Abstract
To test whether binge eating and emotional eating mediate the relationships between self-reported stress, morning cortisol and the homeostatic model of insulin resistance and waist circumference. We also explored the moderators of gender and age. Data were from 249 adults (mean BMI = 26.9 ± 5.1 kg/m2; mean age = 28.3 ± 8.3 years; 54.2 % male; 69.5 % white) recruited from the community who were enrolled in a cross-sectional study. Participants completed a comprehensive assessment panel of psychological and physiological assessments including a morning blood draw for plasma cortisol. We found negative relationships between stress and morning cortisol (r = −0.15 to −0.21; p < 0.05), and cortisol and the homeostatic model of insulin resistance and waist circumference (r = −0.16, −0.25, respectively; p < 0.05). There was not statistical support for binge eating or emotional eating as mediators and no support for moderated mediation for either gender or age; however, gender moderated several paths in the model. These include the paths between perceived stress and emotional eating (B = 0.009, p < 0.001), perceived stress and binge eating (B = 0.01, p = 0.003), and binge eating and increased HOMA-IR (B = 0.149, p = 0.018), which were higher among females. Among women, perceived stress may be an important target to decrease binge and emotional eating. It remains to be determined what physiological and psychological mechanisms underlie the relationships between stress and metabolic abnormalities.
Keywords: Stress, Binge eating, Emotional eating, Insulin resistance, Abdominal obesity
Introduction
Obesity continues to be a leading health issue, impacting 34.9 % of adults in the US (Ogden et al., 2014). This rate is closely tied to the high prevalence of metabolic abnormalities. For example, the prevalence of abdominal obesity is 54.2 % (Ford et al., 2014) and impaired fasting glucose is 26.6 % among adults (Ioannou et al., 2007). These abnormalities are major predictors of coronary heart disease, stroke, and type 2 diabetes (Kannel et al., 1991; Nathan et al., 2007; Rexrode et al., 1998; Santaguida et al., 2005).
Several interventions for prevention and treatment have been developed. These interventions are multifaceted and heterogeneous though many result in only modest and short-term changes in obesity-related metabolic abnormalities (Galani & Schneider, 2007; Lemmens et al., 2008; Seo & Sa, 2008). A more nuanced understanding of the mechanisms related to metabolic abnormalities is necessary to help us amplify, optimize, and target interventions to relevant processes.
Stress and coping
One such relationship in which further clarification is needed is that between stress, coping, and metabolic abnormalities. Stress is a complex and multidimensional concept referring to a real or perceived disruption in homeostasis (Chrousos & Gold, 1992). While there is suggestion that stress increases the risk for obesity-related metabolic abnormalities including insulin resistance and abdominal obesity (Räikkönen et al., 1996, 2007), the mechanisms underlying this process are unclear. There are several potential mechanisms that may contribute to this relationship including decreased physical activity, changes in eating patterns and behaviors, differences in food reward, motivation, and food cravings, and changes in stress-related hormones (Sinha & Jastreboff, 2013). However, the interplay and relationships between these factors have not been well explored, particularly in regards to different types of stress, stress-related hormones, and eating phenotypes such as binge eating and emotional eating.
There is theoretical and empirical support for the thesis that binge eating and emotional eating may be coping mechanisms that mediate the relationship between stress and metabolic abnormalities. Yet, few studies have examined the collective relationships among these variables or the relationships with different types of stress.
Binge eating is a behavior characterized by consumption of an unambiguously large amount of food in a discrete period of time, and a feeling a loss of control over eating (American Psychiatric Association, 2013). Binge eating is common among adults: 5–11 % of adults in wider community samples reporting binge eating (Abraham et al., 2014; Mitchison et al., 2014; Striegel et al., 2012).
Emotional eating is eating that occurs as a response to a range of negative emotions (i.e., stress, anxiety, anger, depression, and loneliness) (Arnow et al., 1995). Although emotional eating is positively associated with binge eating, there is a subgroup of individuals who are emotional eaters but not binge eaters (Ricca et al., 2009). This means that these individuals eat in response to negative affect but may not overeat or feel a loss of control (Linderman & Stark, 2001).
Binge eating and emotional eating may mediate associations between stress and metabolic abnormalities. Eating as a coping mechanism in response to stress is frequently reported among individuals across the weight spectrum, though there is wide variability between studies with estimates of individuals who eat more when stressed ranging from 4 to 55 % (Macht, 2008; Weinstein et al., 1997). This wide variability is not surprising given that the concept of stress-induced eating is not well defined and multiple measures and paradigms of stress have been used when examining these relationships. Examining specific eating phenotypes (i.e., binge eating and emotional eating), will allow for a deeper understanding of these processes.
Cortisol dysfunction has been linked to both binge eating and emotional eating, though there is a paucity of literature examining these relationships and study results have been inconsistent (Lo Sauro et al., 2008). While some researchers have found that females who are obese and have a binge eating disorder have higher basal cortisol compared to females who are obese without binge eating disorder (Gluck et al., 2004), others have found no difference in cortisol levels between women who are obese with and without binge eating disorder (Coutinho et al., 2007). There is also indication that high cortisol reactivity is positively associated with stress-induced eating (Epel et al., 2001).
There is evidence that the characteristics of binge eating are important determinants of metabolic abnormalities including abdominal obesity and hyperglycemia; however, the relationship between emotional eating and metabolic abnormalities is unclear. Evidence suggests that individuals who have binge eating disorder are at increased risk for developing metabolic abnormalities (Blomquist et al., 2012; Hudson et al., 2010; Roehrig et al., 2009). While some researchers have found that emotional eating is associated with increased weight (Geliebter & Aversa, 2003), others have demonstrated no association (Masheb & Grilo, 2006; Nguyen-Rodriguez et al., 2008).
Along with the above evidence, the Transactional Model of Stress and Coping and Selye’s Theory of Stress were used as a framework in this study (Fig. 1). In brief, according to Lazarus and Folkman’s Transactional Model of Stress and Coping, if an individual appraises an event as stressful, coping ensues (Lazarus, 1966; Lazarus & Folk-man, 1984). Individuals may cope or attempt to adapt to a stressful situation by binge eating or emotional eating. Individuals may use eating as a form of emotion-based coping to reduce the negative emotional responses associated with stress. Selye posited that stress results in physiological responses to prepare the body to cope with stress, including activation of the hypothalamic-pituitary-adreno-cortical (HPA) axis (Selye, 1956). Activation of the HPA axis results in the secretion of cortisol, a steroid hormone that regulates eating behaviors and choices (Pacák & Palkovits, 2001). In certain individuals the effects of cortisol result in increased caloric intake, particularly of carbohydrates and fats (Duong et al., 2012; Vicennati et al., 2011; Zellner et al., 2006), which may be classified as binge or emotional eating. Without elucidation and clarification of the role and effects of such mechanisms, the utility of targeting stress as opposed to potential mediators such as binge eating and emotional eating is uncertain.
Fig. 1.
