Abstract
Enoldiazo esters and amides have proven to be versatile reagents for cycloaddition reactions that allow highly efficient construction of various carbocycles and heterocycles. Their versatility is exemplified by (1) [2+n]-cycloadditions (n = 3, 4) by the enol silyl ether units of enoldiazo compounds with retention of the diazo functionality to furnish α-cyclic-α-diazo compounds that are themselves subject to further transformations of the diazo functional group; (2) [3+n]-cycloadditions (n = 1–5) by metallo-enolcarbenes formed by catalytic dinitrogen extrusion from enoldiazo compounds; (3) [2+n]-cycloadditions (n = 3, 4) by donor–acceptor cyclopropenes generated in situ from enoldiazo compounds that produce cyclopropane-fused ring systems. The role of dirhodium(II) and the emergence of copper(I) catalysts are described, as are the different outcomes of reactions initiated with these catalysts. This comprehensive review on cycloaddition reactions of enoldiazo compounds, with emphasis on methodology development, mechanistic insight, and catalyst-controlled chemodivergence, aims to provide inspiration for future discoveries in the field and to catalyze the application of enoldiazo reagents by the wider synthetic community.
Graphical Abstract
A comprehensive review on cycloaddition reactions of enoldiazo compounds is presented with emphasis on methodology development and mechanistic insight.

1. Introduction
Diazo compounds (N2=CRR′) are among the most important reagents in organic synthesis.1–14 One of their major applications is for highly efficient carbon–carbon/carbon–heteroatom bond formation, thus allowing the construction of various carbocycles and heterocycles. In cycloaddition reactions with unsaturated compounds, diazo compounds are directly utilized as N–N- or N–N–C-type dipolar components.15–19 They also serve as effective metal carbene precursors in a wide range of transition metal-catalyzed cyclization processes, including cyclopropanation,20–26 cyclopropenation,27–30 and intramolecular carbene transformations (C–H insertion, O–H insertion, N–H insertion, dimerization, etc.).31–41 Furthermore, several reactive intermediates, such as carbonyl ylides, oxonium ylides, azomethine ylides, and nitrones, can be generated from diazo compounds in the presence of transition metal catalysts and then undergo intermolecular cycloaddition or intramolecular ring closure.16,32,34,39,42–53 Additionally, the successful combination of metal carbene chemistry and other catalytic strategies (e.g., C–H activation,45,54–62 C–C cleavage,63–66 and gold catalysis17,46,67–70) is fully illustrated by recent advances in cycloaddition reactions of diazo compounds.
Bearing both enol and diazo functionalities, enoldiazo compounds have proven to be one of the most versatile tools for the development of cycloaddition reactions. As depicted in Scheme 1, (1) the Enol Silyl Ether (ESE) units of enoldiazo compounds can participate in [2+n]-cycloadditions (n = 3, 4) with retention of the diazo functionality to furnish α-cyclic-α-diazo compounds (ESEC pathway); (2) the Metallo-EnolCarbenes (MEC) formed by dinitrogen extrusion from enoldiazo compounds can act as three-carbon synthons in transition metal-catalyzed [3+n]-cycloadditions (n = 1–5, MECC pathway); (3) the donor–acceptor CycloPropEnes (CPE) generated in situ from enoldiazo compounds can undergo [2+n]-cycloadditions (n = 3, 4) to produce cyclopropane-fused carbocycles and heterocycles (CPEC pathway). Notably, these three reaction pathways can be switched from one to another simply by the choice of catalyst, which allows divergent cycloaddition outcomes from identical reactants and thus demonstrates the controllable versatility of enoldiazo compounds.
Scheme 1.
Cycloaddition reactions of enoldiazo compounds.
Despite tremendous achievements made so far in this area, a comprehensive review on cycloaddition reactions of enoldiazo compounds is still unavailable. Herein, we present a systematic overview of cycloaddition transformations involving enoldiazo compounds, with emphasis on methodology development and mechanistic insight. This review is organized based on the aforementioned cycloaddition pathways in the sequence of ESEC, MECC, and CPEC reactions, followed by a discussion of catalyst-controlled switchable chemoselectivity in these processes. While we focus on representative examples from the past two decades, early pioneering works are included as well. With the present review article we aim to inspire researchers to explore new avenues in this field, and to add enoldiazo compounds, a reagent with controllable versatility, to the toolbox of synthetic chemists.
2. ESEC reactions of enoldiazo compounds
The enol-silyl-ether cycloaddition (ESEC) reactions of enoldiazo compounds, usually facilitated by Lewis or Brønsted acids, are cycloaddition reactions of the enol silyl ether units of enoldiazo compounds that retain the diazo functionality. In the presence of transition metal catalysts, the resulting α-cyclic-α-diazocarbonyl compounds undergo dinitrogen extrusion and selective 1,2-migration to furnish structurally different cyclic products. In these reactions the enol group serves as unsaturated two-carbon component in [2+3]- or [2+4]-cycloaddition transformations that leave the diazo unit intact and adjacent to a quaternary carbon, providing pathways for 1,2-migrations to the metal carbene carbon other than the common 1,2-hydrogen migration. Alternatively, acid catalysis can effect substitution or addition reactions with enoldiazoacetates to form functionalized diazoketoesters that can further undergo intramolecular cyclization processes upon dinitrogen extrusion under catalytic or thermal conditions.
2.1 [2+3]-Cycloaddition
Donor–acceptor cyclopropanes/epoxides
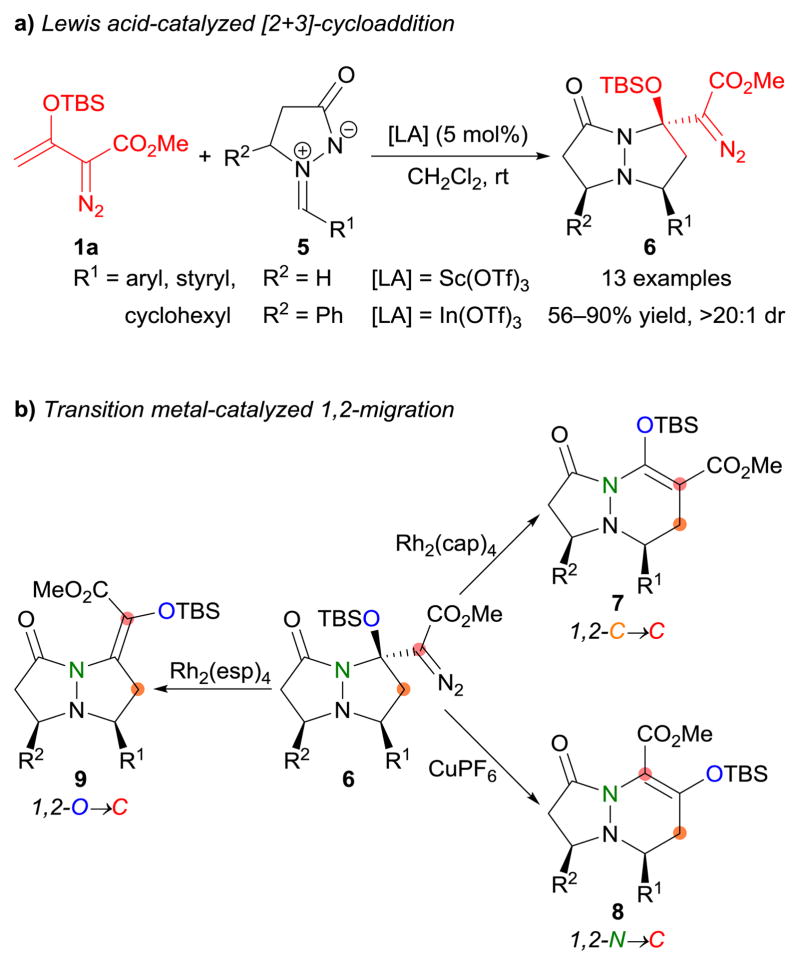
Based on earlier uses of donor–acceptor cyclopropanes as efficient three-carbon synthons in Lewis acid-catalyzed cycloaddition reactions,71–77 the [2+3]-cycloaddition of tert-butyldimethylsilyl (TBS)-protected enoldiazoacetate 1a with donor–acceptor cyclopropanes or epoxides 2 (X = CH2 or O) proceeded smoothly at room temperature with catalysis of ytterbium(III) triflate [Yb(OTf)3], furnishing cycloadducts 3 in good yields with high diastereocontrol (Scheme 2a).78 This cycloaddition process did not interfere with the diazo functionality, thereby presenting a structure that, upon catalytic dinitrogen extrusion, was capable of 1,2-migration with three migration products possible. However, using rhodium(II) caprolactamate [Rh2(cap)4] as the catalyst, highly chemoselective ring expansion of 3 produced six-membered ring products 4 via metal carbene formation and consecutive 1,2-migration of the quaternary carbon (Ca) to the electrophilic carbene carbon (Scheme 2b).78 Note that the migration of secondary carbon (Cb) or silyloxy moiety (TBSO) was not observed in these reactions.
Scheme 2.
Cycloaddition reactions of enoldiazoacetate with donor–acceptor cyclopropanes/epoxides.
Azomethine imines
Lewis acid-catalyzed [2+3]-cycloaddition reactions of enoldiazoacetate 1a with azomethine imines 5 also delivered the expected β-methylene-β-amido-β-silyloxy-α-diazoacetates 6 with exclusive diastereocontrol (Scheme 3a).79 Various transition metal catalysts effected dinitrogen extrusion/1,2-migration from cycloadducts 6, and three catalyst-dependent bond migrations were identified. Intriguingly, rhodium(II) caprolactamate [Rh2(cap)4], copper(I) hexafluorophosphate [Cu(MeCN)4PF6], and rhodium(II) α,α,α′,α′-tetramethyl-1,3-benzenedipropionate [Rh2(esp)2] directed the migration by carbon, nitrogen, and oxygen substituents, respectively, to afford a diverse array of dinitrogen-fused heterocyclic compounds (7–9) in an efficient and controllable manner that was reported to be conformationally dependent on the metal carbene intermediate (Scheme 3b).79
Scheme 3.
Cycloaddition reactions of enoldiazoacetate with azomethine imines.
2.2 [2+4]-Cycloaddition
Besides [2+3]-cycloaddition, the enol silyl ether units of enoldiazo compounds provide ideal polarized two-carbon synthons for potential [2+4]-cycloaddition reactions.
N-Aryl imines
Triflic acid (TfOH)-catalyzed Povarov reactions of vinyldiazoacetates with N-aryl imines have been reported earlier, for which the reaction between enoldiazoacetate 1b and N-benzylideneaniline 10a produced the diazo-containing cycloadduct 11 in 81% yield with excellent diastereoselectivity (Scheme 4a).80 A plausible stepwise mechanism involving a chair-like transition state was proposed to rationalize the stereochemical course, which is differentiated from the concerted pathway for reactions of other vinyldiazoacetates.80 Furthermore, in the presence of gold(I) or dirhodium(II) catalysts, the functionalized diazo compound 11 underwent metal carbene-mediated ring expansion (1,2-migration) to provide seven-membered azacycle (12, Scheme 4b).80
Scheme 4.
Cycloaddition reaction of enoldiazoacetate with N-benzylideneaniline.
Azoalkenes
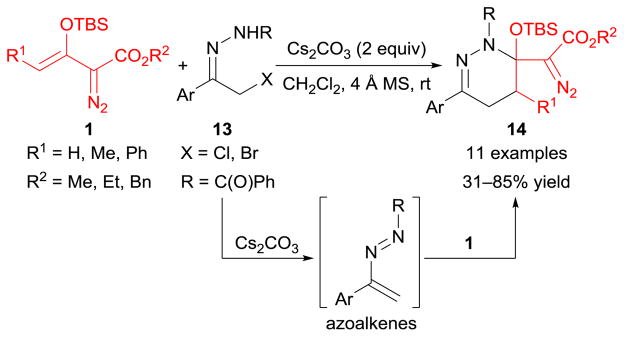
[2+4]-Cycloaddition reactions of enoldiazoacetates with azoalkenes have recently been developed.81 As depicted in Scheme 5, the reactive azoalkenes were readily accessed by treating α-halohydrazones 13 with stoichiometric amounts of inorganic base (Cs2CO3), which subsequently reacted with a wide range of enoldiazoacetates 1 to afford tetrahydropyridazinyl-substituted diazoacetates 14 in moderate to good yields. It is noteworthy that an unexpected tetrahydropyridazine derivative was obtained when cycloadduct 14 was treated with copper(I) catalysts, which was due to acetyl migration from nitrogen, and the formation of an intermediate oxazolium salt accounted for the acetyl migration outcome.81
Scheme 5.
