary responsibility for final content. MWG designed and conducted research, wrote the manuscript, and had primary responsibility for final content. DCL performed statistical analysis, wrote the manuscript, and had primary responsibility for final content. SS designed and conducted research, wrote the manuscript, and had primary responsibility for final content. LB designed and conducted research, wrote the manuscript, and had primary responsibility for final content. KCR designed and conducted research, wrote the manuscript, and had primary responsibility for final content. KH designed and conducted research, wrote the manuscript, and had primary responsibility for final content. DAL designed and conducted research, performed statistical analyses, wrote the manuscript, and had primary responsibility for final content.
Miller TL, Jacobson DL, Somarriba G, et al. A multicenter study of diet quality on birth weight and gestational age in infants of HIV‐infected women. Matern Child Nutr. 2017;13:e12378 10.1111/mcn.12378
Note: The conclusions and opinions in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or US Department of Health and Human Services.
REFERENCES
- Afran, L. , Garcia Knight, M. , Nduati, E. , Urban, B. C. , Heyderman, R. S. , & Rowland‐Jones, S. L. (2014). HIV‐exposed uninfected children: A growing population with a vulnerable immune system? Clinical and Experimental Immunology, 176, 11–22. doi: 10.1111/cei.12251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer Wilson, D. , & Nietert, P. J. (2002). Patterns of fruit, vegetable, and milk consumption among smoking and nonsmoking female teens. American Journal of Preventive Medicine, 22, 240–246. [DOI] [PubMed] [Google Scholar]
- Braun, T. P. , & Marks, D. L. (2010). Pathophysiology and treatment of inflammatory anorexia in chronic disease. Journal of Cachexia, Sarcopenia and Muscle, 1, 135–145. doi: 10.1007/s13539-010-0015-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman, L. F. J. , Olshen, R. A. , & Stone, C. J. (1984). Classification and regression trees. Belmont, CA: Wadsworth. [Google Scholar]
- Brooke, O. G. , Anderson, H. R. , Bland, J. M. , Peacock, J. L. , & Stewart, C. M. (1989). Effects on birth weight of smoking, alcohol, caffeine, socioeconomic factors, and psychosocial stress. BMJ, 298, 795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brussaard, J. H. , van Erp‐Baart, M. A. , Brants, H. A. , Hulshof, K. F. , & Lowik, M. R. (2001). Nutrition and health among migrants in The Netherlands. Public Health Nutrition, 4, 659–664. [DOI] [PubMed] [Google Scholar]
- Burke, V. , Milligan, R. A. , Beilin, L. J. , Dunbar, D. , Spencer, M. , Balde, E. , Gracey, M. P . (1997). Clustering of health‐related behaviors among 18‐year‐old Australians. Preventive Medicine, 26, 724–733. doi: 10.1006/pmed.1997.0198 [DOI] [PubMed] [Google Scholar]
- CDC . Recommendations and Reports. MMWR 1999. 43 (RR1–13): 29–31 http://www.cdc.gov/mmwr/preview/mmwrhtml/rr4813a2.htm
- CDC (2006). Achievements in public health. Reduction in perinatal transmission of HIV infection–United States, 1985–2005. MMWR. Morbidity and Mortality Weekly Report, 55, 592. [PubMed] [Google Scholar]
- Dammann, K. W. , & Smith, C. (2009). Factors affecting low‐income women's food choices and the perceived impact of dietary intake and socioeconomic status on their health and weight. Journal of Nutrition Education and Behavior, 41, 242–253. doi: 10.1016/j.jneb.2008.07.003 [DOI] [PubMed] [Google Scholar]
- Dixon, L. B. , Sundquist, J. , & Winkleby, M. (2000). Differences in energy, nutrient, and food intakes in a US sample of Mexican‐American women and men: Findings from the Third National Health and Nutrition Examination Survey, 1988–1994. American Journal of Epidemiology, 152, 548–557. [DOI] [PubMed] [Google Scholar]