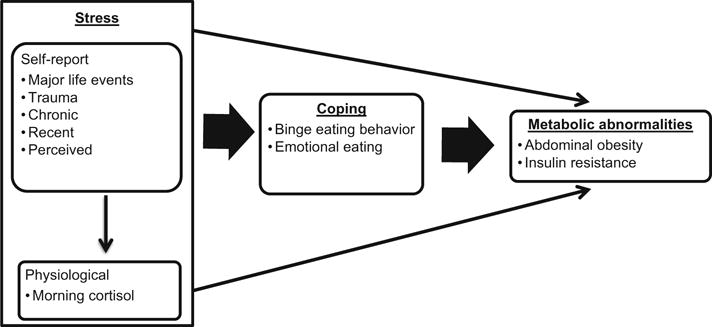
Conceptual framework
The purpose of this study was to examine the relationships among self-reported stress, morning cortisol, binge eating, emotional eating, and the metabolic abnormalities of insulin resistance and abdominal obesity in adults. Insulin resistance was estimated using a well-established surrogate measure, the homeostatic model of insulin resistance (HOMA-IR; Matthews et al., 1985). Based on the above theories and empirical evidence, we hypothesized that: (1) self-reports of stress (major life events, trauma, and chronic, recent, and perceived stress) and cortisol are related to HOMA-IR and waist circumference (WC), (2) binge eating and emotional eating will mediate the relationships between self-reports of stress and HOMA-IR and WC, and (3) binge eating and emotional eating will mediate the relationships between morning cortisol and HOMA-IR and WC. We also explored moderators [gender and age] of these relationships.
Methods
This study is a secondary analysis of data collected from the Interdisciplinary Research Consortium on Stress, Self-Control, and Addiction (IRCSSA; the National Institutes of Health grants PL1-DA024859 and UL1-DE019859). In brief, the IRCSSA is a collaborative, interdisciplinary, cross-sectional set of studies examining the interactions between stress, self-control, and addiction across multiple brain, body, behavioral, and social systems.
Sample and Setting
The sample for the IRCSSA is a convenience sample of men and women who were recruited from online and print advertisements in local newspapers, community centers and churches in New Haven, Connecticut. The sample includes individuals with the addictive behaviors of cigarette smoking, alcohol drinking, and overeating, as well as those without these disorders, from a variety of racial and socioeconomic backgrounds. Inclusion criteria were that participants were between the ages of 18–50 years and were able to read English at least at the sixth grade level. Exclusion criteria were dependence on any drug other than alcohol or nicotine, use of prescribed medications for any psychiatric disorders, pregnancy, and medical conditions or medications that may influence cortisol levels. Additionally, individuals identified on the Eating Disorder Examination-Questionnaire as engaging in compensatory mechanisms (i.e., laxative use, self-induced vomiting) were excluded as biobehavioral processes may differ in this subgroup of individuals. Study procedures were conducted at the Yale Stress Center, and participants received compensation for participation in assessment sessions.
Procedures
The Yale University Institutional Review Board approved this study. Potential participants completed an initial screening over the telephone or in person to determine eligibility based on inclusion and exclusion criteria. Following screening, eligible participants met with a research assistant for a 2-h intake session to obtain informed consent and begin assessments. After the intake session, participants had three to four additional sessions. During these sessions, the research staff performed a comprehensive assessment battery including physical examinations, diagnostics, cognitive and psychological assessments, and conducted blood work. This paper is a secondary data analysis; however, the sample meets the suggested “critical” sample size of 200 for structural equation modeling (Hoelter, 1983).
Measures
Demographics/BMI
Data were collected with self-report forms designed for this study that provided data on age, gender, and race/ethnicity. A research nurse or trained research team member measured heights and weights during the physical examination. These were used to calculate BMI.
Stress
Self-report
Trained interviewers administered the Cumulative Adversity Interview (Turner & Wheaton, 1995; Turner et al., 1995), a well-established, 140-item interview that assesses major life events, traumas, recent life stress, and chronic stress. Major life events are social adversities that are non-violent but severe and have potential for long-term consequences (e.g., loss of a child, significant other with substance use). Recent life stress measures discrete stressful events occurring in the past 12 months to the participant or participant’s immediate family member (e.g., physical assault, abortion or miscarriage, serious accident or injury). Chronic stress measures the subjective experience of continuous stressors or ongoing life problems and hassles. For traumas and recent life stress, items were scored as being present or absent and are summed to form the subscales. For chronic stressors, items were scored using a 3-point Likert scale ranging from not true to very true. Three-month test–retest reliability was measured in subsamples of individuals participating in the IRCSSA (Stults-Kolehmainen et al., 2014). Reliability coefficients for major life events, trauma, recent life events, and chronic stress were 0.91, 0.83, 0.94, and 0.92 respectively (Stults-Kolehmainen et al., 2014). Use of interview techniques is recommended to decrease participant recall bias (Dohrenwend, 2006).
Perceived stress
The Perceived Stress Scale (PSS-14) is a 14-item self-report scale that assesses the degree to which individuals appraise situations in their lives as stressful during the previous month (Cohen et al., 1983). Participants are asked how often they felt or thought a certain way on a 5-point Likert Scale of 0 (never) to 4 (very often). Higher scores indicate greater perceived stress. The PSS has good internal reliability (a = 0.84–0.86) and concurrent validity with the Daily Stress Inventory (r = 0.62) (Cohen et al., 1983; Machulda et al., 1998).
Cortisol
Plasma cortisol levels were collected on participants who fasted for 13 h prior to blood draw and were instructed to come to the study site immediately after waking (Kudielka & Wüst, 2009; Nicolson, 2008). Upon arrival, participants had a peripheral intravenous line inserted, and cortisol levels were collected at 15-min intervals for an hour. Morning cortisol was operationalized as the mean plasma cortisol value of the repeated measurements taken over the hour. Morning cortisol has high intra-individual stability (r = 0.63) (Hucklebridge et al., 2005; Wüst et al., 2000) with a “normal” morning range of 6–23 mcg/dL (Varon & Fromm, 2014).
Coping
Binge eating Binge eating was assessed with the Eating Disorder Examination-Questionnaire with instructions (EDE-Q-I) (Fairburn & Beglin, 1994; Goldfein et al., 2005). The EDE-Q is a self-report version of the interviewer-based EDE that assesses dimensional aspects of eating disorders (i.e. binge eating, vomiting). The 36-item questionnaire uses 7-point, forced-choice Likert scales to assess eating attitudes and also asks about the frequency of overeating episodes that are unusually large (overeating) and accompanied by a sense of loss of control (binge eating) over the past 28 days. The EDE-Q has recently been revised with instructions about the concepts of binge eating (EDE-Q-I) to improve reliability, and has psychometric support for assessing binge eating in both community and clinical populations (Celio et al., 2004; Goldfein et al., 2005; Mond et al., 2004; Reas et al., 2006).
Emotional eating
Emotional eating was measured using the raw score from the emotional eating subscale of the Dutch Eating Behavior Questionnaire (Van Strien et al., 1986). This subscale measures the tendency to overeat in response to negative emotions. The subscale contains 13 questions measured on a 5-point Likert scale ranging from never to very often and has good internal reliability (a = 0.93) in adults (Van Strien et al., 1986).
Metabolic abnormalities
Abdominal obesity
WC is a commonly used index of abdominal obesity because it is predictive of cardiovascular disease, low-cost, and low-burden (Janssen et al., 2004; Zhu et al., 2004). WC was measured following the National Heart, Lung, and Blood Institute and National Health and Nutrition Education Survey protocol, at minimal inspiration midway between the last rib and the iliac crest (Janssen et al., 2002). For men, high risk is considered at a WC > 40 inches. For women, high risk is considered a WC > 35 inches (NHLBI Obesity Education Initiative Expert Panel, 1998).
Insulin resistance screen
Fasting glucose and insulin were measured to calculate a well-accepted screening measure of insulin resistance, HOMA-IR (Wallace et al., 2004). The following formula was used for this calculation: (plasma glucose in mg/dL × plasma insulin in μU/mL)/405 (Ikeda et al., 2001). The HOMA-IR has strong correlations with well-validated methods that assess insulin resistance including euglycemic (Garcia-Estevez et al., 2003) and hyperglycemic clamps (Matthews et al., 1985; Wallace et al., 2004).