Cycloaddition reactions of enoldiazoacetates with α-halohydrazones.
2.3 Miscellaneous
Besides their direct ESEC reactions described above, enoldiazo compounds were also employed in Lewis (or Brønsted) acid-catalyzed nucleophilic substitution82–87 and nucleophilic addition (Mukaiyama aldol,88 Mukaiyama-Michael,89–98 and Mukaiyama-Mannich99 reactions) to furnish complex organodiazo frameworks (the enol silyl ether unit serving as a general nucleophile with the diazo functionality maintained). Subsequent intramolecular cyclization, triggered by dinitrogen extrusion, produced various carbocyclic and heterocyclic ring systems.82–97,99 A representative example is illustrated in Scheme 6:
Scheme 6.
Cyclization reactions of enoldiazoacetate with nitrones.
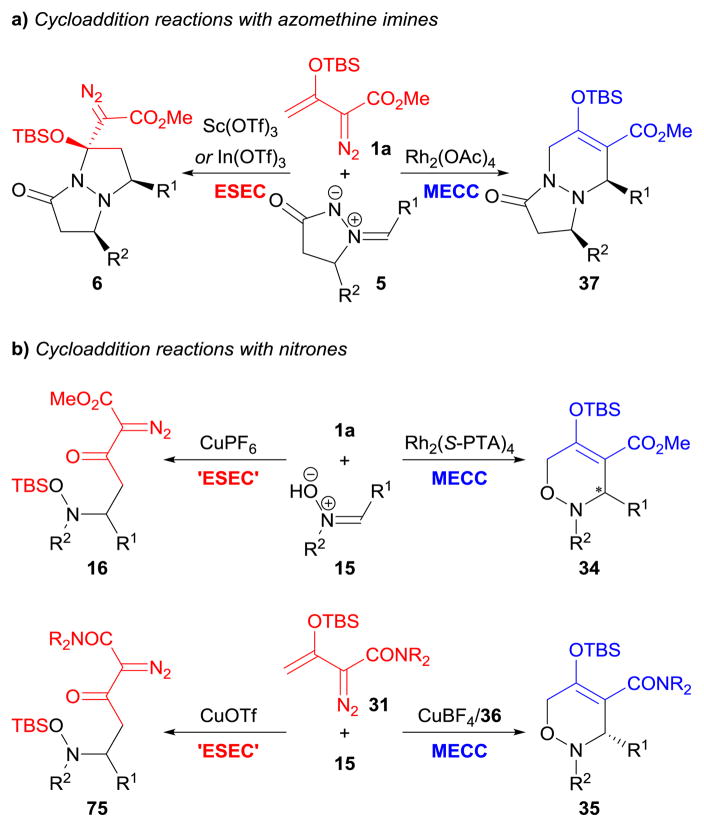
A three-step, one-pot cascade approach to synthesize multifunctionalized 3-hydroxypyrroles 18 from enoldiazoacetate 1a and nitrones 15 demonstrates the importance of the catalyst.99 In this report, copper(I) hexafluorophosphate activated the nitrones through Lewis-acid coordination for electrophilic addition to the enol ether moieties (at 0 °C) that with silyl group transfer to the nitrone oxygen produced stable Mannich addition products (16); subsequent dirhodium(II)-catalyzed N–O bond insertion (at room temperature) followed by acid-promoted deprotection and aromatization (at 70 °C) completed the transformation.99 Catalysis of the same combination of reagents by dirhodium(II) acetate effected dinitrogen extrusion from the enoldiazoacetate without activation of the nitrone, thus allowing [3+3]-cycloaddition between the resulting metal carbene and the nitrone (see Section 3.3). However, use of both catalytic copper(I) hexafluorophosphate and dirhodium(II) acetate in the same pot at the same time gave initial copper(I)-catalyzed Mannich addition, then dirhodium(II)-catalyzed N–O insertion, resulting in the same products (17) as were formed in the two-step process.99 The striking feature of this study is the significant difference in the reactivity of copper(I) and rhodium(II) towards enoldiazoacetates (see also Section 5.1).
3. MECC reactions of enoldiazo compounds
Catalytically generated metallo-enolcarbenes have proven to be efficient and versatile three-carbon synthons in the construction of various carbocycles and heterocycles that are generally formed with high regio- and stereocontrol. As depicted in Scheme 7, the metallo-enolcarbene exhibits vinylogous electrophilic reactivity that enables nucleophilic coupling to form an intermediate dipolar metallo-vinyl complex which completes the intramolecular cyclization with the aid of electron donation by the ether oxygen. This latter step distinguishes reactions of enoldiazo compounds from those of other vinyldiazo compounds (e.g., styryldiazoacetate). The metallo-enolcarbene cycloaddition (MECC) reactions of enoldiazo compounds are cycloaddition reactions of the metallo-enolcarbenes formed by dinitrogen extrusion from enoldiazo compounds, which are facilitated by transition metal catalysts. They include [3+1]-, [3+2]-, [3+3]-, [3+4]-, and [3+5]-cycloaddition reactions, many of which occur with high enantiocontrol.
Scheme 7.
[3+n]-Cycloaddition reactions of enoldiazo compounds.
3.1 [3+1]-Cycloaddition
Cyclobutane and cyclobutene are important structural elements in many natural products and biologically active compounds. As an alternative to the well-established [2+2]-cycloaddition and an attractive approach to structurally diverse four-membered rings, [3+1]-cycloaddition, especially its asymmetric version, has remained underexploited.100,101 The first highly stereoselective [3+1]-cycloaddition between enoldiazoacetates and sulfur ylides has recently been reported.102 As depicted in Scheme 8, a chiral copper(I) catalyst, generated in situ from copper(I) triflate [CuOTf·Tol1/2] and double-sidearmed bisoxazoline ligand 21, facilitated dinitrogen extrusion from triisopropylsilyl (TIPS)-protected enoldiazoacetates 1. Consecutive nucleophilic addition of sulfur ylides 19 to the vinylogous position of the resulting copper-enolcarbenes, followed by intramolecular cyclization with displacement of the thioether (R′2S) and the catalyst produced enantioenriched multifunctionalized cyclobutenes 20. Notably, the first-generation Grubbs catalyst was also successfully employed as stoichiometric one-carbon component to furnish the corresponding [3+1]-cycloaddition product in 71% yield, in which case the cyclization was accomplished by presumed nucleophilic displacement of a ruthenium leaving group.102
Scheme 8.
Cycloaddition reactions of enoldiazoacetates with sulfur ylides.
3.2 [3+2]-Cycloaddition
Polarized alkenes, such as enol ethers103,104 and enamides,105–107 have been widely applied in [3+2]-dipolar cycloaddition reactions. As illustrated in Scheme 9, the resonance contributing structure of catalytically generated metallo-enolcarbenes provides an ideal dipolar three-carbon scaffold for [3+2]-cycloaddition reactions with polarized alkenes. These transformations are proposed to go through a plausible stepwise pathway involving initial nucleophilic addition of polarized alkenes to the vinylogous position of electrophilic metallo-enolcarbenes. Subsequent intramolecular electrophilic addition of oxonium or iminium ions to the metal-bound vinyl carbon is enhanced by the electron-donating silyloxy group originated from enoldiazo compounds, and consecutive release of the ligated metal completes the [3+2]-cycloaddition process.
Scheme 9.
[3+2]-Cycloaddition reactions of enoldiazo compounds.
Enol silyl ethers
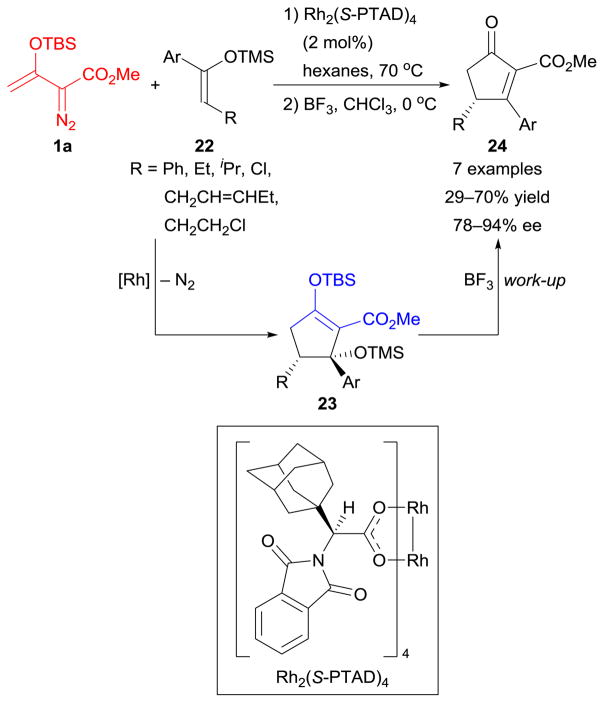
Dirhodium(II)-catalyzed [3+2]-cycloaddition reactions of enoldiazoacetate 1a with enol silyl ethers 22 formed cyclopentene derivatives 23, and subsequent removal of silyl protecting groups (TBS and TMS) produced cyclopentenone derivatives 24 (Scheme 10).108 The chiral dirhodium(II) catalyst [Rh2(S-PTAD)4] allowed high levels of enantiocontrol in this process. Although this transformation was limited to sterically demanding substrates with modest to moderate yields, it demonstrates the capability of catalytically generated rhodium-enolcarbenes to undergo [3+2]-cycloaddition reactions and provided new access to enantioenriched cyclopentenone building blocks.
Scheme 10.
Cycloaddition reactions of enoldiazoacetate with enol silyl ethers.
Silyl ketene imines
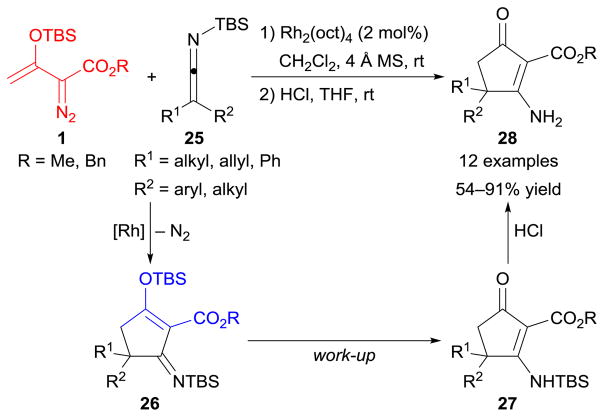
An application of [3+2]-cycloaddition between enoldiazoacetates 1 and silyl ketene imines 25 was subsequently reported.109 As depicted in Scheme 11, rhodium(II) octanoate [Rh2(oct)4] efficiently catalyzed the cycloaddition process to furnish five-membered ring products 26, which further underwent acid-promoted deprotection to produce 3-amino-2-cyclopentenone derivatives 28 in moderate to high overall yields.
Scheme 11.
Cycloaddition reactions of enoldiazoacetates with silyl ketene imines.
Enecarbamates
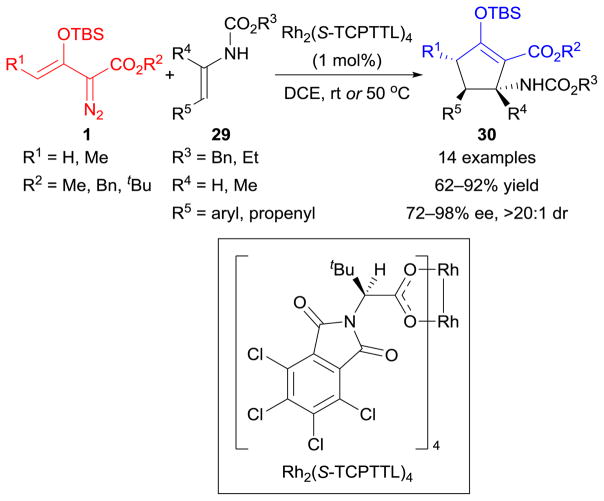
Chiral carbocyclic β-amino acids are key structural elements of many natural products and antibiotics and, also, important building blocks in peptide synthesis. A recent report110 described a highly enantio- and diastereoselective approach to multifunctionalized cyclopentyl β-amino esters 30 through chiral dirhodium(II) [Rh2(S-TCPTTL)4]-catalyzed [3+2]-cycloaddition of enoldiazoacetates 1 with enecarbamates 29 (Scheme 12). Notably, a series of aryl/hydroxyl-substituted cyclopentyl β-amino acids were successfully produced from the cycloadducts via a deprotection/reduction/hydrogenolysis process.110
Scheme 12.