- Dubowitz T, Subramanian SV, Acevedo‐Garcia D, Osypuk TL, Peterson KE. Individual and neighborhood differences in diet among low‐income foreign and U.S.‐born women. Womens Health Issues. 2008. May Jun;18:181–90. DOI: 10.1016/j.whi.2007.11.001. Epub 2008 Jan 28. PubMed PMID: 18222706; PubMed Central PMCID: PMC2760067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffey, K. J. , Gordon‐Larsen, P. , Ayala, G. X. , & Popkin, B. M. (2008, December). Birthplace is associated with more adverse dietary profiles for US‐born than for foreign‐born Latino adults. The Journal of Nutrition, 138, 2428–2435. doi: 10.3945/jn.108.097105 .PubMed PMID:19022968 [DOI] [PubMed] [Google Scholar]
- Fenton, T. R. , & Sauve, R. S. (2007). Using the LMS method to calculate z‐scores for the Fenton preterm infant growth chart. European Journal of Clinical Nutrition, 61, 1380–1385. doi: 10.1038/sj.ejcn.1602667 [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Board. Institute of Medicine (2000). Dietary reference intakes: Applications in dietary assessment. Washington, DC: National Academy of Sciences. [Google Scholar]
- Guenther, P. M. , Reedy, J. , Krebs‐Smith, S. M. , & Reeve, B. B. (2008). Evaluation of the Healthy Eating Index‐2005. Journal of the American Dietetic Association, 108, 1854–1864. doi: 10.1016/j.jada.2008.08.011 [DOI] [PubMed] [Google Scholar]
- Guenther, P. M. , Casavale, K. O. , Reedy, J. , Kirkpatrick, S. I. , Hiza, H. A. , Kuczynski, K. J. , et al. (2013). Update of the Healthy Eating Index: HEI‐2010. Journal of the Academy of Nutrition and Dietetics, 113, 569–580. doi: 10.1016/j.jand.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther, P. M. , Kirkpatrick, S. I. , Reedy, J. , Krebs‐Smith, S. M. , Buckman, D. W. , Dodd, K. W. , … Carroll, R. J . (2014). The Healthy Eating Index‐2010 is a valid and reliable measure of diet quality according to the 2010 Dietary Guidelines for Americans. The Journal of Nutrition, 144, 399–407. doi: 10.3945/jn.113.183079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley, K. , & Eskenazi, B. (2006). Time in the United States, social support and health behaviors during pregnancy among women of Mexican descent. Social Science & Medicine, 62, 3048–3061. doi: 10.1016/j.socscimed.2005.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillesund, E. R. , Bere, E. , Haugen, M. , & Overby, N. C. (2014). Development of a New Nordic Diet score and its association with gestational weight gain and fetal growth – a study performed in the Norwegian Mother and Child Cohort Study (MoBa). Public Health Nutrition, 17, 1909–1918. doi: 10.1017/S1368980014000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imdad, A. , & Bhutta, Z. A. (2012). Maternal nutrition and birth outcomes: Effect of balanced protein‐energy supplementation. Paediatric and Perinatal Epidemiology, 26(Suppl 1), 178–190. doi: 10.1111/j.1365-3016.2012.01308.x [DOI] [PubMed] [Google Scholar]
- Jao, J. , Agwu, A. , Mhango, G. , Kim, A. , Park, K. , Posada, R. , … Sperling R. S. (2015). Growth patterns in the first year of life differ in infants born to perinatally vs. nonperinatally HIV‐infected women. AIDS, 29, 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, E. T. , Ohls, J. , Carlson, S. , & Fleming, K. (1995). The Healthy Eating Index: Design and applications. Journal of the American Dietetic Association, 95, 1103–1108. doi: 10.1016/S0002-8223(95)00300-2 [DOI] [PubMed] [Google Scholar]
- Kiernan, M. , Kraemer, H. C. , Winkleby, M. A. , King, A. C. , & Taylor, C. B. (2001). Do logistic regression and signal detection identify different subgroups at risk? Implications for the design of tailored interventions. Psychological Methods, 6, 35–48. [DOI] [PubMed] [Google Scholar]
- Knudsen, V. K. , Orozova‐Bekkevold, I. M. , Mikkelsen, T. B. , Wolff, S. , & Olsen, S. F. (2008). Major dietary patterns in pregnancy and fetal growth. European Journal of Clinical Nutrition, 62, 463–470. doi: 10.1038/sj.ejcn.1602745 [DOI] [PubMed] [Google Scholar]
- Kvaavik, E. , Andersen, L. F. , & Klepp, K. I. (2005). The stability of soft drinks intake from adolescence to adult age and the association between long‐term consumption of soft drinks and lifestyle factors and body weight. Public Health Nutrition, 8, 149–157. [DOI] [PubMed] [Google Scholar]
