Abstract
Introduction
Vascular grafts, especially in paediatric cases, need to be durable. Common failures such as thrombosis are well documented with research efforts directed towards them. However, there are lesser known causes of graft failure, such as graft calcification, and these also require further research focus.
Report
A paediatric case is described in which a synthetic renovascular graft, implanted for mid-aortic syndrome, became calcified, necessitating surgical intervention to resolve graft malfunction. Significant calcification in the limb of a bifurcated polyethylene terephthalate graft was found to be the cause of resistant stenosis and refractory hypertension. Histology conducted on the explanted limb showed the presence of multinuclear giant cells, indicating a chronic foreign body response.
Discussion
Calcification of vascular grafts is probably more common than previously recognised. Stenosis typically resistant to angioplasty may result in the long term and thus leading to surgical intervention. In young children, this is suboptimal as these grafts need to last throughout adulthood. Explanted prosthetic grafts should be sent to specialist registries such as that in Strasbourg to be optimally assessed so that contributory factors can be identified.
Keywords: Renovascular graft, Paediatric, Calcification
Highlights
-
•
Calcification is found in a renovascular synthetic graft in a paediatric patient.
-
•
Calcification is a lesser known cause of premature vascular graft failure.
-
•
Careful consideration of graft material is required, especially in paediatric cases.
-
•
A registry of vascular graft failures is advocated to identify the causes.
Introduction
The choice of synthetic material for vascular bypass applications remains an important consideration for vascular surgeons. The main vascular graft materials available on the market are polyethylene terephthalate (PET) and polytetrafluoroethylene (PTFE), with both being used with varying degrees of success depending on the vascular application. In paediatric surgery, this is even more challenging, as the graft has to be durable enough to last throughout adulthood as well as adapting to the growth of the child.
A rare case is described of calcification of a synthetic aortobirenal bypass graft that caused subsequent impending failure of the graft requiring surgical intervention and replacement.
Report
At 6 months of age a male child underwent resection and chemotherapy for a Stage 2 neuroblastoma, which was located in the retroperitoneal and peri-adrenal region abutting the distal thoracic aorta. At 7 years old, he developed mid-aortic syndrome with bilateral renal artery stenosis and occlusion of the superior mesenteric axis, which was likely due to post-surgical fibrosis and damage to the vasa vasorum from the surgical resection, and resulting in uncontrolled hypertension.
The hypertension was refractory to medical and interventional treatment and was initially treated by open revascularisation with an end-to-side graft from the supracoeliac aorta, end-to-end to both renal arteries using a bifurcated PET graft (Dialine II, Cardial S.A., Saint Etienne, France). This was routed retroperitoneally, on the right behind the portal structures, and on the left, behind the body of the pancreas. Three years later, he represented with uncontrolled hypertension, because of infrarenal aortic narrowing, and this was treated with angioplasty and aortic stenting. Unfortunately, 4 years later, the hypertension recurred, and further investigations revealed a stenosis at the junction of the proximal and middle thirds of the right limb of the graft with a significant pressure gradient of 44 mmHg (Fig. 1). This stenosis was refractory to angioplasty, even with the employment of cutting balloons. After a year of non-operative management, definitive surgical reintervention was considered when he was 16 years old.
Figure 1.
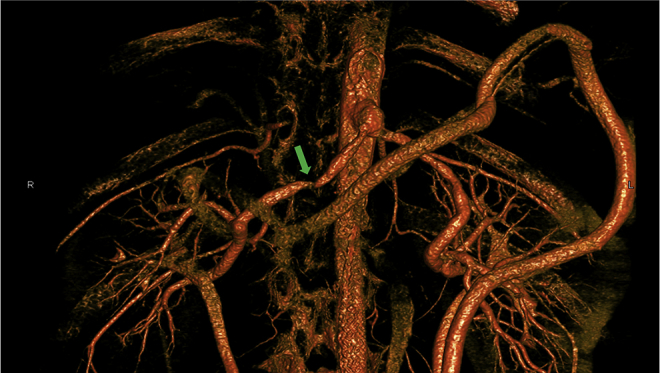
This is a three-dimensional reconstruction of the computed tomography angiogram to show the tight stenosis at the proximal third of the right renal artery (green arrow). Note that due to the mid-aortic syndrome there are large collaterals that have formed as a result.