Cycloaddition reactions of enoldiazoacetates with enecarbamates.
Furthermore, an unprecedented hydrogen-bond association between the intermediate rhodium-enolcarbene and the enecarbamate was proposed to explain the observed geometrical constraints from both reactants.110
Indoles
Besides polarized alkenes, considerable efforts have been devoted to develop [3+2]-cycloaddition of indoles.111–115 In the previous studies, substituents at the C2- and/or C3-positions of indoles were required for appropriate regiocontrol.111–114 For example, the dirhodium(II)-catalyzed [3+2]-cycloaddition reaction between (E)-styryldiazoacetate and N-methylindole 32a only afforded moderate regioselectivity (4:1 rr).112 Recently, this limitation has been overcome by the employment of enoldiazoacetamides 31 in dearomatizing [3+2]-cycloaddition with C2,C3-unsubstituted indoles 32, which provided cyclopentane-fused indoline derivatives 33 with exceptional regio-, diastereo-, and enantiocontrol (Scheme 13).115 Note that the highest enantioselectivity was achieved with the chiral dirhodium(II) catalyst bearing the least sterically encumbered prolinate ligand [Rh2(S-MSP)4] of those that have been previously utilized.115
Scheme 13.
Cycloaddition reactions of enoldiazoacetamides with indoles.
Allenes
The exploration of allenes in [3+2]-cycloaddition with vinyldiazo compounds was also reported recently.116 However, a gold(I)-catalyzed reaction between enoldiazoacetate 1a and 1,1-diphenylallene only afforded the corresponding [3+2]-cycloadduct in 25% yield.
3.3 [3+3]-Cycloaddition
[3+3]-Cycloaddition of enoldiazo compounds, which contributes to the efficient and highly selective synthesis of six-membered heterocyclic compounds, has attracted burgeoning interest over the past six years.117 As depicted in Scheme 14, transition metal complexes [such as those of rhodium(II) and copper(I)] facilitate dinitrogen extrusion from enoldiazo compounds to form metallo-enolcarbenes, which can serve as effective 1,3-dipole equivalents. A subsequent cycloaddition process starts with nucleophilic attack by a 1,3-dipole at the vinylogous position of the electrophilic metallo-enolcarbene. Importantly, the electron-donating silyloxy group enhances electrophilic ring closure to the metal-bound vinyl carbon, and this step demonstrates the unique advantage of enoldiazo compounds for this transformation. Final elimination of the metal catalyst delivers the [3+3]-cycloaddition product.
Scheme 14.
[3+3]-Cycloaddition reactions of enoldiazo compounds.
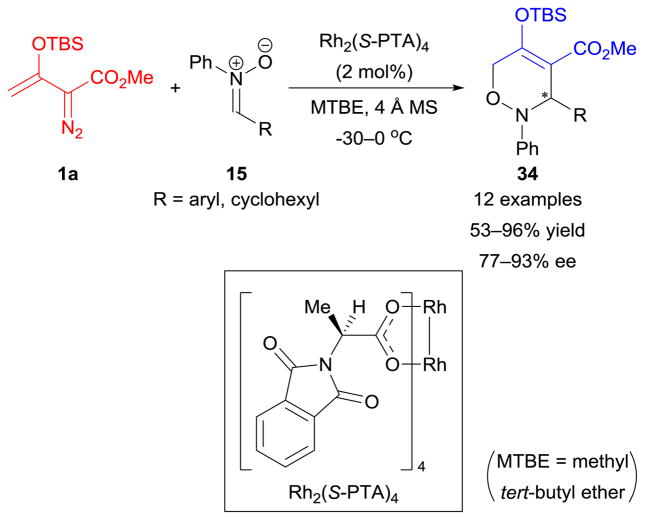
Nitrones
The first [3+3]-cycloaddition of enoldiazo compounds was reported in 2011.118 Under the catalysis of Rh2(S-PTA)4, chiral 3,6-dihydro-1,2-oxazine derivatives 34 were formed from enoldiazoacetate 1a and nitrones 15 in moderate to high yields with good enantiocontrol (Scheme 15). In contrast, [2+3]-cycloaddition products were obtained from dirhodium(II)-catalyzed reactions between β-unsubstituted vinyldiazoacetates and nitrones, in which the metallo-vinylcarbenes participated only as two-carbon components (the carbon–carbon double bond).119 This comparison indicates that the β-silyloxy substituents of enoldiazo compounds play a crucial role in enhancing the [3+3]-cycloaddition process118 and in inhibiting other competing reaction pathways.119–121
Scheme 15.
Cycloaddition reactions of enoldiazoacetate with nitrones.
While the field is dominated by chiral dirhodium(II) catalysts, the first highly enantioselective base metal-catalyzed vinylcarbene transformation was recently uncovered.122 As depicted in Scheme 16, a chiral copper(I) catalyst, generated in situ from copper(I) tetrafluoroborate [Cu(MeCN)4BF4] and bisoxazoline ligand 36, exhibited high catalytic activity and excellent enantiocontrol in [3+3]-cycloaddition reactions between enoldiazoacetamides 31 and nitrones 15, which also furnished the first example of an intermolecular reaction with vinyldiazoacetamides. Notably, dirhodium(II) catalysts [Rh2(OAc)4, Rh2(S-PTA)4, Rh2(S-PTTL)4, and Rh2(S-DOSP)4] were also evaluated in this system, which surprisingly afforded very low reactivities and the recovery of nitrones under otherwise identical conditions.122
Scheme 16.
Cycloaddition reactions of enoldiazoacetamides with nitrones.
Moreover, γ-substituted enoldiazoacetamides122 and enoldiazoacetates123,124 were also successfully employed in [3+3]-cycloaddition reactions with nitrones. Copper(I)122 and copper(II)123 salts, as well as a combination of dirhodium(II) and silver(I) complexes124 were utilized as the catalysts in these transformations. Additionally, dirhodium(II)-catalyzed [3+3]-cycloaddition of enoldiazoacetates with silyl nitronates was presented recently, which produced a series of N-silyloxy-3,6-dihydro-1,2-oxazine derivatives in good yields.125
Azomethine imines
In pioneering studies on transition metal-catalyzed [3+3]-cycloaddition, azomethine imines 5 have proven to be effective dipolar reactants (in palladium-catalyzed reactions with trimethylenemethane126 and gold-catalyzed reactions with propargyl esters127). Their [3+3]-cycloaddition reactions with enoldiazoacetates 1 were also realized in the presence of a dirhodium(II) catalyst [Rh2(OAc)4], which produced bicyclic pyrazolidinone derivatives 37 in moderate to high yields with exclusive diastereocontrol (Scheme 17).128
Scheme 17.
Cycloaddition reactions of enoldiazoacetates with azomethine imines.
N-Acyliminopyridinium ylides
Inspired by previously reported nickel-catalyzed [3+3]-cycloaddition of donor–acceptor cyclopropanes as dipolar reactants with N-iminopyridinium ylides,129,130 catalytic reactions between dipolar metallo-enolcarbene precursors (i.e., enoldiazoacetates 1) and N-acyliminopyridinium ylides 38 were investigated.131 By using appropriate combinations of chiral dirhodium(II) catalyst [Rh2(S-PTTL)4 or Rh2(S-PTAD)4] and solvent (fluorobenzene or toluene), bicyclic tetrahydropyridazine derivatives 39 were formed in a highly enantioselective manner (Scheme 18).
Scheme 18.
Cycloaddition reactions of enoldiazoacetates with N-acyliminopyridinium ylides.
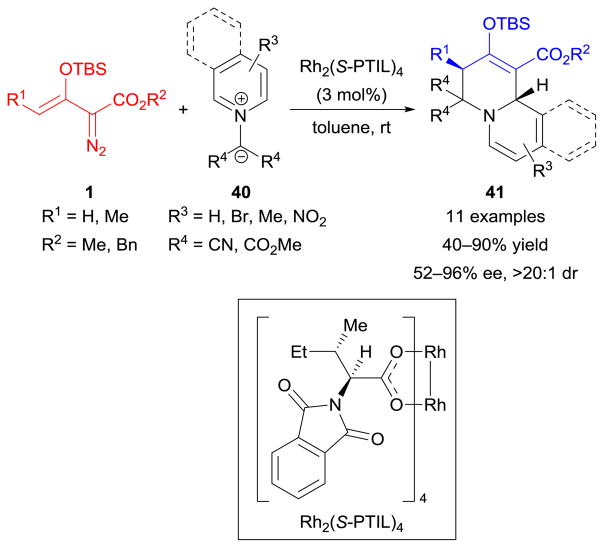
Isoquinolinium/pyridinium methylides
In addition to the aforementioned C–N–O (nitrones 15) and C–N–N (azomethine imines 5 and N-acyliminopyridinium ylides 38) dipolar species, the C–N–C-type dipoles were also evaluated. As illustrated in Scheme 19, isoquinolinium or pyridinium methylides 40 smoothly underwent [3+3]-cycloaddition with enoldiazoacetates 1 in the presence of a chiral dirhodium(II) catalyst [Rh2(S-PTIL)4].132 Dearomatization occurred in these cycloaddition reactions, as well as those with N-acyliminopyridinium ylides (38, Scheme 18),131 furnishing enantioenriched quinolizidine derivatives 41 in moderate to high yields and enantioselectivities.132 It is worth mentioning that the outcome of this reaction was found to be highly catalyst-dependent, and further discussion is presented in Section 5.3.
Scheme 19.
Cycloaddition reactions of enoldiazoacetates with isoquinolinium/pyridinium methylides.
Hydrazones
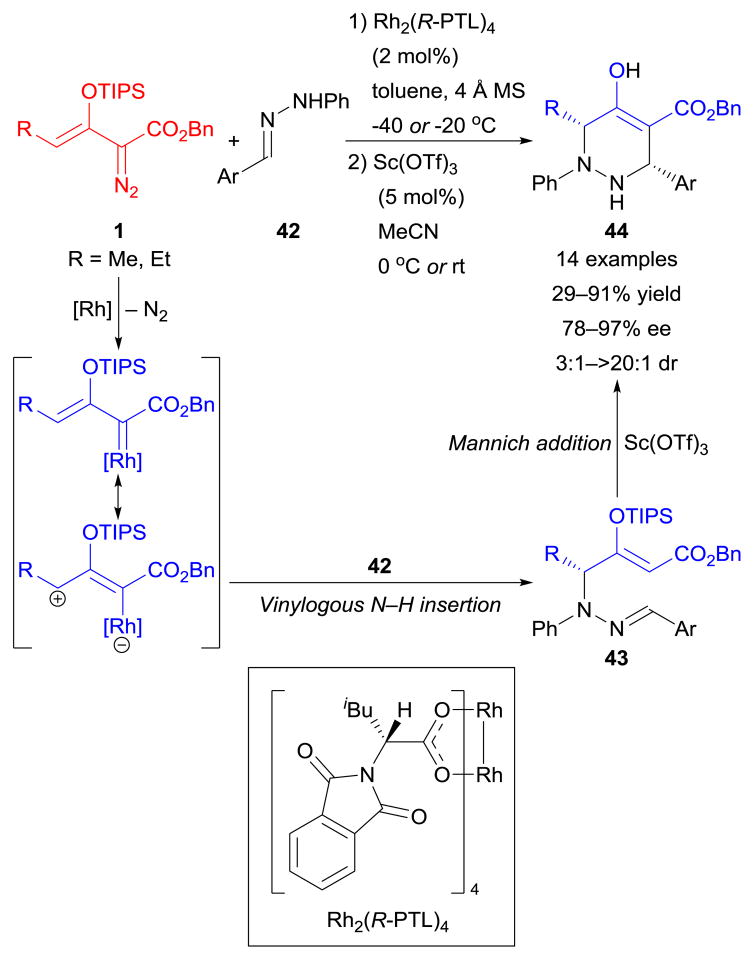
Besides 1,3-dipolar species, hydrazones 42 were also successfully utilized in an overall [3+3]-cyclization process.133 Initially, rhodium-enolcarbenes formed from enoldiazoacetates 1 and chiral dirhodium(II) catalyst [Rh2(R-PTL)4] underwent enantioselective vinylogous N–H bond insertion to furnish intermediate products 43, and subsequent Lewis acid [Sc(OTf)3]-catalyzed intramolecular Mannich addition produced multifunctionalized chiral tetrahydropyridazine derivatives 44 (Scheme 20). Additionally, when donor/acceptor-substituted hydrazones (N-aryl hydrazonoacetates) were used instead of diarylhydrazones 42, a series of pyrazole derivatives were formed via dirhodium(II)-catalyzed vinylogous addition followed by Lewis acid-catalyzed cyclization and aromatization.134
Scheme 20.