Data analysis
We used SPSS version 21.0 and SPSS AMOS version 22.0 to conduct analyses. We began data analysis by calculating univariate statistics for each variable to evaluate potential outliers and the distribution of data. We found a number of variables with a positive skew (major life events, trauma, recent life events, morning cortisol, emotional eating, binge eating, and HOMA-IR). These variables underwent a log transformation which helped to normalize values and are reported as such throughout this paper. Next, we conducted bivariate analyses and used Cohen’s criteria to determine effect sizes (Cohen, 1992). Then we conducted more complex multivariate analyses using structural equation modeling with maximum likelihood estimation following the procedures recommended by Kline (2011). Indirect effects were estimated using 2000 bootstrapped samples and 95 % bias-corrected confidence intervals (Preacher & Hayes, 2008) using both en bloc (i.e., consideration of both mediators) and decomposed approaches for multiple mediators (Daniel et al., 2015). To test model fit we used the fit indices of root mean square error of approximation (RMSEA) and the Comparative Fit Index (CFI). A RMSEA value of ≤ 0.05 indicates a close approximate fit and a RMSEA ≥ 1 suggests a poor fit (Browne et al., 1993). A CFI value ≥ 0.90 and close to 1.00 indicate good fit (Hu & Bentler, 1999).
Moderation by gender was tested using a multi-group path analysis in AMOS. We compared an unconstrained model which assumes that path coefficients differ across groups to a constrained model where coefficients are set to be equal across group using a Chi square difference test. Gender moderation was tested using a parameter level analysis by comparing path coefficients between the two groups using a z-score over 1.96 (Arbuckle, 2013). To test moderation by age, we used an interaction moderation analysis (i.e., moderated mediational effects) (Bollen, 1998; Hayduk, 1988; Kline, 2011). To reduce multicollinearity, first-order terms were standardized before creating cross-product interaction terms. We entered the interaction terms as both first (i.e., each type of stress × age) and second-stage (i.e., emotional eating × age; binge eating × age) moderation (Kline, 2011).
Due to multicollinearity, BMI was not included in any of the models. However as an exploratory and post hoc analysis, we also conducted a mediation analysis testing whether binge eating and emotional eating mediated the relationships between stress and BMI.
Results
Preliminary analysis
The sample included 249 adults. The mean BMI was 26.93 (SD 5.14) kg/m2 with 29.3 % overweight and 27.3 % obese. The mean age was 28.30 (SD 8.31) years. A little over half of the sample was male at 54.2 % and the sample was predominantly white at 69.5 %. The mean binge eating episodes was 0.87 (SD 2.26) with 22.9 % of the sample endorsing at least 1 day with a binge eating episode over the month. The mean emotional eating score was 26.64 (SD 11.89). The mean WC was 34.90 (SD 5.62) inches with 22.9 % meeting gender-specific criteria for abdominal obesity. The mean HOMA-IR was 3.09 (SD 2.00).
Bivariate correlations
The bivariate correlations are show in Table 1. There were small to moderate positive associations among the self-reported stress scales (r = 0.14–0.43). Morning cortisol was significantly correlated with major life events, chronic stress, and trauma with a small effect size (r = −0.15 to −0.21). There were significant relationships between major life events, trauma, and increased HOMA-IR and WC (r = 0.14–0.26). Chronic stress was significantly associated with increased WC (r = 0.14). Morning cortisol was inversely related to HOMA-IR (r = −0.16) and WC (r = −0.25).
Table 1.
Correlations between study variables (N = 249)
| Major life events | Trauma | Recent life | Chronic stress | Perceived stress | Morning cortisol | Emotional eating | Binge eating | HOMA-IR | |
|---|---|---|---|---|---|---|---|---|---|
| Major life events | – | ||||||||
| Trauma | 0.425** | – | |||||||
| Recent life | 0.363** | 0.404** | – | ||||||
| Chronic stress | 0.393** | 0.318** | 0.285** | – | |||||
| Perceived stress | 0.342** | 0.144* | 0.217** | 0.401** | – | ||||
| Morning cortisol | −0.208** | −0.152* | 0.059 | −0.162* | −0.009 | – | |||
| Emotional eating | 0.128* | 0.000 | 0.029 | 0.160* | 0.305** | 0.057 | – | ||
| Binge eating | 0.080 | 0.071 | 0.020 | 0.100 | 0.161* | 0.042 | 0.365** | – | |
| HOMA-IR | 0.140* | 0.162* | 0.047 | 0.109 | 0.040 | −0.160* | −0.005 | 0.019 | – |
| Waist circumference | 0.197** | 0.257** | 0.080 | 0.139* | −0.012 | −0.246** | −0.006 | 0.195** | 0.489** |
Correlation is significant at the 0.05 level (2-tailed)
Correlation is significant at the 0.01 level (2-tailed)
Mediation analysis
We used confirmatory modeling to test our hypotheses about the relationships between self-reported stress, morning cortisol, binge eating, emotional eating, HOMA-IR, and WC as shown in Fig. 1. The results of the model are provided in Table 2. Perceived stress was positively associated with both emotional eating and binge eating (B = 0.006, SE = 0.002, p < 0.001 and B = 0.005, SE = 0.002, p = 0.04, respectively). Binge eating was also associated with increased WC (B = 3.976, SE = 1.249, p = 0.002). Trauma was associated with increased WC (B = 2.928, SE = 1.434, p = 0.041) and lower morning cortisol was associated with increased WC (B = -6.466, SE = 1.992, p = 0.001). However, despite attempts to improve the model fit, there is some evidence of lack of fit with the data with RMSEA = 0.335 and CFI = 0.921 (Kline, 2011). There was no evidence of mediation either en bloc or decomposed with all indirect effects at p > 0.05, two-tailed (p = 0.180–0.881). Similar to the results above, our post hoc analysis using BMI as the outcome variable in lieu of HOMA-IR and WC demonstrated no statistical evidence of mediation either en bloc or decomposed with all indirect effects at p > 0.05 (p = 0.140–0.550; data not shown), two-tailed.
Table 2.