- Landy, D. C. , Kurtz, J. M. , Miller, T. L. , & Ludwig, D. A. (2013). Statistical program to automate the creation of Healthy Eating Index scores using nutrition data system for research output. Journal of the Academy of Nutrition and Dietetics, 112, A14. [Google Scholar]
- Larson, N. I. , Story, M. , Perry, C. L. , Neumark‐Sztainer, D. , & Hannan, P. J. (2007). Are diet and physical activity patterns related to cigarette smoking in adolescents? Findings from Project EAT. Preventing Chronic Disease, 4, A51. [PMC free article] [PubMed] [Google Scholar]
- Liese, A. D. , Krebs‐Smith, S. M. , Subar, A. F. , George, S. M. , Harmon, B. E. , Neuhouser, M. L. , … Reedy, J . (2015). The dietary patterns methods project: Synthesis of findings across cohorts and relevance to dietary guidance. The Journal of Nutrition, 145, 393–402. doi: 10.3945/jn.114.205336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DA, Landy, D.C. , Kurtz, J.M. , Miller, T.L. (2013). Using SAS to expand the application of standard measures and guide statistical exploration: creating healthy eating index scores using nutrition data system for research output. In: SAS Global International Conference 2013; San Francisco, CA. Available at: http://support.sas.com/resources/papers/proceedings13/216-2013.pdf
- Lukmanji, Z. , Hertzmark, E. , Spiegelman, D. , & Fawzi, W. W. (2013). Dietary patterns, nutrient intake, and sociodemographic characteristics in HIV‐infected Tanzanian pregnant women. Ecology of Food and Nutrition, 52, 34–62. [DOI] [PubMed] [Google Scholar]
- McGuire, S. (2011). U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010 7th Edition, Washington, DC: U.S. Government Printing Office, January 2011. Advances in Nutrition, 2, 293–294. doi: 10.3945/an.111.000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, T. L. , Somarriba, G. , Orav, E. J. , Mendez, A. J. , Neri, D. , Schaefer, N. , … Lipshultz, S. E . (2010). Biomarkers of vascular dysfunction in children infected with human immunodeficiency virus‐1. Journal of Acquired Immune Deficiency Syndromes, 55, 182–188. doi: 10.1097/QAI.0b013e3181e222c9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshfegh, A. J. , Rhodes, D. G. , Baer, D. J. , Murayi, T. , Clemens, J. C. , Rumpler, W. V. , … Cleveland, L. E . (2008). The US Department of Agriculture Automated Multiple‐Pass Method reduces bias in the collection of energy intakes. The American Journal of Clinical Nutrition, 88, 324–332. [DOI] [PubMed] [Google Scholar]
- Neri, D. , Somarriba, G. A. , Schaefer, N. N. , Chaparro, A. I. , Scott, G. B. , Lopez Mitnik, G. , … Miller, T. L . (2013). Growth and body composition of uninfected children exposed to human immunodeficiency virus: Comparison with a contemporary cohort and United States National Standards. The Journal of Pediatrics, 163, 249–254. e1‐2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaou, M. , van Dam, R. M. , & Stronks, K. (2006). Acculturation and education level in relation to quality of the diet: A study of Surinamese South Asian and Afro‐Caribbean residents of the Netherlands. Journal of Human Nutrition and Dietetics, 19, 383–393. [DOI] [PubMed] [Google Scholar]
- Obenchain , R. L. (2006). Identifying meaningful patient subgroups via clustering‐sensitivity graphics. Proceedings of the American Statistical Association Joint Statistical Meeting, Alexandria, VA. Available at: http://localcontrolstatistics.org/other/MeanSG.PDF
- Pomerleau, J. , Ostbye, T. , & Bright‐See, E. (1998a). Place of birth and dietary intake in Ontario. II. Protein and selected micronutrients. Preventive Medicine, 27, 41–49. doi: 10.1006/pmed.1997.0257 [DOI] [PubMed] [Google Scholar]
- Pomerleau, J. , Ostbye, T. , & Bright‐See, E. (1998b). Place of birth and dietary intake in Ontario. I. Energy, fat, cholesterol, carbohydrate, fiber, and alcohol. Preventive Medicine, 27, 32–40. doi: 10.1006/pmed.1997.0256 [DOI] [PubMed] [Google Scholar]