Cycloaddition reactions of enoldiazoacetates with hydrazones.
3.4 [3+4]-Cycloaddition
[3+4]-Cycloaddition of enoldiazo compounds has become an attractive tool for the establishment of seven-membered cycles, which are abundant in natural products and, hence, of interest to many synthetic chemists. Since the disclosure of tandem cyclopropanation/Cope rearrangement reactions,135–153 the Davies group has been a major contributor to this area. Recently, they154 and others155 also developed [3+4]-methodologies that shared similar mechanistic pathways (vinylogous addition/ring closure) with the aforementioned [3+n]-cycloaddition reactions (n = 1–3). Like the chemistry described in the previous two subsections, this field is dominated by dirhodium(II) catalysts,135–152,154 although cooperation between rhodium and copper153 as well as a gold-catalyzed reaction155 have been observed and are included in the following subsection.
Dienes
Two mechanistically different [3+4]-cycloaddition pathways between enoldiazoacetates and dienes have been discovered. In the first approach, the rhodium-enolcarbene generated from enoldiazoacetate 1a and Rh2(S-PTAD)4 underwent enantioselective cyclopropanation with dienes 45, and subsequent Cope rearrangement of the resulting cis-1,2-divinylcyclopropanes delivered chiral 1,4-cycloheptadiene derivatives 46 with excellent stereocontrol (Scheme 21).138 Notably, in a comparison among structurally different vinyldiazo compounds, the reactions of enoldiazoacetates exhibited higher enantioselectivities (up to 91% ee) than those of β-unsubstituted vinyldiazoacetates (up to 57% ee).138 Moreover, this strategy has already found applications in the total synthesis of natural products, such as (−)-5-epi-vibsanin E,138 (+)-barekoxide,139 and (−)-barekol.139
Scheme 21.
Rh2(S-PTAD)4-catalyzed cycloaddition reactions of enoldiazoacetate with dienes.
Whereas Rh2(S-DOSP)4137,154 and Rh2(S-PTAD)4138,154 preferentially induced rhodium-enolcarbenes to be attacked at their carbene carbon (to undergo the tandem cyclopropanation/Cope rearrangement sequences), sterically crowded tetrakis(triarylcyclopropanecarboxylate) dirhodium(II) catalysts have proven to be effective promoters of their vinylogous reactivity. The use of Rh2(S-BTPCP)4 in cycloaddition reactions of enoldiazoacetates 1 with 2-silyloxy-1,3-dienes 47 is illustrative (Scheme 22).154 Nucleophilic attack by the dienes at the vinylogous position of rhodium-enolcarbenes produced enantioenriched 1,4-cycloheptadiene derivatives 48, rather than forming their regioisomers (46-type cycloadducts) by reaction at the carbene carbon.
Scheme 22.
Rh2(S-BTPCP)4-catalyzed cycloaddition reactions of enoldiazoacetates with dienes.
Furans
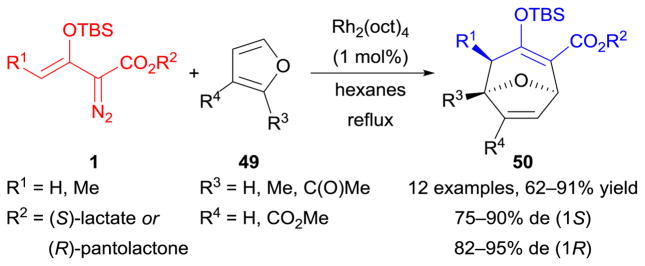
Analogous to dienes 45 (Scheme 21), furans 49 are also suitable substrates for dirhodium(II)-catalyzed [3+4]-cycloaddition reactions of enoldiazoacetates 1 (tandem cyclopropanation/Cope rearrangement pathway). The employment of (S)-lactate or (R)-pantolactone as a chiral auxiliary allowed the efficient construction of stereoenriched 8-oxabicyclo[3.2.1]octene derivatives 50 (Scheme 23).140 Additionally, this method served as the key step in the formal synthesis of (−)-englerin A.143,144
Scheme 23.
Cycloaddition reactions of enoldiazoacetates with furans.
Pyrroles
Related to their oxygen-analogues (furans 49, Scheme 23), pyrroles 51, along with enoldiazoacetate 1a, have proven to be excellent building blocks for the 8-azabicyclo[3.2.1]octane (tropane) skeleton (52, Scheme 24).147 It is worth mentioning that simple vinyldiazoacetate (in the absence of β-silyloxy group) only gave moderate yields and enantioselectivities (up to 58% yield and 65% ee).147 The potential of this strategy was fully illustrated by upscaling to multi-gram quantities146 and by the syntheses of (−)-anhydroecgonine methyl ester,146 (−)-ferruginine,146 isostemofoline,147 (+)-batzelladine B,148 and scopolamine.149
Scheme 24.
Cycloaddition reactions of enoldiazoacetate with pyrroles.
Cinnamaldehydes
In comparison with dienes, furans, and pyrroles (Schemes 21, 23, and 24), cinnamaldehydes 53 underwent epoxidation (in preference to cyclopropanation) with rhodium-enolcarbenes formed from enoldiazoacetates 1 and rhodium(II) acetate, furnishing trans-2,3-divinylepoxides 54 that are more stable than the aforementioned cis-1,2-divinylcyclopropane intermediates. Subsequent Cope rearrangement was facilitated by copper(II) hexafluoroacetylacetonate [Cu(hfacac)2] under thermal conditions, which completed the overall [3+4]-cyclization process (Scheme 25).153 This two-step, one-pot approach allowed the formation of 4,5-dihydrooxepine derivatives 55 in high yields with exclusive regio- and diastereocontrol.
Scheme 25.
Cycloaddition reactions of enoldiazoacetates with cinnamaldehydes.
Hexahydro-s-triazines
In the [3+4]-cycloaddition reactions described so far, the use of vinyldiazoacetates that are devoid of β-silyloxy substituents had a negative influence on product yields and selectivities. A more profound effect was observed in gold(I)-catalyzed cycloaddition reactions between vinyldiazo compounds and hexahydro-s-triazines 56.155 Gold-enolcarbenes generated from either enoldiazoacetates 1 or enoldiazoacetamide 31a furnished [3+4]-cycloadducts 57 via vinylogous addition and consecutive ring closure (Scheme 26). By contrast, gold-vinylcarbenes formed from β-unsubstituted vinyldiazoacetates participated only as one-carbon components (the carbene carbon) to afford the corresponding five-membered ring products.155
Scheme 26.
Cycloaddition reactions of enoldiazo compounds with hexahydro-s-triazines.
3.5 [3+5]-Cycloaddition
To the best of our knowledge, there exists only a single report on [3+5]-cycloaddition involving enoldiazo compounds, and this report was only recently published. Inspired by their previous observation156 that isolable pyridinium zwitterions 58 could participate in cycloaddition reactions with activated alkynes as 1,5-dipole equivalents, Yoo et al.157 accomplished the first [3+5]-cycloaddition of enoldiazo compounds. As illustrated in Scheme 27, rhodium(II) pivalate [Rh2(piv)4] facilitated dinitrogen extrusion from enoldiazoacetate 1a to form rhodium-enolcarbene, and consecutive vinylogous addition/ring closure yielded diazocine derivatives 59. Furthermore, the asymmetric version of this process was preliminarily attempted, and high enantioselectivity (90% ee) was achieved with chiral dirhodium(II) catalyst [Rh2(S-PTAD)4].157 Although the field is clearly still in its infancy, the few results available are promising and merit further investigation.
Scheme 27.
Cycloaddition reactions of enoldiazoacetate with pyridinium zwitterions.
3.6 Miscellaneous
Besides all of the [3+n]-cycloaddition reactions (n = 1–5) described above, enoldiazo compounds were also employed as carbene precursors in several well-established metal carbene transformations, such as cyclopropanation,158–165 cyclopropenation,166 and the Buchner reaction.167,168 In these [1+n]-cycloaddition processes (n = 2, 6), catalytically generated metallo-enolcarbenes served as one-carbon synthons (the carbene carbon) to furnish the corresponding cyclopropane,158–165 cyclopropene,166 and cycloheptatriene167,168 derivatives.
4. CPEC reactions of enoldiazo compounds
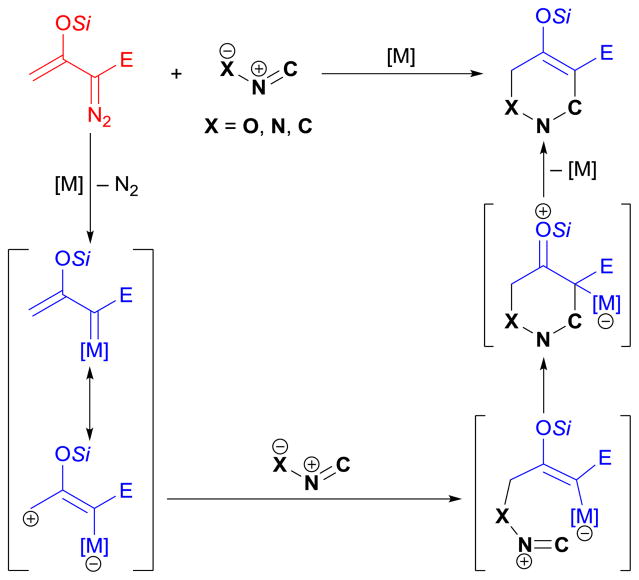
The cyclopropene cycloaddition (CPEC) reactions of enoldiazo compounds are cycloaddition reactions of the donor–acceptor cyclopropenes catalytically generated from enoldiazo compounds. The resulting highly strained cyclopropane-fused cyclic scaffolds provide further access to other ring systems via selective ring opening and rearrangement. The link between donor–acceptor cyclopropenes and metallo-enolcarbenes was discovered in the investigation of dirhodium(II)-catalyzed [3+3]-cycloaddition reactions between enoldiazoacetates and Isoquinolinium/pyridinium methylides.132 In these reactions, the methylides trapped the donor–acceptor cyclopropene by [2+3]-cycloaddition at low catalyst loading, but this process did not occur at higher catalyst loading. The conclusion drawn from this investigation was that the donor–acceptor cyclopropene was a carbene resting state that was rapidly formed from the metallo-enolcarbene and was rapidly reconverted to the metallo-enolcarbene by reacting with an electrophilic metal catalyst. The efficacy of donor–acceptor cyclopropenes as metal carbene precursors compared to the corresponding diazo-precursors was also investigated.132 Moreover, the metal-free thermal conversion of enoldiazoacetates and enoldiazoacetamides to donor–acceptor cyclopropenes has been reported to occur under mild conditions (50 °C),165 but the donor–acceptor cyclopropenes are quite stable at room temperature.
4.1 [2+3]-Cycloaddition
Isoquinolinium methylide
During the catalyst screening of the cycloaddition reaction between enoldiazoacetate 1a and isoquinolinium methylide 40a, [2+3]-cycloadduct 61a was obtained as the sole reaction outcome when rhodium(II) trifluoroacetate [Rh2(tfa)4] was utilized as the catalyst.132 As illustrated in Scheme 28, rhodium-enolcarbene, formed through dinitrogen extrusion from the enoldiazoacetate, underwent intramolecular cyclization with elimination of the dirhodium(II) catalyst to deliver donor–acceptor cyclopropene 60a; and consecutive uncatalyzed cycloaddition between 1,3-dipole equivalent 40a and highly activated carbon–carbon double bond of the cyclopropene completed this transformation.
Scheme 28.
Cycloaddition reaction of enoldiazoacetate with isoquinolinium methylide.
Carbonyl ylides
Recently, a novel cycloaddition reaction between two structurally different diazo compounds has been reported (Scheme 29).169 By adding the mixture of enoldiazoacetamides 31 and α-diazoketones 63 to the solution of rhodium(II) perfluorobutyrate [Rh2(pfb)4], catalytically generated cyclopropenes 62 and carbonyl ylides (via carbene formation/intramolecular cyclization from 31 and 63, respectively) underwent [2+3]-cycloaddition to furnish donor–acceptor cyclopropane-fused benzoxa[3.2.1]octane scaffolds 64 with excellent chemo-, regio-, and diastereoselectivities. The key feature of this efficient transformation is the rapid conversion of 31 to 62 to create a reservoir of donor–acceptor cyclopropenes for [2+3]-cycloaddition with the carbonyl ylides formed from 63. With rhodium(II) perfluorobutyrate as the catalyst, the rhodium-enolcarbenes were significantly diluted relative to other reaction components so that [3+3]-cycloaddition products were not observed. Moreover, the cycloadducts (64) can be readily transformed into benzoxa[3.3.1]nonane (65) and hexahydronaphthofuran (66) derivatives with exact stereocontrol.169 This synthetic methodology allowed the efficient construction of three fused and bridged ring systems, all of which are important skeletons of numerous biologically active natural products.