Results of the structural equation model testing the relationships between self-report stress, morning cortisol, binge eating, emotional eating, insulin resistance, and waist circumference (N = 249)
| Variables of interest | B | SE | β | p value | 95 % Bias-corrected confidence interval |
|---|---|---|---|---|---|
| Major life events → emotional eating | 0.049 | 0.053 | 0.069 | 0.350 | −0.103, 0.129 |
| Major life events → binge eating | 0.027 | 0.084 | 0.024 | 0.751 | −0.121, 0.217 |
| Trauma → emotional eating | −0.033 | 0.046 | −0.052 | 0.466 | −0.095, 0.096 |
| Trauma → binge eating | 0.061 | 0.073 | 0.062 | 0.400 | −0.052, 0.230 |
| Recent life events → emotional eating | −0.039 | 0.044 | −0.063 | 0.369 | −0.127, 0.060 |
| Recent life events → binge eating | −0.059 | 0.070 | −0.061 | 0.396 | −0.284, 0.088 |
| Chronic stress → emotional eating | 0.002 | 0.002 | 0.064 | 0.373 | −0.003, 0.008 |
| Chronic stress → binge eating | 0.002 | 0.004 | 0.043 | 0.565 | −0.004, 0.012 |
| Perceived stress → emotional eating | 0.006 | 0.002 | 0.280 | <0.001 | 0.003, 0.010 |
| Perceived stress → binge eating | 0.005 | 0.002 | 0.142 | 0.044 | 0.001, 0.011 |
| Morning cortisol → emotional eating | 0.083 | 0.063 | 0.084 | 0.190 | −0.031, 0.216 |
| Morning cortisol → binge eating | 0.109 | 0.101 | 0.071 | 0.281 | −0.051, 0.311 |
| Emotional eating → HOMA-IR | −0.010 | 0.060 | −0.012 | 0.862 | −0.132, 0.127 |
| Emotional eating → waist circumference | −1.600 | 2.011 | −0.051 | 0.427 | −4.719, 3.345 |
| Binge eating → HOMA-IR | 0.008 | 0.037 | 0.013 | 0.835 | −0.078, 0.117 |
| Binge eating → waist circumference | 3.976 | 1.249 | 0.196 | 0.002 | 1.195, 6.915 |
| Major life events → HOMA-IR | 0.041 | 0.049 | 0.063 | 0.406 | −0.108, 0.103 |
| Major life events → waist circumference | 2.409 | 1.459 | 0.109 | 0.145 | −2.023, 5.771 |
| Trauma → HOMA-IR | 0.067 | 0.043 | 0.115 | 0.118 | 0.017, 0.199 |
| Trauma → waist circumference | 2.928 | 1.434 | 0.147 | 0.041 | 0.791, 6.397 |
| Recent life events → HOMA-IR | −0.014 | 0.041 | −0.025 | 0.733 | −0.104, 0.075 |
| Recent life events → waist circumference | −0.516 | 1.370 | −0.027 | 0.707 | −3.712, 1.988 |
| Chronic stress → HOMA-IR | 0.001 | 0.002 | 0.037 | 0.617 | −0.004, 0.006 |
| Chronic stress → waist circumference | 0.051 | 0.074 | 0.050 | 0.487 | −0.079, 0.188 |
| Perceived stress → HOMA-IR | −0.002 | 0.001 | −0.008 | 0.909 | −0.003, 0.002 |
| Perceived stress → waist circumference | −0.068 | 0.047 | −0.103 | 0.149 | −0.175, 0.013 |
| Morning cortisol → HOMA-IR | −0.108 | 0.059 | −0.120 | 0.070 | −0.206, 0.059 |
| Morning cortisol → waist circumference | −6.466 | 1.992 | −0.209 | 0.001 | −10.624, 21.561 |
The following variables are log transformed: major life events, trauma, recent life events, morning cortisol, emotional eating, binge eating, and HOMA-IR
Tests significant at the 0.05 level are shown in bold; tests with trend-significance at the 0.10 level are shown in italics
Values are from 95 % bias-corrected confidence intervals from 2000 bootstrapped samples
Moderation analyses
Gender
Next we examined the extent to which gender moderated these relationships. The Chi square difference test between the completely unconstrained and constrained models was significant, Δχ2(10) = 32.15, p < 0.001, suggesting the model is different across gender. Differences were then tested between the parameter estimates from the full model, demonstrating some significant differences in some paths by gender (Table 3). For females only, there was a significant relationship between perceived stress and emotional eating (B = 0.009, p < 0.001), and perceived stress and binge eating (B = 0.01, p = 0.003). There was an inverse relationship between recent life events and binge eating (B = −0.214, p = 0.026). There was a significant relationship between binge eating and increased HOMA-IR among females (B = 0.149, p = 0.013). For males only, there was a significant relationship between trauma and increased HOMA-IR (B = 0.124, p = 0.011). Also, lower morning cortisol was associated with higher WC among males (B = −10.126, p < 0.001). When mediation analyses were conducted separated by gender, we did not find statistical support for mediation.
Table 3.
Results of the structural equation model testing moderation by gender (N = 249)
| Variables of interest | Male
|
Female
|
z score | ||
|---|---|---|---|---|---|
| Estimate | p value | Estimate | p value | ||
| Major life events → emotional eating | 0.087 | 0.216 | −0.060 | 0.409 | −1.455 |
| Major life events → binge eating | 0.062 | 0.591 | 0.008 | 0.946 | −0.326 |
| Trauma → emotional eating | 0.001 | 0.987 | −0.028 | 0.690 | −0.321 |
| Trauma → binge eating | 0.069 | 0.461 | 0.009 | 0.940 | −0.403 |
| Recent life events → emotional eating | −0.041 | 0.493 | −0.009 | 0.876 | 0.380 |
| Recent life events → binge eating | 0.064 | 0.513 | −0.214 | 0.026 | −2.031** |
| Chronic stress → emotional eating | 0.006 | 0.110 | −0.002 | 0.579 | −1.570 |
| Chronic stress → binge eating | 0.002 | 0.736 | 0.005 | 0.340 | 0.375 |
| Perceived stress → emotional eating | 0.004 | 0.060 | 0.009 | 0.000 | 1.918* |
| Perceived stress → binge eating | 0.001 | 0.728 | 0.010 | 0.003 | 1.868* |
| Morning cortisol → emotional eating | −0.026 | 0.805 | 0.094 | 0.187 | 0.947 |
| Morning cortisol → binge eating | 0.144 | 0.405 | 0.106 | 0.369 | −0.180 |
| Emotional eating → HOMA-IR | 0.125 | 0.099 | −0.212 | 0.034 | 2.683*** |
| Emotional eating → waist circumference | 3.568 | 0.154 | −2.622 | 0.416 | −1.517 |
| Binge eating → HOMA-IR | −0.103 | 0.023 | 0.149 | 0.013 | 3.353*** |
| Binge eating → waist circumference | 2.010 | 0.179 | 5.162 | 0.007 | 1.292 |
| Major life events → HOMA-IR | 0.050 | 0.416 | 0.015 | 0.847 | −0.359 |
| Major life events → waist circumference | 1.483 | 0.463 | 4.073 | 0.097 | 0.814 |
| Trauma → HOMA-IR | 0.124 | 0.011 | −0.048 | 0.515 | −1.943* |
| Trauma → waist circumference | 4.057 | 0.012 | −0.664 | 0.781 | −1.638 |
| Recent life events → HOMA-IR | −0.016 | 0.759 | 0.032 | 0.608 | 0.591 |
| Recent life events → Waist circumference | −1.864 | 0.269 | 0.885 | 0.661 | 1.046 |
| Chronic stress → HOMA-IR | −0.002 | 0.610 | 0.004 | 0.252 | 1.182 |
| Chronic stress → Waist circumference | 0.007 | 0.945 | 0.171 | 0.092 | 1.160 |
| Perceived stress → HOMA-IR | −0.001 | 0.650 | 0.001 | 0.703 | 0.575 |
| Perceived stress → waist circumference | −0.042 | 0.455 | −0.116 | 0.124 | −0.797 |
| Morning cortisol → HOMA-IR | −0.212 | 0.019 | −0.026 | 0.737 | 1.577 |
| Cortisol → Waist circumference | −10.126 | 0.000 | −2.923 | 0.234 | 1.862* |
The following variables are log transformed: major life events, trauma, recent life events, morning cortisol, emotional eating, binge eating, and HOMA-IR
Tests showing a significant difference between genders at the 0.10 level are shown in bold
*** p value <0.01; ** p value <0.05; * p value <0.10
Age
We did not find support for evidence of moderated mediation (i.e., conditional indirect effects) at either the first or second stage in the model. There were main effects of age on WC (B = 0.11 SE = 0.04, p = 0.008). There was an interaction effect between age and perceived stress on the relationship with HOMA-IR (B = −0.022, SE = 0.009, p = 0.018). All other interaction terms were not statistically significant (p > 0.05, two-tailed; p values ranged from 0.103 to 0.987).