- Poon, A. K. , Yeung, E. , Boghossian, N. , Albert, P. S. , & Zhang, C. (2013). Maternal dietary patterns during third trimester in association with birthweight characteristics and early infant growth. Scientifica (Cairo), 2013, 786409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit, V. , Rapaka, R. S. , & Shurtleff, D. (2010). Mother‐to‐child transmission (MTCT) of HIV and drugs of abuse in post‐highly active antiretroviral therapy (HAART) era. Journal of Neuroimmune Pharmacology, 5, 507–515. doi: 10.1007/s11481-010-9242-7 [DOI] [PubMed] [Google Scholar]
- Rifas‐Shiman, S. L. , Rich‐Edwards, J. W. , Kleinman, K. P. , Oken, E. , & Gillman, M. W. (2009). Dietary quality during pregnancy varies by maternal characteristics in Project Viva: A US cohort. Journal of the American Dietetic Association, 109, 1004–1011. doi: 10.1016/j.jada.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Bernal, C. L. , Rebagliato, M. , Iniguez, C. , Vioque, J. , Navarrete‐Munoz, E. M. , Murcia, M. , … Ballester F. (2010). Diet quality in early pregnancy and its effects on fetal growth outcomes: The Infancia y Medio Ambiente (Childhood and Environment) Mother and Child Cohort Study in Spain. The American Journal of Clinical Nutrition, 91, 1659–1666. doi: 10.3945/ajcn.2009.28866 [DOI] [PubMed] [Google Scholar]
- Rosenthal, T. (2014). The effect of migration on hypertension and other cardiovascular risk factors: A review. Journal of the American Society of Hypertension, 8, 171–191. doi: 10.1016/j.jash.2013.12.007 [DOI] [PubMed] [Google Scholar]
- Saltelli A, Chan K, Scott M. (Eds.) Sensitivity Analysis. Wiley Series in Probability and Statistics New York: John Wiley and Sons, 2000. [Google Scholar]
- Satia‐Abouta, J. , Patterson, R. E. , Neuhouser, M. L. , & Elder, J. (2002). Dietary acculturation: Applications to nutrition research and dietetics. Journal of the American Dietetic Association, 102, 1105–1118. [DOI] [PubMed] [Google Scholar]
- Schwender, H. , Zucknick, M. , Ickstadt, K. , & Bolt, H. M. (2004). A pilot study on the application of statistical classification procedures to molecular epidemiological data. Toxicology Letters, 151, 291–299. doi: 10.1016/j.toxlet.2004.02.021 [DOI] [PubMed] [Google Scholar]
- Siegfried, N. , Irlam, J. H. , Visser, M. E. , & Rollins, N. N. (2012). Micronutrient supplementation in pregnant women with HIV infection. Cochrane Database of Systematic Reviews, 3, CD009755. doi: 10.1002/14651858.CD009755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, P , Gossett, J. M. , Parker, J. G. , & Hall, R. A . (2003). Logistic regression modeling – JMP start your analysis with a tree. Proceedings of the Twenty‐Eighth Annual SAS Users Group International Conference. Cary, NC: SAS Institute Inc. Available at: http://www2.sas.com/proceedings/sugi28/257-28.pdf
- The Healthy Eating Index 1995. Center for Nutritional Policy and Promotion, United States Department of Agriculture, October 1995.
- UNAIDS 2013. UN AIDS Report on the Global AIDS Epidemic. http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. (accessed 5 April 2015).
- U.S. Department of Agriculture, Agricultural Research Service (2014). USDA National Nutrient Database for Standard Reference, Release 27. Center for Nutrition Policy and Promotion Home Page, http://www.cnpp.usda.gov/
- Vyas, A. , Greenhalgh, A. , Cade, J. , Sanghera, B. , Riste, L. , Sharma, S. , Cruickshank, K . (2003). Nutrient intakes of an adult Pakistani, European and African‐Caribbean community in inner city Britain. Journal of Human Nutrition and Dietetics, 16, 327–337. [DOI] [PubMed] [Google Scholar]
- Westreich, D. , Lessler, J. , & Funk, M. J. (2010). Propensity score estimation: Neural networks, support vector machines, decision trees (CART), and meta‐classifiers as alternatives to logistic regression. Journal of Clinical Epidemiology, 63, 826–833. doi: 10.1016/j.jclinepi.2009.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]