Scheme 29.
Cycloaddition reactions of enoldiazoacetamides with α-diazoketones.
4.2 [2+4]-Cycloaddition
Cyclopentadiene
Early investigations by Davies et al.170–173 indicated the catalytic formation of donor–acceptor cyclopropenes from a series of vinyldiazo compounds. Among them, cyclopropenes generated in situ from enoldiazo compounds were successfully trapped by cyclopentadiene to deliver the corresponding [2+4]-cycloadducts.172 The synthetic application of these intriguing donor–acceptor cyclopropenes was later explored by Doyle and co-workers.
N-Aryl imines
As depicted in Scheme 30, rhodium/Lewis acid-catalyzed [2+4]-cycloaddition reactions of enoldiazoacetates 1 with N-aryl imines 5 were accomplished via a two-step, one-pot approach.174 Initially, cyclopropenes 60 were formed from the enoldiazoacetates with catalysis of rhodium(II) acetate; subsequent treatment with scandium(III) triflate catalyzed the Povarov reaction between the donor–acceptor cyclopropenes and the imines to produce cyclopropane-fused tetrahydroquinoline derivatives 67. In addition, cyclopropane ring opening of the cycloadducts was triggered by the removal of tert-butyldimethylsilyl group, furnishing benzazipine derivatives 68 in good yields.174
Scheme 30.
Cycloaddition reactions of enoldiazoacetates with N-aryl imines.
Azoalkenes
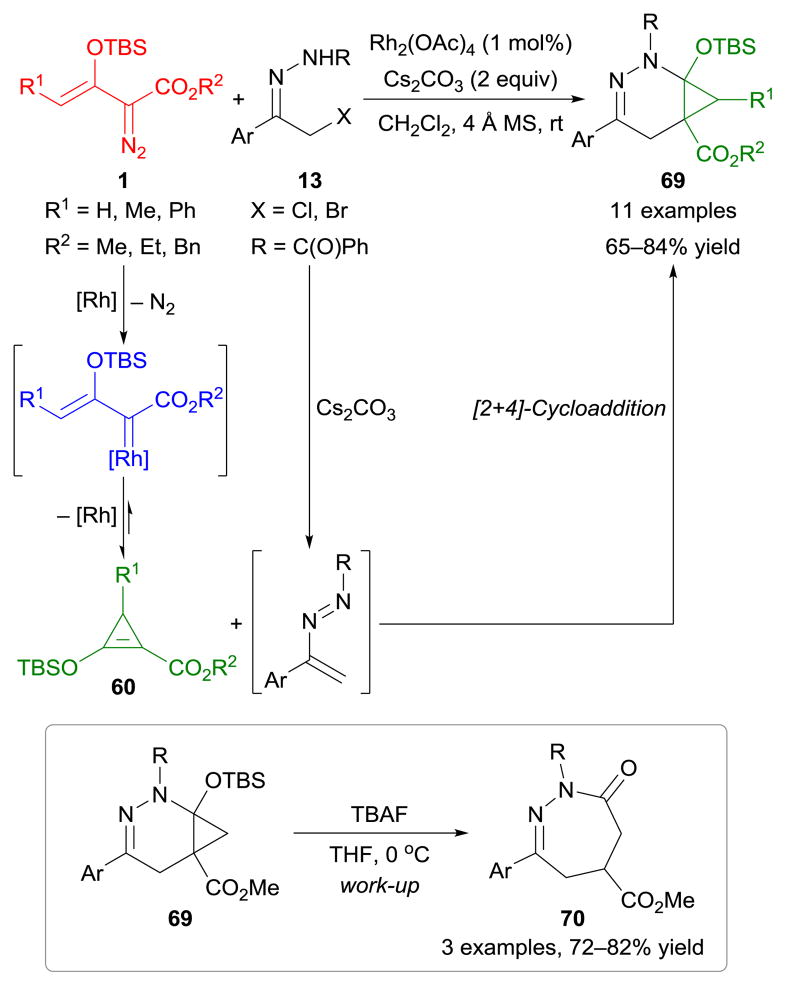
As part of their continuous efforts to explore the synthetic potential of enoldiazo compounds, Doyle, Xu, and co-workers81 recently developed dirhodium(II)-catalyzed/base-promoted [2+4]-cycloaddition reactions of enoldiazoacetates 1 with α-halohydrazones 13 (Scheme 31). This transformation occurred through [2+4]-cycloaddition between donor–acceptor cyclopropenes 60 and azoalkenes, which were generated in situ from 1 and 13 in the presence of rhodium(II) acetate and caesium carbonate, respectively. Moreover, the resulting cyclopropane-fused tetrahydropyridazine scaffolds 69 were readily transformed into tetrahydrodiazepine derivatives 70 upon deprotection.81
Scheme 31.
Cycloaddition reactions of enoldiazoacetates with α-halohydrazones.
4.3 Miscellaneous
Besides their direct CPEC reactions described above, enoldiazo compounds were also employed in composite cyclization processes, triggered by the formation of donor–acceptor cyclopropenes, to afford a diverse array of carbocyclic and heterocyclic ring systems.175–177 A representative example is illustrated in Scheme 32:
Scheme 32.
Cycloaddition reactions of enoldiazoacetates with nitrile oxides.
An unexpected product duality was obtained from dirhodium(II)-catalyzed cyclization reactions of enoldiazoacetates 1 with nitrile oxides 71.176,177 Unstable compounds 72, formed by [2+3]-cycloaddition between catalytically generated donor–acceptor cyclopropenes and nitrile oxides, were proposed as plausible intermediates; and consecutive rearrangements were found to be highly substrate-dependent. Electron-donating substituents of the nitrile oxides directed the formation of ketenimine intermediates via the Lossen rearrangement, furnishing multifunctionalized 5-aminofuran-2(3H)-ones 73;176 whereas the electron-withdrawing substituents favored the Neber rearrangement to deliver azirine intermediates, which further produced 2-oxa-6-azabicyclo[3.1.0]hexan-3-one derivatives 74.177
5. Catalyst-controlled switchable chemoselectivity
Complete control over the product distribution of catalytic reactions is a significant and long-standing goal in synthetic organic chemistry. Under similar reaction conditions, divergent products can be obtained from identical reactants solely controlled by different catalysts.178–180 In previous studies, α-diazocarbonyl compounds, such as α-hydro-,181 α-alkyl-,182 α-vinyl-,183,184 and α-aryl-α-diazoacetates,185 have proven to be versatile reagents in several catalyst-dependent processes. The controllable versatility of enoldiazo compounds (catalyst-controlled switchable chemoselectivity) in cycloaddition reactions is discussed in this section.
5.1 MECC vs. ESEC pathways
In cycloaddition reactions between enoldiazo compounds and dipolar species, transition metal catalysts facilitate dinitrogen extrusion from the enoldiazo compounds to form metallo-enolcarbenes, and their vinylogous position is then attacked by the nucleophilic site of the dipoles (MECC pathway). In contrast, Lewis acid catalysts promote vinylogous association of the enoldiazo compounds with the dipole’s electrophilic site, and the diazo functionality is maintained (ESEC pathway). For example, rhodium(II) acetate directs the formation of [3+3]-cycloadducts 37 from enoldiazoacetate 1a and azomethine imines 5;128 whereas scandium(III) or indium(III) triflate-catalyzed reactions produce [2+3]-cycloadducts 6 (Scheme 33a).79 Similarly, [3+3]-cycloaddition118 and Mannich addition99 reactions of enoldiazoacetate 1a with nitrones 5 are efficiently catalyzed by Rh2(S-PTA)4118 and copper(I) hexafluorophosphate,99 respectively (Scheme 33b). More interestingly, when the diazo reagent is changed to enoldiazoacetamides 31, copper(I) catalysts exhibit their unique advantages by switching the reaction pathway between [3+3]-cycloaddition and Mannich addition (Scheme 33b).122 The catalyst with more open coordination sites [copper(I) triflate] favors the latter pathway by facilitating the migration of tert-butyldimethylsilyl group to the nitrone oxygen.122
Scheme 33.
MECC vs. ESEC pathways in cycloaddition reactions of enoldiazo compounds.
5.2 ESEC vs. CPEC pathways
As documented in Sections 2 and 4, the enol silyl ether units of enoldiazo compounds participate in [2+n]-cycloadditions with retention of the diazo functionality to furnish α-cyclic-α-diazocarbonyl compounds (ESEC pathway); and the donor–acceptor cyclopropenes catalytically generated from enoldiazo compounds also serve as two-carbon components in cycloaddition reactions to produce cyclopropane-fused carbocycles and heterocycles (CPEC pathway). As illustrated in Scheme 34a, Povarov reactions of enoldiazoacetates 1 with N-aryl imines 10 are achieved by using triflic acid as the catalyst.80 Alternatively, donor–acceptor cyclopropenes are formed from the enoldiazoacetates in the presence of the dirhodium(II) catalyst, which further undergo scandium(III) triflate-catalyzed Povarov reactions with the N-aryl imines.174
Scheme 34.
ESEC vs. CPEC pathways in cycloaddition reactions of enoldiazoacetates.
An analogous catalyst-dependent process has also been discovered in [2+4]-cycloaddition reactions between enoldiazoacetates 1 and α-halohydrazones 13 (Scheme 34b).81 In the absence of transition metal catalysts, tetrahydropyridazinyl-substituted diazoacetates 14 are obtained under basic conditions; whereas cyclopropane-fused tetrahydropyridazines 69 are produced with catalysis of rhodium(II) acetate under otherwise identical conditions.
5.3 CPEC vs. MECC pathways
Donor–acceptor cyclopropenes,186 generated in situ from enoldiazo compounds, are not only direct participants in CPEC reactions81,132,169–177 but also important intermediates (in equilibrium with the corresponding metallo-enolcarbenes) in MECC reactions (detected during 1H NMR monitoring).102,122,132,152,167,168 Furthermore, the cyclopropenes, preformed from enoldiazo compounds under catalytic or thermal conditions, have proven to be effective metallo-enolcarbene precursors in several MECC reactions.102,115,122,124,132,165,167,168 As depicted in Scheme 35, catalyst-controlled divergent outcomes are obtained from cycloaddition reactions of enoldiazoacetate 1a with Isoquinolinium methylide 40a.132 Coordination of the Lewis basic methylide to the carbene-bound dirhodium catalyst promotes the formation of donor–acceptor cyclopropene from rhodium-enolcarbene. Thus, the more Lewis acidic catalyst [rhodium(II) trifluoroacetate] preferentially provides [2+3]-CPEC product 61a. Alternatively, the in-situ generated cyclopropene reforms the rhodium-enolcarbene in the presence of Rh2(S-PTIL)4, which further delivers [3+3]-MECC product 41a. Note that when the cyclopropene, preformed from 1a, is subjected to the same reaction conditions, the results are the same (product ratio and enantioselectivity) as those obtained with 1a.132
Scheme 35.
CPEC vs. MECC pathways in cycloaddition reactions of enoldiazoacetate.
6. Conclusions
Enoldiazo compounds have enormous flexibility in their chemical reactions. Their enol silyl ether unit is active for cycloaddition that occurs on the activated carbon–carbon double bond, producing a quaternary carbon and leaving the adjacent diazo functionality intact for subsequent reactions (Schemes 2–4). Enoldiazo compounds have a directive advantage over other vinyldiazo compounds for cycloaddition reactions of metal carbene intermediates. The silyloxy group plays a crucial role in enhancing the nucleophilic character of the original carbenic carbon in order to complete the two-step cycloaddition process (Schemes 7, 9, and 14). Enoldiazoacetates and enoldiazoacetamides are stable at room temperature and below for weeks or months, yet they undergo thermal dinitrogen extrusion at temperatures as low as 50 °C (3–4 h) to quantitatively form donor–acceptor cyclopropenes that are precursors to the same metallo-enolcarbenes which are generated from the corresponding enoldiazo compounds. These donor–acceptor cyclopropenes are themselves highly reactive dipolarophiles or dienophiles that form bi- or tricyclic structures which are subject to further transformations (Schemes 29–31). Enoldiazoacetamides are more stable than are enoldiazoacetates, and so are their corresponding donor–acceptor cyclopropenes. In these intermolecular transformations of enoldiazo compounds, their basicity relative to that of the dipolar reactant determines product formation, so that the Lewis acidity of the catalyst can be used to direct the enoldiazo compound selectively to different products. Although, highly selective cycloaddition reactions of enoldiazo compounds have been established with dirhodium(II) catalysts, recent efforts have shown advantages of copper(I) catalysts for several of these processes (Schemes 8, 16, and 33). Besides the exploration of other dipolar reactants in cycloaddition reactions with enoldiazo compounds, the development and investigation of new subclasses of enoldiazo compounds, as well as the application of these cycloaddition reactions in the syntheses of natural products and pharmaceutical analogues, should be expected in the near future.