Discussion
Our study assesses the relationships among self-reported stress, morning cortisol, binge eating, emotional eating, HOMA-IR, and WC in a sample of adults recruited from the community. Several key contributions emerge from our results. Our first hypothesis, that self-reports of stress and morning cortisol are related to metabolic abnormalities, was partially supported. Similar to previous studies, we found significant associations between increased major life events, trauma and increased HOMA-IR and WC (Heppner et al., 2009; Lee et al., 2014). Chronic stress was associated with increased WC. We also found that lower morning cortisol was correlated with higher HOMA-IR and WC. These results corroborate the association between lower morning cortisol and metabolic disturbances, which may be indicative of impaired cortisol regulation (Bruehl et al., 2009; Lasikiewicz et al., 2008). Contrary to our hypothesis, we did not find evidence that binge eating or emotional eating statistically mediated the relationship between any of the types of stress and HOMA-IR or WC.
Our results support the importance of perceived stress as a correlate of binge eating and emotional eating (Greeno & Wing, 1994; Groesz et al., 2012; Polivy & Herman, 1993), and our results extend this literature by demonstrating a gender moderation effect. Similar to our results, researchers have found associations between perceived stress and binge and emotional eating in samples with only women (Tomiyama et al., 2011). Few studies have examined correlates of eating disorder pathology in samples that include men. However, researchers have found no significant difference in perceived stress between males who did and did not binge eat (Rosenberger & Dorflinger, 2013). Additionally, our results are congruent with a prior cross-sectional study demonstrating that there is a gender moderation effect in the relationship between perceived stress and emotional eating among middle school students (Nguyen-Rodriguez et al., 2009). Our results also support results from a large, cross-sectional study of adults who were participating in a health risk self-assessment screening. One item from the Perceived Stress Scale was used to assess perceived stress and this study demonstrated a similar moderation effect as our results, with women having a higher association between stress and binge eating (Striegel et al., 2012). The results presented add to the growing body of literature on stress and eating phenotypes through use of the complete Perceived Stress Scale and also demonstrate agender moderation effect for emotional eating.
Numerous measures have been used to measure and quantify stress throughout the literature, with no clear gold standard. In this study we incorporate environmental, psychological, and biological perspectives related to stress measurement (Cohen et al., 1997). While we found a significant relationship between binge and emotional eating and perceived stress as assessed by the Perceived Stress Scale (Cohen et al., 1983), we did not find consistent relationships with major life events, trauma, recent life events, and chronic stress as assessed by the Cumulative Adversity Interview (Turner & Wheaton, 1995). The Perceived Stress Scale is a global measure that assesses the degree to which one appraises situations in one’s life as stressful, unpredictable, overloading, and uncontrollable (Cohen et al., 1983). This originates from a more psychological perspective (i.e., focusing on an individuals’ subjective abilities to cope with stress) (Cohen, 2000; Cohen et al., 1997). On the other hand, the Cumulative Adversity Interview asks more specific and event-specific questions about stress exposure in terms of interpersonal, social and financial issues, work and home environment and relationships with family and significant others. This originates from the environmental perspective, which focuses on environmental events and experiences, which objectively are related with substantial adaptive burdens (Cohen, 2000). Further exploration of the convergent and discriminant validity of these two measures, particularly related to the concept of chronic stress, is warranted.
Lower morning cortisol was associated with higher HOMA-IR and WC, especially in men. This is congruent with prior studies suggesting that men cope with stress differently than females (Ljung et al., 2000). These results also may signal a perturbed function of the hypothalamic– pituitary–adrenal axis due to “burn-out” (Björntorp, 1999) or “allostatic overload” (McEwen, 2005). Though we did not include other measures of allostasis, there is suggestion that with allostatic overload there are disturbances in other systems (e.g., gonadal, sympathetic or autonomic nervous systems). Thus, the associations with HOMA-IR and abdominal obesity may be a result of perturbations in those systems given that morning cortisol was low (Björntorp & Rosmond, 2000).
There have been few studies on the relationships between biological markers of stress in binge and emotional eaters, and results have been mixed (Lo Sauro et al., 2008). In this study, we found no statistically significant relationship between morning cortisol levels and emotional and binge eating. Another type of cortisol response that has been frequently examined is cortisol reactivity; however, there is no consensus regarding the relationships. In studies examining cortisol reactivity, some have demonstrated increased cortisol reactivity (Gluck et al., 2004; Rosenberger & Dorflinger, 2013), others have demonstrated a blunted cortisol response (Rosenberg et al., 2013), while others have demonstrated no difference (Schulz et al., 2011) based on binge eating. There is a paucity of studies examining the relationships between biological markers of stress and emotional eating. The studies that have been conducted have been mainly with undergraduate, female-only populations. Of the studies that have examined cortisol and emotional eating, most have looked at cortisol reactivity and found no difference in cortisol reactivity between emotional and non-emotional eaters (Raspopow et al., 2014; van Strien et al., 2013). Some of these discrepancies in the literature are likely due to variable measures and cortisol reactivity paradigms such as the dexamethasone suppressor test, Trier Social Stress Test (Kirschbaum et al., 1993) and further replication with larger samples is necessary. Additionally, the physiological mechanisms related to stress are complex and imaging techniques have demonstrated that a complex integration of brain networks influence feeding behavior (Dallman, 2010). It is possible that other physiological stress pathways play a role related to obesity-related eating phenotypes, and exploration of the relationships between other stress biomarkers is needed. For example, a study examining aspects of sympathetic nervous system functioning demonstrated that among obese women with binge eating disorder, stress-induced changes in hunger were associated with greater stress-induced changes in systolic and diastolic blood pressure (r = 0.76, 0.78, respectively). These relationships were not seen in obese or normal weight women without binge eating disorder (Klatzkin et al., 2015). Additionally, the influence of stress on other areas in the brain such as the amygdala and hippocampus, which are involved in learning and memory, may play an important role in these relationships (Dallman, 2010).
Similar to previous literature demonstrating a relationship between binge eating and increased risks of metabolic abnormalities (Roehrig et al., 2009), our findings support that individuals who binge eat have an increased WC. Yet, the relationship between binge eating and HOMA-IR was significant in females only. This may be related to the types of food consumed during binges as well as the rapid speed that is common during a binge. Females tend to favor high carbohydrate/high fat foods whereas males tend to prefer high protein/fat foods (Drewnowski et al., 1992). Thus, the types of foods consumed and resultant effects on physiology may account for this moderation effect.
Among phenomena that may explain the relationship between stress and metabolic abnormalities, hedonic eating (i.e., eating for pleasure and not due to energy deprivation) is a prime candidate. Nevertheless, our hypothesis that two types of hedonic eating, binge eating and emotional eating, would mediate the relationship between stress and HOMA-IR and WC was not supported. It is possible that there is a relationship, though it may be weak and thus require a larger sample size to detect. Additionally, these effects may be restricted to specific subgroups. For example, while we did not find any moderation effect of age, the mean age of our population was fairly young at 28.3 years. Since the risk of diabetes increases with age, a longitudinal approach or a sample of older participants may be necessary to see these relationships. Also, we included individuals across the weight spectrum which may have attenuated significant results. Future studies may benefit from directly studying these relationships among individuals who are overweight or obese. Furthermore, a combination of multiple mediators—such as physical activity and sedentary behavior— acting jointly may be involved.