At surgery, after division of dense hepatic and right renal bed adhesions, the right limb of the graft was mobilised with no evidence of kinking or extrinsic compression. At the junction of the proximal and mid-segments of the vascular graft, the region of recurrent stenosis was identified and noted to be bony hard. The right limb was removed and replaced with an “end-to-end” 8-mm PET graft (Hemashield, Atrium, Hudson, NH, USA) restoring excellent renal flow assessed by completion intraoperative Doppler. The left limb, shown preoperatively and confirmed intraoperatively as functioning normally, was not replaced.
The patient made an uneventful recovery from this operation with significantly improved hypertension and continues to do well 3 years 10 months after this operation. His blood pressure remains under good control with dual antihypertensive drug therapy.
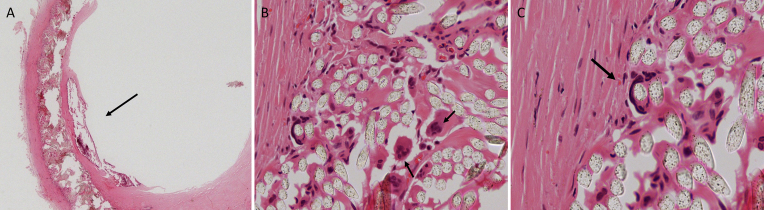
Histological examination, using haematoxylin and eosin staining, of the explanted graft confirmed the presence of chronic thrombosis and marked calcification at the site of the stenosis. Macrophages and multinuclear giant cells were visualised around the polyethylene terephthalate graft material, indicating the presence of a chronic foreign body response (Fig. 2).
Figure 2.
Histological analysis was conducted using haematoxylin and eosin staining. (A) A low power magnification (×25) showing the calcified plaque in the lumen of the synthetic graft (arrow). (B) The infiltration of the multinuclear giant cells (arrow) within the Dacron graft. (C) A multinuclear giant cell which has “engulfed” one of the fibrils of the Dacron graft into its cytoplasm. (B,C) High-power magnifications (×200) of details of the graft.
Since presentation the patient has maintained normal renal function throughout despite the recurrent renal stenoses, with no episodes of renal failure or possible hyperparathyroidism.
Discussion
Mid-aortic syndrome has been described as a “clinical condition caused by segmental narrowing of the abdominal or distal descending thoracic aorta.”1 Renovascular disease secondary to renal artery stenoses is the most frequent presentation, with associated problems including visceral stenosis and lower limb ischaemia being less common. The indications for correction of mid-aortic syndrome are uncontrolled resistant hypertension, preservation of renal function, claudication, lower limb growth deficiency, and, rarely, mesenteric ischaemia. Uncontrolled hypertension was therefore the main reason for intervention in this child. The use of prosthetic grafts plays an important but problematic role in young children as there is usually no adequate autologous option, especially as the saphenous vein poses a significant lifetime risk of aneurysmal change. With this in mind, selection of a durable prosthetic vascular graft is of paramount importance, especially one that is as adaptable as possible to the child's growth with the aim of functioning throughout their lifetime.
A number of hypotheses for graft calcification exist, with the most likely by Levy et al.,2 who propose a mechanism of direct adsorption of calcium and phosphate by the polymer surface with subsequent sub-surface crystallization. Within degraded cellular components, calcification is commonly initiated. Collagen moieties can also act as additional nucleation sites of calcium phosphate minerals, independent of cellular components; this mechanism is possibly highly relevant because of the collagen sealing of knitted PET grafts. Haemodynamic stress and turbulent flow can further stimulate calcium and phosphate mineralisation, as can the pro-inflammatory foreign body reaction from the time of implantation. There is a correlation between the presence of foreign body type giant cells and calcified vascular grafts with a metaplastic response and severe neointimal hyperplasia being predominant factors. Also, macrophages on the surface of biomaterials can undergo phenotypic changes into mesenchymal cells such as osteoid cells depending on their microenvironment.3 There are other reports of non-vascular implants becoming calcified interestingly in ear, nose, and throat implants.4
Vascular graft failure due to calcification of prosthetic material has only been described once before.5 However, Mehta et al.6 systematically analysed a series of explanted synthetic vascular grafts finding significant calcification in many. Calcification contributes to graft failure by reducing compliance, increasing stiffness, and generating compliance mismatch and mechanically predisposing to material fracture. Stenosis caused by the calcification can generate abnormal haemodynamic flow patterns and shear stress, exacerbating endothelial dysfunction and further promoting intimal hyperplasia. However, the explanted vascular grafts in this series were from adults, and, as far as the authors are aware, there is no comparable information in children.