Acknowledgments
We are grateful to the National Science Foundation (CHE-1464690 and CHE-1212446), the National Institutes of Health (GM46503), and Rita and John Feik for their support of this research. The authors of the published research are the innovators who have developed and expanded these discoveries.
Biographies
 Qing-Qing Cheng was born in Binzhou of Shandong province, China in 1987. He received his B.Sc. from Tianjin University in 2009. Then he began his graduate study at Nankai University under the supervision of Professor Qi-Lin Zhou, and obtained his Ph.D. in organic chemistry in 2014. Subsequently, he joined the research group of Professor Michael P. Doyle at the University of Texas at San Antonio as a postdoctoral fellow. His research interests include the development of synthetic methodologies and their application in the syntheses of biologically active products, asymmetric catalysis, and medicinal chemistry. His current research in the Doyle group has focused on highly selective catalytic metal carbene transformations, in particular the cycloaddition reactions of enoldiazo compounds.
Qing-Qing Cheng was born in Binzhou of Shandong province, China in 1987. He received his B.Sc. from Tianjin University in 2009. Then he began his graduate study at Nankai University under the supervision of Professor Qi-Lin Zhou, and obtained his Ph.D. in organic chemistry in 2014. Subsequently, he joined the research group of Professor Michael P. Doyle at the University of Texas at San Antonio as a postdoctoral fellow. His research interests include the development of synthetic methodologies and their application in the syntheses of biologically active products, asymmetric catalysis, and medicinal chemistry. His current research in the Doyle group has focused on highly selective catalytic metal carbene transformations, in particular the cycloaddition reactions of enoldiazo compounds.
 Yongming Deng received his B.Sc. from Shandong University in 2009. In 2014, he obtained his Ph.D. from Miami University (Ohio) under the supervision of Dr. Hong Wang. At MIAMI, he was engaged in the development of enamine/metal Lewis acid cooperative catalysis for new chemical reactions discovery. In the same year, he began his postdoctoral studies with Professor Michael P. Doyle at University of Maryland then moved to the University of Texas at San Antonio. His research in the Doyle group has focused on developing assessments of new catalysts and reagents to access catalytic metal carbene reactions and on investigating selective catalytic transformations of enoldiazo compounds.
Yongming Deng received his B.Sc. from Shandong University in 2009. In 2014, he obtained his Ph.D. from Miami University (Ohio) under the supervision of Dr. Hong Wang. At MIAMI, he was engaged in the development of enamine/metal Lewis acid cooperative catalysis for new chemical reactions discovery. In the same year, he began his postdoctoral studies with Professor Michael P. Doyle at University of Maryland then moved to the University of Texas at San Antonio. His research in the Doyle group has focused on developing assessments of new catalysts and reagents to access catalytic metal carbene reactions and on investigating selective catalytic transformations of enoldiazo compounds.
 Marianne Lankelma (1994) obtained her B.S. and M.S. degrees from the University of Amsterdam, with a specialization in molecular design, synthesis, and catalysis. After her undergraduate project in the group of Prof. Dr. Olivia Reinaud (Université Paris Descartes) and her graduate project in the group of Prof. Dr. Joost N. H. Reek (University of Amsterdam), she completed her studies with an extracurricular internship in the group of Prof. Dr. Michael P. Doyle at the University of Texas at San Antonio. Currently she investigates rhodium-mediated carbene (C1) polymerization as a Ph.D. student under the supervision of Prof. Dr. Bas de Bruin at the University of Amsterdam.
Marianne Lankelma (1994) obtained her B.S. and M.S. degrees from the University of Amsterdam, with a specialization in molecular design, synthesis, and catalysis. After her undergraduate project in the group of Prof. Dr. Olivia Reinaud (Université Paris Descartes) and her graduate project in the group of Prof. Dr. Joost N. H. Reek (University of Amsterdam), she completed her studies with an extracurricular internship in the group of Prof. Dr. Michael P. Doyle at the University of Texas at San Antonio. Currently she investigates rhodium-mediated carbene (C1) polymerization as a Ph.D. student under the supervision of Prof. Dr. Bas de Bruin at the University of Amsterdam.
 Michael P. (Mike) Doyle is the Rita and John Feik Distinguished University Chair in Medicinal Chemistry at the University of Texas at San Antonio. He is a graduate of the College of St. Thomas and Iowa State University, has had academic appointments at undergraduate institutions (Hope College and Trinity University) and graduate universities (University of Arizona and University of Maryland), as well as being Vice President, then President, of a science foundation (Research Corporation) before taking his current position. Doyle is a Fellow of the American Chemical Society, the American Association for the Advancement of Science, and the Royal Society of Chemistry, and he is widely recognized for his research in catalytic methods for metal carbene transformations.
Michael P. (Mike) Doyle is the Rita and John Feik Distinguished University Chair in Medicinal Chemistry at the University of Texas at San Antonio. He is a graduate of the College of St. Thomas and Iowa State University, has had academic appointments at undergraduate institutions (Hope College and Trinity University) and graduate universities (University of Arizona and University of Maryland), as well as being Vice President, then President, of a science foundation (Research Corporation) before taking his current position. Doyle is a Fellow of the American Chemical Society, the American Association for the Advancement of Science, and the Royal Society of Chemistry, and he is widely recognized for his research in catalytic methods for metal carbene transformations.
References
- 1.Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds: From Cyclopropanes to Ylides. Wiley; New York: 1998. [Google Scholar]
- 2.Doyle MP. Chem Rev. 1986;86:919. [Google Scholar]
- 3.Ye T, McKervey MA. Chem Rev. 1994;94:1091. [Google Scholar]
- 4.Doyle MP, Forbes DC. Chem Rev. 1998;98:911. doi: 10.1021/cr940066a. [DOI] [PubMed] [Google Scholar]
- 5.Ford A, Miel H, Ring A, Slattery CN, Maguire AR, McKervey MA. Chem Rev. 2015;115:9981. doi: 10.1021/acs.chemrev.5b00121. [DOI] [PubMed] [Google Scholar]
- 6.Marinozzi M, Pertusati F, Serpi M. Chem Rev. 2016;116:13991. doi: 10.1021/acs.chemrev.6b00373. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Q-Q, Doyle MP. Adv Organomet Chem. 2016;66:1. [Google Scholar]
- 8.Thumar NJ, Wei Q, Hu W. Adv Organomet Chem. 2016;66:33. [Google Scholar]
- 9.Jellema E, Jongerius AL, Reek JNH, de Bruin B. Chem Soc Rev. 2010;39:1706. doi: 10.1039/b911333a. [DOI] [PubMed] [Google Scholar]
- 10.Davies HML, Morton D. Chem Soc Rev. 2011;40:1857. doi: 10.1039/c0cs00217h. [DOI] [PubMed] [Google Scholar]
- 11.Gillingham D, Fei N. Chem Soc Rev. 2013;42:4918. doi: 10.1039/c3cs35496b. [DOI] [PubMed] [Google Scholar]
- 12.Liao K, Negretti S, Musaev DG, Bacsa J, Davies HML. Nature. 2016;533:230. doi: 10.1038/nature17651. [DOI] [PubMed] [Google Scholar]
- 13.Key HM, Dydio P, Clark DS, Hartwig JF. Nature. 2016;534:534. doi: 10.1038/nature17968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kan SBJ, Lewis RD, Chen K, Arnold FH. Science. 2016;354:1048. doi: 10.1126/science.aah6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Back TG, Clary KN, Gao D. Chem Rev. 2010;110:4498. doi: 10.1021/cr1000546. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto T, Maruoka K. Chem Rev. 2015;115:5366. doi: 10.1021/cr5007182. [DOI] [PubMed] [Google Scholar]
- 17.Xu G, Zhu C, Gu W, Li J, Sun J. Angew Chem, Int Ed. 2015;54:883. doi: 10.1002/anie.201409845. [DOI] [PubMed] [Google Scholar]
- 18.Qiu H, Srinivas HD, Zavalij PY, Doyle MP. J Am Chem Soc. 2016;138:1808. doi: 10.1021/jacs.5b12877. [DOI] [PubMed] [Google Scholar]
- 19.Duan A, Yu P, Liu F, Qiu H, Gu FL, Doyle MP, Houk KN. J Am Chem Soc. 2017;139:2766. doi: 10.1021/jacs.6b12371. [DOI] [PubMed] [Google Scholar]
- 20.Lebel H, Marcoux J-F, Molinaro C, Charette AB. Chem Rev. 2003;103:977. doi: 10.1021/cr010007e. [DOI] [PubMed] [Google Scholar]
- 21.Maas G. Chem Soc Rev. 2004;33:183. doi: 10.1039/b309046a. [DOI] [PubMed] [Google Scholar]
- 22.Chanthamath S, Iwasa S. Acc Chem Res. 2016;49:2080. doi: 10.1021/acs.accounts.6b00070. [DOI] [PubMed] [Google Scholar]
- 23.Lindsay VNG, Fiset D, Gritsch PJ, Azzi S, Charette AB. J Am Chem Soc. 2013;135:1463. doi: 10.1021/ja3099728. [DOI] [PubMed] [Google Scholar]
- 24.Cao Z-Y, Wang X, Tan C, Zhao X-L, Zhou J, Ding K. J Am Chem Soc. 2013;135:8197. doi: 10.1021/ja4040895. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, Zhu S, Cui X, Wojtas L, Zhang XP. Angew Chem, Int Ed. 2013;52:11857. doi: 10.1002/anie.201305883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan K-H, Guan X, Lo VK-Y, Che C-M. Angew Chem, Int Ed. 2014;53:2982. doi: 10.1002/anie.201309888. [DOI] [PubMed] [Google Scholar]
- 27.Marek I, Simaan S, Masarwa A. Angew Chem, Int Ed. 2007;46:7364. doi: 10.1002/anie.200604774. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Z-B, Wei Y, Shi M. Chem Soc Rev. 2011;40:5534. doi: 10.1039/c1cs15074j. [DOI] [PubMed] [Google Scholar]