Lastly, researchers have been exploring different types of psychologically-motivated and hedonic eating types including binge eating, emotional eating, and stress-induced eating (Lowe & Butryn, 2007). Future research is needed to examine the potential physiological and behavioral mechanisms and conceptual overlaps between different types of eating phenotypes (e.g., binge eating, emotional eating, stress-induced eating). It is possible that only a subset of individuals who are binge or emotional eaters would also be classified as “stress-eaters”. Further research is needed to examine whether subtyping binge eating based on stress or physiological stress responses would aid with phenomenology, etiology, outcomes, course and treatment. For example, researchers have used the opioidergic antagonist, naltrexone, to examine changes in hedonic eating following a mindfulness-based weight loss intervention. Participants who reported naltrexone-induced nausea and who were randomized to a mindfulness group had greater improvements in hedonic eating following the mindfulness intervention compared to individuals randomized to the control group (Mason et al., 2015). This finding was also observed in another sample of obese women (Daubenmier et al., 2014).
Limitations
This study is not without its limitations. First, the data are cross-sectional and non-experimental, thus this study does not lead to casual inferences. Second, there are limitations resulting from the sampling methods used in the IRCSSA study. The sample is a convenience sample and individuals with medical conditions or taking medications that may disrupt cortisol levels were excluded. This was done to control the homogeneity of the sample; however, decreases external generalizability. Third, morning cortisol was used in this study and is not representative of the daily fluctuations of cortisol, the cortisol awakening response or of cortisol reactivity; however, these may be important potential areas for further exploration. Fourth, due to multicollinearity, BMI was not included in this model. Thus the independent effects on WC and HOMA-IR beyond the effect on BMI are not known. Despite these limitations, the results from this study provide the foreground for additional longitudinal and experimental studies exploring these variables.
In conclusion, we did not find evidence that binge eating and emotional eating statistically mediate the relationship between stress and the metabolic abnormalities of insulin resistance and abdominal obesity. We found significant negative relationships between stress and morning cortisol levels, and cortisol and HOMA-IR and WC. Our results support previous research demonstrating gender moderation effects among the relationships of perceived stress, and binge and emotional eating and the relationship with binge eating and increased HOMA-IR. These findings suggest that interventions that target perceived stress targeted may improve binge and emotional eating among females.
Acknowledgments
We would like to thank all of our study participants and our funding sources. The National Institute on Drug Abuse/National Institute of Health (NIH) grants PL1-DA024859 and UL1-DE019859 funded this study. AC was funded by pre-doctoral fellowships from the Jonas Center for Nursing Excellence and the National Institute of Nursing Research/NIH (F31-NR014375; T32-NR008346). CMG was funded, in part, by the National Institute of Diabetes and Digestive and Kidney Disease/NIH (K24-DK070052). These funding sources did not participate in designing the study, collecting data, analyzing and interpreting data, writing this report, or submitting the article for publication.
Footnotes
Author contributions Study concept and design: AC, MG, RW, JRS, CMG, RS. Acquisition and collection of data: RS. Analysis of data: AC. Obtained funding for study: AC, RS. Administrative, technical, and material support: RS. All authors were involved in writing and revising the paper, and provided final approval of the manuscript.
Compliance with ethical standards
Conflict of interest Ariana Chao, Margaret Grey, Robin Whittemore, Jonathan Reuning-Scherer, Carlos M. Grilo and Rajita Sinha declare that they have no conflict of interest.
Human and animal rights and Informed consent All procedures followed were in accordance with ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
References
- Abraham TM, Massaro JM, Hoffmann U, Yanovski JA, Fox CS. Metabolic characterization of adults with binge eating in the general population: The Framingham Heart Study. Obesity. 2014;22:2441–2449. doi: 10.1002/oby.20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. The diagnostic and statistical manual of mental disorders: DSM 5. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Arbuckle JL. IBM SPSS Amos 22 user’s guide. Chicago, IL: SPSS; 2013. [Google Scholar]
- Arnow B, Kenardy J, Agras WS. The Emotional Eating Scale: The development of a measure to assess coping with negative affect by eating. International Journal of Eating Disorders. 1995;18:79–90. doi: 10.1002/1098-108x(199507)18:1<79::aid-eat2260180109>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Björntorp P. Neuroendocrine perturbations as a cause of insulin resistance. Diabetes/Metabolism Research and Reviews. 1999;15:427–441. doi: 10.1002/(sici)1520-7560(199911/12)15:6<427::aid-dmrr68>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Björntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000;16:924–936. doi: 10.1016/s0899-9007(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Blomquist KK, Milsom VA, Barnes RD, Boeka AG, White MA, Masheb RM, Grilo CM. Metabolic syndrome in obese men and women with binge eating disorder: Developmental trajectories of eating and weight-related behaviors. Comprehensive Psychiatry. 2012;53:1021–1027. doi: 10.1016/j.comppsych.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen KA. Structural equation models. New York, NY: Wiley Online Library; 1998. [Google Scholar]
- Browne MW, Cudeck R, Bollen KA. Alternative ways of assessing model fit. Sage Focus Editions. 1993;154:136. [Google Scholar]
- Bruehl H, Wolf OT, Convit A. A blunted cortisol awakening response and hippocampal atrophy in type 2 diabetes mellitus. Psychoneuroendocrinology. 2009;34:815–821. doi: 10.1016/j.psyneuen.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio AA, Wilfley DE, Crow SJ, Mitchell J, Walsh BT. A comparison of the binge eating scale, questionnaire for eating and weight patterns-revised, and eating disorder examination questionnaire with instructions with the eating disorder examination in the assessment of binge eating disorder and its symptoms. International Journal of Eating Disorders. 2004;36:434–444. doi: 10.1002/eat.20057. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cohen S. Measures of psychological stress. 2000 Retrieved from http://www.macses.ucsf.edu/research/psychosocial/stress.php.