In particular, young people can suffer accelerated calcification of vascular vessels, especially in the presence of renal disease, a possible factor in this case which was characterised by renovascular hypertension but at no point renal failure.7, 8 Careful consideration of the graft material to be used should include recognition of likely calcification in the long term, especially in the presence of predisposing features. Sites prone to stenosis such as at kinks and anastomoses can propagate calcification, as well as intergraft regions, as shown by this case. In this case, the calcification was sited within the PET network and leads us to highlight that the graft material itself is an important factor to calcification. The “sealing” material used in making knitted grafts may also be an important contributor.
In this case, there were no common factors identified, such as hypercalcaemia, to have caused the calcification. Furthermore, it is not completely clear whether the stenosis propagated the calcification or vice versa. It is likely that this process is multifactorial, more frequent than currently recognised and requires highlighting so that research focus can be directed to further our understanding of graft failure.
Conclusion
The problem of calcification of prosthetic grafts can be most optimally assessed by sending explanted grafts to specialist registries such as that in Strasbourg.9 It is only by such meticulous and professional scrutiny that potential contributory factors to calcification such as the graft structure and composition can be identified to allow development of new materials resistant to calcification.9 This will be particularly invaluable information in paediatric vascular surgery.
Acknowledgements
The authors would like to acknowledge the help of the Department of Cellular Pathology, Royal Free London NHS Foundation Trust, especially Dr Paul Bass.
Conflicts of Interest
None.
Funding
None.
References
- 1.Delis K.T., Gloviczki P. Middle aortic syndrome: from presentation to contemporary open surgical and endovascular treatment. Perspect Vasc Surg Endovasc Ther. 2005;17(3):187–203. doi: 10.1177/153100350501700302. [DOI] [PubMed] [Google Scholar]
- 2.Levy R.J., Schoen F.J., Anderson H.C., Harasaki H., Koch T.H. Cardiovascular implant calcification: a survey and update. Biomaterials. 1991;12(8):707–714. doi: 10.1016/0142-9612(91)90017-5. [DOI] [PubMed] [Google Scholar]
- 3.Jay S.M., Skokos E.A., Zeng J., Knox K., Kyriakides T.R. Macrophage fusion leading to foreign body giant cell formation persists under phagocytic stimulation by microspheres in vitro and in vivo in mouse models. J Biomed Mater Res A. 2010;93(1):189–199. doi: 10.1002/jbm.a.32513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jang T.Y., Choi J.Y., Jung D.H., Park H.J., Lim S.C. Histologic study of Gore-Tex removed after rhinoplasty. Laryngoscope. 2009;119(4):620–627. doi: 10.1002/lary.20158. [DOI] [PubMed] [Google Scholar]
- 5.Padmakumar R., Krishnamoorthy K.M., Tharakan J.A. Calcific stenotic jump graft. Ann R Coll Surg Engl. 2004;86(6):W36–W37. doi: 10.1308/14787080492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta R.I., Mukherjee A.K., Patterson T.D., Fishbein M.C. Pathology of explanted polytetrafluoroethylene vascular grafts. Cardiovasc Pathol. 2011;20(4):213–221. doi: 10.1016/j.carpath.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Schoen F.J., Levy R.J. Calcification of tissue heart valve substitutes: progress toward understanding and prevention. Ann Thorac Surg. 2005;79(3):1072–1080. doi: 10.1016/j.athoracsur.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 8.Goodman W.G., Goldin J., Kuizon B.D., Yoon C., Gales B., Sider D. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. New Engl J Med. 2000;342(20):1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 9.Georg Y., Settembre N., Marchand C., Lejay A., Thaveau F., Durand B. Poor long-term stability of the Corvita abdominal stentgraft. Eur J Vasc Endovasc Surg. 2014;47(2):160–163. doi: 10.1016/j.ejvs.2013.10.010. [DOI] [PubMed] [Google Scholar]