- 29.Briones JF, Hansen J, Hardcastle KI, Autschbach J, Davies HML. J Am Chem Soc. 2010;132:17211. doi: 10.1021/ja106509b. [DOI] [PubMed] [Google Scholar]
- 30.Briones JF, Davies HML. J Am Chem Soc. 2012;134:11916. doi: 10.1021/ja304506g. [DOI] [PubMed] [Google Scholar]
- 31.Davies HML, Beckwith REJ. Chem Rev. 2003;103:2861. doi: 10.1021/cr0200217. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura I, Yamamoto Y. Chem Rev. 2004;104:2127. doi: 10.1021/cr020095i. [DOI] [PubMed] [Google Scholar]
- 33.Doyle MP, Duffy R, Ratnikov M, Zhou L. Chem Rev. 2010;110:704. doi: 10.1021/cr900239n. [DOI] [PubMed] [Google Scholar]
- 34.Gulevich AV, Dudnik AS, Chernyak N, Gevorgyan V. Chem Rev. 2013;113:3084. doi: 10.1021/cr300333u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X, Doyle MP. Aust J Chem. 2014;67:365. [Google Scholar]
- 36.Zhang D, Song H, Qin Y. Acc Chem Res. 2011;44:447. doi: 10.1021/ar200004w. [DOI] [PubMed] [Google Scholar]
- 37.Zhu S-F, Zhou Q-L. Acc Chem Res. 2012;45:1365. doi: 10.1021/ar300051u. [DOI] [PubMed] [Google Scholar]
- 38.Burtoloso ACB, Dias RMP, Bernardim B. Acc Chem Res. 2015;48:921. doi: 10.1021/ar500433t. [DOI] [PubMed] [Google Scholar]
- 39.DeAngelis A, Panish R, Fox JM. Acc Chem Res. 2016;49:115. doi: 10.1021/acs.accounts.5b00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J-C, Zhang Y, Xu Z-J, Lo VK-Y, Che C-M. ACS Catal. 2013;3:1144. [Google Scholar]
- 41.Cui X, Xu X, Jin L-M, Wojtas L, Zhang XP. Chem Sci. 2015;6:1219. doi: 10.1039/c4sc02610a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padwa A, Hornbuckle SF. Chem Rev. 1991;91:263. [Google Scholar]
- 43.Padwa A, Weingarten MD. Chem Rev. 1996;96:223. doi: 10.1021/cr950022h. [DOI] [PubMed] [Google Scholar]
- 44.Hodgson DM, Pierard FYTM, Stupple PA. Chem Soc Rev. 2001;30:50. [Google Scholar]
- 45.Chen J-R, Hu X-Q, Lu L-Q, Xiao W-J. Chem Rev. 2015;115:5301. doi: 10.1021/cr5006974. [DOI] [PubMed] [Google Scholar]
- 46.Liu L, Zhang J. Chem Soc Rev. 2016;45:506. doi: 10.1039/c5cs00821b. [DOI] [PubMed] [Google Scholar]
- 47.Xiao Q, Zhang Y, Wang J. Acc Chem Res. 2013;46:236. doi: 10.1021/ar300101k. [DOI] [PubMed] [Google Scholar]
- 48.Guo X, Hu W. Acc Chem Res. 2013;46:2427. doi: 10.1021/ar300340k. [DOI] [PubMed] [Google Scholar]
- 49.Liao S, Sun X-L, Tang Y. Acc Chem Res. 2014;47:2260. doi: 10.1021/ar800104y. [DOI] [PubMed] [Google Scholar]
- 50.Nicolle SM, Lewis W, Hayes CJ, Moody CJ. Angew Chem, Int Ed. 2015;54:8485. doi: 10.1002/anie.201502484. [DOI] [PubMed] [Google Scholar]
- 51.Liu K, Zhu C, Min J, Peng S, Xu G, Sun J. Angew Chem, Int Ed. 2015;54:12962. doi: 10.1002/anie.201507122. [DOI] [PubMed] [Google Scholar]
- 52.Alamsetti SK, Spanka M, Schneider C. Angew Chem, Int Ed. 2016;55:2392. doi: 10.1002/anie.201509247. [DOI] [PubMed] [Google Scholar]
- 53.Nicolle SM, Lewis W, Hayes CJ, Moody CJ. Angew Chem, Int Ed. 2016;55:3749. doi: 10.1002/anie.201511433. [DOI] [PubMed] [Google Scholar]
- 54.Chan W-W, Lo S-F, Zhou Z, Yu W-Y. J Am Chem Soc. 2012;134:13565. doi: 10.1021/ja305771y. [DOI] [PubMed] [Google Scholar]
- 55.Hyster TK, Ruhl KE, Rovis T. J Am Chem Soc. 2013;135:5364. doi: 10.1021/ja402274g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi Z, Koester DC, Boultadakis-Arapinis M, Glorius F. J Am Chem Soc. 2013;135:12204. doi: 10.1021/ja406338r. [DOI] [PubMed] [Google Scholar]
- 57.Cui S, Zhang Y, Wang D, Wu Q. Chem Sci. 2013;4:3912. [Google Scholar]
- 58.Yu S, Liu S, Lan Y, Wan B, Li X. J Am Chem Soc. 2015;137:1623. doi: 10.1021/ja511796h. [DOI] [PubMed] [Google Scholar]
- 59.Zhao D, Kim JH, Stegemann L, Strassert CA, Glorius F. Angew Chem, Int Ed. 2015;54:4508. doi: 10.1002/anie.201411994. [DOI] [PubMed] [Google Scholar]
- 60.Zhou B, Chen Z, Yang Y, Ai W, Tang H, Wu Y, Zhu W, Li Y. Angew Chem, Int Ed. 2015;54:12121. doi: 10.1002/anie.201505302. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y, Wang X, Li Y, Zhou B. Angew Chem, Int Ed. 2015;54:15400. doi: 10.1002/anie.201508702. [DOI] [PubMed] [Google Scholar]
- 62.Gutiérrez-Bonet Á, Juliá-Hernández F, de Luis B, Martin R. J Am Chem Soc. 2016;138:6384. doi: 10.1021/jacs.6b02867. [DOI] [PubMed] [Google Scholar]
- 63.Candeias NR, Paterna R, Gois PMP. Chem Rev. 2016;116:2937. doi: 10.1021/acs.chemrev.5b00381. [DOI] [PubMed] [Google Scholar]
- 64.Moebius DC, Kingsbury JS. J Am Chem Soc. 2009;131:878. doi: 10.1021/ja809220j. [DOI] [PubMed] [Google Scholar]
- 65.Xia Y, Liu Z, Liu Z, Ge R, Ye F, Hossain M, Zhang Y, Wang J. J Am Chem Soc. 2014;136:3013. doi: 10.1021/ja500118w. [DOI] [PubMed] [Google Scholar]
- 66.Yada A, Fujita S, Murakami M. J Am Chem Soc. 2014;136:7217. doi: 10.1021/ja502229c. [DOI] [PubMed] [Google Scholar]
- 67.Pagar VV, Jadhav AM, Liu R-S. J Am Chem Soc. 2011;133:20728. doi: 10.1021/ja209980d. [DOI] [PubMed] [Google Scholar]
- 68.Kalepu J, Katukojvala S. Angew Chem, Int Ed. 2016;55:7831. doi: 10.1002/anie.201600878. [DOI] [PubMed] [Google Scholar]
- 69.Yu Z, Qiu H, Liu L, Zhang J. Chem Commun. 2016;52:2257. doi: 10.1039/c5cc08880a. [DOI] [PubMed] [Google Scholar]
- 70.López E, González J, López LA. Adv Synth Catal. 2016;358:1428. [Google Scholar]
- 71.Reissig H-U, Zimmer R. Chem Rev. 2003;103:1151. doi: 10.1021/cr010016n. [DOI] [PubMed] [Google Scholar]
- 72.Yu M, Pagenkopf BL. Tetrahedron. 2005;61:321. [Google Scholar]
- 73.Carson CA, Kerr MA. Chem Soc Rev. 2009;38:3051. doi: 10.1039/b901245c. [DOI] [PubMed] [Google Scholar]
- 74.De Simone F, Waser J. Synthesis. 2009;41:3353. [Google Scholar]
- 75.Schneider TF, Kaschel J, Werz DB. Angew Chem, Int Ed. 2014;53:5504. doi: 10.1002/anie.201309886. [DOI] [PubMed] [Google Scholar]
- 76.Cavitt MA, Phun LH, France S. Chem Soc Rev. 2014;43:804. doi: 10.1039/c3cs60238a. [DOI] [PubMed] [Google Scholar]
- 77.Grover HK, Emmett MR, Kerr MA. Org Biomol Chem. 2015;13:655. doi: 10.1039/c4ob02117g. [DOI] [PubMed] [Google Scholar]
- 78.Cheng Q-Q, Qian Y, Zavalij PY, Doyle MP. Org Lett. 2015;17:3568. doi: 10.1021/acs.orglett.5b01674. [DOI] [PubMed] [Google Scholar]
- 79.Xu X, Qian Y, Zavalij PY, Doyle MP. J Am Chem Soc. 2013;135:1244. doi: 10.1021/ja311392m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jadhav AM, Pagar VV, Liu R-S. Angew Chem, Int Ed. 2012;51:11809. doi: 10.1002/anie.201205692. [DOI] [PubMed] [Google Scholar]
- 81.Deng Y, Pei C, Arman H, Dong K, Xu X, Doyle MP. Org Lett. 2016;18:5884. doi: 10.1021/acs.orglett.6b02965. [DOI] [PubMed] [Google Scholar]
- 82.Hart DJ, Lee C-S. J Am Chem Soc. 1986;108:6054. doi: 10.1021/ja00279a072. [DOI] [PubMed] [Google Scholar]
- 83.Cainelli G, Panunzio M. J Am Chem Soc. 1988;110:6879. [Google Scholar]
- 84.Bodner MJ, Phelan RM, Townsend CA. Org Lett. 2009;11:3606. doi: 10.1021/ol901269d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qian Y, Shanahan CS, Doyle MP. Eur J Org Chem. 2013:6032. [Google Scholar]
- 86.Liu Y, Liu Y, Shanahan CS, Xu X, Doyle MP. Org Biomol Chem. 2014;12:5227. doi: 10.1039/c4ob00709c. [DOI] [PubMed] [Google Scholar]
- 87.Liu Y, Deng Y, Zavalij PY, Liu R, Doyle MP. Chem Commun. 2015;51:565. doi: 10.1039/c4cc08255a. [DOI] [PubMed] [Google Scholar]
- 88.Doyle MP, Kundu K, Russell AE. Org Lett. 2005;7:5171. doi: 10.1021/ol052003s. [DOI] [PubMed] [Google Scholar]
- 89.Lee DJ, Kim K, Park YJ. Org Lett. 2002;4:873. doi: 10.1021/ol016995n. [DOI] [PubMed] [Google Scholar]
- 90.Lee DJ, Kim K. J Org Chem. 2004;69:4867. doi: 10.1021/jo049769a. [DOI] [PubMed] [Google Scholar]
- 91.Liu Y, Bakshi K, Zavalij PY, Doyle MP. Org Lett. 2010;12:4304. doi: 10.1021/ol101744h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jaber DM, Burgin RN, Hepler M, Zavalij PY, Doyle MP. Chem Commun. 2011;47:7623. doi: 10.1039/c1cc12443a. [DOI] [PubMed] [Google Scholar]
- 93.Liu Y, Doyle MP. Org Biomol Chem. 2012;10:6388. doi: 10.1039/c2ob25776a. [DOI] [PubMed] [Google Scholar]
- 94.Jaber DM, Burgin RN, Hepler M, Zavalij PY, Doyle MP. Org Lett. 2012;14:1676. doi: 10.1021/ol300213u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Truong PM, Shanahan CS, Doyle MP. Org Lett. 2012;14:3608. doi: 10.1021/ol301317a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Del Bel M, Rovira A, Guerrero CA. J Am Chem Soc. 2013;135:12188. doi: 10.1021/ja4054866. [DOI] [PubMed] [Google Scholar]
- 97.Hou S-H, Tu Y-Q, Liu L, Zhang F-M, Wang S-H, Zhang X-M. Angew Chem, Int Ed. 2013;52:11373. doi: 10.1002/anie.201306369. [DOI] [PubMed] [Google Scholar]
- 98.Xu X, Hu W-H, Doyle MP. Angew Chem, Int Ed. 2011;50:6392. doi: 10.1002/anie.201102405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu X, Ratnikov MO, Zavalij PY, Doyle MP. Org Lett. 2011;13:6122. doi: 10.1021/ol2026125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barluenga J, Riesgo L, López LA, Rubio E, Tomás M. Angew Chem, Int Ed. 2009;48:7569. doi: 10.1002/anie.200903902. [DOI] [PubMed] [Google Scholar]
- 101.Barluenga J, Riesgo L, Lonzi G, Tomás M, López LA. Chem – Eur J. 2012;18:9221. doi: 10.1002/chem.201200998. [DOI] [PubMed] [Google Scholar]
- 102.Deng Y, Massey LA, Zavalij PY, Doyle MP. Angew Chem, Int Ed. 2017;56:7479. doi: 10.1002/anie.201704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Davies HML, Xiang B, Kong N, Stafford DG. J Am Chem Soc. 2001;123:7461. doi: 10.1021/ja0160546. [DOI] [PubMed] [Google Scholar]
- 104.Briones JF, Davies HML. J Am Chem Soc. 2013;135:13314. doi: 10.1021/ja407179c. [DOI] [PubMed] [Google Scholar]
- 105.Carbery DR. Org Biomol Chem. 2008;6:3455. doi: 10.1039/b809319a. [DOI] [PubMed] [Google Scholar]
- 106.Gopalaiah K, Kagan HB. Chem Rev. 2011;111:4599. doi: 10.1021/cr100031f. [DOI] [PubMed] [Google Scholar]
- 107.Bernadat G, Masson G. Synlett. 2014;25:2842. [Google Scholar]