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Cohen S, Kessler RC, Gordon LU. Measuring stress: A guide for health and social scientists. Oxford: Oxford University Press; 1997. [Google Scholar]
- Coutinho WF, Moreira RO, Spagnol C, Appolinario JC. Does binge eating disorder alter cortisol secretion in obese women? Eating Behaviors. 2007;8:59–64. doi: 10.1016/j.eatbeh.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Stress-induced obesity and the emotional nervous system. Trends in Endocrinology and Metabolism. 2010;21:159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R, De Stavola B, Cousens S, Vansteelandt S. Causal mediation analysis with multiple mediators. Biometrics. 2015;71:1–14. doi: 10.1111/biom.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Lustig RH, Hecht FM, Kristeller J, Woolley J, Adam T, et al. A new biomarker of hedonic eating? A preliminary investigation of cortisol and nausea responses to acute opiod blockade. Appetite. 2014;74:92–100. doi: 10.1016/j.appet.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend BP. Inventorying stressful life events as risk factors for psychopathology: Toward resolution of the problem of intracategory variability. Psychological Bulletin. 2006;132:477. doi: 10.1037/0033-2909.132.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A, Kurth C, Holden-Wiltse J, Saari J. Food preferences in human obesity: Carbohydrates versus fats. Appetite. 1992;18:207–221. doi: 10.1016/0195-6663(92)90198-f. [DOI] [PubMed] [Google Scholar]
- Duong M, Cohen JI, Convit A. High cortisol levels are associated with low quality food choice in type 2 diabetes. Endocrine. 2012;41:76–81. doi: 10.1007/s12020-011-9527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? International Journal of Eating Disorders. 1994;16:363–370. [PubMed] [Google Scholar]
- Ford ES, Maynard LM, Li C. Trends in mean waist circumference and abdominal obesity among US adults, 1999–2012. JAMA. 2014;312:1151–1153. doi: 10.1001/jama.2014.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani C, Schneider H. Prevention and treatment of obesity with lifestyle interventions: Review and meta-analysis. International Journal of Public Health. 2007;52:348–359. doi: 10.1007/s00038-007-7015-8. [DOI] [PubMed] [Google Scholar]
- Garcia-Estevez D, Araujo-Vilar D, Fiestras-Janeiro G, Saavedra-Gonzalez A, Cabezas-Cerrato J. Comparison of several insulin sensitivity indices derived from basal plasma insulin and glucose levels with minimal model indices. Hormone and Metabolic Research. 2003;35:13–17. doi: 10.1055/s-2003-38385. [DOI] [PubMed] [Google Scholar]
- Geliebter A, Aversa A. Emotional eating in overweight, normal weight, and underweight individuals. Eating Behaviors. 2003;3:341–347. doi: 10.1016/s1471-0153(02)00100-9. [DOI] [PubMed] [Google Scholar]
- Gluck ME, Geliebter A, Hung J, Yahav E. Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosomatic Medicine. 2004;66:876–881. doi: 10.1097/01.psy.0000143637.63508.47. [DOI] [PubMed] [Google Scholar]
- Goldfein JA, Devlin MJ, Kamenetz C. Eating Disorder Examination-Questionnaire with and without instruction to assess binge eating in patients with binge eating disorder. International Journal of Eating Disorders. 2005;37:107–111. doi: 10.1002/eat.20075. [DOI] [PubMed] [Google Scholar]
- Greeno CG, Wing RR. Stress-induced eating. Psychological Bulletin. 1994;115:444. doi: 10.1037/0033-2909.115.3.444. [DOI] [PubMed] [Google Scholar]
- Groesz LM, McCoy S, Carl J, Saslow L, Stewart J, Adler N, Epel E. What is eating you→ Stress and the drive to eat. Appetite. 2012;58:717–721. doi: 10.1016/j.appet.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayduk LA. Structural equation modeling with LISREL: Essentials and advances. Baltimore, MD: JHU Press; 1988. [Google Scholar]
- Heppner PS, Crawford EF, Haji UA, Afari N, Hauger RL, Dashevsky BA, Baker DG. The association of posttraumatic stress disorder and metabolic syndrome: A study of increased health risk in veterans. BMC Medicine. 2009;7:1. doi: 10.1186/1741-7015-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelter JW. The analysis of covariance structures. Sociological Methods & Research. 1938;11(3):325–344. [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999;6:1–55. [Google Scholar]
- Hucklebridge F, Hussain T, Evans P, Clow A. The diurnal patterns of the adrenal steroids cortisol and dehydroepiandrosterone (DHEA) in relation to awakening. Psychoneuroendocrinology. 2005;30:51–57. doi: 10.1016/j.psyneuen.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Lalonde JK, Coit CE, Tsuang MT, McElroy SL, Crow SJ, Rosenthal NR. Longitudinal study of the diagnosis of components of the metabolic syndrome in individuals with binge-eating disorder. The American Journal of Clinical Nutrition. 2010;91:1568–1573. doi: 10.3945/ajcn.2010.29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Suehiro T, Nakamura T, Kumon Y, Hashimoto K. Clinical significance of the insulin resistance index as assessed by homeostasis model assessment. Endocrine Journal. 2001;48:81–86. doi: 10.1507/endocrj.48.81. [DOI] [PubMed] [Google Scholar]
- Ioannou GN, Bryson CL, Boyko EJ. Prevalence and trends of insulin resistance, impaired fasting glucose, and diabetes. Journal of Diabetes and Its Complications. 2007;21:363–370. doi: 10.1016/j.jdiacomp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: Evidence in support of current National Institutes of Health guidelines. Archives of Internal Medicine. 2002;162:2074–2079. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. The American Journal of Clinical Nutrition. 2004;79:379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Adrienne Cupples L, Ramaswami R, Stokes J, III, Kreger BE, Higgins M. Regional obesity and risk of cardiovascular disease; The Framingham Study. Journal of Clinical Epidemiology. 1991;44:183–190. doi: 10.1016/0895-4356(91)90265-b. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Gaffney S, Cyrus K, Bigus E, Brownley KA. Binge eating disorder and obesity: Preliminary evidence for distinct cardiovascular and psychological phenotypes. Physiology & Behavior. 2015;142:20–27. doi: 10.1016/j.physbeh.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. New York, NY: Guilford Press; 2011. [Google Scholar]
- Kudielka BM, Wüst S. Human models in acute and chronic stress: Assessing determinants of individual hypothala-mus-pituitary-adrenal axis activity and reactivity. Stress: The International Journal on the Biology of Stress. 2009;13:1–14. doi: 10.3109/10253890902874913. [DOI] [PubMed] [Google Scholar]
- Lasikiewicz N, Hendrickx H, Talbot D, Dye L. Exploration of basal diurnal salivary cortisol profiles in middle-aged adults: Associations with sleep quality and metabolic parameters. Psychoneuroendocrinology. 2008;33:143–151. doi: 10.1016/j.psyneuen.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Psychological stress and the coping process. New York, NY: MGraw-Hill; 1966. [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York, NY: Springer; 1984. [Google Scholar]
- Lee C, Tsenkova V, Carr D. Childhood trauma and metabolic syndrome in men and women. Social Science and Medicine. 2014;105:122–130. doi: 10.1016/j.socscimed.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens VEPP, Oenema A, Klepp KI, Henriksen HB, Brug J. A systematic review of the evidence regarding efficacy of obesity prevention interventions among adults. Obesity Reviews. 2008;9:446–455. doi: 10.1111/j.1467-789X.2008.00468.x. [DOI] [PubMed] [Google Scholar]
- Linderman M, Stark K. Emotional eating and eating disorder psychopathology. Eating Disorders. 2001;9:251–259. doi: 10.1080/10640260127552. [DOI] [PubMed] [Google Scholar]
- Ljung T, Holm G, Friberg P, Andersson B, Bengtsson BÅ, Svensson J, Björntorp P. The activity of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system in relation to waist/hip circumference ratio in men. Obesity Research. 2000;8:487–495. doi: 10.1038/oby.2000.61. [DOI] [PubMed] [Google Scholar]
- Lo Sauro C, Ravaldi C, Cabras PL, Faravelli C, Ricca V. Stress, hypothalamic–pituitary–adrenal axis and eating disorders. Neuropsychobiology. 2008;57:95–115. doi: 10.1159/000138912. [DOI] [PubMed] [Google Scholar]
- Lowe MR, Butryn ML. Hedonic hunger: A new dimension of appetite? Physiology & Behavior. 2007;91:432–439. doi: 10.1016/j.physbeh.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Macht M. How emotions affect eating: A five-way model. Appetite. 2008;50:1–11. doi: 10.1016/j.appet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Bergquist TF, Ito V, Chew S. Relationship between stress, coping, and postconcussion symptoms in a healthy adult population. Archives of Clinical Neuropsychology. 1998;13:415–424. [PubMed] [Google Scholar]
- Masheb RM, Grilo CM. Emotional overeating and its associations with eating disorder psychopathology among overweight patients with binge eating disorder. International Journal of Eating Disorders. 2006;39:141–146. doi: 10.1002/eat.20221. [DOI] [PubMed] [Google Scholar]
- Mason AE, Lustig RH, Brown RR, Acree M, Bacchetti P, Moran PJ, et al. Acute responses to opioidergic blockade as a biomarker of hedonic eating among obese women enrolled in a mindfulness-based weight loss intervention. Appetite. 2015;91:311–320. doi: 10.1016/j.appet.2015.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stressed or stressed out: What is the difference? Journal of Psychiatry and Neuroscience. 2005;30:315. [PMC free article] [PubMed] [Google Scholar]
- Mitchison D, Hay P, Slewa-Younan S, Mond J. The changing demographic profile of eating disorder behaviors in the community. BMC Public Health. 2014;14:943. doi: 10.1186/1471-2458-14-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond JM, Hay PJ, Rodgers B, Owen C, Beumont P. Validity of the Eating Disorder Examination Questionnaire (EDE-Q) in screening for eating disorders in community samples. Behaviour Research and Therapy. 2004;42:551–567. doi: 10.1016/S0005-7967(03)00161-X. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Davidson MB, Defronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B. Impaired fasting glucose and impaired glucose tolerance implications for care. Diabetes Care. 2007;30:753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- Nguyen-Rodriguez ST, Chou CP, Unger JB, Spruijt-Metz D. BMI as a moderator of perceived stress and emotional eating in adolescents. Eating Behaviors. 2008;9:238–246. doi: 10.1016/j.eatbeh.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Rodriguez ST, Unger JB, Spruijt-Metz D. Psychological determinants of emotional eating in adolescence. Eating Disorders. 2009;17:211–224. doi: 10.1080/10640260902848543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHLBI Obesity Education Initiative Expert Panel. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Obesity Research. 1998;6(suppl 2):51–209S. [PubMed] [Google Scholar]
- Nicolson NA. Measurement of cortisol. In: Luecken LJ, Gallo LC, editors. Handbook of physiological research methods in health psychology. Thousand Oaks, California: Sage Publications; 2008. pp. 37–74. [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacák K, Palkovits M. Stressor specificity of central neuroendocrine responses: Implications for stress-related disorders. Endocrine Reviews. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- Polivy J, Herman CP. Etiology of binge eating: Psychological mechanisms 1993 [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Räikkönen K, Keltikangas-Järvinen L, Adlercreutz H, Hautanen A. Psychosocial stress and the insulin resistance syndrome. Metabolism. 1996;45:1533–1538. doi: 10.1016/s0026-0495(96)90184-5. [DOI] [PubMed] [Google Scholar]
- Räikkönen K, Matthews KA, Kuller LH. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women a comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care. 2007;30:872–877. doi: 10.2337/dc06-1857. [DOI] [PubMed] [Google Scholar]
- Raspopow K, Abizaid A, Matheson K, Anisman H. Anticipation of a psychosocial stressor differentially influences ghrelin, cortisol and food intake among emotional and nonemotional eaters. Appetite. 2014;74:35–43. doi: 10.1016/j.appet.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Reas DL, Grilo CM, Masheb RM. Reliability of the Eating Disorder Examination-Questionnaire in patients with binge eating disorder. Behaviour Research and Therapy. 2006;44:43–51. doi: 10.1016/j.brat.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Manson JE. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- Ricca V, Castellini G, Lo Sauro C, Ravaldi C, Lapi F, Mannucci E, Faravelli C. Correlations between binge eating and emotional eating in a sample of overweight subjects. Appetite. 2009;53:418–421. doi: 10.1016/j.appet.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Roehrig M, Masheb RM, White MA, Grilo CM. The metabolic syndrome and behavioral correlates in obese patients with binge eating disorder. Obesity. 2009;17:481–486. doi: 10.1038/oby.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N, Bloch M, Ben Avi I, Rouach V, Schreiber S, Stern N, Greenman Y. Cortisol response and desire to binge following psychological stress: Comparison between obese subjects with and without binge eating disorder. Psychiatry Research. 2013;208:156–161. doi: 10.1016/j.psychres.2012.09.050. [DOI] [PubMed] [Google Scholar]
- Rosenberger PH, Dorflinger L. Psychosocial factors associated with binge eating among overweight and obese male veterans. Eating Behaviors. 2013;14:401–404. doi: 10.1016/j.eatbeh.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Santaguida PL, Balion C, Hunt D, Morrison K, Gerstein H, Raina P, et al. AHRQ Evidence Report Summaries. Rockville, MD: Agency for Healthcare Research and Quality; 2005. Diagnosis, prognosis, and treatment of impaired glucose tolerance and impaired fasting glucose; pp. 1–11. [PMC free article] [PubMed] [Google Scholar]
- Schulz S, Laessle R, Hellhammer D. No evidence of increased cortisol stress response in obese women with binge eating disorder. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity. 2011;16:209–211. doi: 10.1007/BF03325134. [DOI] [PubMed] [Google Scholar]
- Selye H. The stress of life. New York, NY: McGraw-Hill; 1956. [Google Scholar]
- Seo DC, Sa J. A meta-analysis of psycho-behavioral obesity interventions among US multiethnic and minority adults. Preventive Medicine. 2008;47:573–582. doi: 10.1016/j.ypmed.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Sinha R, Jastreboff AM. Stress as a common risk factor for obesity and addiction. Biological Psychiatry. 2013;73:827–835. doi: 10.1016/j.biopsych.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striegel RH, Bedrosian R, Wang C, Schwartz S. Why men should be included in research on binge eating. International Journal of Eating Disorders. 2012;45(2):233–240. doi: 10.1002/eat.20962. [DOI] [PubMed] [Google Scholar]
- Stults-Kolehmainen MA, Tuit K, Sinha R. Lower cumulative stress is associated with better health for physically active adults in the community. Stress. 2014;17:157–168. doi: 10.3109/10253890.2013.878329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama AJ, Dallman MF, Epel ES. Comfort food is comforting to those most stressed: evidence of the chronic stress response network in high stress women. Psychoneuroendocrinology. 2011;36:1513–1519. doi: 10.1016/j.psyneuen.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RJ, Wheaton B. (Measuring stress: A guide for health and social scientists).Checklist measurement of stressful life events. 1995:29–58. [Google Scholar]
- Turner RJ, Wheaton B, Lloyd DA. The epidemiology of social stress. American Sociological Review. 1995;60:104–125. [Google Scholar]
- Van Strien T, Frijters JE, Bergers G, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5:295–315. [Google Scholar]
- van Strien T, Roelofs K, de Weerth C. Cortisol reactivity and distress-induced emotional eating. Psychoneuroendocrinology. 2013;38:677–684. doi: 10.1016/j.psyneuen.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Varon J, Fromm RE. Endocrinology and metabolism facts and formulas. In: Varon J, Fromm RE, editors. Acute and critical care formulas and laboratory values. Berlin: Springer; 2014. pp. 25–31. [Google Scholar]
- Vicennati V, Pasqui F, Cavazza C, Garelli S, Casadio E, di Dalmazi G, Pasquali R. Cortisol, energy intake, and food frequency in overweight/obese women. Nutrition. 2011;27:677–680. doi: 10.1016/j.nut.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- Weinstein SE, Shide DJ, Rolls BJ. Changes in food intake in response to stress in men and women: Psychological factors. Appetite. 1997;28:7–18. doi: 10.1006/appe.1996.0056. [DOI] [PubMed] [Google Scholar]
- Wüst S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25:707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, Wolf A. Food selection changes under stress. Physiology & Behavior. 2006;87:789–793. doi: 10.1016/j.physbeh.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Zhu S, Heshka S, Wang Z, Shen W, Allison DB, Ross R, Heymsfield SB. Combination of BMI and waist circumference for identifying cardiovascular risk factors in whites. Obesity Research. 2004;12:633–645. doi: 10.1038/oby.2004.73. [DOI] [PubMed] [Google Scholar]