- 108.Smith AG, Davies HML. J Am Chem Soc. 2012;134:18241. doi: 10.1021/ja3092399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu X, Leszczynski JS, Mason SM, Zavalij PY, Doyle MP. Chem Commun. 2014;50:2462. doi: 10.1039/c3cc48993k. [DOI] [PubMed] [Google Scholar]
- 110.Deng Y, Yglesias MV, Arman H, Doyle MP. Angew Chem, Int Ed. 2016;55:10108. doi: 10.1002/anie.201605438. [DOI] [PubMed] [Google Scholar]
- 111.Barluenga J, Tudela E, Ballesteros A, Tomás M. J Am Chem Soc. 2009;131:2096. doi: 10.1021/ja809919t. [DOI] [PubMed] [Google Scholar]
- 112.Lian Y, Davies HML. J Am Chem Soc. 2010;132:440. doi: 10.1021/ja9078094. [DOI] [PubMed] [Google Scholar]
- 113.Xiong H, Xu H, Liao S, Xie Z, Tang Y. J Am Chem Soc. 2013;135:7851. doi: 10.1021/ja4042127. [DOI] [PubMed] [Google Scholar]
- 114.Zhu J, Liang Y, Wang L, Zheng Z-B, Houk KN, Tang Y. J Am Chem Soc. 2014;136:6900. doi: 10.1021/ja503117q. [DOI] [PubMed] [Google Scholar]
- 115.Jing C, Cheng Q-Q, Deng Y, Arman H, Doyle MP. Org Lett. 2016;18:4550. doi: 10.1021/acs.orglett.6b02192. [DOI] [PubMed] [Google Scholar]
- 116.López E, Lonzi G, González J, López LA. Chem Commun. 2016;52:9398. doi: 10.1039/c6cc04106j. [DOI] [PubMed] [Google Scholar]
- 117.Xu X, Doyle MP. Acc Chem Res. 2014;47:1396. doi: 10.1021/ar5000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang X, Xu X, Zavalij PY, Doyle MP. J Am Chem Soc. 2011;133:16402. doi: 10.1021/ja207664r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qin C, Davies HML. J Am Chem Soc. 2013;135:14516. doi: 10.1021/ja4069003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang X, Abrahams QM, Zavalij PY, Doyle MP. Angew Chem, Int Ed. 2012;51:5907. doi: 10.1002/anie.201201917. [DOI] [PubMed] [Google Scholar]
- 121.Pagar VV, Liu R-S. Angew Chem, Int Ed. 2015;54:4923. doi: 10.1002/anie.201500340. [DOI] [PubMed] [Google Scholar]
- 122.Cheng Q-Q, Yedoyan J, Arman H, Doyle MP. J Am Chem Soc. 2016;138:44. doi: 10.1021/jacs.5b10860. [DOI] [PubMed] [Google Scholar]
- 123.Qian Y, Xu X, Wang X, Zavalij PY, Hu W, Doyle MP. Angew Chem, Int Ed. 2012;51:5900. doi: 10.1002/anie.201202525. [DOI] [PubMed] [Google Scholar]
- 124.Xu X, Zavalij PY, Doyle MP. Chem Commun. 2013;49:10287. doi: 10.1039/c3cc46415f. [DOI] [PubMed] [Google Scholar]
- 125.Shved AS, Tabolin AA, Novikov RA, Nelyubina YV, Timofeev VP, Ioffe SL. Eur J Org Chem. 2016:5569. [Google Scholar]
- 126.Shintani R, Hayashi T. J Am Chem Soc. 2006;128:6330. doi: 10.1021/ja061662c. [DOI] [PubMed] [Google Scholar]
- 127.Shapiro ND, Shi Y, Toste FD. J Am Chem Soc. 2009;131:11654. doi: 10.1021/ja903863b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Qian Y, Zavalij PY, Hu W, Doyle MP. Org Lett. 2013;15:1564. doi: 10.1021/ol400339c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Perreault C, Goudreau SR, Zimmer LE, Charette AB. Org Lett. 2008;10:689. doi: 10.1021/ol702414e. [DOI] [PubMed] [Google Scholar]
- 130.Zhou Y-Y, Li J, Ling L, Liao S-H, Sun X-L, Li Y-X, Wang L-J, Tang Y. Angew Chem, Int Ed. 2013;52:1452. doi: 10.1002/anie.201207576. [DOI] [PubMed] [Google Scholar]
- 131.Xu X, Zavalij PY, Doyle MP. Angew Chem, Int Ed. 2013;52:12664. doi: 10.1002/anie.201305539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu X, Zavalij PY, Doyle MP. J Am Chem Soc. 2013;135:12439. doi: 10.1021/ja406482q. [DOI] [PubMed] [Google Scholar]
- 133.Xu X, Zavalij PY, Doyle MP. Angew Chem, Int Ed. 2012;51:9829. doi: 10.1002/anie.201203962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xu X, Zavalij PY, Hu W, Doyle MP. J Org Chem. 2013;78:1583. doi: 10.1021/jo302696y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Davies HML. Tetrahedron. 1993;49:5203. [Google Scholar]
- 136.Davies HML, Peng Z-Q, Houser JH. Tetrahedron Lett. 1994;35:8939. [Google Scholar]
- 137.Davies HML, Stafford DG, Doan BD, Houser JH. J Am Chem Soc. 1998;120:3326. [Google Scholar]
- 138.Schwartz BD, Denton JR, Lian Y, Davies HML, Williams CM. J Am Chem Soc. 2009;131:8329. doi: 10.1021/ja9019484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lian Y, Miller LC, Born S, Sarpong R, Davies HML. J Am Chem Soc. 2010;132:12422. doi: 10.1021/ja103916t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Davies HML, Ahmed G, Churchill MR. J Am Chem Soc. 1996;118:10774. [Google Scholar]
- 141.Davies HML, Calvo R, Ahmed G. Tetrahedron Lett. 1997;38:1737. [Google Scholar]
- 142.Davies HML, Calvo RL, Townsend RJ, Ren P, Churchill MR. J Org Chem. 2000;65:4261. doi: 10.1021/jo991959b. [DOI] [PubMed] [Google Scholar]
- 143.Xu J, Caro-Diaz EJE, Theodorakis EA. Org Lett. 2010;12:3708. doi: 10.1021/ol1015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu J, Caro-Diaz EJE, Batova A, Sullivan SDE, Theodorakis EA. Chem – Asian J. 2012;7:1052. doi: 10.1002/asia.201101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Davies HML, Matasi JJ, Ahmed G. J Org Chem. 1996;61:2305. [Google Scholar]
- 146.Davies HML, Matasi JJ, Hodges LM, Huby NJS, Thornley C, Kong N, Houser JH. J Org Chem. 1997;62:1095. [Google Scholar]
- 147.Reddy RP, Davies HML. J Am Chem Soc. 2007;129:10312. doi: 10.1021/ja072936e. [DOI] [PubMed] [Google Scholar]
- 148.Parr BT, Economou C, Herzon SB. Nature. 2015;525:507. doi: 10.1038/nature14902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nocquet P-A, Opatz T. Eur J Org Chem. 2016:1156. [Google Scholar]
- 150.Davies HML, Hodges LM, Thornley CT. Tetrahedron Lett. 1998;39:2707. [Google Scholar]
- 151.Davies HML, Hodges LM. J Org Chem. 2002;67:5683. doi: 10.1021/jo025697g. [DOI] [PubMed] [Google Scholar]
- 152.Xu X, Wang X, Zavalij PY, Doyle MP. Org Lett. 2015;17:790. doi: 10.1021/ol503498n. [DOI] [PubMed] [Google Scholar]
- 153.Xu X, Hu W-H, Zavalij PY, Doyle MP. Angew Chem, Int Ed. 2011;50:11152. doi: 10.1002/anie.201105557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Guzmán PE, Lian Y, Davies HML. Angew Chem, Int Ed. 2014;53:13083. doi: 10.1002/anie.201406440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhu C, Xu G, Sun J. Angew Chem, Int Ed. 2016;55:11867. doi: 10.1002/anie.201606139. [DOI] [PubMed] [Google Scholar]
- 156.Lee DJ, Han HS, Shin J, Yoo EJ. J Am Chem Soc. 2014;136:11606. doi: 10.1021/ja5061609. [DOI] [PubMed] [Google Scholar]
- 157.Lee DJ, Ko D, Yoo EJ. Angew Chem, Int Ed. 2015;54:13715. doi: 10.1002/anie.201506764. [DOI] [PubMed] [Google Scholar]
- 158.Davies HML, Hu B. Heterocycles. 1993;35:385. [Google Scholar]
- 159.Davies HML, Ahmed G, Calvo RL, Churchill MR, Churchill DG. J Org Chem. 1998;63:2641. doi: 10.1021/jo972189b. [DOI] [PubMed] [Google Scholar]
- 160.Müller P, Bernardinelli G, Allenbach YF, Ferri M, Flack HD. Org Lett. 2004;6:1725. doi: 10.1021/ol049554n. [DOI] [PubMed] [Google Scholar]
- 161.Ghanem A, Lacrampe F, Schurig V. Helv Chim Acta. 2005;88:216. [Google Scholar]
- 162.Müller P, Bernardinelli G, Allenbach YF, Ferri M, Grass S. Synlett. 2005;16:1397. [Google Scholar]
- 163.Müller P, Allenbach YF, Grass S. Tetrahedron: Asymmetry. 2005;16:2007. [Google Scholar]
- 164.Valette D, Lian Y, Haydek JP, Hardcastle KI, Davies HML. Angew Chem, Int Ed. 2012;51:8636. doi: 10.1002/anie.201204047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Deng Y, Jing C, Doyle MP. Chem Commun. 2015;51:12924. doi: 10.1039/c5cc05006e. [DOI] [PubMed] [Google Scholar]
- 166.Briones JF, Davies HML. Tetrahedron. 2011;67:4313. [Google Scholar]
- 167.Xu X, Deng Y, Yim DN, Zavalij PY, Doyle MP. Chem Sci. 2015;6:2196. doi: 10.1039/c4sc03991b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Deng Y, Jing C, Arman H, Doyle MP. Organometallics. 2016;35:3413. [Google Scholar]
- 169.Cheng Q-Q, Yedoyan J, Arman H, Doyle MP. Angew Chem, Int Ed. 2016;55:5573. doi: 10.1002/anie.201601260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Davies HML, Clark DM, Alligood DB, Eiband GR. Tetrahedron. 1987;43:4265. [Google Scholar]
- 171.Davies HML, Hu B, Saikali E, Bruzinski PR. J Org Chem. 1994;59:4535. [Google Scholar]
- 172.Davies HML, Houser JH, Thornley C. J Org Chem. 1995;60:7529. [Google Scholar]
- 173.Nikolaev VA, Supurgibekov MB, Davies HML, Sieler J, Zakharova VM. J Org Chem. 2013;78:4239. doi: 10.1021/jo302726m. [DOI] [PubMed] [Google Scholar]
- 174.Truong PM, Mandler MD, Zavalij PY, Doyle MP. Org Lett. 2013;15:3278. doi: 10.1021/ol401308d. [DOI] [PubMed] [Google Scholar]
- 175.Deng Y, Jing C, Zavalij PY, Doyle MP. Org Lett. 2015;17:4312. doi: 10.1021/acs.orglett.5b02129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xu X, Shabashov D, Zavalij PY, Doyle MP. Org Lett. 2012;14:800. doi: 10.1021/ol203331r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Xu X, Shabashov D, Zavalij PY, Doyle MP. J Org Chem. 2012;77:5313. doi: 10.1021/jo3006733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mahatthananchai J, Dumas AM, Bode JW. Angew Chem, Int Ed. 2012;51:10954. doi: 10.1002/anie.201201787. [DOI] [PubMed] [Google Scholar]
- 179.Xu X, Doyle MP. Chin Chem Lett. 2015;26:227. [Google Scholar]
- 180.Miller LC, Sarpong R. Chem Soc Rev. 2011;40:4550. doi: 10.1039/c1cs15069c. [DOI] [PubMed] [Google Scholar]
- 181.Xu B, Tambar UK. J Am Chem Soc. 2016;138:12073. doi: 10.1021/jacs.6b08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Panne P, Fox JM. J Am Chem Soc. 2007;129:22. doi: 10.1021/ja0660195. [DOI] [PubMed] [Google Scholar]
- 183.Doyle MP, Yan M, Hu W, Gronenberg LS. J Am Chem Soc. 2003;125:4692. doi: 10.1021/ja029745q. [DOI] [PubMed] [Google Scholar]
- 184.Hansen JH, Davies HML. Chem Sci. 2011;2:457. [Google Scholar]
- 185.Ivanova MV, Bayle A, Besset T, Pannecoucke X, Poisson T. Angew Chem, Int Ed. 2016;55:14141. doi: 10.1002/anie.201608294. [DOI] [PubMed] [Google Scholar]
- 186.Deng Y, Doyle MP. Isr J Chem. 2016;56:399. [Google Scholar]